Comparative Method Between Eddy Current and Optical Microscopy in the Determination of Thickness of 6063 Aluminum Alloy Anodization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Anodization Process
2.3. Optical Microscopy
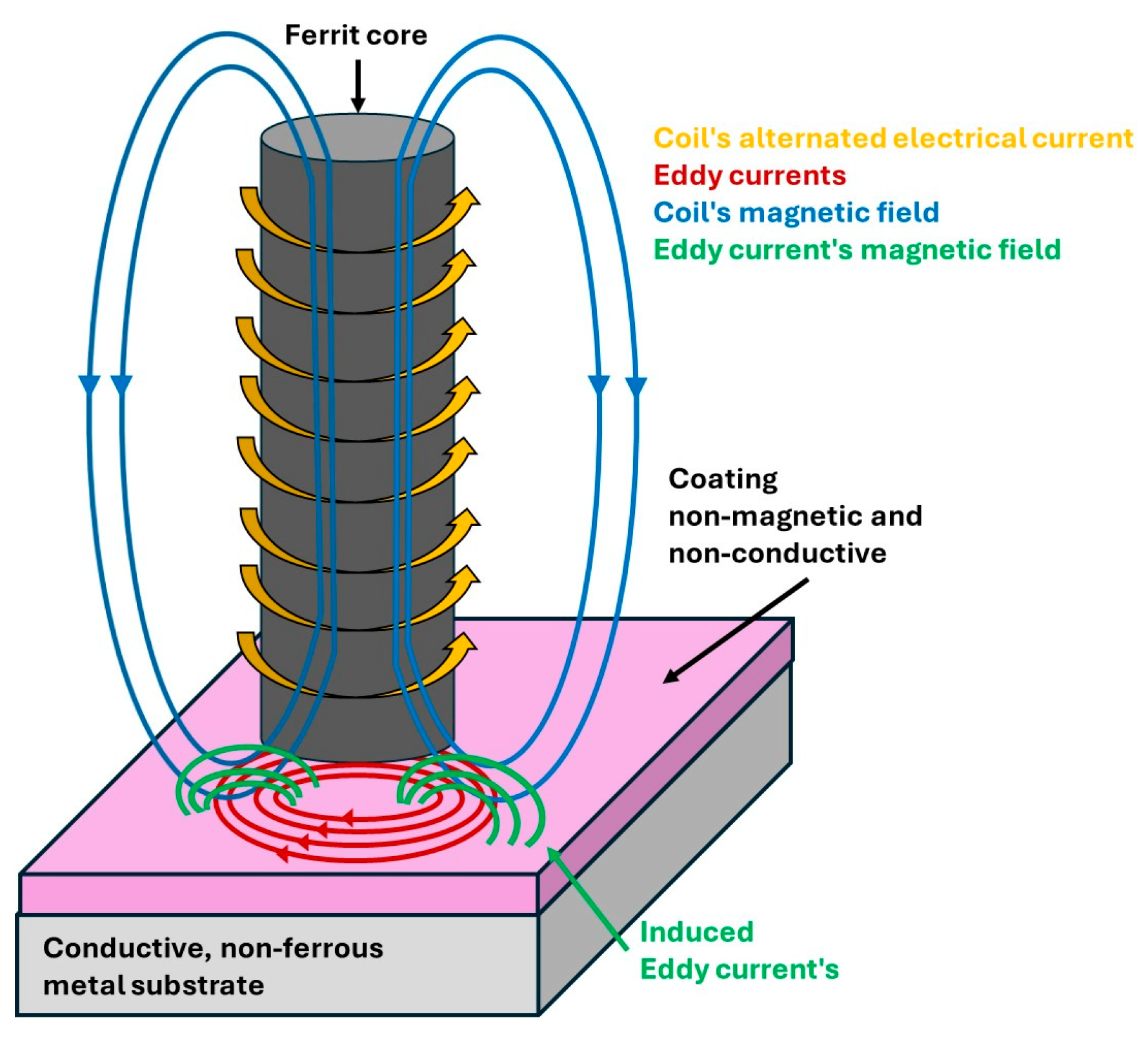
2.4. Eddy Current
3. Results and Discussion
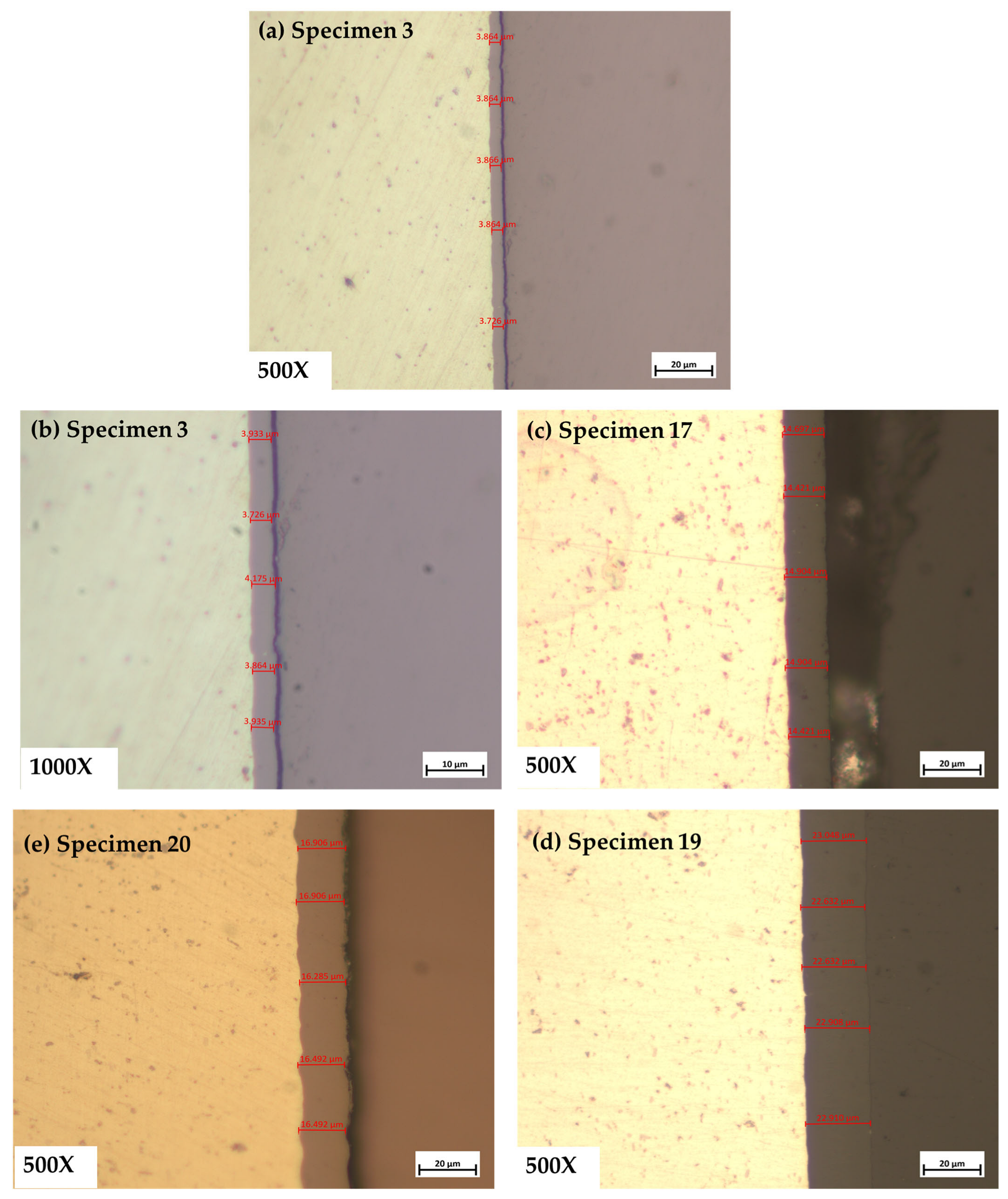
3.1. Thickness Measurement Using Optical Microscopy
3.2. Thickness Measurement via Eddy Current
3.2.1. Measurement with Calibration of Each Specimen
3.2.2. Measurement with Calibration Every 10 Specimens
3.2.3. Error Percentage of Thickness Measurement for the Different Techniques

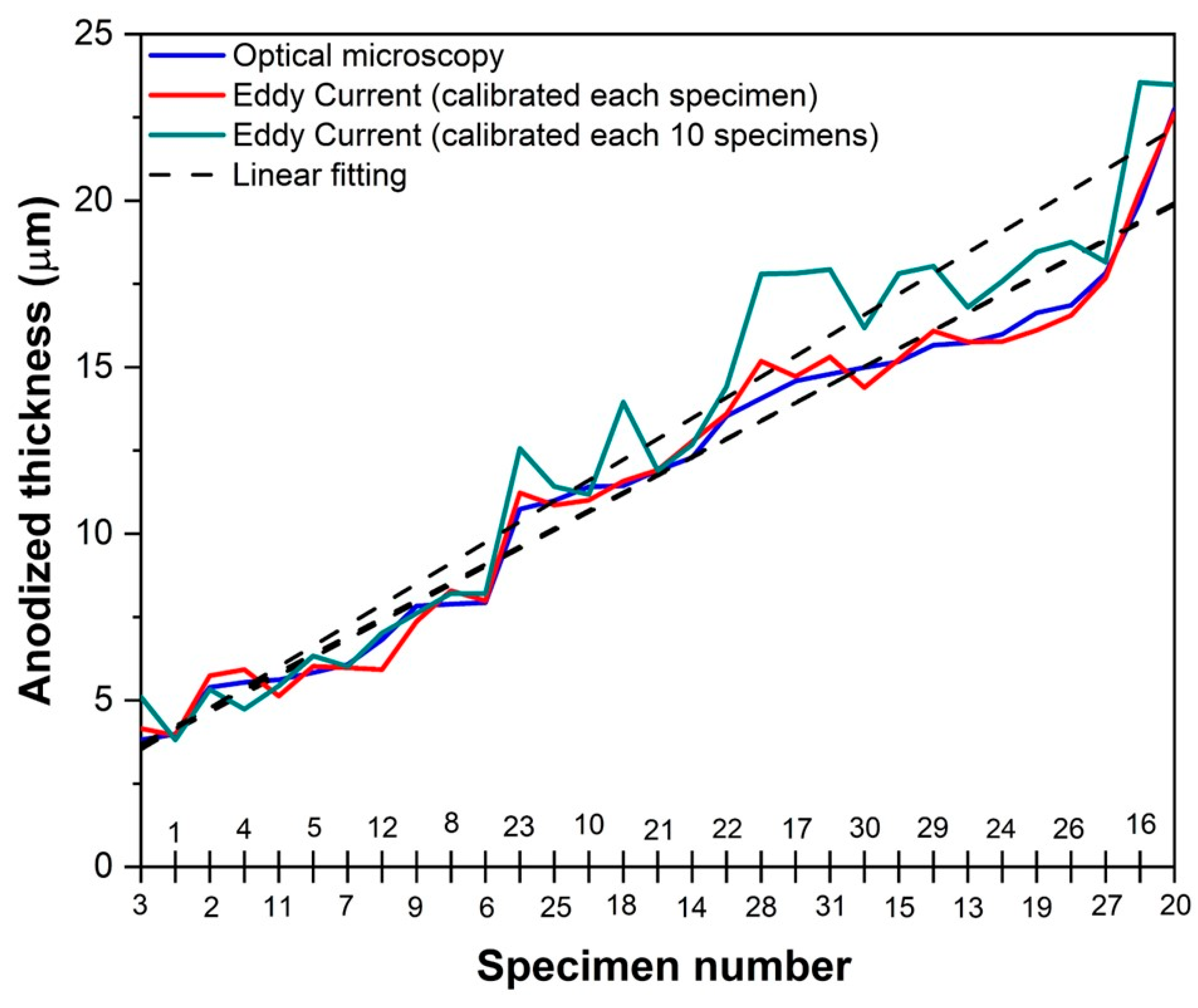
3.3. Comparison of Measurement Methods
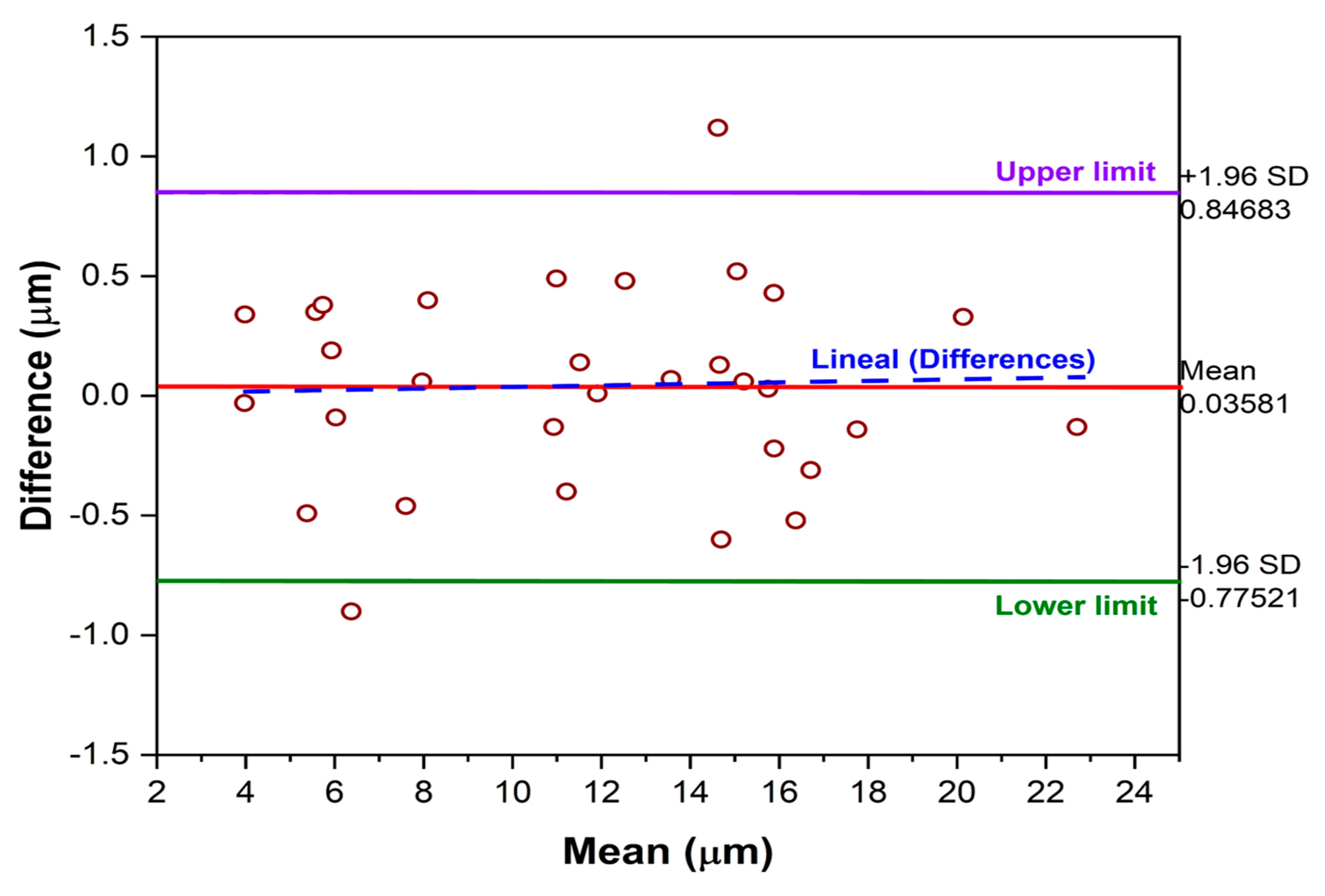
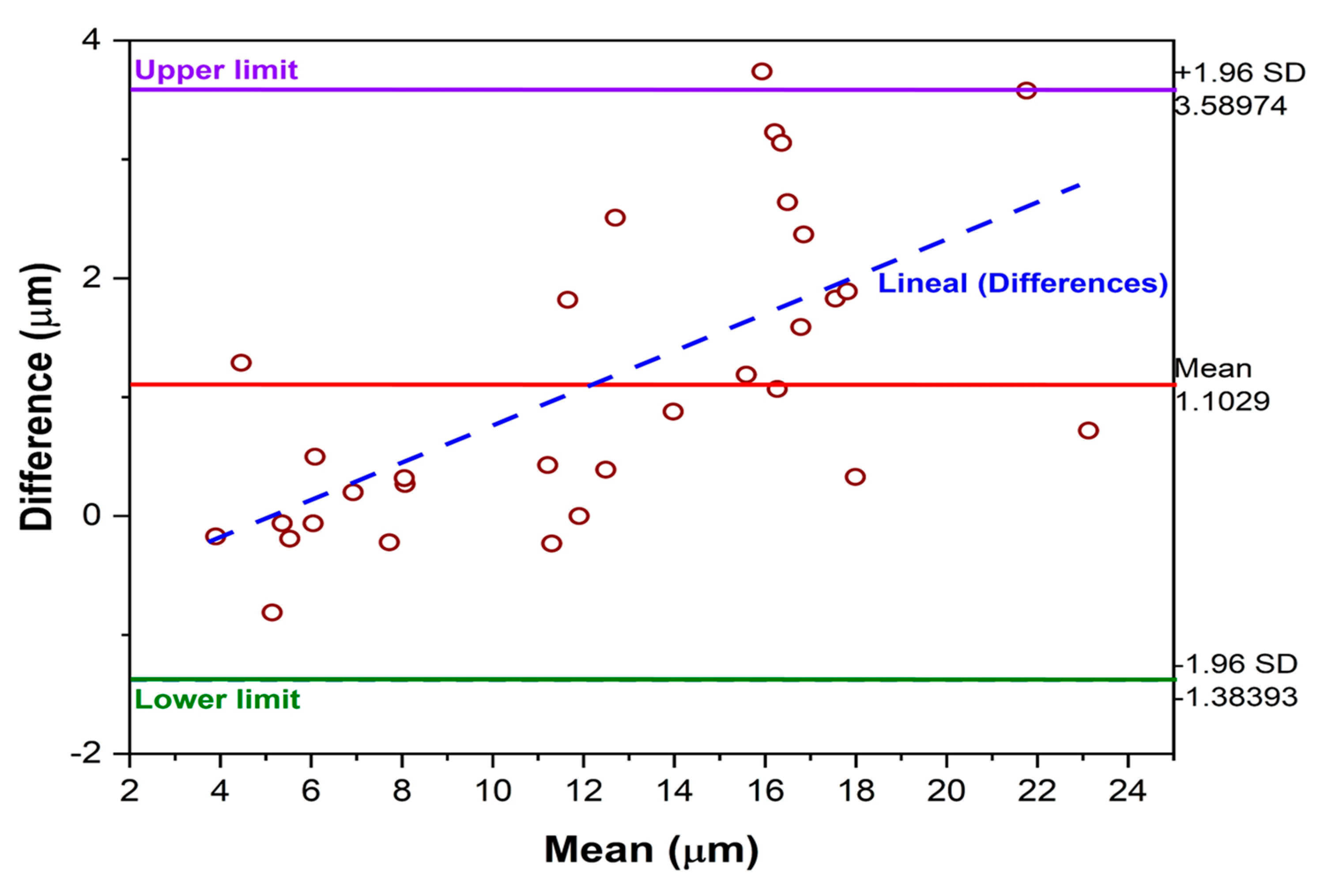
3.3.1. Bland–Altman Method for Measurement with Calibration
3.3.2. Bland–Altman Method for Measurement with Calibration Every 10 Specimens
