Featured Application
The findings of this review offer valuable insights into the microbiological air quality of healthcare environments, with practical implications for hospital administrators, infection control specialists, and policymakers. By identifying the most prevalent airborne microorganisms associated with healthcare-associated infections, along with their antibiotic resistance profiles, this study provides evidence for the development of more effective air quality management strategies.
Abstract
Exposure to microorganisms can significantly impact well-being and, more importantly, human health. A frequently overlooked aspect of indoor air quality (IAQ) research is the risk posed by harmful biological agents transported through the air in the form of biological aerosols. Given that healthcare facilities create environments with an increased risk of infection transmission, monitoring IAQ and reducing microbiological contamination have become global public health challenges. This paper presents a literature review, focusing on the current state of knowledge regarding microbiological air quality in healthcare settings. The analysis confirms that Escherichia coli and Staphylococcus aureus are among the most prevalent airborne pathogens in healthcare facilities. The review also underlines the necessity for harmonized guidelines and integrated air quality management strategies to reduce microbial contamination effectively. Finally, the review compiles data on microorganism concentration levels and influencing factors. The present study highlights that implementing standardized monitoring and effective air filtration and disinfection methods is essential to improving microbiological air quality and enhancing patient safety. The sources analyzed in this review were collected from databases such as PubMed, ScienceDirect, ResearchGate, and Web of Science, considering only English-language publications. The studies cited were conducted in multiple countries across different regions, providing a comprehensive global perspective on the issue.
1. Introduction
One of the key parameters determining indoor air quality (IAQ) is the concentration of pollutants. These pollutants comprise various categories, including biological contaminants, which may originate from both indoor and outdoor sources. Exposure to microorganisms such as fungi, bacteria, or viruses can significantly affect human well-being and, more importantly, health. Researchers often overlook, yet must address, the presence of harmful biological agents transmitted in the form of bioaerosols as a critical component of IAQ. Bioaerosols represent a crucial category of airborne agents that require special attention, especially in indoor environments. They consist of airborne particles composed of or derived from biological entities, typically ranging in size from a few nanometers to over 100 μm [1]. Bioaerosols include microorganisms such as bacteria, fungi, and viruses, as well as pollen, spores, and fragments of plant or animal origin [2]. Both natural and anthropogenic sources, such as vegetation, soil, water, human activity, and indoor environments, release these particles. Smaller particles can remain suspended in the air for extended periods and penetrate more deeply into the respiratory tract, which increases their potential to harm human health [3].
In healthcare settings, bioaerosols significantly contribute to the spread of infection. They play an essential role in transmitting healthcare-associated infections (HAIs), which present a significant challenge for healthcare systems [4,5,6]. HAIs develop as a result of a patient’s stay in a medical facility or medical procedures performed there, with symptoms typically appearing at least 48 h after admission [7]. Studies estimate that airborne bioaerosols cause approximately 10–20% of HAIs and hospital contamination [8]. These pathogens threaten the health of patients, healthcare workers, and visitors. Limited public awareness of biological contamination in indoor environments and potential transmission routes further exacerbates the problem [9].
Epidemiological data reflect the scale of the problem. The World Health Organization (WHO) reports that HAIs affect approximately one in ten patients worldwide, with higher rates in low- and middle-income countries. High-risk patient groups experience similar trends. The European Centre for Disease Prevention and Control (ECDC) reports that, on any given day, at least 1 in 15 hospitalized patients and 1 in 26 residents of long-term care facilities acquire HAI. Across Europe, HAIs occur in an estimated 8.9 million cases annually [4,5,7].
Not all microorganisms are pathogenic. Three principal parameters, i.e., species composition, concentration, and aerodynamic diameter, determine how bioaerosols affect human health. The aerodynamic diameter influences how deeply inhaled particles are deposited in specific regions of the respiratory system and how these particles interact with human cells [10,11,12].
Given that the unique environment of healthcare facilities elevates the risk of infection transmission, controlling IAQ and reducing microbiological contamination have emerged as global priorities in public health. Consequently, ensuring safe IAQ is now widely recognized as a critical worldwide challenge.
This paper presents a literature review on microbiological air quality in healthcare environments, with a focus on associated health risks, commonly used monitoring techniques, and identified research gaps. Sources were retrieved from PubMed, ScienceDirect, ResearchGate, and Web of Science, considering only publications in English from reputable scientific journals to ensure substantive quality and credibility. No strict publication date limits were applied; however, the reviewed studies spanned the years 2000–2025, with most originating from the last two decades.
Studies were selected based on predefined inclusion criteria to ensure methodological rigor and thematic relevance. These criteria encompassed publications in peer-reviewed and reputable scientific journals, direct relevance to the core research topic, geographic diversity of study locations, the use of active microbiological sampling techniques, and full-text availability in an accessible language. Keywords used for the literature search included “bioaerosols,” “airborne microorganisms,” “hospital,” “air quality,” “indoor air,” “healthcare facilities,” and “infections.” The detailed criteria are summarized in Table 1.

Table 1.
Criteria for article selection.
This methodological approach contributed to a coherent organization and interpretation of the reviewed literature. The consistent application of standardized methodologies in future studies, combined with larger datasets, may facilitate more robust comparisons and provide a basis for meta-analyses or causal inference. In line with these methods, the present review aimed to (1) synthesize current knowledge on microbiological air quality in healthcare environments; (2) evaluate associated health risks; (3) summarize monitoring approaches; and (4) identify gaps requiring further research and propose corresponding priorities.
2. Bioaerosols
Humans are the primary source of bioaerosols in indoor air [13,14,15]. During sneezing, coughing, and even speaking, microorganisms are released into the air, where they can remain suspended, settle on various surfaces, or be inhaled by individuals nearby [16]. Inhalation of polluted air has been shown to impair the respiratory system’s defense mechanisms, thereby increasing susceptibility to infections from airborne pathogens [17,18]. Consequently, in addition to exposure to contaminated indoor air, infection may also occur through contact with contaminated surfaces or direct interaction with an infected individual. The presence of bioaerosols in indoor air can lead to serious health concerns [19]. Studies have shown that the concentration of bacteria in ambient air can decrease when speaking because the human respiratory system can retain some of the bacteria and particles [20]. Airborne biological particles, depending on their size, can reach different regions of the respiratory system. However, special attention must be given to the respirable fraction, as particles with a diameter ranging from 1.1 to 3.3 μm penetrate the secondary and tertiary bronchi, while the smallest, not exceeding 1.1 μm, can reach the pulmonary bronchioles, which are the areas constituting the lower parts of the respiratory system (Figure 1) [10,21]. Such exposure promotes the development of serious diseases, including pneumonia and tuberculosis, due to the penetration of bacteria into the deeper parts of the respiratory tract. Furthermore, the presence of bacteria and fungi in the air can contribute to allergic and chronic conditions such as hay fever, asthma, sinusitis, conjunctivitis, and bronchitis [22,23].
