Mechanistic Investigation of Machine-Made Sand Methylene Blue Value Effects on Mortar Performance
Abstract
1. Introduction
2. Test Progress
2.1. Mix Proportion Design
2.2. Experimental Setup and Measurements
3. Influence of MB Value on Macrostructural Properties of Mortar
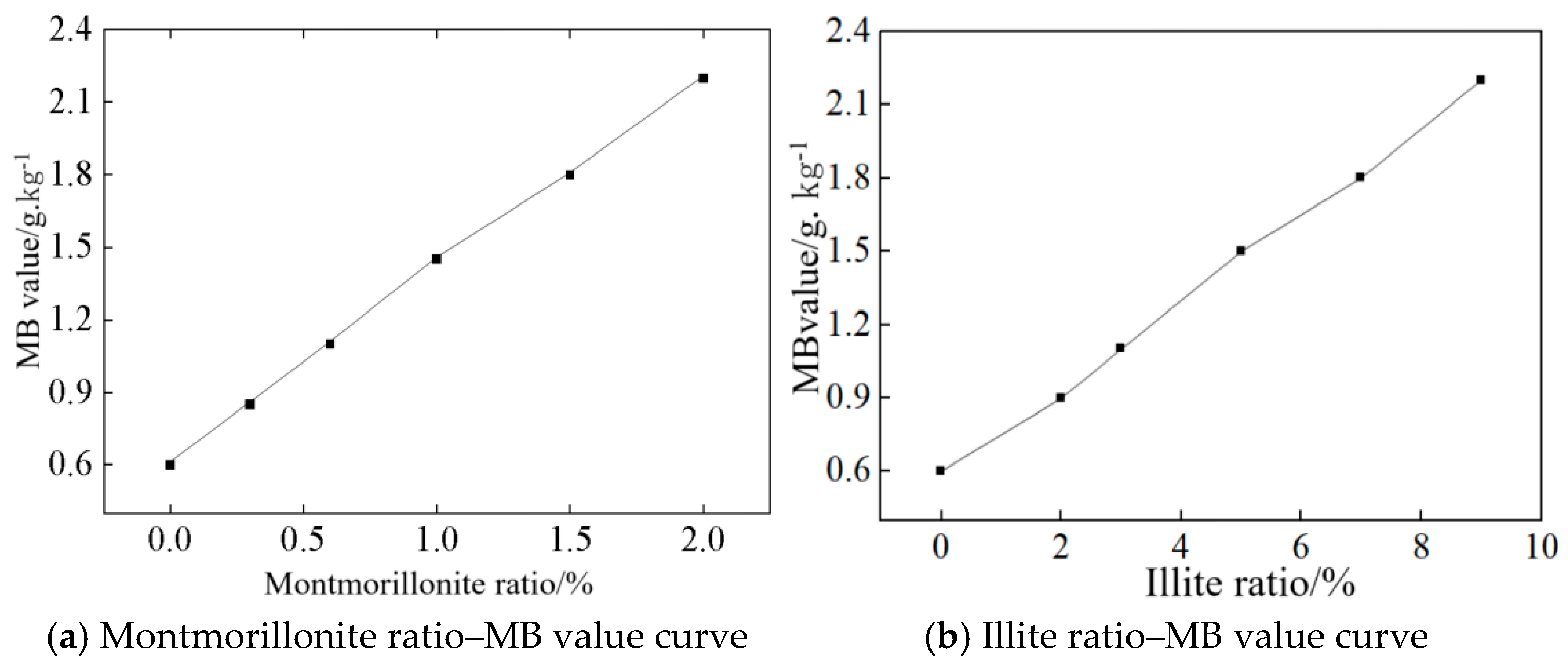
3.1. Effects of Clay Content Types and Concentrations on Mb Value
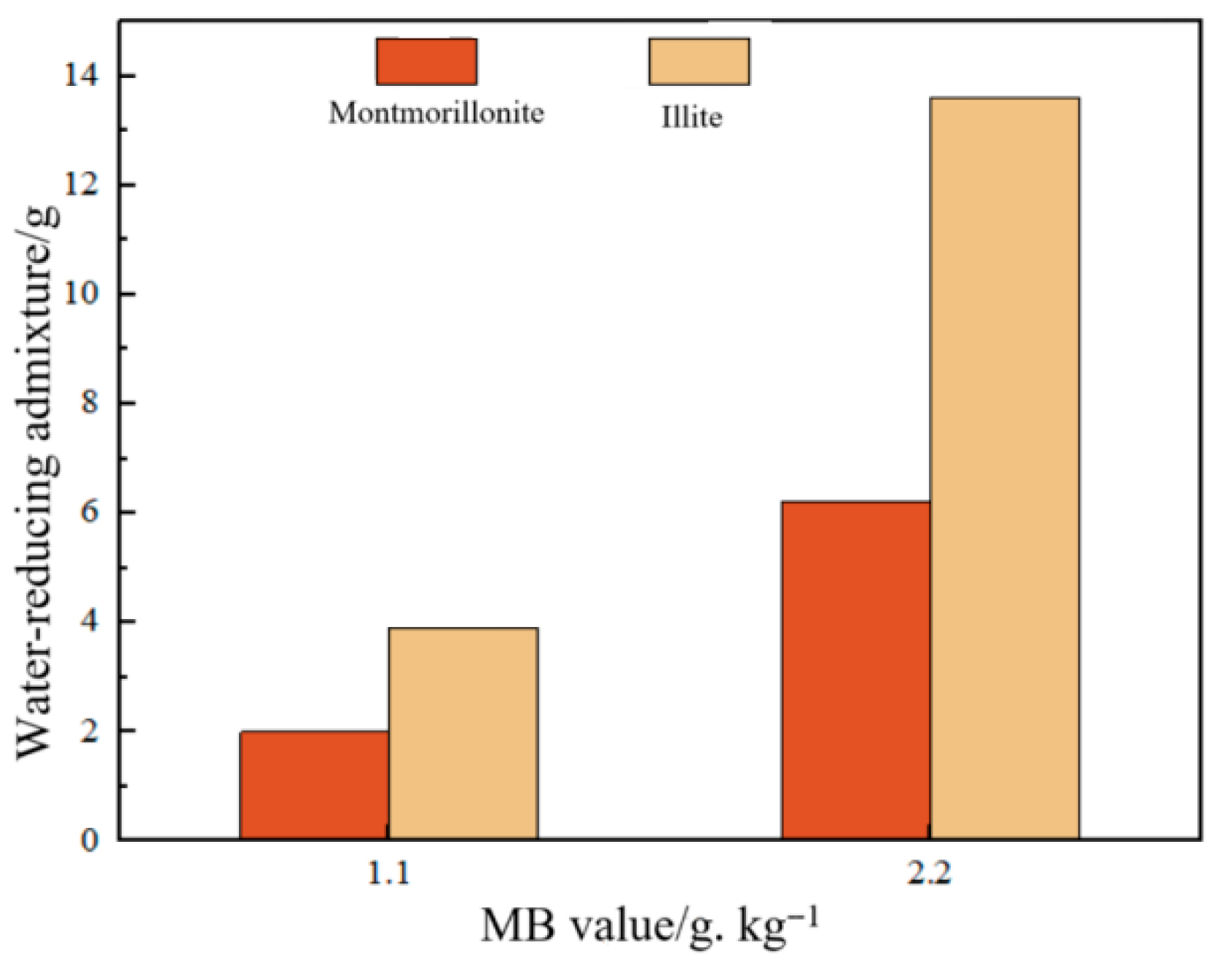
3.2. Effect of MB Value on Mortar Workability
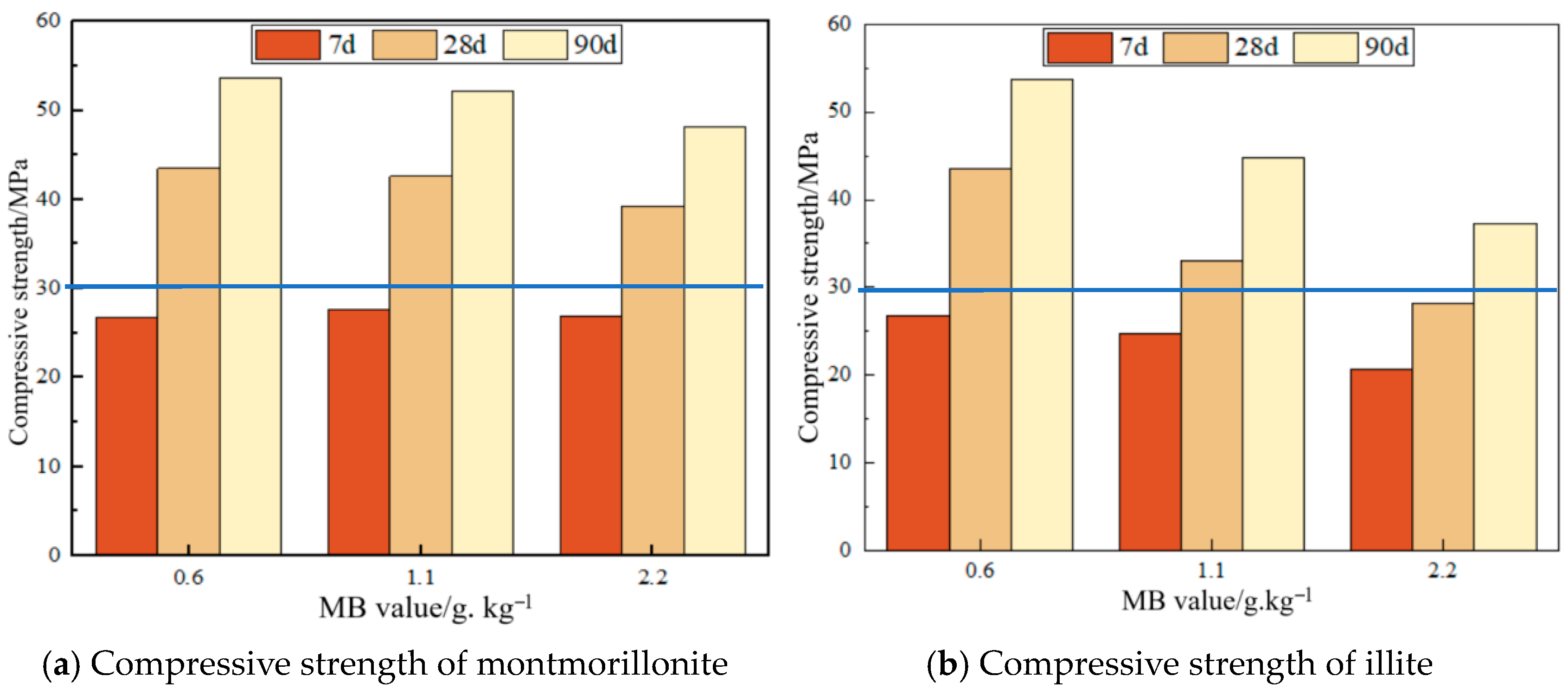
3.3. Effect of MB Value on Mortar Compressive Strength
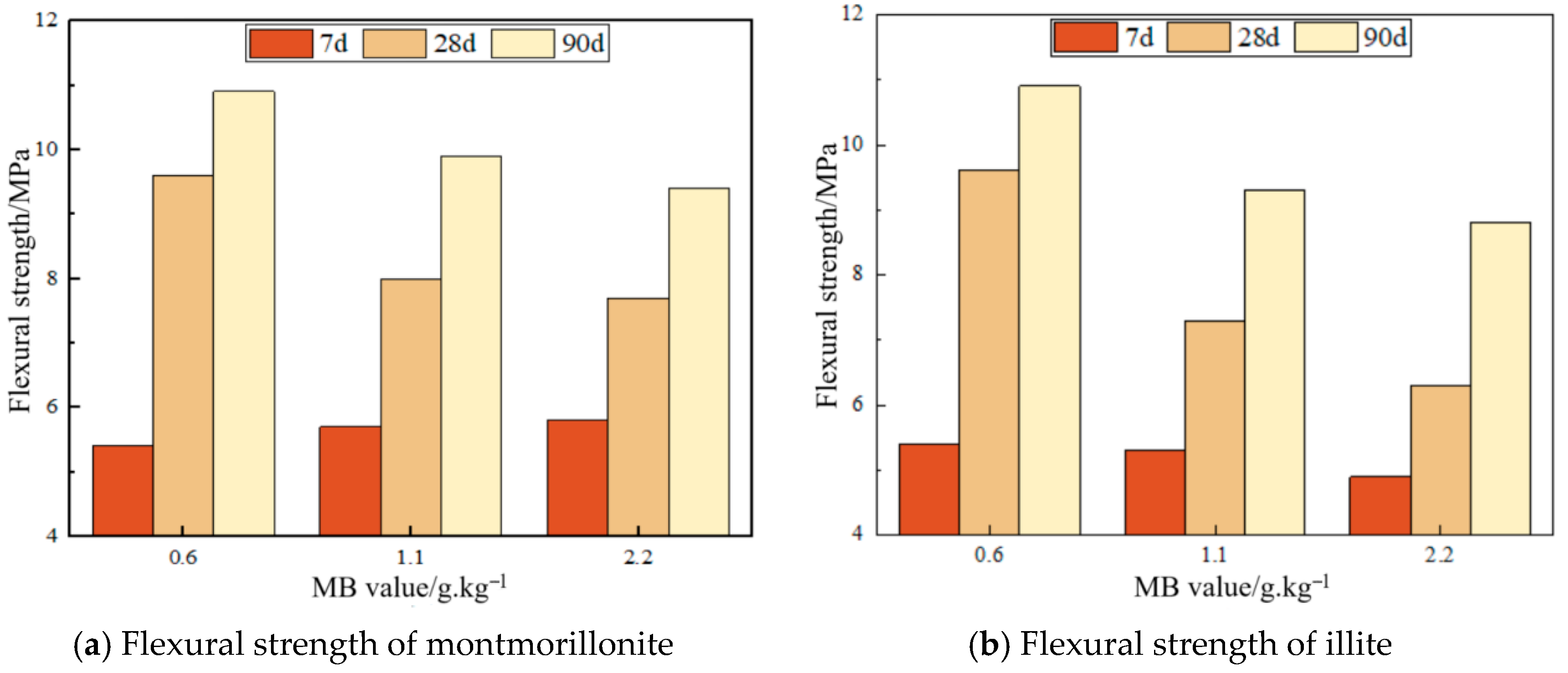