4. Conclusions
- A comparative analysis of the traditional optical microscopy measurement method and the Eddy current method, utilizing various calibration frequencies to ascertain the anodized layer thickness in a 6063 aluminum alloy, facilitated a thorough assessment of the concordance, precision and applicability of the novel method under controlled conditions.
- Since optical microscopy can only measure a certain portion of the specimen that has undergone metallographic preparation, it cannot offer a comprehensive assessment of the thickness of the anodized layer.
- The results obtained using the Eddy current method demonstrate that calibrating the device before each measurement is the most accurate option, since it presents an error percentage of 3.4%.
- Calibrating the device every 10 samples yields an error rate of 9.9%, indicating that the first methodology is approximately three times more accurate.
- The use of the Eddy current device enables the determination of anodized layer thickness at multiple locations along the specimen, which facilitates a more effective detection of layer uniformity than the optical microscopy method.
- The results show that calibrating the device prior to each measurement is the most accurate option, since the Eddy current method yields an error percentage of 3.4%.
- The method of calibrating Eddy currents every ten samples presented a greater error with respect to the reference method and overestimated the thickness by 1.10 μm with respect to the reference value obtained via optical microscopy.
- The Bland–Altman analysis for the Eddy current method calibrated every 10 specimens revealed an increasing trend in the discrepancy between methods as the thickness of the anodized layer increased.
- Calibration is a determining factor in the accuracy of the alternative method; the results are acceptable for lower thicknesses, since with thicknesses greater than 15 μm, the deviations in the measurements become more noticeable.
Research Limitations
- For this research, only anodized 6063 aluminum alloy materials were used, where the current density, time and concentration of the solution were varied, and the effect of each parameter on the coating thickness was not evaluated.
- This work only presents data from optical microscopy and Eddy current thickness measurements in a 6063 aluminum alloy. The results may not be applicable to other aluminum alloys due to the composition of the alloys, the heat treatments applied, the anodization parameters, such as time, solution concentration and applied current density, and the parameters involved in the measurements.
- The thickness measurement range of between 3 and 25 μm is derived from the anodization conditions used on the samples. These anodization conditions are presented in Table 2 of this document.