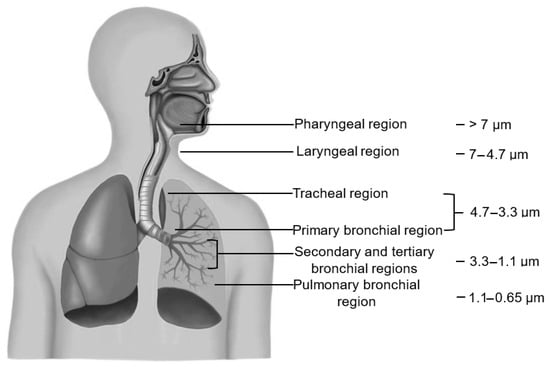
Figure 1.
Regions of respiratory system with particle size settling at specific depths. Based on [24].
In healthcare facilities, a significant number of individuals, including many patients with pre-existing infections, move through the premises daily. This dynamic environment increases the risk of infection transmission, making air quality control and the reduction of bioaerosols a key public health challenge.
A significant influence on the presence and concentration of biological aerosols in the hospital environment is exerted by external factors. While some studies do not indicate a correlation, the majority of studies in the literature suggest their considerable impact [25,26,27]. Among external factors, atmospheric conditions and seasonal variability are the most important. Changes in weather and the resulting alterations in air parameters directly influence contamination levels, as different microorganisms increase under distinct environmental conditions. Another significant factor is the facility’s location; urban areas often exhibit different types and concentrations of contaminants compared to greener, less industrialized regions. Finally, the age of the building also affects contamination levels, as advances in construction materials, building technologies, and the condition of ventilation systems over time have a direct impact on their operational efficiency [14,28,29]. Although atmospheric conditions and seasonal factors are frequently examined in scientific studies, the influence of a building’s structural characteristics is far less commonly considered. The properties of building materials, such as porosity and surface roughness, can promote the settlement of microorganisms and create favorable conditions for their growth. In the case of older buildings, analyzing the chemical composition of the materials used is also crucial, as they may influence the proliferation of microorganisms. Research indicates that the technical condition of healthcare facilities can significantly affect the frequency of hospital-acquired infections. Many facilities, particularly older ones, fail to meet current hygienic and technical standards for medical premises, thereby increasing the risk of microbiological contamination in the indoor environment [5,14,30,31].
Microorganisms present in the indoor air of hospital facilities are characterized by a different composition. In analyses concerning the morphological assessment of bacteria and fungi, individual species were identified in various hospital wards. The most common microorganism species associated with infections acquired in healthcare settings and identified in long-term care facilities are Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Clostridium difficile, as well as species of Enterococcus, Enterobacter, Klebsiella, and Candida [7,32,33,34]. Although not all healthcare-associated infections are transmitted via the airborne route, many of the pathogens listed in Table 2 have been identified in hospital air samples. This overview therefore provides an essential clinical context for understanding the potential implications of airborne microbiological contamination in healthcare environments. Bacteria are responsible for nearly 90% of HAIs, while infections caused by viruses, fungi, or mycobacteria are much less common [35,36].

Table 2.
Distribution of healthcare-associated infections (HAIs) in European hospitals and the most frequently identified microorganisms responsible for these infections [7,27,31,33,36,37,38,39,40,41,42,43].
3. Control and Reduction Strategies
The airborne transmission of microorganisms in indoor environments depends on several factors. Firstly, these include the duration of the agent’s activity—pathogenic microorganisms exhibit different survival capacities, which influence their persistence in the air and their potential to cause infection. The risk of infection increases when pathogens remain in the air for extended periods or at higher concentrations. Therefore, another critical factor is the duration of contact with the agent. A further key factor is the size of the particles. Depending on their size and weight, some can remain airborne for extended periods.
In contrast, others may settle on surfaces, which affects both the manner and duration of exposure to these microorganisms. Another consideration is the presence of additional pollutants, which can lead to an elevated concentration of microorganisms in the air. Polluted air can disrupt the respiratory microflora, which, in turn, increases the risk of infection [44]. Another highly significant consideration is the efficiency of methods for reducing airborne pathogens. The implementation of ventilation systems, air filtration, purification techniques, or disinfection processes plays a vital role in mitigating the risk of pathogen transmission [45,46]. Research is also being conducted on spatial planning and the allocation of healthcare resources to enhance access to medical care [47,48]. Such an approach may simultaneously mitigate the burden on healthcare systems and improve the management of air pollution-related issues.
Furthermore, the American Society of Heating, Refrigerating, and Air-Conditioning Engineers (ASHRAE) outlines several strategies to effectively reduce microbiological contaminants in indoor environments. In addition to appropriate design requirements, ASHRAE emphasizes the role of increasing the air exchange rate to lower microbial loads. The use of airlocks is recommended to control airflow between adjacent rooms, while maintaining pressure differentials helps enforce directional airflow. Adjusting the temperature and humidity levels to the specific requirements of each space also prevents the development of conditions conducive to the growth of pathogens, while ensuring user comfort. Finally, zoning—using separate HVAC units—allows indoor air quality standards to be tailored to the needs of individual rooms [49].