3.4. Effect of MB Value on Mortar Flexural Strength
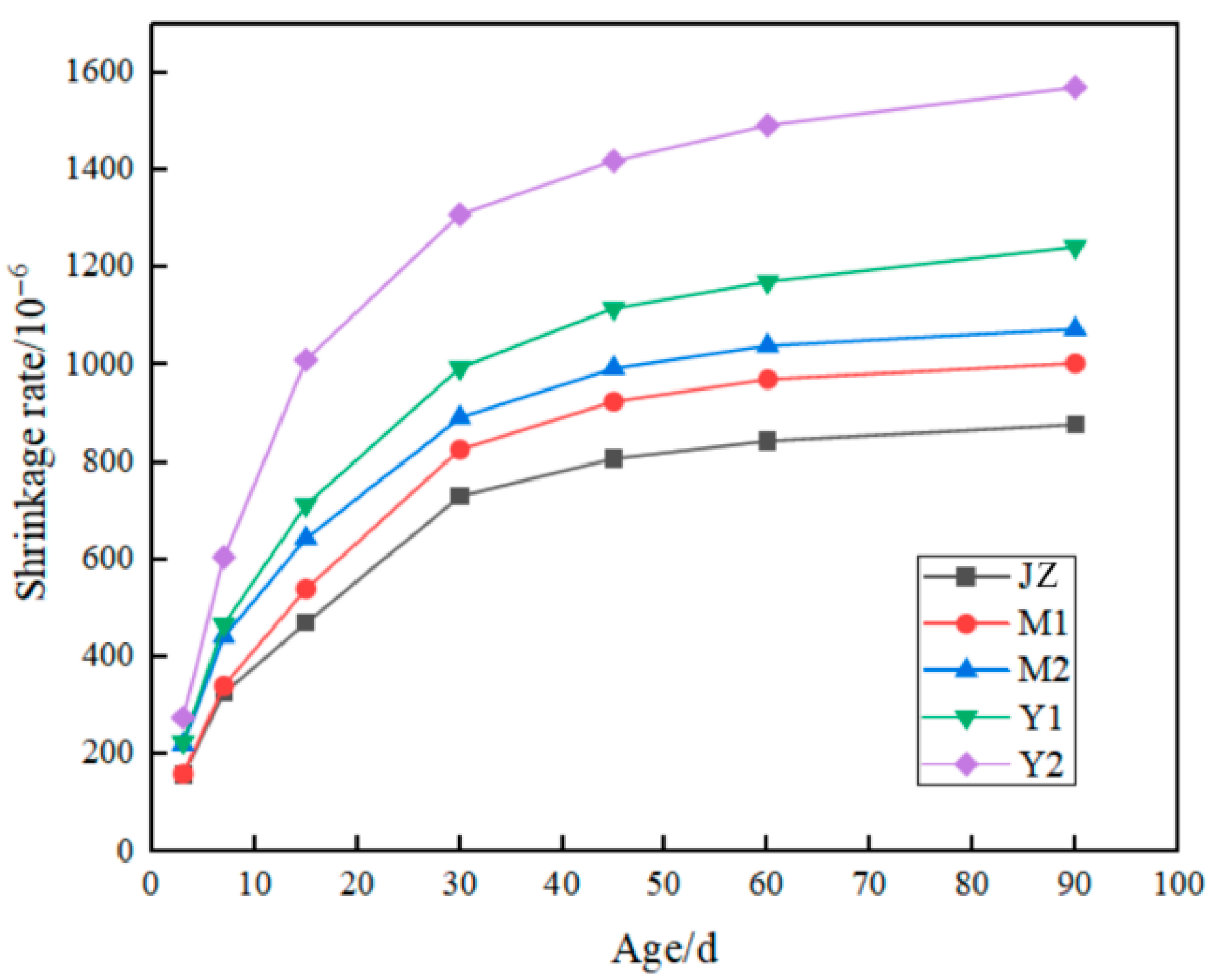
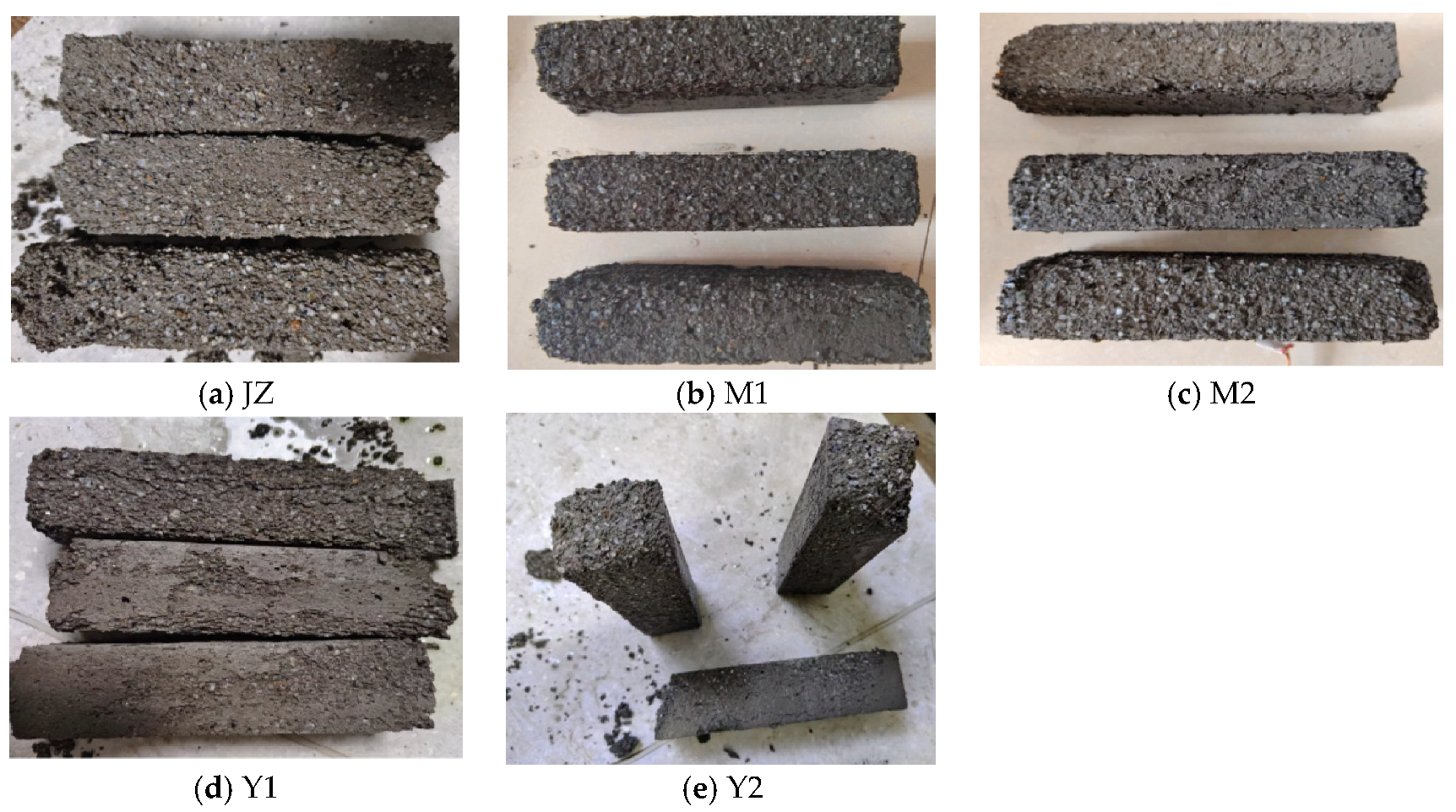
3.5. Effect of MB Value on Mortar Drying Shrinkage
3.6. Effect of MB Value on Mortar Frost Resistance
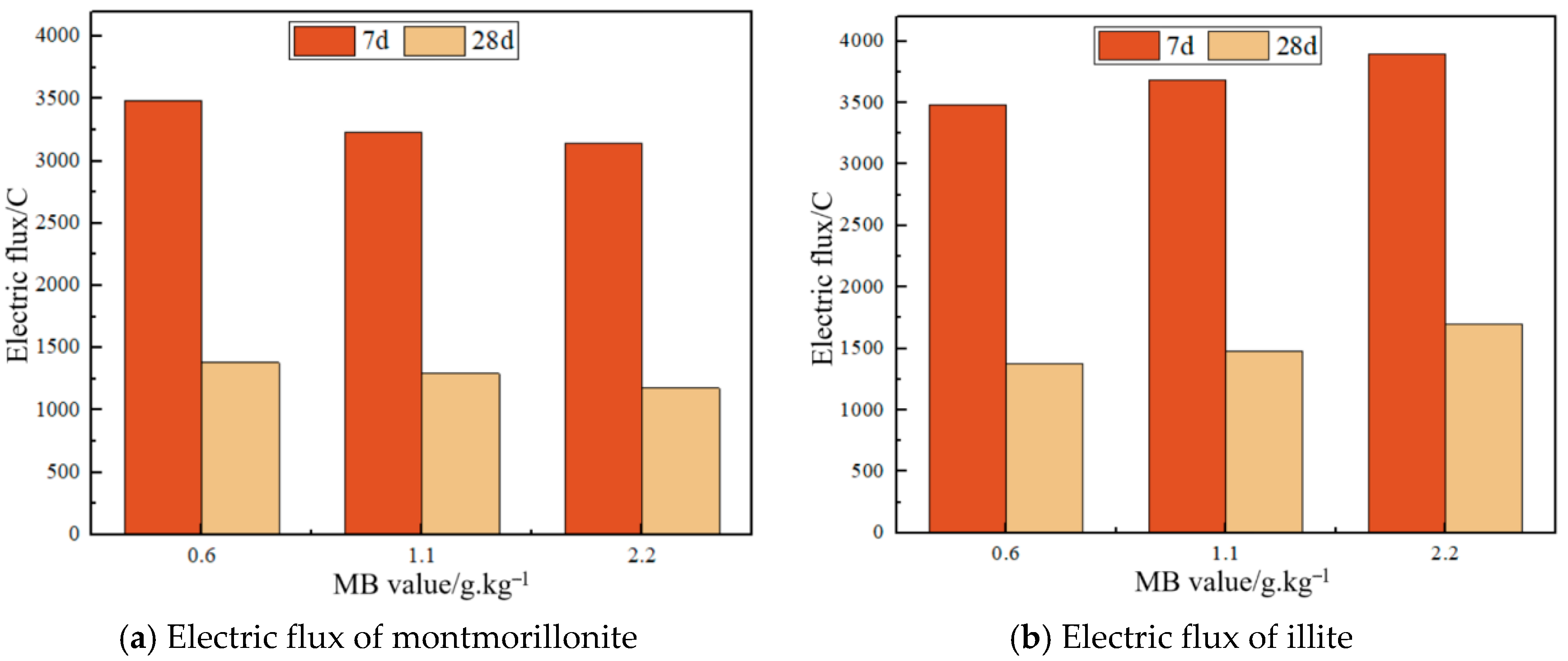
3.7. Effect of MB Value on Mortar Impermeability
4. Effect of MB Value on Mortar Microstructure
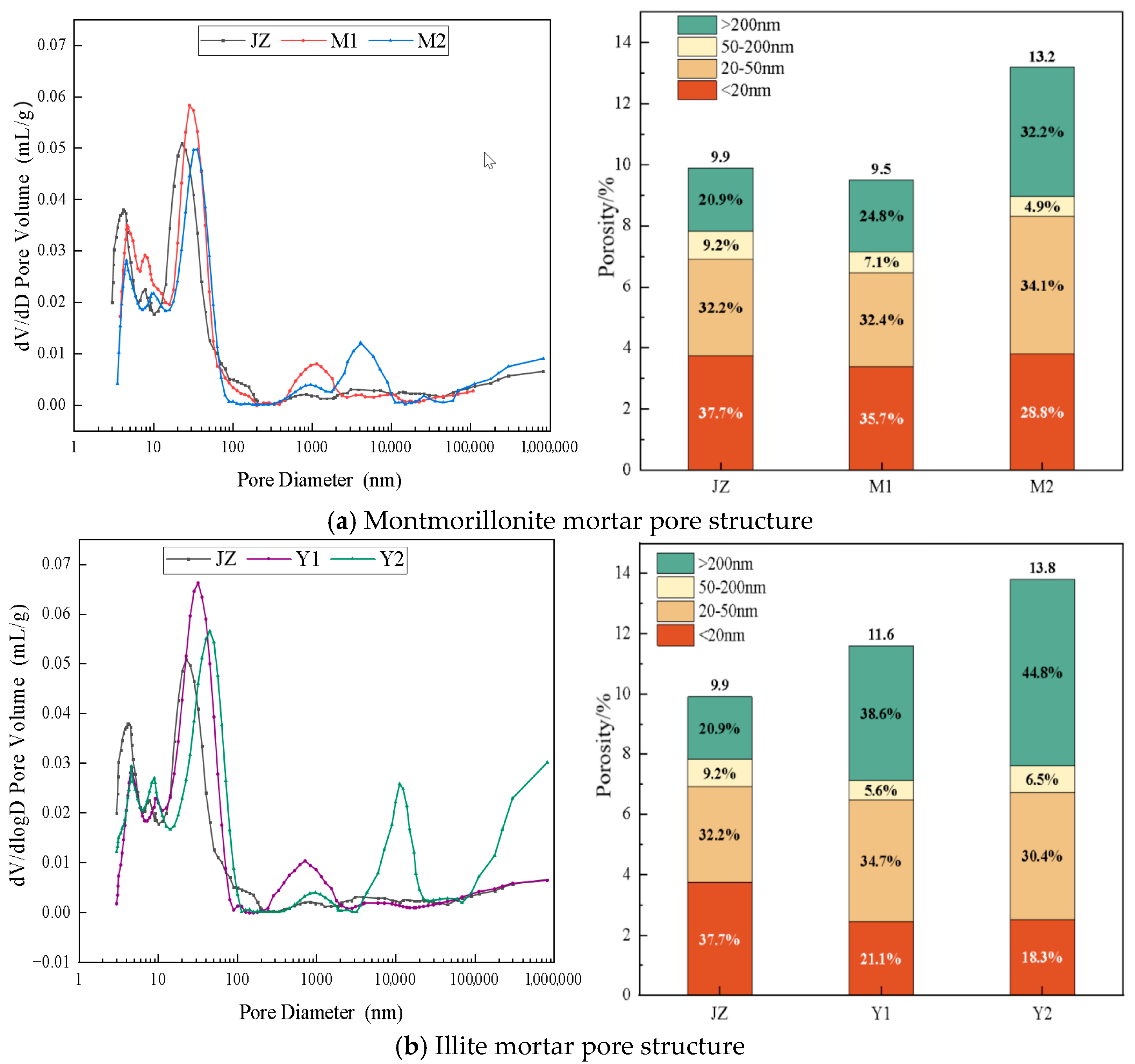
4.1. Influence of MB Value on Mortar Pore Structure
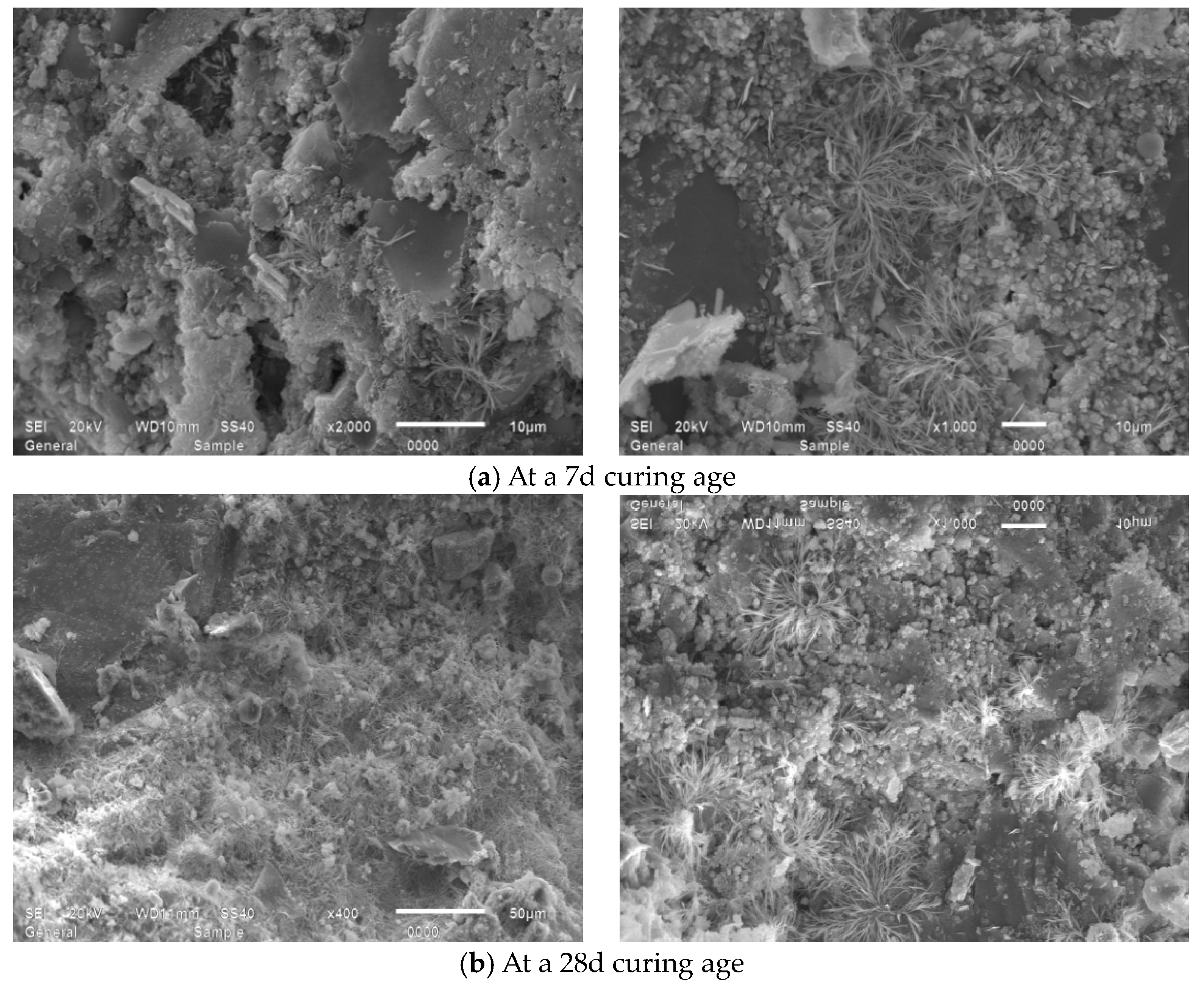
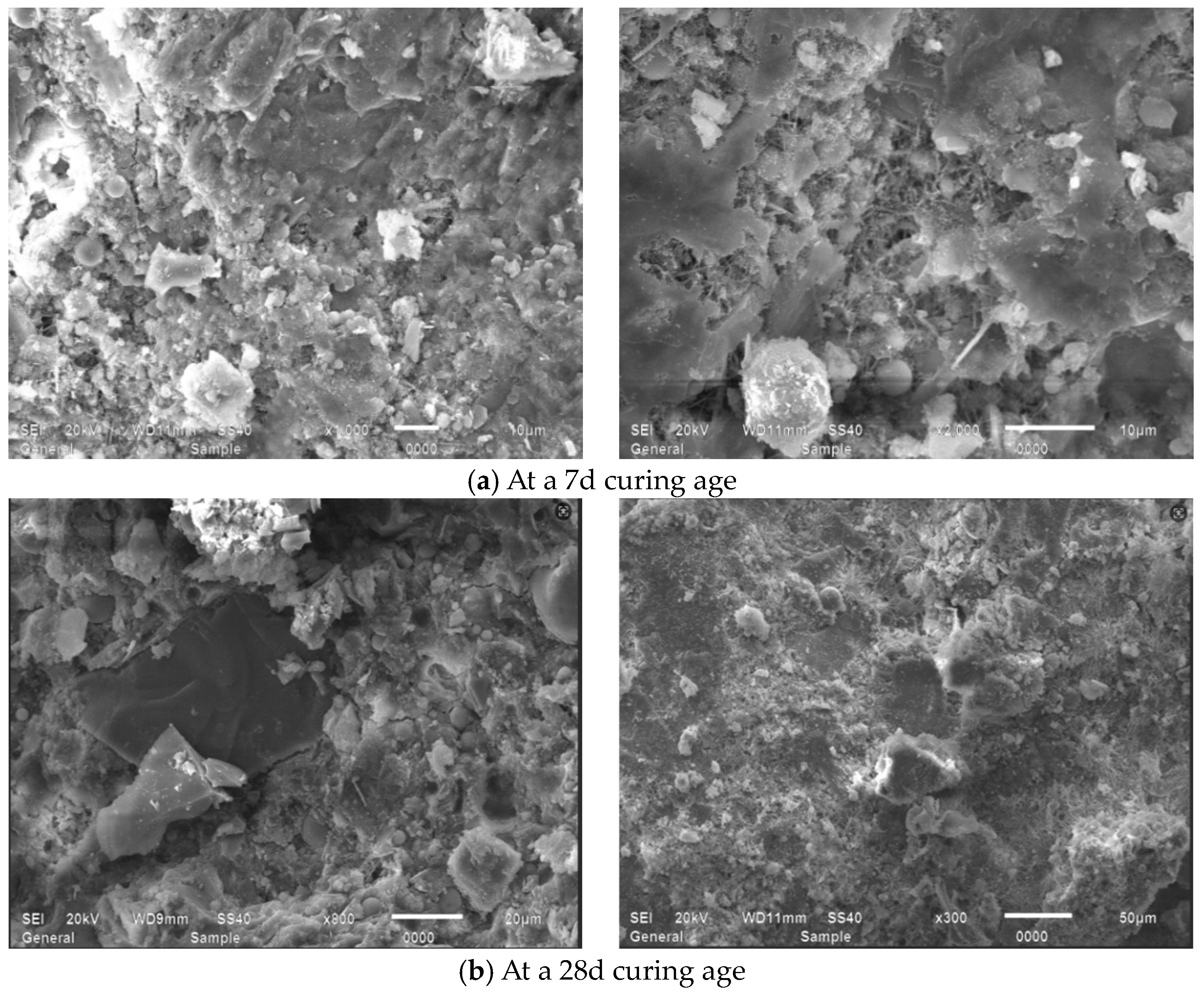
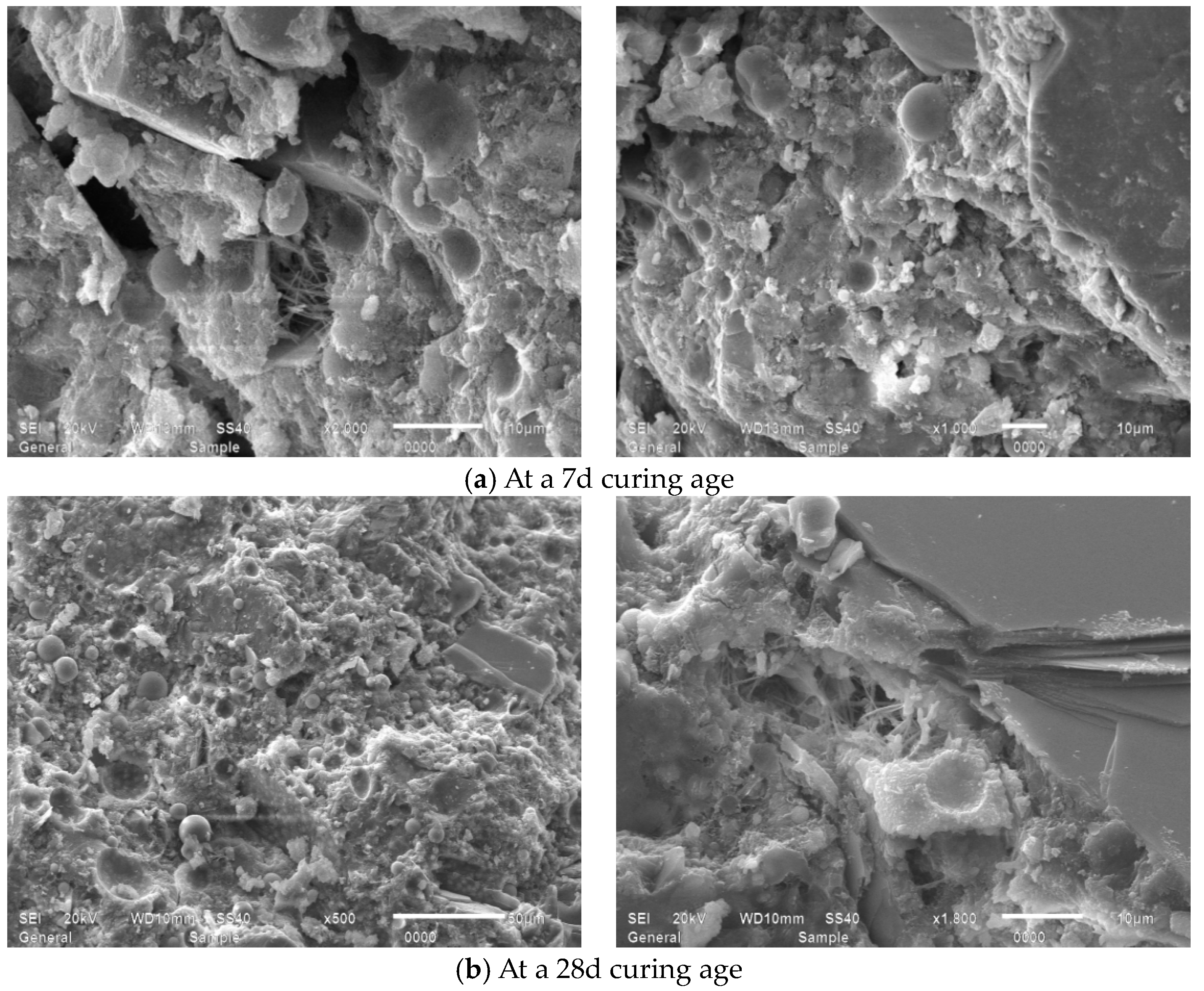
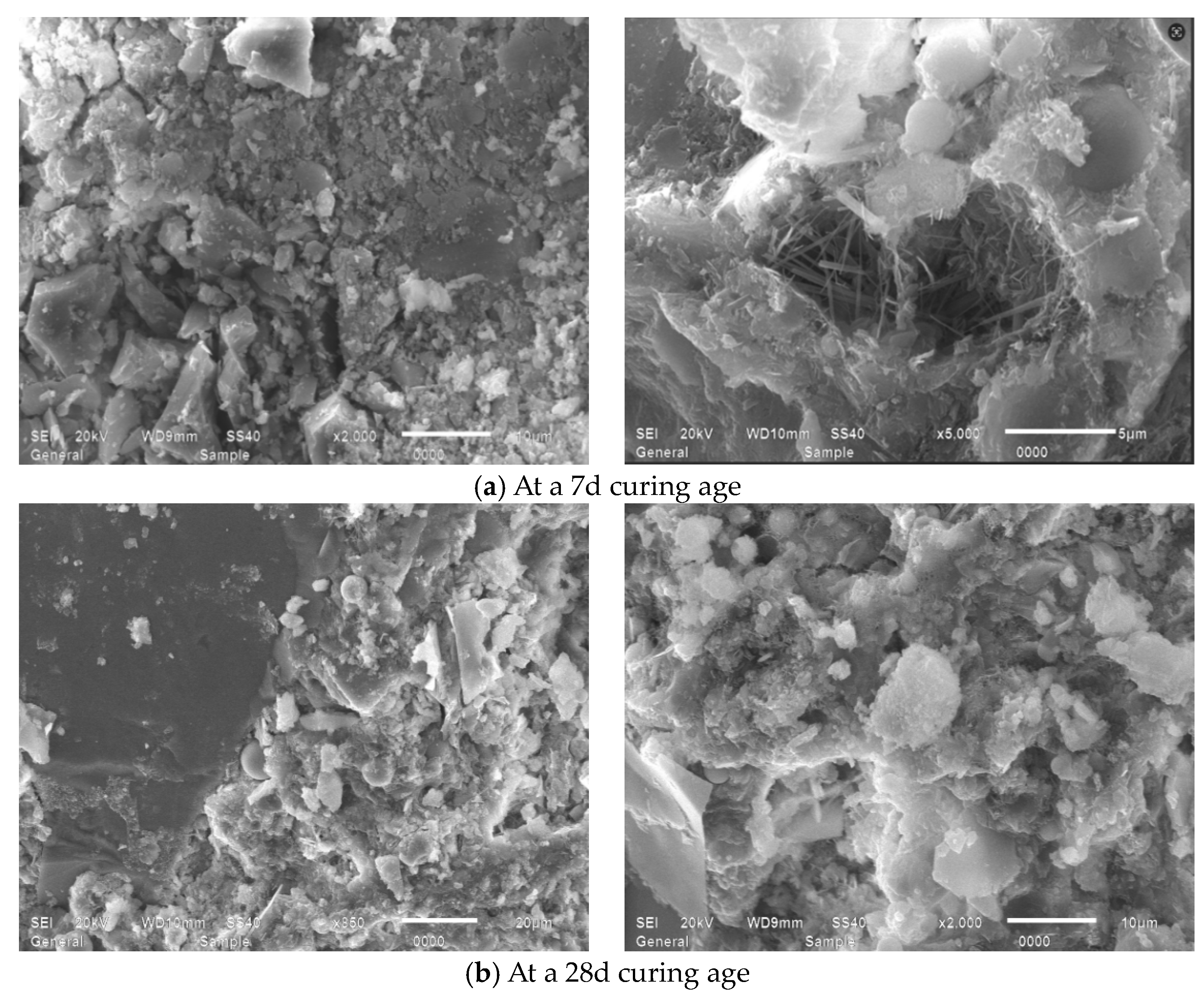