- The number of samples used in this study is limited, as they are samples used in the design of experiments focused on evaluating the effects of process variables on the characteristics of anodization, which is not the objective of this research study.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muñoz, J.A.; Komissarov, A.; Avalos, M.; Bolmaro, R.E. Heat Treatment effect on an AA6063 alloy. Mater. Lett. 2020, 277, 128338. [Google Scholar] [CrossRef]
- Muñoz, J.A.; Khelfa, T.; Duarte, G.A.; Avalos, M.; Bolmaro, R.; Cabrera, J.M. Plastic Behavior and Microstructure Heterogeneity of an AA6063-T6 Aluminum Alloy Processed by Symmetric and Asymmetric Rolling. Metals 2022, 12, 1551. [Google Scholar] [CrossRef]
- Bensalah, W.; Elleuch, K.; Feki, M.; Gigandet, M.P.; Ayedi, H.F. Optimization of mechanical and chemical properties of sulphuric anodized aluminium using statistical experimental methods. Mat. Chem. Phys. 2008, 108, 296–305. [Google Scholar] [CrossRef]
- Martínez-Ramos, C.; Olguin-Coca, J.; Lopez-Leon, L.D.; Gaona-Tiburcio, C.; Lara-Banda, M.; Maldonado-Bandala, E.; Castañeda-Robles, I.; Jaquez-Muñoz, J.M.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; et al. Electrochemical Noise Analysis Using Experimental Chaos Theory, Power Spectral Density and Hilbert–Huang Transform in Anodized Aluminum Alloys in Tartaric–Phosphoric–Sulfuric Acid Solutions. Metals 2023, 13, 1850. [Google Scholar] [CrossRef]
- Vergara-Guillén, L.E.; Nerey-Carvajal, L.M.; Guedez Torcates, V.M. Oxide film thickness and microhardness prediction model of Al3003-B14 and Al6063-T6 anodized aluminum using multifactorial analysis. Ingeniare Rev. Chil. De Ing. 2011, 19, 186–195. [Google Scholar] [CrossRef]
- Thompson, G.E. Porous anodic alumina: Fabrication, characterization and applications. Thin Solid Films 1997, 297, 192–201. [Google Scholar] [CrossRef]
- ASTM B487-85; Standard Test Method for Measurement of Metal and Oxide Coating Thickness by Microscopical Examination of Cross Section. ASTM International: West Conshohocken, PA, USA, 2020.
- Chen, L.; Zhou, Y.; Zhou, R.; Hong, M. Microsphere-Toward future of optical microscopes. Iscience 2020, 23, 101211. [Google Scholar] [CrossRef]
- Giurlani, W.; Berretti, E.; Innocenti, M.; Lavacchi, A. Measuring the Thickness of Metal Coatings: A Review of the Methods. Coatings 2020, 10, 1211. [Google Scholar] [CrossRef]
- Machado, M.A. Eddy Currents Probe Design for NDT Applications: A Review. Sensors 2024, 24, 5819. [Google Scholar] [CrossRef]
- Sitko, R. Quantitative X-ray fluorescence analysis of samples of less than “infinite thickness”: Difficulties and possibilities. Spectrochim. Acta Part B At. Spectrosc. 2009, 64, 1161–1172. [Google Scholar] [CrossRef]
- Lopes, F.; Cardozo Amorin, L.H.; da Silva Martins, L.; Urbano, A.; Roberto Appoloni, C.; Cesareo, R. Thickness measurement of V2O5 nanometric thin films using a portable XRF. J. Spectrosc. 2016, 2016, 9509043. [Google Scholar] [CrossRef]
- Criss, J.W.; Birks, L.S. Calculation methods for fluorescent X-ray spectrometry: Empirical coefficients vs. fundamental parameters. Anal. Chem. 1968, 40, 1080–1086. [Google Scholar] [CrossRef]
- Giurlani, W.; Berretti, E.; Lavacchi, A.; Innocenti, M. Thickness determination of metal multilayers by ED-XRF multivariate analysis using Monte Carlo simulated standards. Anal. Chim. Acta 2020, 1130, 72–79. [Google Scholar] [CrossRef]
- Giurlani, W.; Berretti, E.; Innocenti, M.; Lavacchi, A. Coating Thickness determination using X-ray fluorescence spectroscopy: Monte Carlo simulations as an alternative to the use of standards. Coatings 2019, 9, 79. [Google Scholar] [CrossRef]
- Pessanha, S.; Manso, M.; Antunes, V.; Carvalho, M.L.; Sampaio, J.M. Monte Carlo simulation of portable X-ray fluorescence setup: Non-invasive determination of gold leaf thickness in indo-Portuguese panel paintings. Spectrochim. Acta Part B At. Spectrosc. 2019, 156, 1–6. [Google Scholar] [CrossRef]
- Malarde, D.; Powell, M.J.; Quesada-Cabrera, R.; Wilson, R.L.; Carmalt, C.J.; Sankar, G.; Parkin, I.P.; Palgrave, R.G. Optimized atmospheric-pressure chemical vapor deposition thermochromic VO2 thin films for intelligent window applications. ACS Omega 2017, 2, 1040–1046. [Google Scholar] [CrossRef]
- Krumrey, M.; Gleber, G.; Scholze, F.; Wernecke, J. Synchrotron radiation-based X-ray reflection and scattering techniques for dimensional nanometrology. Meas. Sci. Technol. 2011, 22, 094032. [Google Scholar] [CrossRef]
- Serafi’nczuk, J.; Pietrucha, J.; Schroeder, G.; Gotszalk, T.P. Thin film thickness determination using X-ray reflectivity and Savitzky-Golay algorithm. Opt. Appl. 2011, 41, 315–322. [Google Scholar]
- Sokolov, S.A.; Milovanov, R.A.; Sidorov, L.N. Determination of the thickness of thin films based on scanning electron microscopy and energy dispersive X-ray analysis. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2019, 13, 836–847. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.M.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-Ray Microanalysis; Springer Publishing: New York, NY, USA, 2018; ISBN 978-1-4939-6674-5. [Google Scholar]
- Jablonski, A. Evaluation of procedures for overlayer thickness determination from XPS intensities. Surf. Sci. 2019, 688, 14–24. [Google Scholar] [CrossRef]
- Walton, J.; Alexander, M.R.; Fairley, N.; Roach, P.; Shard, A.G. Film thickness measurement and contamination layer correction for quantitative XPS. Surf. Interface Anal. 2016, 48, 164–172. [Google Scholar] [CrossRef]
- Cumpson, P.J. The thickogram: A method for easy film thickness measurement in XPS. Surf. Interface Anal. 2000, 29, 403–406. [Google Scholar] [CrossRef]
- Nave, F.; Nave, J. Técnicas estadísticas utilizadas en la comparación de métodos cuantitativos de medición. Cienc. Tecnol. Sal. 2023, 10, 53–75. [Google Scholar] [CrossRef]
- Myung, J.I.; Pitt, M.A. Model comparison methods. Methods Enzymol. 2004, 383, 351–366. [Google Scholar] [CrossRef]
- Deng, W.; Wang, J.; Zhang, R. Measures of concordance and testing of independence in multivariant structure. J. Multivar. Anal. 2022, 191, 105035. [Google Scholar] [CrossRef]
- Tommervik, L.; Shreiber, D.; Pope, D. Comparing Thickness Measurement Methods Using Bland–Altman Analysis. DEVCOM ARL 2021, 9326. Available online: https://apps.dtic.mil/sti/trecms/pdf/AD1149580.pdf (accessed on 12 April 2025).