3.1. Air Filtration
The most commonly used method for reducing contamination and minimizing the risk of pathogen transmission is air exchange through a ventilation system. However, this form of air circulation has certain limitations, particularly in terms of maintaining thermal comfort and ensuring adequate air distribution. An additional challenge arises from the specific nature of medical facilities. The ventilation system must be adapted to the specific tasks performed in individual rooms, while also considering the number and health status of people staying in these rooms [31]. HVAC air treatment systems are considered a more effective approach for controlling and reducing pollution. Their primary function is to regulate temperature, humidity, and air quality within the buildings they serve. In this context, filters are essential for ensuring the proper quality of indoor air in these systems. HEPA (high-efficiency particulate air) filters are the most widely used and are particularly recommended for controlling hospital-acquired infections. They remove 99.97% of particles with a diameter of ≥0.3 μm. ULPA (ultra-low penetration into air) filters, used in environments with the highest cleanliness requirements, achieve an efficiency of 99.9995% in removing particles with a diameter of ≥0.12 μm. However, due to the higher pressure drop that they generate compared with HEPA filters, their application is less common [50,51].
Several key factors, including airflow velocity, particle characteristics, and the properties of the filtration material, determine the efficiency of HEPA and ULPA filters. In general, filtration efficiency increases with greater particle size and, to a certain extent, with higher airflow velocity. It is important to emphasize that systems equipped with HEPA and ULPA filters require regular maintenance and frequent filter replacement. Cleaning these filters is generally not recommended, as it may compromise their performance. Furthermore, their service life can be reduced in environments with elevated temperatures or humidity levels [52].
3.2. Air Disinfection
An alternative to the mechanical removal of solid particles is air disinfection. However, to maximize the effectiveness of IAQ improvement, such techniques should be applied in combination with filtration. In the context of the risk of microbial growth on filter surfaces, this approach may also enhance the durability of HVAC system components [53]. Several methods used in healthcare facilities have been described in the literature, which differ in terms of effectiveness, application, and mechanism of action. Air disinfection methods are presented in Table 3. They can be applied directly to the surfaces of HVAC system filters to inhibit the growth of microorganisms and extend the life of the filters or applied directly in ventilation ducts or indoor spaces. Their specific application depends on the technology used and the hygiene requirements of the facility.

Table 3.
Selected methods of indoor air disinfection.
The methods presented in Table 3 are used to inactivate pathogens in the hospital environment. However, their effectiveness depends on certain factors, such as the type of microorganisms, environmental conditions, and technical parameters. In addition, their use is associated with significant limitations. UV radiation can pose a health risk to staff and patients if used improperly, and photocatalysis generates reactive oxygen species that can lead to secondary chemical pollution. These compounds, although effective in disinfection, can adversely affect air quality and human health, requiring further detailed research. In addition, the solutions described often encounter technical limitations due to the complexity of the installation design, as well as challenges related to effective system maintenance and associated costs, including high levels of energy consumption.
The combination of filtration systems with air disinfection methods effectively reduces the risk of infection. However, there are still no clearly defined parameters necessary for effective control of HAIs [50,53,59].
4. Formation of Microbial Contamination Levels
There is a lack of standardized guidelines for monitoring and assessing parameters related to IAQ, including microbiological analysis [19,60]. WHO experts recommend 1000 CFU/m3 as the permissible threshold for microorganism concentrations in indoor air. Alternative guidelines propose upper limits of 300 CFU/m3 for fungi and 750 CFU/m3 for bacteria. In turn, the Interdepartmental Commission for the Maximum Permissible Concentrations and Intensities of Health-Harmful Factors in the Work Environment suggested a threshold of less than 5000 CFU/m3 for both bacteria and fungi [61,62,63]. Therefore, the literature reviews in this field typically do not provide direct references to specific numerical values. Instead, studies primarily focus on the impact of internal and external factors. Table 4 and Table 5 present data on bacteria and fungi in healthcare facilities, specifying their concentration with the type of facility, ward, and season. Furthermore, this review examines the air sampling method, the presence of other pollutants, the number of occupants, the type of ventilation system, and the volume of analyzed space.

Table 4.
A review of bacterial air quality in healthcare environments.

Table 5.
A review of fungal air quality in healthcare environments.
A review of the scientific literature revealed that the majority of available studies originated from Iran. Several potential explanations for this observation can be proposed. This trend may indicate a growing interest in the topic within that region, potentially linked to local socio-cultural factors, public health priorities, or the high level of research activity in Iranian academic institutions.
In contrast, locating relevant articles from countries such as the United States or Australia proved significantly more challenging. In the case of the United States, many of the available publications date to before the year 2000, which may suggest that academic interest in the subject was more pronounced in the past and has since declined. Possible explanations include a shift in research priorities over recent decades, a reduced relevance of the topic within the current public health landscape, or its broader integration into interdisciplinary research domains.
The reviewed studies assessing the concentrations of airborne bacteria and fungi in hospital environments exhibited several essential differences. Firstly, the investigations were conducted in a variety of hospital wards, often equipped with different types of ventilation systems. This variation may affect both the efficiency of air exchange and the removal of microbiological contaminants. In general, wards with modern filtration systems and positive air pressure should demonstrate lower levels of airborne microbial contamination compared to those with outdated or passive ventilation.
Moreover, the number of individuals present in the examined spaces, as well as the volume of the rooms, likely varied across the studies; however, in most cases, precise data on these parameters were not reported. This limitation can significantly hinder the direct comparability of results across studies, as occupant density influences the emission of microorganisms (originating from skin surfaces, clothing, and the respiratory tract). At the same time, room volume affects the degree to which airborne contaminants are diluted.
Another important factor differentiating the studies was the time of day and season during sample collection, as well as the geographical location of the facilities, corresponding to different climatic zones. These factors are critical to microbial dynamics because temperature, humidity, and sunlight exposure influence the survival, metabolic activity, and dispersal capacity of both bacteria and fungi. For example, increased humidity favors fungal growth, whereas bacteria may remain more active under moderate temperature conditions. Consequently, direct comparisons between data obtained in different seasons or climate zones are difficult without appropriate standardization. Nevertheless, the available data indicate a persistent risk of exposure for both patients and healthcare staff.