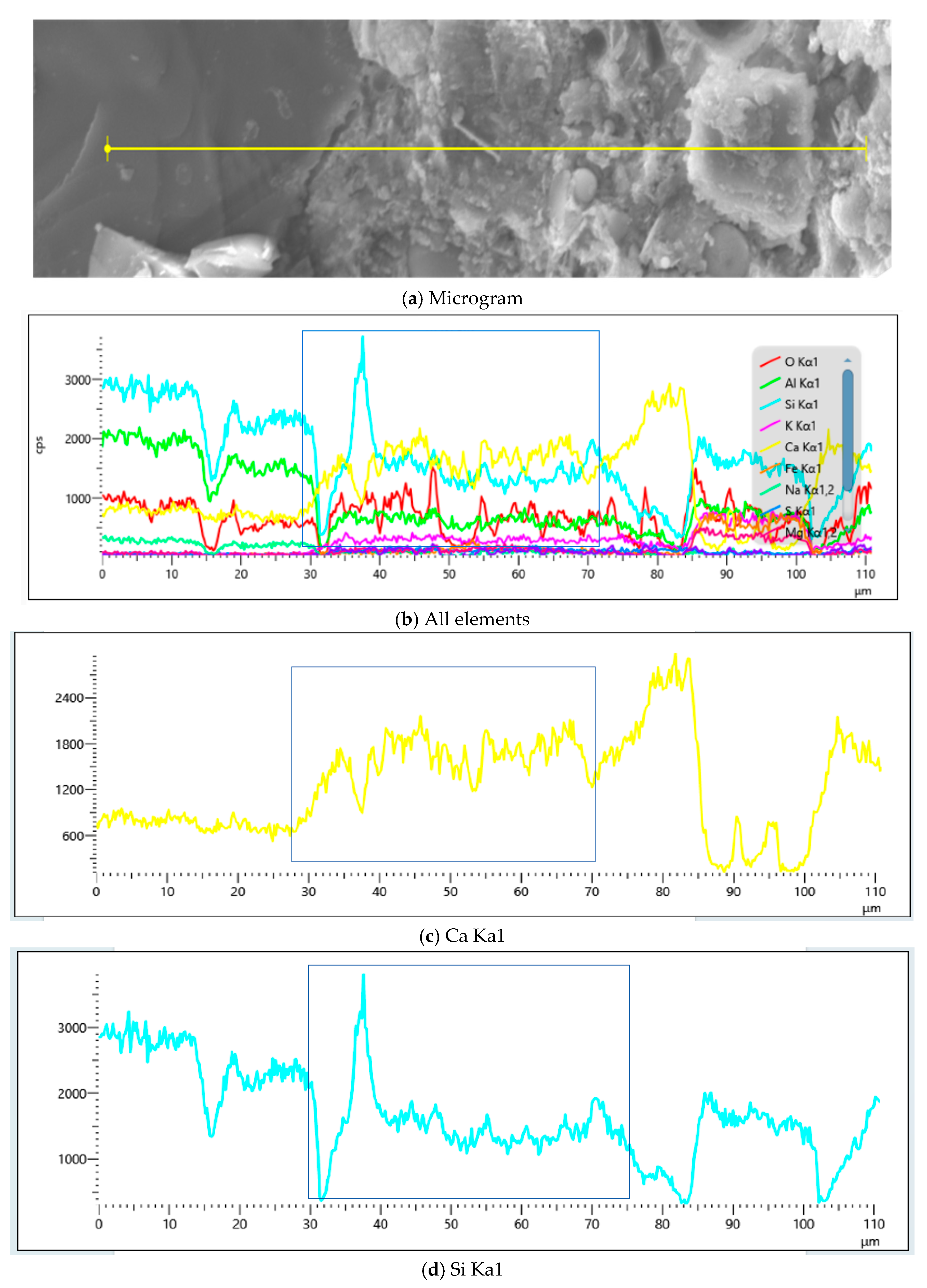
4.2. Microscopic Morphology Analysis
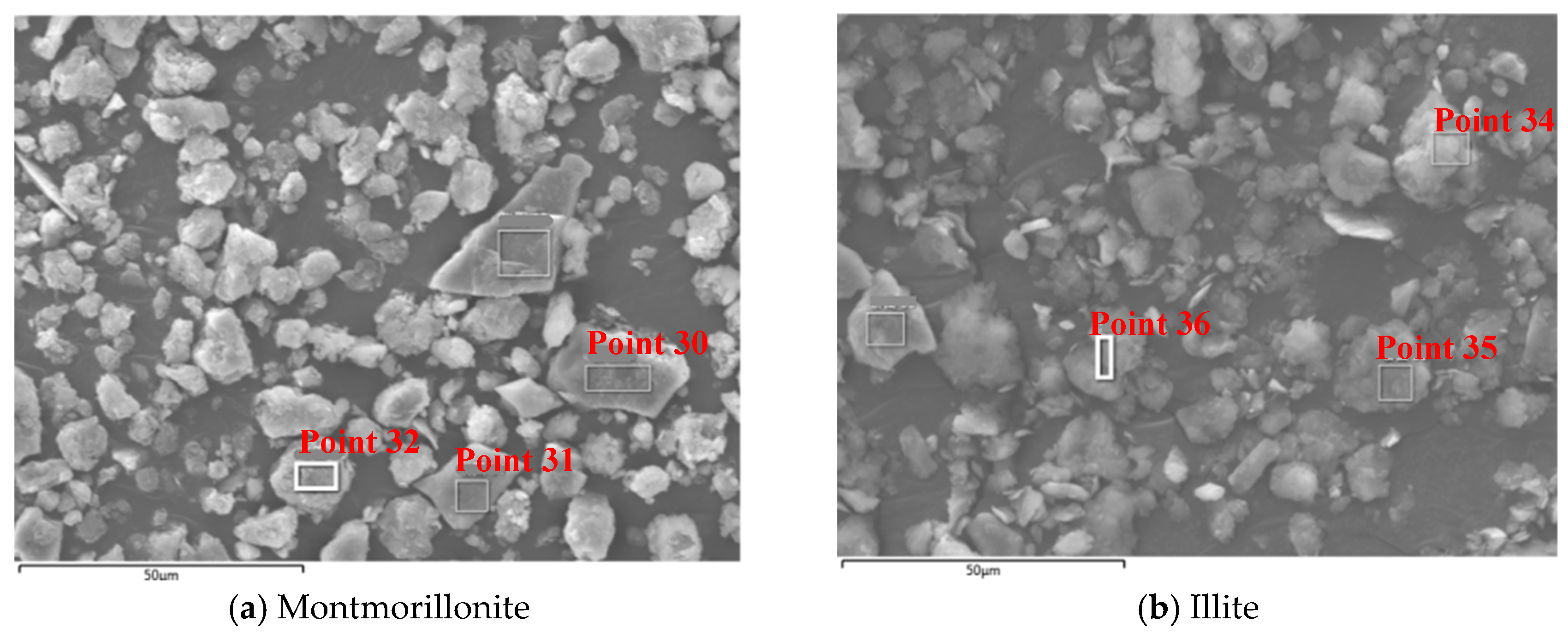
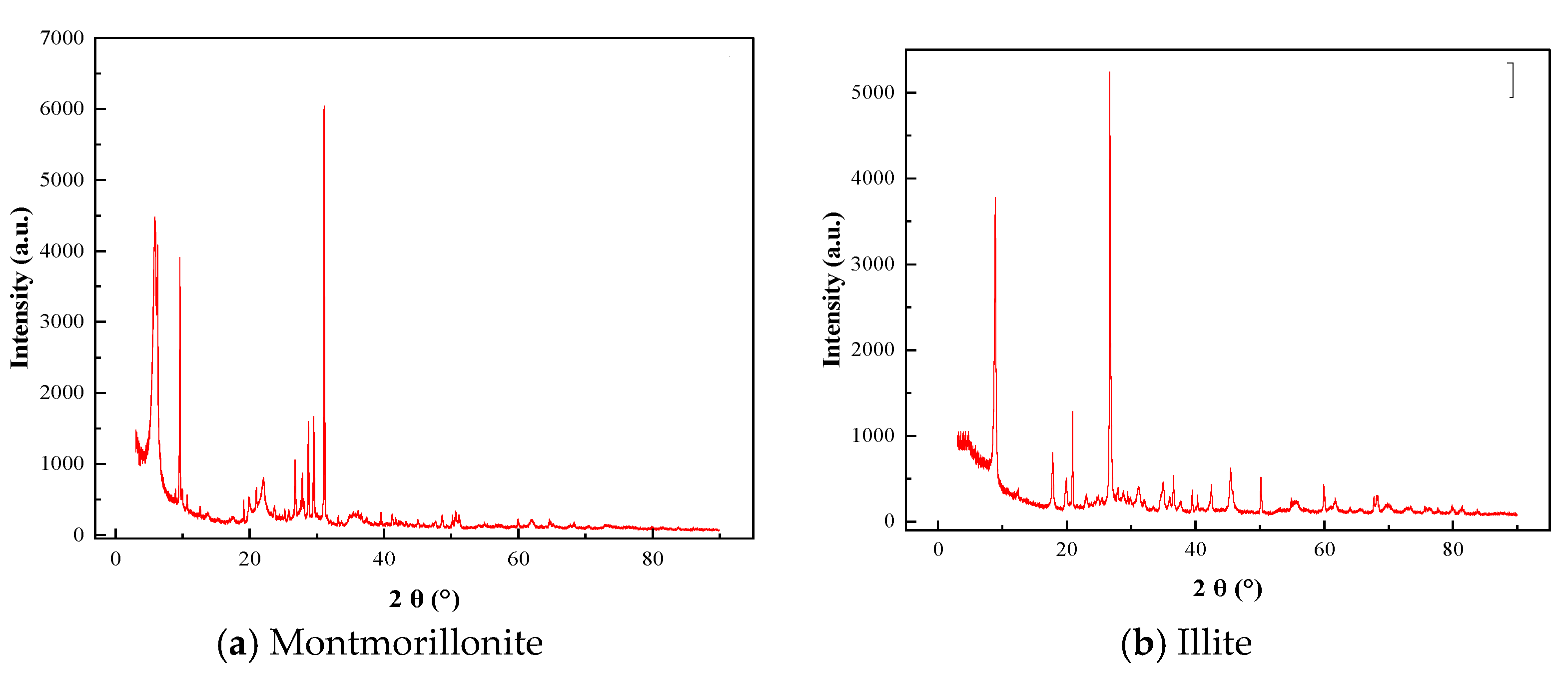
4.2.1. Microscopic Morphology Analysis of Montmorillonite and Illite
4.2.2. Influence of MB Value on the Micro-Morphology of Mortars
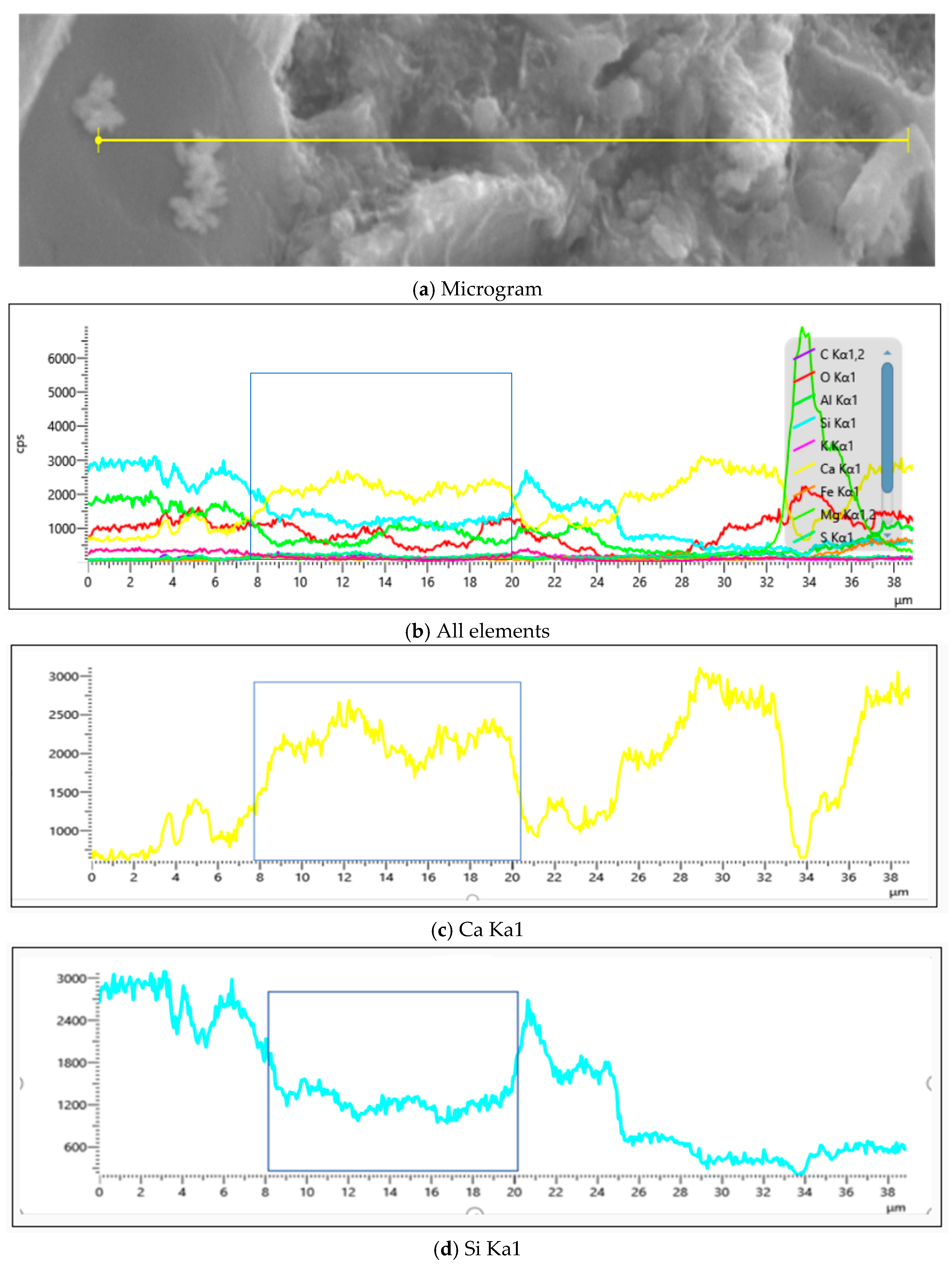
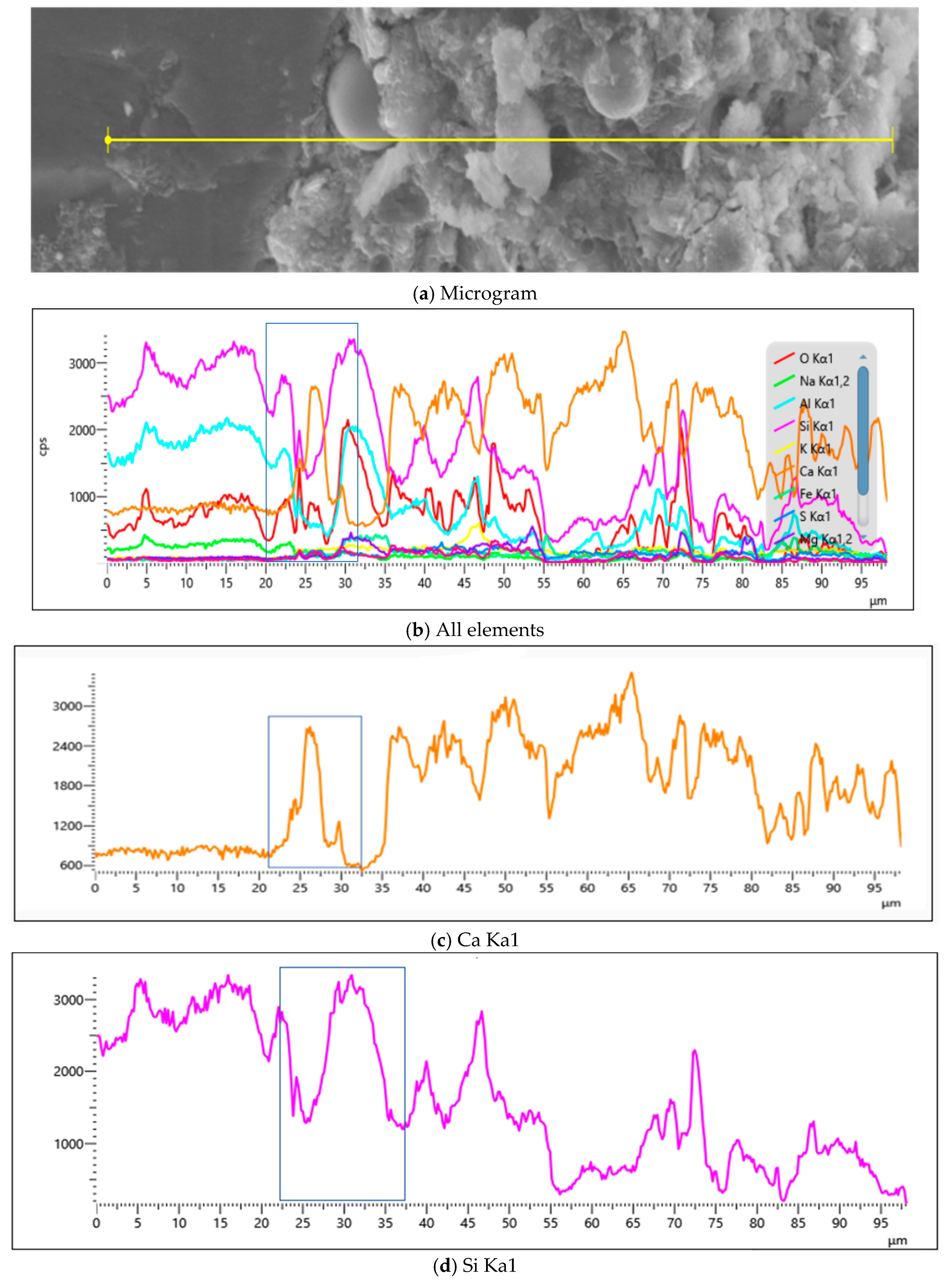
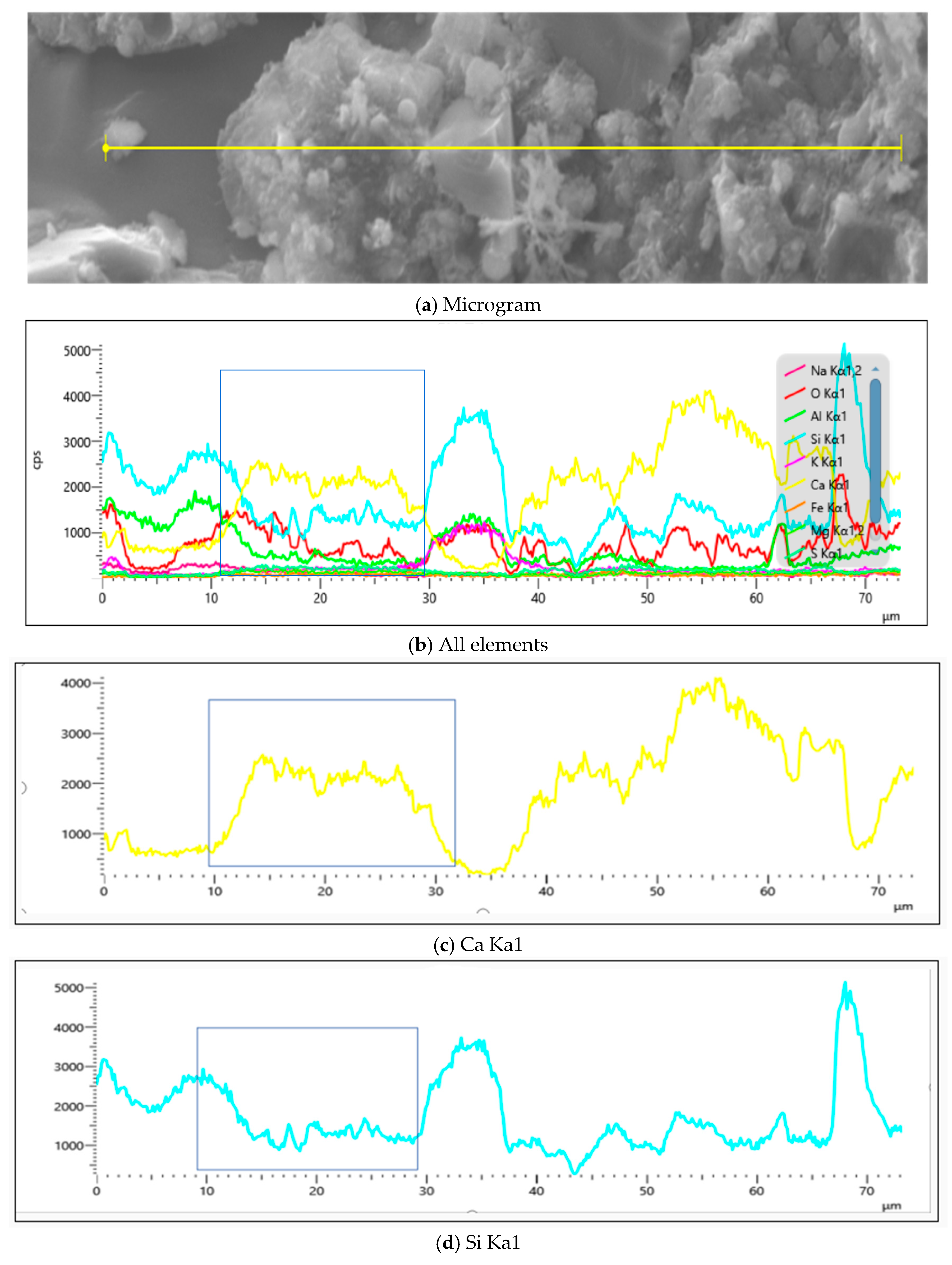
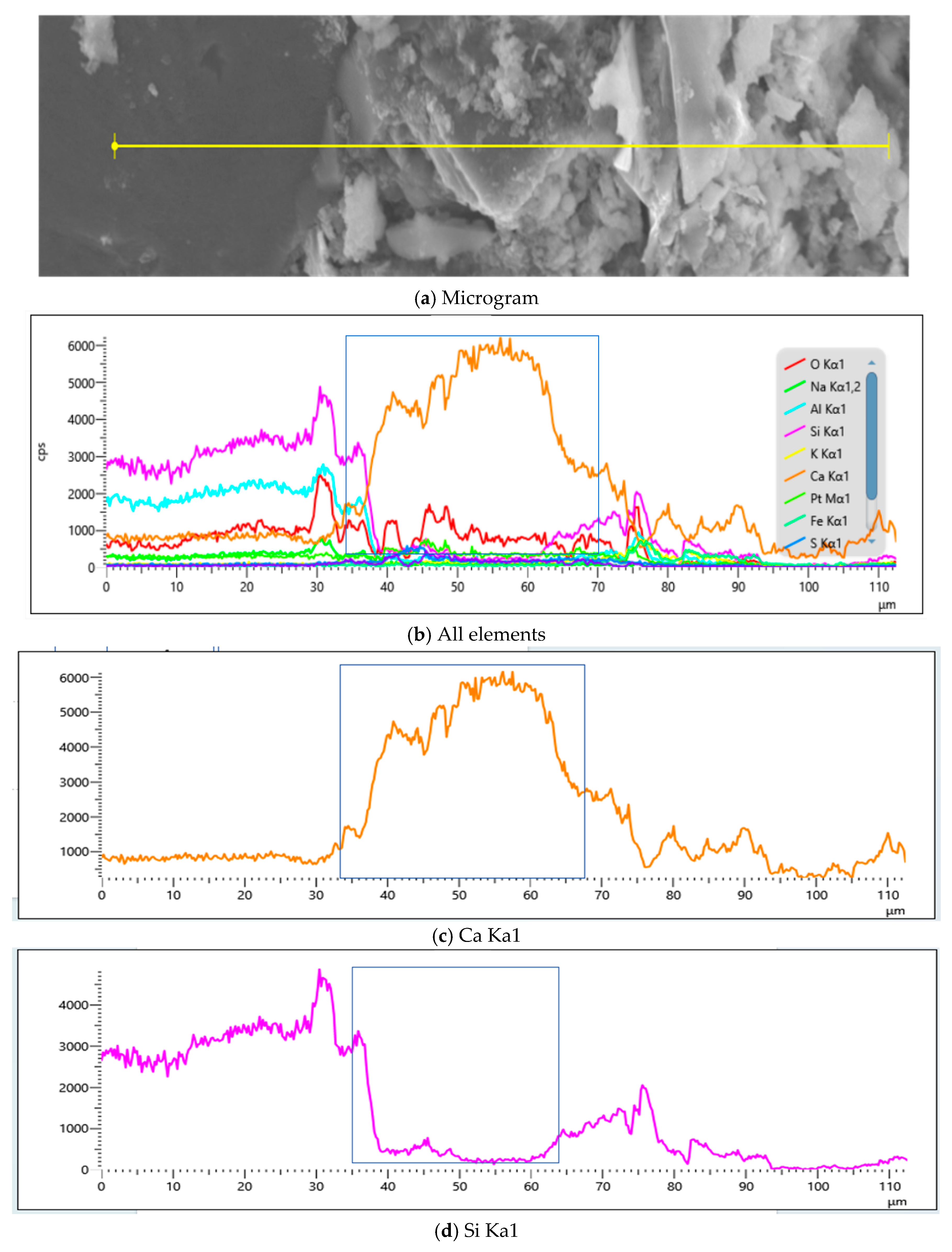
4.2.3. Influence of MB Value on Chemical Composition of Mortar
- (1)