- Cabral Miramontes, J.C.; Gaona Tiburcio, C.; García Mata, E.; Esneider Alcála, M.Á.; Maldonado-Bandala, E.; Lara-Banda, M.; Nieves-Mendoza, D.; Olguín-Coca, J.; Zambrano-Robledo, P.; López-León, L.D.; et al. Corrosion Resistance of Aluminum Alloy AA2024 with Hard Anodizing in Sulfuric Acid-Free Solution. Materials 2022, 15, 6401. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.; Almeraya-Calderón, F.; López, F.E.; Lara Banda, M.; Olguín-Coca, J.; López-León, L.D.; Castañeda-Robles, I.; Alcalá, M.Á.E.; Zambrano-Robledo, P.; Gaona-Tiburcio, C. Citric Acid as an Alternative to Sulfuric Acid for the Hard-Anodizing of AA6061. Metals 2021, 11, 1838. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Chacón-Nava, J.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.M.; Delgado, A.D.; Flores-De Los Rios, J.P.; Bocchetta, P.; Almeraya-Calderón, F. Electrochemical Corrosion of Titanium and Titanium Alloys Anodized in H2SO4 and H3PO4 Solutions. Coatings 2022, 12, 325. [Google Scholar] [CrossRef]
- ASTM E3-95; Standard Practice for Preparation of Metallographic Specimens. ASTM International: West Conshohocken, PA, USA, 1995.
- Sardellitti, A.; Milano, F.; Laracca, M.; Ventre, S.; Ferrigno, L.; Tamburrino, A. An Eddy-Current Testing Method for Measuring the Thickness of Metallic Plates. IEEE Trans. Instrum. Meas. 2023, 72, 1–10. [Google Scholar] [CrossRef]
- Liu, P.; Singh, V.P.; Rajaputra, S. Barrier layer non-uniformity effects in anodized aluminum oxide nanopores on ITO substrates. Nanotechnology 2010, 21, 115303. Available online: https://iopscience.iop.org/article/10.1088/0957-4484/21/11/115303 (accessed on 12 February 2025). [CrossRef]
- Pu, Y.; Hu, J.; Yao, T.; Li, L.; Zhao, J.; Guo, Y. Influence of anodization parameters on film thickness and volume expansion of thick- and large-sized anodic aluminum oxide film. J. Mater. Sci. Mater. Electron. 2021, 32, 13708–13718. [Google Scholar] [CrossRef]
- Villegas-Tovar, J.; Gaona-Tiburcio, C.; Lara-Banda, M.; Maldonado-Bandala, E.; Baltazar-Zamora, M.A.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Olguin-Coca, J.; Estupiñan-Lopez, F.; Almeraya-Calderón, F. Electrochemical Corrosion Behavior of Passivated Precipitation Hardening Stainless Steels for Aerospace Applications. Metals 2023, 13, 835. [Google Scholar] [CrossRef]
- Rodger, J.; Bartlett, S.; Atrens, A. Corrosion of the galvanizing of galvanized-steel electricity transmission towers. Mater. Corros. 2017, 68, 902–910. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statical methods for assessing agreement between two methods of clinical measurements. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Yellareddygari, S.K.R.; Gudmestad, N.C. Bland-Altman comparison of two methods for assessing severity of Verticillium wilt of potato. Crop Prot. 2017, 101, 68–75. [Google Scholar] [CrossRef]
- Tiptona, E.; Shusterb, J. A framework for the meta-analysis of Bland–Altman studies based on a limits of agreement approach. Stat. Med. 2017, 36, 3621–3635. [Google Scholar] [CrossRef]
- Goedhart, J.; Rishniw, M. BA-plotteR—A web tool for generating Bland-Altman plots and constructing limits of agreement. Res. Vet. Sci. 2021, 137, 281–286. [Google Scholar] [CrossRef]
- Moore, A.R. A review of Bland–Altman difference plot analysis in the veterinary clinical pathology laboratory. Vet. Clin. Pathol. 2024, 53, 75–85. [Google Scholar] [CrossRef]
- Mansournia, M.; Waters, R.; Nazemipour, M.; Bland, M.; Altman, D. Bland-Altman methods for comparing methods of measurement and response to criticisms. Glob. Epidemiol. 2021, 3, 100045. [Google Scholar] [CrossRef]
- Haghayegh, S.; Kang, H.; Khoshnevis, S.; Smolensky, M.; Diller, K. A comprehensive guideline for Bland–Altman and intra class correlation calculations to properly compare two methods of measurement and interpret findings. Physiol. Meas. 2020, 41, 055012. [Google Scholar] [CrossRef]