Low concentrations of microorganisms, such as those reported in a dental office in France (0–11 CFU/m3) [65] or during operations in Italy (13–82 CFU/500 L) [69] may indicate a high standard of sterility, adequate ventilation, and air filtration, which is crucial in rooms requiring special hygiene, such as operating rooms or intensive care units. The concentration of microorganisms in such environments may serve as an indicator of the effectiveness and regularity of cleaning procedures, including the application of appropriate disinfectants and the continuous monitoring of sanitary and hygienic conditions. High concentrations of microorganisms, such as those reported in a hospital in Thailand (880.19 CFU/m3) in female medicine [68] or in a women’s infectious disease ward in Iran (835 CFU/m3) [64] may suggest the need for more regular cleaning, as well as the need to improve ventilation or the air filtration system. Elevated values of CFU/m3 may also result from overcrowded conditions, which complicate air quality management efforts. Moreover, high concentrations, observed in an orthopedic ward at an Indian hospital (ranging from 3788 to 191,111 CFU/m3) [70], highlight the challenges of maintaining a controlled indoor environment in regions characterized by high temperature and humidity conditions that promote microbial proliferation. Comparable variability has also been documented in hospitals located in other areas. For instance, in the Korean hospital lobbies, the levels ranged from 50 to 2300 CFU/m3 [30], indicating potential difficulties in controlling both internal and external sources of contamination. Additionally, observed variability may result from emission sources, humans, and environmental conditions that favor their multiplication, or the ability to generate bioaerosols. Such fluctuations may reflect the influence of outdoor air pollution. Air inadequately filtered can significantly alter IAQ. Similar trends have been observed for fungal contamination.
Some studies suggest a correlation between air quality parameters and microorganism concentrations [27,67], while others have found no such association [67,68,75]. Additionally, increased movement within healthcare facilities and a higher number of visitors may contribute to elevated bioaerosol levels and facilitate their spread [27,75]. Furthermore, factors such as inadequate disinfection procedures, poor hygiene practices, improper use of personal protective equipment by staff, ineffective ventilation, external sources of microorganisms, and seasonal fluctuations have been identified as potential contributors to this phenomenon [70,75,76,77,78,79]. Other studies have reported that HEPA filters effectively reduce microbial load [80,81], whereas one did not confirm this effect [82]. Notably, in studies where strain identification was performed, the detected microorganisms almost invariably corresponded to those listed in Table 2. This finding confirms their widespread presence in hospital settings and underscores the importance of monitoring these specific pathogens as indicators in the assessment of microbiological air quality.
Due to the substantial heterogeneity of the available data and the absence of standardized measurement indicators, conducting a reliable statistical analysis was not feasible at this stage. Nevertheless, the authors recognize a clear need to strengthen the quantitative dimension in future research by incorporating empirical data obtained through direct measurements and analyses. Such efforts would enable the application of appropriate effect measures, facilitate methodological standardization, and enhance the comparability of results.
At the current stage of analysis, the causes of the discrepancies among individual study results cannot be determined, despite the selection of studies being based on specific criteria. These differences may arise from variations in geographical location and potential seasonal influences. The limited availability of information on other relevant parameters further constrains interpretation. The presence of such inconsistencies underscores the need for systematic, standardized investigations in this field. Conducting such research would facilitate causal inference and provide a valuable contribution to the existing body of knowledge.
The inability to interpret the results is a barrier to research in this field, posing a significant challenge to minimizing the risk of infections and ensuring a safe indoor environment for both medical staff and patients. This issue is particularly relevant in the context of antimicrobial resistance among airborne microorganisms, as the airborne-droplet route is considered one of the primary pathways for the spread of antibiotic-resistant pathogens.
Antibiotic resistance refers to the ability of bacteria to survive or multiply despite exposure to one or more antibiotics due to intrinsic or acquired resistance mechanisms. Acquired resistance is of particular concern, as it often leads to infections that are more difficult to treat, resulting in prolonged hospital stays and increased mortality rates [4,81,82,83].
A specific and more severe form of this problem is multidrug resistance (MDR), defined as resistance to at least three different classes of antibiotics. It is estimated that in some regions of the world, up to 75% of hospital-acquired infections are caused by MDR bacteria [84,85]. According to the European Antimicrobial Resistance Surveillance Network, MDR bacteria were responsible for over 800,000 infections in the European Union and European Economic Area (EU/EEA) in 2020 [86].
On a global scale, bacterial resistance to antimicrobial agents was estimated to have directly caused 1.14 million deaths in 2021 [87]. At the same time, the incidence of antibiotic-resistant bacteria continues to rise worldwide [88,89,90].
5. Conclusions
This review synthesizes current knowledge on microbiological air quality in healthcare environments, evaluates associated health risks, summarizes monitoring approaches, and identifies gaps requiring further research. According to the reviewed studies, bacterial concentrations ranged from below detection (<1 CFU/m3) to 191,111 CFU/m3 (in an orthopedic ward in a tropical hospital), with a median of approximately 300 CFU/m3 and a mean of 4729 CFU/m3. Fungal concentrations ranged from <1 CFU/m3 to 15,150 CFU/m3, with a median of 44.7 CFU/m3 and a mean of 514 CFU/m3. These findings address the first objective by demonstrating the substantial variability that occurs in microbial loads, which are affected by facility type, ventilation performance, environmental conditions, and infection control practices. The most frequently identified pathogens in hospital air included Staphylococcus aureus (associated with six infection types), Escherichia coli (five types), Enterobacter spp., and Klebsiella spp. (four types), Pseudomonas aeruginosa and Enterococcus spp. (three types), many of which are known for antibiotic resistance and significant airborne transmission potential.
In relation to the third objective, our synthesis confirms that active air sampling remains the most widely used method for detecting airborne microorganisms, with methodological diversity often limiting comparability between studies. The lack of harmonized sampling protocols, limited data on permissible indoor concentrations, and insufficient consideration of influencing factors such as room volume, occupancy, and other air quality parameters have emerged as major obstacles to robust monitoring.
Finally, we address the fourth objective to identify clear research and policy priorities. These include the development of standardized international guidelines for microbial air contamination, the expansion of research into underrepresented healthcare settings, such as hospices and long-term care facilities, and the implementation of longitudinal, multicenter studies that account for regional and seasonal variability. Achieving these goals will require integrated, multi-layered strategies combining architectural design, electrification of environmental controls, afforestation around facilities, and the application of effective air filtration and disinfection technologies.
Beyond technical measures, raising public and professional awareness of indoor air hygiene is essential. Even relatively simple interventions—such as limiting visitor numbers during outbreaks, ensuring correct use of personal protective equipment, and maintaining ventilation and filtration systems—can significantly reduce airborne infection risks. By directly aligning these findings and recommendations with the stated objectives, this review provides both a comprehensive knowledge base and a practical framework for improving microbial air quality in healthcare environments.