- EDS analyses of specific products within JZ’s SEM images, as established through Figure 21 and Table 7, demonstrate that the 28d mortar calcium-to-silicon ratios significantly exceeded their 7d counterparts, increasing from 2.88 and 2.25 at 7 days to 3.73 and 4.49 at 28 days, thereby confirming more extensive hydration.
- (2)
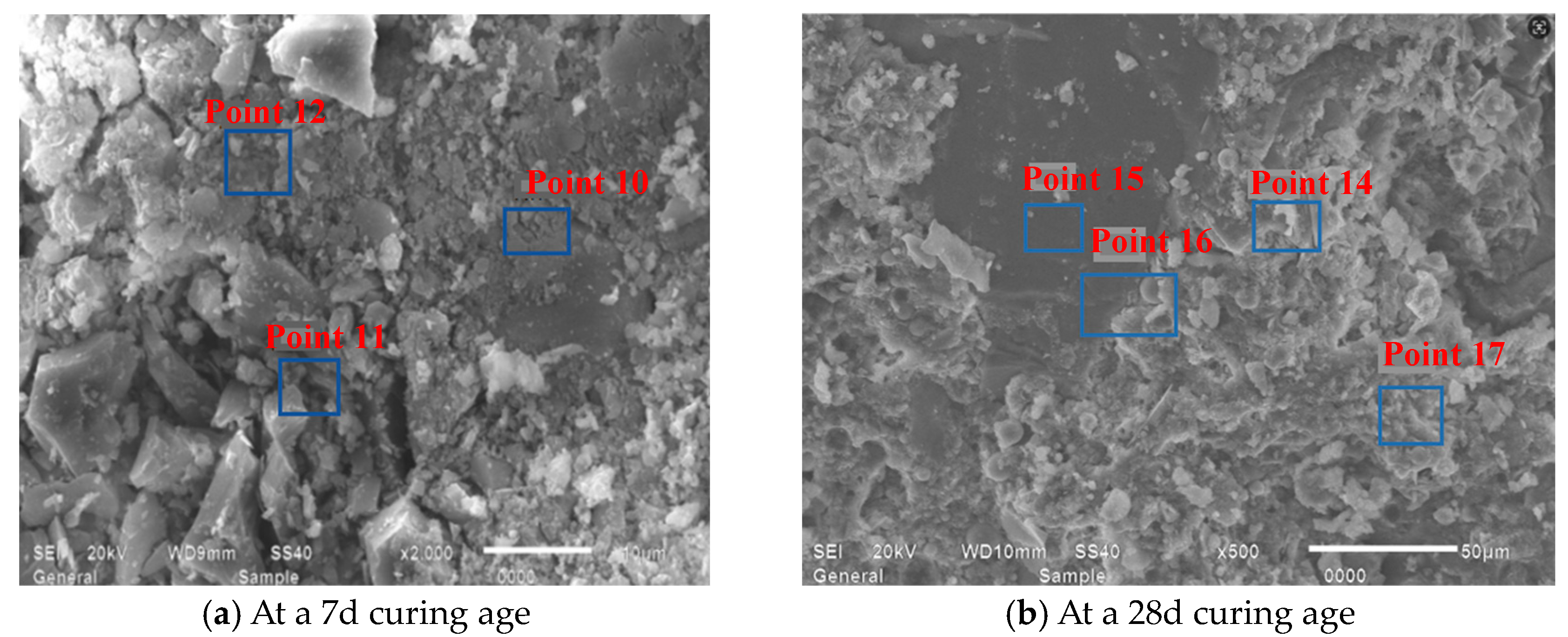
- EDS analyses of products across M1’s SEM, as demonstrated through Figure 22 and Table 8, revealed that the 28d mortar calcium-to-silicon ratios surpassed their 7d counterparts, with point 4 identified as montmorillonite, while other points, excluding point 5, represented calcium silicate aluminate hydrates at varying hydration stages alongside portlandite.