- Doğan, N.O. Bland-Altman analysis: A paradigm to understand correlation and agreement. TJEM 2018, 18, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef]
- Almeida, G.; Gonzalez, J.; Rosado, L.; Vilaça, P.; Santos, T.G. Advances in NDT and materials characterization by eddy currents. Procedia CIRP 2013, 7, 359–364. [Google Scholar] [CrossRef]
- Xie, Y.; Li, J.; Tao, Y.; Wang, S.; Yin, W.; Xu, L. Edge Effect Analysis and Edge Defect Detection of Titanium Alloy Based on Eddy Current Testing. Appl. Sci. 2020, 10, 8796. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, Q.; Du, W.; Zhang, B. Edge Effect on Eddy Current Detection for Subsurface Defects in Titanium Alloys. In Proceedings of the 8th International Conference on Computational Methods (ICCM2017), Guilin, China, 25–29 July 2017. [Google Scholar]
- Gerke, O. Reporting Standards for a Bland–Altman Agreement Analysis: A Review of Methodological Reviews. Diagnostics 2020, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Graubard, B.; Ioannidis, J.P.A. Use and reporting of Bland–Altman analyses in studies of self-reported versus measured weight and height. IJO 2020, 44, 1311–1318. [Google Scholar] [CrossRef]
- ISO 21968:2019; Non-magnetic metallic coatings on metallic and non-metallic basis materials—Measurement of coating thickness—Phase-sensitive eddy-current method. International Organization for Standardization: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/73285.html (accessed on 4 March 2025).
- Francq, B.G.; Govaerts, B. How to regress and predict in a Bland–Altman plot? Review and contribution based on tolerance intervals and correlated-errors-in-variables models. Stat. Med. 2016, 35, 2328–2358. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Miyata, K.; Yoshikawa, K.; Mizukami, M. A comparison between the shoulder shifting test and seated reaching test by bland–altman plot: A pilot study. J. Spinal Cord Med. 2022, 47, 522–529. [Google Scholar] [CrossRef]
- Cabral, J.; Pedraza, G.K.; Gaona, C.; Zambrano, P.; Poblano, C.A.; Almeraya, F. Coatings characterization of Ni-based alloy applied by HVOF. Aircr. Eng. Aerosp. Tec. 2018, 90, 336–343. [Google Scholar] [CrossRef]
- Gaona-Tiburcio, C.; Montoya, R.M.; Cabral, M.J.A.; Estupiñan, L.F.; Zambrano, R.P.; Orozco, C.R.; Chacon-Nava, J.G.; Baltazar, Z.M.A.; Almeraya-Calderon, F. Corrosion Resistance of Multilayer Coatings Deposited by PVD on Inconel 718 Using Electrochemical Impedance Spectroscopy Technique. Coatings 2020, 10, 521. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Comparing methods of measurement: Why plotting difference against standard method is misleading. Lancet 1995, 346, 1085–1087. [Google Scholar] [CrossRef]
- Carrasco, J.L.; Jover, L. Métodos estadísticos para evaluar la concordancia. Med. Clin. 2004, 122 (Suppl. 1), 28–34. Available online: https://www.elsevier.es/es-revista-medicina-clinica-2-articulo-metodos-estadisticos-evaluar-concordancia-13057543 (accessed on 12 February 2025). [CrossRef]
- Samaniego-Gámez, P.; Almeraya-Calderón, F.; Martin, U.; Ress, J.; Gaona-Tiburcio, C.; Silva-Vidaurri, L.; Cabral-Miramontes, J.; Bastidas, J.M.; Chacón-Nava, J.G.; Bastidas, D.M. Efecto del tratamiento de sellado en el comportamiento frente a corrosión de la aleación anodizada de aluminio-litio AA2099. Rev. Met. 2020, 56, e180. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Comparing two methods of clinical measurement: A personal history. Int. J. Epidem. 1995, 24 (Suppl. 1), S7–S14. [Google Scholar] [CrossRef] [PubMed]


| Elements | Al | Si | Fe | Cu | Mn | Mg | Cr | Ti | Zn |
|---|---|---|---|---|---|---|---|---|---|
| AA 6063 | Bal | 0.55 | 0.06 | 0.004 | 0.006 | 0.83 | 0.002 | 0.002 | 0.007 |
| Nominal | Bal | 0.20–0.60 | 0.0–0.35 | 0.0–0.10 | 0.0–0.10 | 0.45–0.90 | 0.0–0.10 | 0.0–0.10 | 0.0–0.10 |