Author Contributions
Conceptualization, K.K., E.B. and A.M.; writing—original draft preparation, K.K.; writing—review and editing, E.B. and A.M.; supervision, E.B. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Faculty of Energy and Environmental Engineering, Silesian University of Technology through a statutory research grant for young scientists (BKM 585/RIE2/2025, 08/020/BKM25/0048).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IAQ | Indoor air quality |
| HAI | Healthcare-associated infections |
| WHO | World Health Organization |
| EDCD | The European Center for Disease Prevention and Control |
| ASHARE | American Society of Heating, Refrigerating, and Air-Conditioning Engineers |
| HVAC | Heating, ventilation, and air conditioning |
| HEPA | High-efficiency particulate air |
| ULPA | Ultra-low penetration air |
| UV | Ultraviolet radiation |
| CFU | Colony forming unit |
| N/A | Not applicable |
| MDR | Multidrug-resistant bacteria |
| EU/EEA | European Union and European Economic Area |
References
- Rogerson, A.; Detwiler, A. Abundance of Airborne Heterotrophic Protists in Ground Level Air of South Dakota. Atmos. Res. 1999, 51, 35–44. [Google Scholar] [CrossRef]
- Brągoszewska, E.; Biedroń, I.; Kozielska, B.; Pastuszka, J.S. Microbiological Indoor Air Quality in an Office Building in Gliwice, Poland: Analysis of the Case Study. Air Qual. Atmos. Health 2018, 11, 729–740. [Google Scholar] [CrossRef]
- Matthias-Maser, S.; Jaenicke, R. The Size Distribution of Primary Biological Aerosol Particles in the Multiphase Atmosphere. Aerobiologia 2000, 16, 207–210. [Google Scholar] [CrossRef]
- ECDC. Healthcare-Associated Infections. Available online: https://www.ecdc.europa.eu/en/healthcare-associated-infections (accessed on 10 May 2025).
- WHO. Key Facts and Figures. High-Level Messaging on the HAI and AMR Burden. Available online: https://www.who.int/campaigns/world-hand-hygiene-day/key-facts-and-figures (accessed on 10 May 2025).
- Maugeri, A.; Casini, B.; Esposito, E.; Bracaloni, S.; Scarpaci, M.; Patanè, F.; Milazzo, G.; Agodi, A.; Barchitta, M. Impact of Ultraviolet Light Disinfection on Reducing Hospital-Associated Infections: A Systematic Review in Healthcare Environments. J. Hosp. Infect. 2025, 159, 32–41. [Google Scholar] [CrossRef]
- Szabó, S.; Feier, B.; Capatina, D.; Tertis, M.; Cristea, C.; Popa, A. An Overview of Healthcare Associated Infections and Their Detection Methods Caused by Pathogen Bacteria in Romania and Europe. J. Clin. Med. 2022, 11, 3204. [Google Scholar] [CrossRef]
- Memarzadeh, F.; Xu, W. Role of Air Changes per Hour (ACH) in Possible Transmission of Airborne Infections. Build. Simul. 2012, 5, 15–28. [Google Scholar] [CrossRef]
- Kumar, P.; Kausar, M.A.; Singh, A.B.; Singh, R. Biological Contaminants in the Indoor Air Environment and Their Impacts on Human Health. Air Qual. Atmos. Health 2021, 14, 1723–1736. [Google Scholar] [CrossRef]
- Owen, M.K.; Ensor, D.S.; Sparks, L.E. Airborne Particle Sizes and Sources Found in Indoor Air. Atmos. Environ. Part A General. Top. 1992, 26, 2149–2162. [Google Scholar] [CrossRef]
- Gołofit-Szymczak, M.; Górny, R. Bioaerozole w Budynkach Biurowych. Kosmos 2017, 66, 491–502. [Google Scholar]
- Brągoszewska, E.; Mainka, A. Assessment of Personal Deposited Dose and Particle Size Distribution of Bacterial Aerosol in Kindergarten Located in Southern Poland. Environ. Pollut. 2024, 343, 123208. [Google Scholar] [CrossRef] [PubMed]
- Tomar, S.; Sharma, H. Role of Bioaerosols in Indoor Air Quality and Respiratory Diseases. IJRTI 2025, 10, 320. [Google Scholar]
- Verdier, T.; Coutand, M.; Bertron, A.; Roques, C. A Review of Indoor Microbial Growth across Building Materials and Sampling and Analysis Methods. Build. Environ. 2014, 80, 136–149. [Google Scholar] [CrossRef]
- Feng, X.; Xu, X.; Yao, X.; Zhao, Y.; Tang, Y.; Zhao, Z.; Wei, Y.; Mehmood, T.; Luo, X.S. Sources, Compositions, Spatio-Temporal Distributions, and Human Health Risks of Bioaerosols: A Review. Atmos. Res. 2024, 305, 107453. [Google Scholar] [CrossRef]
- Pertegal, V.; Lacasa, E.; Cañizares, P.; Rodrigo, M.A.; Sáez, C. Understanding the Influence of the Bioaerosol Source on the Distribution of Airborne Bacteria in Hospital Indoor Air. Environ. Res. 2023, 216, 114458. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liang, L.; Lyu, B.; Cai, Y.S.; Zuo, Y.; Su, J.; Tong, Z. Double Trouble: The Interaction of PM2.5 and O3 on Respiratory Hospital Admissions. Environ. Pollut. 2023, 338, 122665. [Google Scholar] [CrossRef]
- Kirwa, K.; Eckert, C.M.; Vedal, S.; Hajat, A.; Kaufman, J.D. Ambient Air Pollution and Risk of Respiratory Infection among Adults: Evidence from the Multiethnic Study of Atherosclerosis (MESA). BMJ Open Respir. Res. 2021, 8, e000866. [Google Scholar] [CrossRef]
- Chawla, H.; Anand, P.; Garg, K.; Bhagat, N.; Varmani, S.G.; Bansal, T.; McBain, A.J.; Marwah, R.G. A Comprehensive Review of Microbial Contamination in the Indoor Environment: Sources, Sampling, Health Risks, and Mitigation Strategies. Front. Public Health 2023, 11, 1285393. [Google Scholar] [CrossRef] [PubMed]
- Heo, K.J.; Lim, C.E.; Kim, H.B.; Lee, B.U. Effects of Human Activities on Concentrations of Culturable Bioaerosols in Indoor Air Environments. J. Aerosol Sci. 2017, 104, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Kauch, K.; Halladin, M.; Patoń, N.; Rytczak, J.; Szczęsny, M.; Palmowska, A.; Brągoszewska, E. Ocena Efektywności Działania Urządzeń Oczyszczających Powietrze Wewnętrzne w Zakresie Usuwania Szkodliwych Czynników Biologicznych (SCB). In Nowoczesne Rozwiązania w Ochronie Środowiska. Zagadnienia Wybrane; Wydawnictwo Naukowe TYGIEL: Lublin, Poland, 2023; pp. 230–238. [Google Scholar]
- Pastuszka, J.S. Narażenie Na Aerozole Ziarniste, Włókniste i Biologiczne (Bakterie i Grzyby Mikroskopijne) Populacji Generalnej Górnośląskiego Okręgu Przemysłowego. Pr. Nauk. Inst. Inżynierii Ochr. Sr. Politech. Wrocławskiej Monogr. 2001, 73, 7–131. [Google Scholar]
- Song, L.; Zhou, J.; Wang, C.; Meng, G.; Li, Y.; Jarin, M.; Wu, Z.; Xie, X. Airborne Pathogenic Microorganisms and Air Cleaning Technology Development: A Review. J. Hazard. Mater. 2022, 424, 127429. [Google Scholar] [CrossRef]
- Układ Oddechowy—Opis Układu i Jego Chorób. Available online: https://vitamarket.pl/g6-Uklad-oddechowy.html (accessed on 19 February 2025).