- (3)
- EDS analyses of products across M2’s SEM, as revealed through Figure 23 and Table 9, showed significantly greater calcium-to-silicon ratios in the 28d mortar compared to the 7d mortar, with points 7 and 11 constituting sand particles, whereas other points represented calcium silicate aluminate hydrates at distinct hydration degrees alongside portlandite.
- (4)
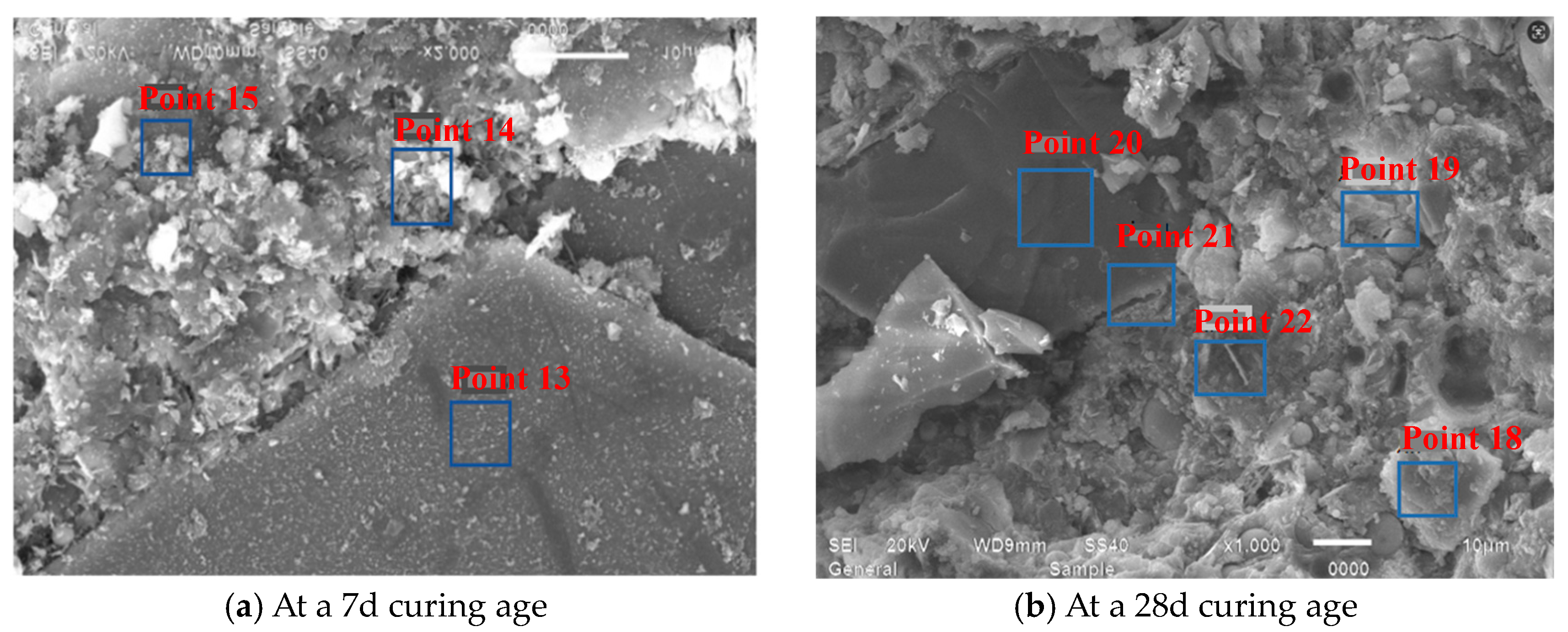
- EDS analyses of products across Y1’s SEM, as established through Figure 24 and Table 10, showed significantly greater calcium-to-silicon ratios in the 28d mortar compared to the 7d mortar, with point 15 constituting sand particles, whereas other points represented calcium silicate aluminate hydrates at distinct hydration degrees alongside portlandite.
- (5)
- EDS analyses of products across Y2’s SEM, as established through Figure 25 and Table 11, revealed significantly greater calcium-to-silicon ratios in the 28d mortar compared to its 7d counterpart, with points 20 and 13 constituting sand particles that demonstrated significantly lower calcium-to-silicon ratios than other mortar groups, thereby indicating the lowest hydration degree in Y2.