| Specimen Number | Time (min) | Current Density of Anodized Samples (A/dm2) | Concentration of H2SO4 (g/L) | Specimen Number | Time (min) | Current Density of Anodized Samples (A/dm2) | Concentration of H2SO4 (g/L) |
|---|---|---|---|---|---|---|---|
| 1 | 10 | 1.27 | 180 | 17 | 15 | 2.5 | 180 |
| 2 | 10 | 1.79 | 180 | 18 | 15 | 2.5 | 350 |
| 3 | 10 | 1.27 | 350 | 19 | 15 | 3.5 | 350 |
| 4 | 10 | 1.79 | 350 | 20 | 15 | 3.5 | 180 |
| 5 | 15 | 1.27 | 180 | 21 | 26 | 1.5 | 180 |
| 6 | 15 | 1.79 | 180 | 22 | 30 | 1.5 | 180 |
| 7 | 15 | 1.27 | 350 | 23 | 13.5 | 3.0 | 265 |
| 8 | 15 | 1.79 | 350 | 24 | 21.7 | 3.0 | 265 |
| 9 | 20 | 1.27 | 350 | 25 | 17.5 | 2.15 | 265 |
| 10 | 20 | 1.79 | 350 | 26 | 17.5 | 3.84 | 265 |
| 11 | 26 | 0.76 | 180 | 27 | 17.5 | 3.84 | 2.65 |
| 12 | 30 | 0.76 | 180 | 28 | 17.5 | 3.0 | 122 |
| 13 | 20 | 2.5 | 180 | 29 | 17.5 | 3.0 | 407 |
| 14 | 20 | 2.5 | 350 | 30 | 17.5 | 3.0 | 265 |
| 15 | 20 | 3.5 | 350 | 31 | 17.5 | 3.0 | 2.65 |
| 16 | 20 | 3.5 | 180 |
| Specimen Number | Optical Microscopy (μm) | Eddy Current Calibrated for Each Specimen (μm) | Eddy Current Calibrated Every 10 Specimens (μm) |
|---|---|---|---|
| 1 | 3.98 | 3.95 | 3.81 |
| 2 | 5.39 | 5.74 | 5.33 |
| 3 | 3.81 | 4.15 | 5.1 |
| 4 | 5.54 | 5.92 | 4.73 |
| 5 | 5.83 | 6.02 | 6.33 |
| 6 | 7.93 | 7.99 | 8.2 |
| 7 | 6.07 | 5.98 | 6.01 |
| 8 | 7.89 | 8.29 | 8.21 |
| 9 | 7.83 | 7.37 | 7.61 |
| 10 | 11.41 | 11.01 | 11.18 |
| 11 | 5.62 | 5.13 | 5.43 |
| 12 | 6.82 | 5.92 | 7.02 |
| 13 | 15.73 | 15.76 | 16.8 |
| 14 | 12.29 | 12.77 | 12.68 |
| 15 | 15.17 | 15.23 | 17.81 |
| 16 | 19.97 | 20.3 | 23.55 |
| 17 | 14.59 | 14.72 | 17.82 |
| 18 | 11.44 | 11.58 | 13.95 |
| 19 | 16.63 | 16.11 | 18.46 |
| 20 | 22.76 | 22.63 | 23.48 |
| 21 | 11.9 | 11.91 | 11.9 |
| 22 | 13.53 | 13.6 | 14.41 |
| 23 | 10.74 | 11.23 | 12.56 |
| 24 | 15.99 | 15.77 | 17.58 |
| 25 | 10.99 | 10.86 | 11.42 |
| 26 | 16.86 | 16.55 | 18.75 |
| 27 | 17.82 | 17.68 | 18.15 |
| 28 | 14.06 | 15.18 | 17.8 |
| 29 | 15.66 | 16.09 | 18.03 |
| 30 | 14.99 | 14.39 | 16.18 |
| 31 | 14.79 | 15.31 | 17.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabral-Miramontes, J.; Gaona-Tiburcio, C.; Maldonado-Bandala, E.; Vera Cervantes, D.; Nieves-Mendoza, D.; Mendez-Ramirez, C.T.; Lara-Banda, M.; Baltazar-Zamora, M.A.; Olguin-Coca, J.; Almeraya-Calderon, F. Comparative Method Between Eddy Current and Optical Microscopy in the Determination of Thickness of 6063 Aluminum Alloy Anodization. Appl. Sci. 2025, 15, 9025. https://doi.org/10.3390/app15169025
Cabral-Miramontes J, Gaona-Tiburcio C, Maldonado-Bandala E, Vera Cervantes D, Nieves-Mendoza D, Mendez-Ramirez CT, Lara-Banda M, Baltazar-Zamora MA, Olguin-Coca J, Almeraya-Calderon F. Comparative Method Between Eddy Current and Optical Microscopy in the Determination of Thickness of 6063 Aluminum Alloy Anodization. Applied Sciences. 2025; 15(16):9025. https://doi.org/10.3390/app15169025
Chicago/Turabian StyleCabral-Miramontes, Jose, Citlalli Gaona-Tiburcio, Erick Maldonado-Bandala, Daniel Vera Cervantes, Demetrio Nieves-Mendoza, Ce Tochtli Mendez-Ramirez, Maria Lara-Banda, Miguel Angel Baltazar-Zamora, Javier Olguin-Coca, and Facundo Almeraya-Calderon. 2025. "Comparative Method Between Eddy Current and Optical Microscopy in the Determination of Thickness of 6063 Aluminum Alloy Anodization" Applied Sciences 15, no. 16: 9025. https://doi.org/10.3390/app15169025
APA StyleCabral-Miramontes, J., Gaona-Tiburcio, C., Maldonado-Bandala, E., Vera Cervantes, D., Nieves-Mendoza, D., Mendez-Ramirez, C. T., Lara-Banda, M., Baltazar-Zamora, M. A., Olguin-Coca, J., & Almeraya-Calderon, F. (2025). Comparative Method Between Eddy Current and Optical Microscopy in the Determination of Thickness of 6063 Aluminum Alloy Anodization. Applied Sciences, 15(16), 9025. https://doi.org/10.3390/app15169025