- El-Sharkawy, M.; Noweir, M. Indoor Air Quality Levels in a University Hospital in the Eastern Province of Saudi Arabia. J. Fam. Community Med. 2014, 21, 39. [Google Scholar] [CrossRef]
- Ekhaise, F.O.; Blessing, O. Microbiological Indoor and Outdoor Air Quality of Two Major Hospitals in Benin City, Nigeria. Sierra Leone J. Biomed. Res. 2011, 3, 169–174. [Google Scholar]
- Sivagnanasundaram, P.; Amarasekara, R.W.K.; Madegedara, R.M.D.; Ekanayake, A.; Magana-Arachchi, D.N. Assessment of Airborne Bacterial and Fungal Communities in Selected Areas of Teaching Hospital, Kandy, Sri Lanka. Biomed. Res. Int. 2019, 2019, 7393926. [Google Scholar] [CrossRef]
- Augustowska, M.; Dutkiewicz, J. Variability of Airborne Microflora in a Hospital Ward within a Period of One Year. Ann. Agric. Environ. Med. 2006, 13, 99–106. [Google Scholar]
- Cabo Verde, S.; Almeida, S.M.; Matos, J.; Guerreiro, D.; Meneses, M.; Faria, T.; Botelho, D.; Santos, M.; Viegas, C. Microbiological Assessment of Indoor Air Quality at Different Hospital Sites. Res. Microbiol. 2015, 166, 557–563. [Google Scholar] [CrossRef]
- Park, D.U.; Yeom, J.K.; Lee, W.J.; Lee, K.M. Assessment of the Levels of Airborne Bacteria, Gram-Negative Bacteria, and Fungi in Hospital Lobbies. Int. J. Environ. Res. Public Health 2013, 10, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M.; Ślosarczyk, A.; Klapiszewska, I.; Riha, J.; Jesionowski, T.; Klapiszewski, Ł. Airborne Bioaerosols in Healthcare Facilities—Transmission Routes and Mitigation Strategies: A Review. J. Build. Eng. 2024, 97, 111015. [Google Scholar] [CrossRef]
- Voidazan, S.; Albu, S.; Toth, R.; Grigorescu, B.; Rachita, A.; Moldovan, I. Healthcare Associated Infections—A New Pathology in Medical Practice? Int. J. Environ. Res. Public. Health 2020, 17, 760. [Google Scholar] [CrossRef] [PubMed]
- Facciolà, A.; Pellicanò, G.F.; Visalli, G.; Paolucci, I.A.; Rullo, E.V.; Ceccarelli, M.; D’Aleo, F.; Di Pietro, A.; Squeri, R.; Nunnari, G.; et al. The Role of the Hospital Environment in the Healthcare-Associated Infections: A General Review of the Literature. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1266–1278. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Most Frequently Isolated Microorganisms. Available online: https://www.ecdc.europa.eu/en/all-topics-z/healthcare-associated-infections-long-term-care-facilities/surveillance-and-disease-5 (accessed on 9 May 2025).
- Bereket, W.; Hemalatha, K.; Getenet, B.; Wondwossen, T.; Solomon, A.; Zeynudin, A.; Kannan, S. Update on Bacterial Nosocomial Infections—PubMed. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1039–1044. [Google Scholar]
- Haque, M.; Sartelli, M.; McKimm, J.; Bakar, M.A. Health Care-Associated Infections—An Overview. Infect. Drug Resist. 2018, 11, 2321–2333. [Google Scholar] [CrossRef]
- Garg, S. Staphylococcus Cohnii: Not so Innocuous. J. Acute Dis. 2017, 6, 239. [Google Scholar] [CrossRef]
- Khan, H.A.; Ahmad, A.; Mehboob, R. Nosocomial Infections and Their Control Strategies. Asian Pac. J. Trop. Biomed. 2015, 5, 509–514. [Google Scholar] [CrossRef]
- Motta, J.C.; Forero-Carreño, C.; Arango, Á.; Sánchez, M. Staphylococcus Cohnii Endocarditis in Native Valve. New Microbes New Infect. 2020, 38, 100825. [Google Scholar] [CrossRef]
- Szymanski, M.; Skiba, M.M.; Piasecka, M.; Olender, A. A Rare Case of Invasive Enterococcus Cecorum Infection and Related Diagnostic Difficulties. Clin. Case Rep. 2024, 12, e9386. [Google Scholar] [CrossRef]
- Tsantes, A.G.; Papadopoulos, D.V.; Markou, E.; Zarokostas, K.; Sokou, R.; Trikoupis, I.; Mavrogenis, A.F.; Houhoula, D.; Piovani, D.; Bonovas, S.; et al. Aspergillus Spp. Osteoarticular Infections: An Updated Systematic Review on the Diagnosis, Treatment and Outcomes of 186 Confirmed Cases. Med. Mycol. 2022, 60, myac052. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Healthcare-Associated Infections in European Hospitals (PPS Survey) 2022–2023. Available online: https://www.ecdc.europa.eu/en/publications-data/healthcare-associated-infections-european-hospitals-pps-survey-2022-2023 (accessed on 11 May 2025).