4.2.4. Influence of MB Value on ITZ Properties in Mortar
4.3. XRD Diffraction Analysis
4.3.1. XRD of Montmorillonite and Illite
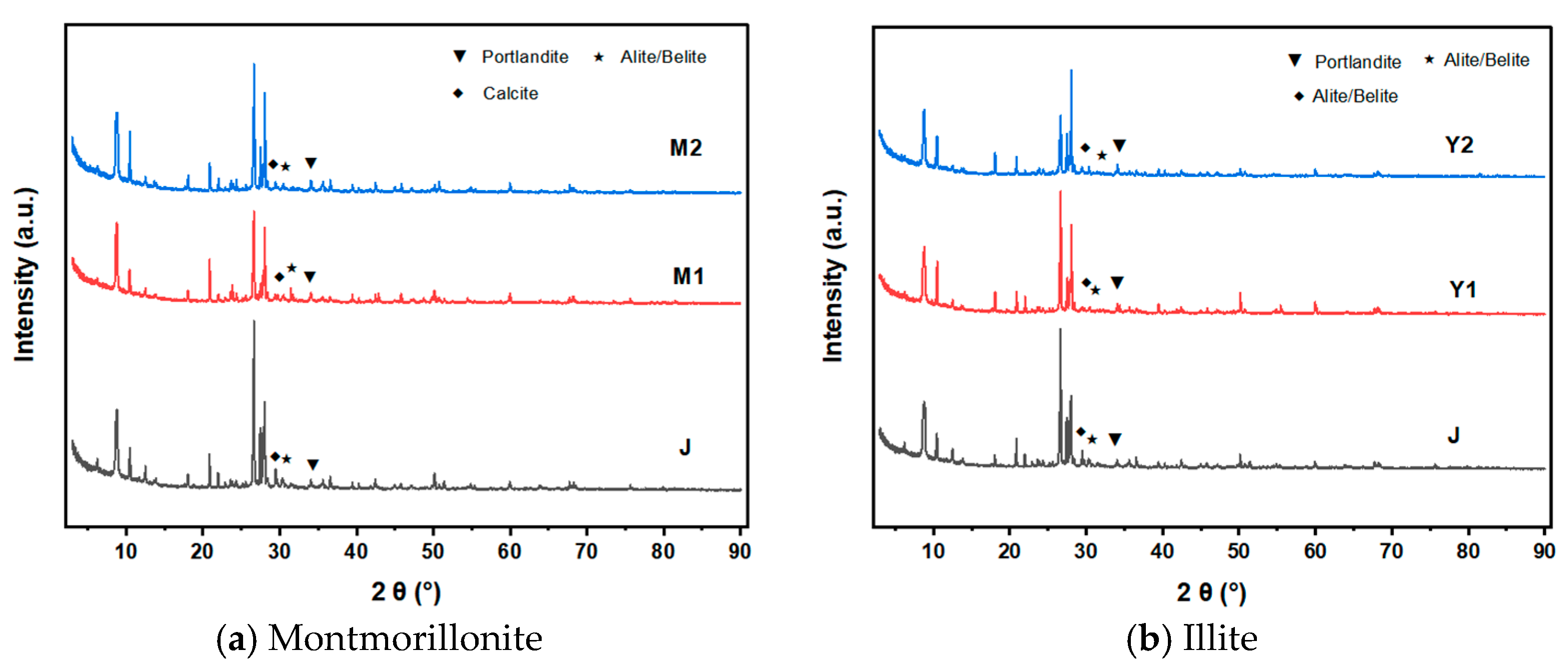
4.3.2. XRD Analysis of Different MB Values
4.4. Thermogravimetric Analysis
5. Conclusions
- (1)
- MB testing reveals that montmorillonite and illite exhibit markedly distinct MB sensitivity, where 1% montmorillonite yields an MB value of 1.42, exceeding the specification limit of 1.40, whereas illite requires a 5% content to attain an MB of 1.50.
- (2)
- Under constant fluidity and mix proportion, equivalent MB values necessitate only 50% water reducer demand for montmorillonite mortars relative to their illite counterparts, yet an equivalent clay powder content conversely demands a significantly higher water reducer for montmorillonite mortars.
- (3)
- Compressive strength of montmorillonite mortars initially increases then decreases with rising MB values at 7d, while flexural strength increases persistently; both parameters decline at 28d and 90d. Across all curing ages (7d, 28d, and 90d), illite mortars exhibit progressive declines in both compressive and flexural strength with an increasing MB, where montmorillonite mortar with an MB of 2.2 surpasses illite mortar with an MB of 1.1 in strength. Given a constant clay type, mortar drying shrinkage escalates with ascending MB values; nevertheless, the montmorillonite mortar with an MB of 2.2 demonstrates superior shrinkage performance compared to the illite mortar with an MB of 1.1, indicating that clay content exerts greater influence than MB value on drying shrinkage.
- (4)
- M1 and M2 exhibit enhanced frost resistance and impermeability relative to JZ. Y1 shows reduced impermeability and marginally inferior frost resistance versus JZ, whereas Y2 displays significantly compromised frost resistance and impermeability. Regarding frost-impermeability impacts, clay content demonstrates greater influence dominance than MB value, with a low clay content, below 2%, improving both properties.
- (5)
- Increasing MB values correlate with reduced harmless and marginally harmful pores, increased multi-harm pores, diminished hydration degrees, decreased hydration products, compromised structural compactness, thickened ITZ, and overall microstructural deterioration, consequently inducing macro-performance degradation.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weerdt, K.D.; Justnes, H. The effect of sea water on the phase assemblage of hydrated cement paste. Cem. Concr. Comp. 2015, 55, 215–222. [Google Scholar] [CrossRef]
- Tadesse, D.M.; Jeon, J.; Kim, S.; Park, S. The effect of seawater on the phase assemblage of hydrated cement paste: A study on PC, CAC and CSA. Dev. Built. Environ. 2023, 15, 100183. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, Y.; Zhang, J.; Hu, Z.; Li, H.; Wang, Y.; Liu, J.; Li, Z. Effect of clay content on plastic shrinkage cracking of cementitious materials. Constr. Build. Mater. 2022, 342, 127989. [Google Scholar] [CrossRef]
- Ghourchian, S.; Wyrzykowski, M.; Plamondon, M.; Lura, P. On the mechanism of plastic shrinkage cracking in fresh cementitious materials. Cem. Concr. Res. 2019, 115, 251–263. [Google Scholar] [CrossRef]
- Nehdi, M. Clay in cement-based materials: Critical overview of state-of-the-art. Constr. Build. Mater. 2014, 51, 372–382. [Google Scholar] [CrossRef]
- Jiao, D.; De Schryver, R.; Shi, C.; De Schutter, G. Thixotropic structural build-up of cement-based materials: A state-of-the-art review. Cem. Concr. Comp. 2021, 122, 104152. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, Y.; Qiao, H.; Liu, H. Damage constitutive model of manufactured sand concrete for bridges under composite salt freeze-thaw cycles. Constr. Build. Mater. 2025, 483, 141339. [Google Scholar] [CrossRef]
- Cortes, D.D.; Kim, H.K.; Palomino, A.; Santamarina, J. Rheological and mechanical properties of mortars prepared with natural and manufactured sand. Cem. Concr. Res. 2008, 38, 1142–1147. [Google Scholar] [CrossRef]
- Yool, A.I.G.; Lees, T.P.; Fried, A. Improvements to the methylene blue dye test for harmful clay in aggregates for concrete and mortar. Cem. Concr. Res. 1998, 28, 1417–1428. [Google Scholar] [CrossRef]
- SL/T 352-2020; Test Code for Hydraulic Concrete. China Water & Power Press: Beijing, China, 2020.
- Liang, T.; Ahmed, W.; Duan, Z.; Qu, Z.; Zhang, B.; Jiao, D. A critical review on the applications of modified recycled coarse aggregate in conventional concrete and high-performance concrete. J. Build. Eng. 2025, 106, 112601. [Google Scholar] [CrossRef]
- Muñoz, J.F.; Gullerud, K.J.; Cramer, S.M.; Tejedor, M.I.; Anderson, M.A. Effects of coarse aggregate coatings on concrete performance. J. Mater. Civ. Eng. 2010, 22, 96–103. [Google Scholar] [CrossRef]
- Courard, L.; Michel, F.; Pierard, J. Influence of clay in limestone fillers for self-compacting cement based composites. Constr. Build. Mater. 2011, 25, 1356–1361. [Google Scholar] [CrossRef]
- Dong, W.; Ahmed, A.H.; Liebscher, M.; Li, H.; Guo, Y.; Pang, B.; Adresi, M.; Li, W.; Mechtcherine, V. Electrical resistivity and self-sensing properties of low-cement limestone calcined clay cement (LC3) mortar. Mater. Design 2025, 252, 113790. [Google Scholar] [CrossRef]
- Chen, X.; Yuguang, G.; Li, B.; Zhou, M.; Li, B.; Liu, Z.; Zhou, J. Coupled effects of the content and methylene blue value (MBV) of microfines on the performance of manufactured sand concrete. Constr. Build. Mater. 2020, 20, 117953. [Google Scholar] [CrossRef]
- Du, W.; Liu, B.; Ding, Q.; Jiang, L.; Zuo, D.; Liu, Q.; Feng, Z. Research on improving the frost resistance and self-repair performance of limestone calcined clay cement concrete by microcapsules. J. Build. Eng. 2024, 94, 109960. [Google Scholar] [CrossRef]
- Jiao, Y.; Yang, H.; Wang, J.; Fang, M.; Shi, H. Effects of fiber properties on flexural and fracture characteristics of manufactured sand concrete based on acoustic emission. J. Build. Eng. 2024, 84, 108308. [Google Scholar] [CrossRef]
- Pedro, D.; De Brito, J.; Evangelista, L. Mechanical characterization of high performance concrete prepared with recycled aggregates and silica fume from precast industry. J. Clean. Prod. 2017, 164, 939–949. [Google Scholar] [CrossRef]
- Xie, J.; Fang, C.; Lu, Z.; Li, Z.; Li, L. Effects of the addition of silica fume and rubber particles on the compressive behaviour of recycled aggregate concrete with steel fibres. J. Clean. Prod. 2018, 197, 656–667. [Google Scholar] [CrossRef]
- Kurad, R.; Silvestre, J.D.; de Brito, J.; Ahmed, H. Effect of incorporation of high volume of recycled concrete aggregates and fly ash on the strength and global warming potential of concrete. J. Clean. Prod. 2017, 166, 485–502. [Google Scholar] [CrossRef]
- Shi, C.; Li, Y.; Zhang, J.; Li, W.; Chong, L.; Xie, Z. Performance enhancement of recycled concrete aggregate—A review. J. Clean. Prod. 2016, 112, 466–472. [Google Scholar] [CrossRef]
- Cai, J.W. Research of effects and mechanism of micro fines on manufactured fine aggregate concrete. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2006, 16, 331–337. [Google Scholar]
- Gonçalves, J.P.; Tavares, L.M.; Toledo Filho, R.D.; Fairbairn, E.M.R.; Cunha, E.R. Comparison of natural and manufactured fine aggregates in cement mortars. Cem. Concr. Res. 2007, 37, 924–932. [Google Scholar] [CrossRef]
- USACE EM 1110-2-2000; Design of Gravity Dams. U.S. Army Corps of Engineers (USACE): Washington, DC, USA, 2000.
- SL 677-2014; Specifications for Hydraulic Concrete Construction. China Water & Power Press: Beijing, China, 2014.
- Li, B.; Ke, G.; Zhou, M. Influence of manufactured sand characteristics on strength and abrasion resistance of pavement cement concrete. Constr. Build. Mater. 2011, 25, 3849–3853. [Google Scholar] [CrossRef]
- Ilangovan, R.; Nagamani, K.; Swamy, P.G. Recycling of quarry waste as an alternative material in concrete manufacturing. Indian Constr. 2007, 40, 7–12. [Google Scholar]
- Li, B.; Wang, J.; Zhou, M. Effect of limestone fines content in manufactured sand on durability of low-and high-strength concretes. Constr. Build. Mater. 2009, 23, 2846–2850. [Google Scholar] [CrossRef]
- GB 8076-2008; Concrete Admixture. Standards Press of China: Beijing, China, 2009.
- GB 175-2023; Common Portland Cement Standard. Standards Press of China: Beijing, China, 2023.
- DL/T 5055-2007; Technical Specification for Fly Ash Used in Hydraulic Concrete. China Electric Power Press: Beijing, China, 2007.
- GB/T 2419-2005; Test Method for Fluidity of Cement Mortar. Standards Press of China: Beijing, China, 2005.
- GB/T 17671-2021; Method for Testing Cement Mortar Strength (ISO Method). Standards Press of China: Beijing, China, 2021.
- JC/T 603-2004; Test Method for Drying Shrinkage of Cement Mortar. China Building Materials Industry Press: Beijing, China, 2004.
- Nanthagopalan, P.; Santhanam, M. Fresh and hardened properties of self-compacting concrete produced with manufactured sand. Cem. Concr. Compos. 2011, 33, 353–358. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, J.; Zhu, L.; He, T. Effects of Manufactured-sand on dry shrinkage and creep of high-strength concrete. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2008, 23, 249–253. [Google Scholar] [CrossRef]
- GB/T 50082-2009; Standard for Test Methods of Long-term Performance and Durability of Ordinary Concrete. China Architecture & Building Press: Beijing, China, 2009.