- Wu, M.J.; Feng, Y.S.; Sung, W.P.; Surampalli, R.Y. Quantification and Analysis of Airborne Bacterial Characteristics in a Nursing Care Institution. J. Air Waste Manag. Assoc. 2011, 61, 732–739. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Niu, Y.; Chen, R.; Wang, C.; Wang, W.; Jiang, J.; Wu, W.; Cai, J.; Zhao, Z.; Xu, X.; Kan, H. Ozone Exposure Leads to Changes in Airway Permeability, Microbiota and Metabolome: A Randomised, Double-Blind, Crossover Trial. Eur. Respir. J. 2020, 56, 2000165. [Google Scholar] [CrossRef] [PubMed]
- Narodowy Instytut Zdrowia Publicznego PZH. Zalecenia Dot. Działań Mających Na Celu Ograniczenie Ryzyka Związanego z Przenoszeniem Się Wirusa SARS-CoV-2 Za Pośrednictwem Systemów Wentylacyjno-Klimatyzacyjnych Wewnątrz Budynków Użyteczności Publicznej Oraz Wielkopowierzchniowych Obiektów Handlowych. Available online: https://www.gov.pl/web/psse-aleksandrow-kujawski/zalecenia-dot-dzialan-majacych-na-celu-ograniczenie-ryzyka-zwiazanego-z-przenoszeniem-sie-wirusa-sars-cov-2-za-posrednictwem-systemow-wentylacyjno-klimatyzacyjnych-wewnatrz-budynkow-uzytecznosci-publicznej-oraz-wielkopowierzchniowych-obiektow-handlowych (accessed on 10 May 2025).
- Wei, J.; Li, Y. Airborne Spread of Infectious Agents in the Indoor Environment. Am. J. Infect. Control 2016, 44, S102. [Google Scholar] [CrossRef]
- Pan, J.; Deng, Y.; Yang, Y.; Zhang, Y. Location-Allocation Modelling for Rational Health Planning: Applying a Two-Step Optimization Approach to Evaluate the Spatial Accessibility Improvement of Newly Added Tertiary Hospitals in a Metropolitan City of China. Soc. Sci. Med. 2023, 338, 116296. [Google Scholar] [CrossRef]
- Wang, X.; Yang, H.; Duan, Z.; Pan, J. Spatial Accessibility of Primary Health Care in China: A Case Study in Sichuan Province. Soc. Sci. Med. 2018, 209, 14–24. [Google Scholar] [CrossRef]
- ASHARE. Health Care Facilities. In ASHRAE Handbook—HVAC Applications; American Society of Heating, Refrigerating and Air-Conditioning Engineers, Inc.: Atlanta, GA, USA, 2019. [Google Scholar]
- Bonadonna, L.; Briancesco, R.; Coccia, A.M.; Meloni, P.; La Rosa, G.; Moscato, U. Microbial Air Quality in Healthcare Facilities. Int. J. Environ. Res. Public. Health 2021, 18, 6226. [Google Scholar] [CrossRef] [PubMed]
- Bolashikov, Z.D.; Melikov, A.K. Methods for Air Cleaning and Protection of Building Occupants from Airborne Pathogens. Build. Environ. 2008, 44, 1378. [Google Scholar] [CrossRef] [PubMed]
- EPA. What Is a HEPA Filter? Available online: https://www.epa.gov/indoor-air-quality-iaq/what-hepa-filter (accessed on 10 May 2025).
- Atkinson, J.; Chartier, Y.; Lúcia Pessoa-Silva, C.; Jensen, P.; Li, Y.; Seto, H. Natural Ventilation for Infection Control in Health-Care Settings; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Ko, G.; First, M.W.; Burge, H.A. The Characterization of Upper-Room Ultraviolet Germicidal Irradiation in Inactivating Airborne Microorganisms. Environ. Health Perspect. 2002, 110, 95. [Google Scholar] [CrossRef]
- Wang, C.; Lu, S.; Zhang, Z. Inactivation of Airborne Bacteria Using Different UV Sources: Performance Modeling, Energy Utilization, and Endotoxin Degradation. Sci. Total Environ. 2019, 655, 787–795. [Google Scholar] [CrossRef]
- Pigeot-Remy, S.; Lazzaroni, J.C.; Simonet, F.; Petinga, P.; Vallet, C.; Petit, P.; Vialle, P.J.; Guillard, C. Survival of Bioaerosols in HVAC System Photocatalytic Filters. Appl. Catal. B 2014, 144, 654–664. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.S. Photocatalytic Oxidation Technology for Indoor Environment Air Purification: The State-of-the-Art. Appl. Catal. B 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Li, J.; Gao, H.; Lan, C.; Nie, L.; Liu, D.; Lu, X.; (Ken) Ostrikov, K. Plasma Air Filtration System for Intercepting and Inactivation of Pathogenic Microbial Aerosols. J. Environ. Chem. Eng. 2023, 11, 110728. [Google Scholar] [CrossRef]
- Olmedo, I.; Nielsen, P.V.; Ruiz de Adana, M.; Jensen, R.L.; Grzelecki, P. Distribution of Exhaled Contaminants and Personal Exposure in a Room Using Three Different Air Distribution Strategies. Indoor Air 2012, 22, 64–76. [Google Scholar] [CrossRef]
- Goyal, R.; Khare, M. Indoor Air Quality: Current Status, Missing Links and Future Road Map for India. J. Civ. Environ. Eng. 2012, 2, 1000118. [Google Scholar] [CrossRef]
- Cappitelli, F.; Fermo, P.; Vecchi, R.; Piazzalunga, A.; Valli, G.; Zanardini, E.; Sorlini, C. Chemical-Physical and Microbiological Measurements for Indoor Air Quality Assessment at the ca’ Granda Historical Archive, Milan (Italy). Water Air Soil. Pollut. 2009, 201, 109–120. [Google Scholar] [CrossRef]