- Jaturapitakkul, C.; Tangpagasit, J.; Songmue, S.; Kiattikomol, K. Filler effect of fine particle sand on the compressive strength of mortar. Int. J. Miner. Metall. Mater. 2010, 18, 240–246. [Google Scholar] [CrossRef]
- Westerholm, M.; Lagerblad, B.; Silfwerbrand, J.; Forssberg, E. Influence of fine aggregate characteristics on the rheological properties of mortars. Cem. Concr. Compos. 2008, 30, 274–282. [Google Scholar] [CrossRef]
- Ji, T.; Chen, C.Y.; Zhuang, Y.Z.; Chen, J.F. A mix proportion design method of manufactured sand concrete based on minimum paste theory. Constr. Build. Mater. 2013, 44, 422–426. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, C.S.; Park, C.K.; Eo, S.H. The fracture characteristics of crushed limestone sand concrete. Cem. Concr. Res. 1997, 27, 1719–1729. [Google Scholar] [CrossRef]
- Li, B.; Zhou, M.; Wang, J. Effect of the methylene blue value of manufactured sand on performances of concrete. J. Ad. Con. Technol. 2011, 9, 127–132. [Google Scholar]
- Zhan, A.L.; Zhou, M.K. Effect of clay-contaminated microfines on properties of low-and high-strength concrete. Arab. J. Sci. Eng. 2015, 11, 13–17. [Google Scholar]
- Zhan, A.L.; Zhou, M.K.; Yao, C.K. Relationship between methylene blue value of manufactured sand and mortar properties. Key Eng. Mater. 2015, 629, 612–617. [Google Scholar]
- Zhan, A.L.; Zhou, M.K.; Li, B.X. Relationships between modified methylene blue value of microfines in manufactured sand and concrete properties. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2016, 31, 574–581. [Google Scholar]
- Wang, L.H.; Liu, J. Effect of particle shape of limestone manufactured sand and natural sand on concrete. In Proceedings of the 2013 International Conference on Material Science and Environmental Engineering (MSEE2013), Wuhan, China, 21–22 December 2013; pp. 463–468. [Google Scholar]
- Marinoni, N.; Pavese, A.; Foi, M.; Trombino, L. Characterisation of mortar morphology in thin sections by digital image processing. Cem. Concr. Res. 2005, 35, 1613–1619. [Google Scholar] [CrossRef]
- Garboczi, E.J.; Bullard, J.W. Contact function, uniform-thickness shell volume, and convexity measure for 3-D star-shaped random particles. Powder Technol. 2013, 237, 191–201. [Google Scholar] [CrossRef]
- Hestnes, K.; Sørensen, B. Evaluation of quantitative X-ray diffraction for possible use in the quality control of granitic pegmatite in mineral production. Miner. Eng. 2012, 39, 239–247. [Google Scholar] [CrossRef]
- Guan, M.S.; Lai, Z.C.; Xiao, Q.; Du, H.B.; Zhang, K. Bond behavior of concrete-filled steel tube columns using manufactured sand (MS-CFT). Eng. Str. 2019, 187, 199–208. [Google Scholar] [CrossRef]
- Yang, H.F.; Liang, D.Y.; Deng, Z.H.; Qin, Y.H. Effect of limestone powder in manufactured sand on the hydration products and microstructure of recycled aggregate concrete. Constr. Build. Mater. 2018, 188, 1045–1049. [Google Scholar] [CrossRef]










| Specimen | Water–Cement Ratio | MB Value/g·kg−1 | Sand | Clay Content/% | Montmorillonite/g | Illite /g | Water /kg | Cement /kg | Fly Ash /kg | Manufactured Sand/kg | Water Reducer/% | Fluidity /mm | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Montmorillonite Ratio | Illite Ratio | ||||||||||||
| JZ | 0.48 | 0.6 | Manufactured Sand | 0 | 0 | 0 | 0 | 216 | 315 | 135 | 1350 | 0 | 175 |
| M1 | 0.48 | 1.1 | Manufactured Sand | 0.6 | 3 | 8.1 | 40.5 | 216 | 315 | 135 | 1350 | 0.1 | 170 |
| M2 | 0.48 | 2.2 | Manufactured Sand | 2 | 9 | 27 | 121.5 | 216 | 315 | 135 | 1350 | 0.31 | 175 |
| Y1 | 0.48 | 1.1 | Manufactured Sand | 0.6 | 3 | 8.1 | 40.5 | 216 | 315 | 135 | 1350 | 0.2 | 170 |
| Y2 | 0.48 | 2.2 | Manufactured Sand | 2 | 9 | 27 | 121.5 | 216 | 315 | 135 | 1350 | 0.68 | 170 |
| Chemical Composition | SiO2 | AL2O3 | CaO | Fe2O3 | MgO | Na2O | K2O |
|---|---|---|---|---|---|---|---|
| Ratio/% | 71.47 | 16.45 | 3.83 | 2.28 | 2.25 | 0.65 | 0.91 |
| Chemical Composition | SiO2 | AL2O3 | CaO | Fe2O3 | MgO | Na2O | K2O |
|---|---|---|---|---|---|---|---|
| Ratio/% | 49.95 | 29.45 | - | 3.28 | 1.25 | - | 7.91 |
| Specimen | Curing Age /d | MB Value | Porosity /% | Pore Size Distribution/% | |||||
|---|---|---|---|---|---|---|---|---|---|
| <20 nm | 20~50 nm | 50~200 nm | >200 nm | Average Pore Diameter/nm | Median Aperture/nm | ||||
| JZ | 28 | 0.6 | 9.9 | 37.7 | 32.2 | 9.2 | 20.9 | 14.2 | 29.6 |
| M1 | 28 | 1.1 | 9.5 | 35.7 | 32.4 | 7.1 | 24.8 | 16.9 | 31.9 |
| M2 | 28 | 2.2 | 13.2 | 28.8 | 34.1 | 4.9 | 32.2 | 18.6 | 34.5 |
| Y1 | 28 | 1.1 | 11.6 | 21.1 | 34.7 | 5.6 | 38.6 | 26.8 | 41.4 |
| Y2 | 28 | 2.2 | 13.8 | 18.3 | 30.4 | 6.5 | 44.8 | 31.3 | 48.2 |
| Location | Ca | O | Mg | Si | Al | Fe |
|---|---|---|---|---|---|---|
| Point 30 | 2.81 | 50.7 | - | 37.66 | 6.72 | - |
| Point 31 | 1.10 | 50.85 | 0.72 | 37.22 | 6.79 | 0.71 |
| Point 32 | 1.91 | 50.5 | 0.88 | 37.85 | 6.68 | 0.66 |
| Location | O | Si | Al | K | Fe | Mg |
|---|---|---|---|---|---|---|
| Point 34 | 46.37 | 24.83 | 17.03 | 8.65 | 2.39 | 0.73 |
| Point 35 | 46.82 | 24.82 | 17.79 | 7.93 | 1.75 | 0.66 |
| Point 36 | 46.81 | 25.17 | 17.29 | 7.83 | 2.05 | 0.85 |
| Age | Location | O | Ca | Si | Al | Fe | Calcium–Silicate Ratio | Hydration Product |
|---|---|---|---|---|---|---|---|---|
| 7d | Point 1 | 38.56 | 48.16 | 3.31 | 1.54 | 0.82 | 14.58 | Calcium hydroxide |
| Point 2 | 38.31 | 30.14 | 13.28 | 6.79 | 5.93 | 2.88 | - | |
| Point 3 | 39.12 | 30.86 | 13.71 | 4.89 | 3.33 | 2.25 | Hydrated calcium (alumino) silicate | |
| 28d | Point 1 | 56.36 | 5.04 | 15.28 | 9.17 | 0.19 | 0.33 | Hydrated calcium (alumino) silicate |
| Point 2 | 55.05 | 21.18 | 5.67 | 1.23 | 1.31 | 3.73 | - | |
| Point 3 | 58.37 | 17.1 | 3.81 | 1.41 | 0.89 | 4.49 | - | |
| Point 4 | 46.51 | 40.79 | 1.21 | 0.36 | 0.34 | 33.7 | Partial calcium hydroxide |
| Age | Location | O | Ca | Si | Al | Fe | Calcium–Silicate Ratio | Hydration Product |
|---|---|---|---|---|---|---|---|---|
| 7d | Point 4 | 41.96 | 1.86 | 31.96 | 11.45 | 3.49 | 0.058 | Montmorillonite |
| Point 5 | 36.47 | 20.86 | 23.41 | 2.70 | 2.21 | 0.89 | Hydrated calcium (alumino) silicate | |
| Point 6 | 38.71 | 31.32 | 15.52 | 6.08 | 2.31 | 2.03 | Hydrated calcium (alumino) silicate | |
| 28d | Point 5 | 52.31 | 5.26 | 18.78 | 12.14 | 0.2 | 0.28 | Silica aggregate |
| Point 6 | 47.64 | 11.22 | 3.21 | 8.24 | 2.33 | 3.49 | - | |
| Point 7 | 51.25 | 12.52 | 4.51 | 4.10 | 2.46 | 2.76 | - | |
| Point 8 | 47.04 | 7.80 | 2.42 | 3.9 | 3.77 | 3.22 | - |
| Age | Location | O | Ca | Si | Al | Fe | Calcium–Silicate Ratio | Hydration Product |
|---|---|---|---|---|---|---|---|---|
| 7d | Point 7 | 44.68 | 6.73 | 33.02 | 8.65 | 0.89 | 0.20 | Silica aggregate |
| Point 8 | 39.96 | 21.62 | 19.16 | 8.71 | 3.08 | 1.08 | Hydrated calcium (alumino) silicate | |
| Point 9 | 42.86 | 18.33 | 18.89 | 10.12 | 4.25 | 0.97 | Hydrated calcium (alumino) silicate | |
| 28d | Point 9 | 58.44 | 12.14 | 7.13 | 2.77 | 1.15 | 1.70 | Hydrated calcium (alumino) silicate |
| Point 10 | 27.84 | 13.82 | 6.8 | 2.54 | 1.1 | 2.03 | Hydrated calcium (alumino) silicate | |
| Point 11 | 52.31 | 6.26 | 19.78 | 12.14 | 0.2 | 0.32 | Silica aggregate | |
| Point 12 | 58.17 | 16.00 | 6.00 | 1.48 | 0.80 | 2.67 | - | |
| Point 13 | 58.68 | 15.47 | 4.72 | 1.6 | 1.02 | 3.28 | - |
| Age | Location | O | Ca | Si | Al | Fe | Calcium–Silicate Ratio | Hydration Product |
|---|---|---|---|---|---|---|---|---|
| 7d | Point 10 | 38.68 | 33.73 | 13.12 | 3.68 | 3.19 | 2.57 | Hydrated calcium (alumino) silicate |
| Point 11 | 41.22 | 27.64 | 11.22 | 1.28 | 1.24 | 2.46 | Hydrated calcium (alumino) silicate | |
| Point 12 | 37.84 | 31.25 | 12.52 | 1.58 | 3.10 | 2.50 | Hydrated calcium (alumino) silicate | |
| 28d | Point 14 | 48.16 | 27.04 | 6.80 | 2.22 | 3.13 | 3.98 | - |
| Point 15 | 53.04 | 6.71 | 18.98 | 11.59 | - | 0.58 | Silica aggregate | |
| Point 16 | 58.24 | 10.53 | 8.78 | 5.40 | 0.3 | 1.20 | - | |
| Point 17 | 41.18 | 35.39 | 4.92 | 1.75 | 7.36 | 7.19 | - |
| Age | Location | O | Ca | Si | Al | Fe | Calcium–Silicate Ratio | Hydration Product |
|---|---|---|---|---|---|---|---|---|
| 7d | Point 13 | 43.51 | 4.67 | 33.08 | 2.18 | 2.05 | 0.14 | Silica aggregate |
| Point 14 | 39.98 | 8.81 | 17.34 | 8.77 | 15.53 | 0.51 | Hydrated calcium (alumino) silicate | |
| Point 15 | 39.73 | 7.55 | 17.17 | 8.7 | 15.27 | 0.43 | Hydrated calcium (alumino) silicate | |
| 28d | Point 18 | 52.63 | 3.67 | 9.63 | 4.89 | 9.2 | 0.38 | - |
| Point 19 | 56.51 | 9.27 | 9.32 | 6.42 | 2.12 | 0.99 | Hydrated calcium (alumino) silicate | |
| Point 20 | 53.03 | 5.56 | 19.27 | 12.04 | 0.17 | 0.29 | Silica aggregate | |
| Point 21 | 56.83 | 11.49 | 9.50 | 5.17 | 0.84 | 1.21 | - | |
| Point 22 | 56.88 | 15.34 | 6.28 | 3.27 | 0.82 | 2.44 | Hydrated calcium (alumino) silicate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Huo, J.; Wang, Y.; Lin, Y.; Deng, Q.; Peng, S. Mechanistic Investigation of Machine-Made Sand Methylene Blue Value Effects on Mortar Performance. Appl. Sci. 2025, 15, 9115. https://doi.org/10.3390/app15169115
Shi Y, Huo J, Wang Y, Lin Y, Deng Q, Peng S. Mechanistic Investigation of Machine-Made Sand Methylene Blue Value Effects on Mortar Performance. Applied Sciences. 2025; 15(16):9115. https://doi.org/10.3390/app15169115
Chicago/Turabian StyleShi, Yan, Jinyang Huo, Yuanyi Wang, Yuqiang Lin, Qingpeng Deng, and Sheng Peng. 2025. "Mechanistic Investigation of Machine-Made Sand Methylene Blue Value Effects on Mortar Performance" Applied Sciences 15, no. 16: 9115. https://doi.org/10.3390/app15169115
APA StyleShi, Y., Huo, J., Wang, Y., Lin, Y., Deng, Q., & Peng, S. (2025). Mechanistic Investigation of Machine-Made Sand Methylene Blue Value Effects on Mortar Performance. Applied Sciences, 15(16), 9115. https://doi.org/10.3390/app15169115