- WHO. WHO Guidelines for Indoor Air Quality: Dampness and Mould; Heseltine, E., Rosen, J., Eds.; WHO: Geneva, Switzerland, 2009; ISBN 978 92 890 4168 3. [Google Scholar]
- Bielawska-Drózd, A.; Cieślik, P.; Bohacz, J.; Korniłłowicz-Kowalska, T.; Żakowska, D.; Bartoszcze, M.; Wlizło-Skowronek, B.; Winnicka, I.; Brytan, M.; Kubiak, L.; et al. Microbiological Analysis of Bioaerosols Collected from Hospital Emergency Departments and Ambulances. Ann. Agric. Environ. Med. 2018, 25, 274–279. [Google Scholar] [CrossRef]
- Hosseini, S.; Kafil, H.S.; Mousavi, S.; Gholampour, A. Seasonal and Spatial Variations of Bioaerosols and Antibiotic Resistance Bacteria in Different Wards of the Hospital. J. Air Pollut. Health 2022, 7, 409–422. [Google Scholar] [CrossRef]
- Baudet, A.; Baurès, E.; Guegan, H.; Blanchard, O.; Guillaso, M.; Le Cann, P.; Gangneux, J.P.; Florentin, A. Indoor Air Quality in Healthcare and Care Facilities: Chemical Pollutants and Microbiological Contaminants. Atmosphere 2021, 12, 1337. [Google Scholar] [CrossRef]
- Božić, J.; Ilić, P.; ilić, S. Indoor Air Quality in the Hospital: The Influence of Heating, Ventilating and Conditioning Systems. Braz. Arch. Biol. Technol. 2019, 62, e19180295. [Google Scholar] [CrossRef]
- Tselebonis, A.; Nena, E.; Panopoulou, M.; Kontogiorgis, C.; Bezirtzoglou, E.; Constantinidis, T. Air Contamination in Different Departments of a Tertiary Hospital: Assessment of Microbial Load and of Antimicrobial Susceptibility. Biomedicines 2020, 8, 163. [Google Scholar] [CrossRef] [PubMed]
- Onmek, N.; Kongcharoen, J.; Singtong, A.; Penjumrus, A.; Junnoo, S. Environmental Factors and Ventilation Affect Concentrations of Microorganisms in Hospital Wards of Southern Thailand. J. Environ. Public. Health 2020, 2020, 7292198. [Google Scholar] [CrossRef]
- Scaltriti, S.; Cencetti, S.; Rovesti, S.; Marchesi, I.; Bargellini, A.; Borella, P. Risk Factors for Particulate and Microbial Contamination of Air in Operating Theatres. J. Hosp. Infect. 2007, 66, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Sudharsanam, S.; Swaminathan, S.; Ramalingam, A.; Thangavel, G.; Annamalai, R.; Steinberg, R.; Balakrishnan, K.; Srikanth, P. Characterization of Indoor Bioaerosols from a Hospital Ward in a Tropical Setting. Afr. Health Sci. 2012, 12, 217. [Google Scholar] [CrossRef]
- de Oliveira, M.T.; Cunha, L.M.S.; Cruz, F.C.; Batista, N.K.R.; Gil, E. de S.; Alves, V.F.; Bara, M.T.F.; Torres, I.M.S. Potentially Pathogenic Bacteria Isolated from Neglected Air and Surfaces in Hospitals. Braz. J. Pharm. Sci. 2021, 57, e18989. [Google Scholar] [CrossRef]
- Ortiz, G.; Yagüe, G.; Segovia, M.; Catalán, V. A Study of Air Microbe Levels in Different Areas of a Hospital. Curr. Microbiol. 2009, 59, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Curtis, L.; Cali, S.; Conroy, L.; Baker, K.; Ou, C.H.; Hershow, R.; Norlock-Cruz, F.; Scheff, P. Aspergillus Surveillance Project at a Large Tertiary-Care Hospital. J. Hosp. Infect. 2005, 59, 188–196. [Google Scholar] [CrossRef]
- Marchand, G.; Duchaine, C.; Lavoie, J.; Veillette, M.; Cloutier, Y. Bacteria Emitted in Ambient. Air during Bronchoscopy—A Risk to Health Care Workers? Am. J. Infect. Control 2016, 44, 1634–1638. [Google Scholar] [CrossRef]
- Veysi, R.; Heibati, B.; Jahangiri, M.; Kumar, P.; Latif, M.T.; Karimi, A. Indoor Air Quality-Induced Respiratory Symptoms of a Hospital Staff in Iran. Environ. Monit. Assess. 2019, 191, 50. [Google Scholar] [CrossRef]
- Montazer, M.; Soleimani, N.; Vahabi, M.; Abtahi, M.; Etemad, K.; Zendehdel, R. Assessment of Bacterial Pathogens and Their Antibiotic Resistance in the Air of Different Wards of Selected Teaching Hospitals in Tehran. Indian. J. Occup. Environ. Med. 2021, 25, 78. [Google Scholar] [CrossRef]
- Nourmoradi, H.; Amin, M.M.; Hatamzadeh, M.; Nikaeen, M. Evaluation of Bio-Aerosols Concentration in the Different Wards of Three Educational Hospitals in Iran. Int. J. Environ. Health Eng. 2012, 1, 47. [Google Scholar] [CrossRef]
- Shokri, S.; Nikpey, A.; Varyani, A.S. Evaluation of Hospital Wards Indoor Air Quality: The Particles Concentration. J. Air Pollut. Health 2016, 1, 205–214. [Google Scholar]
- Viegas, C.; Sabino, R.; Veríssimo, C.; Rosado, L. Assessment of Fungal Contamination in a Portuguese Maternity Unit. WIT Trans. Biomed. Health 2011, 15, 127–133. [Google Scholar] [CrossRef]
- ECDC. Factsheet for the General Public—Antimicrobial Resistance. Available online: https://www.ecdc.europa.eu/en/antimicrobial-resistance/facts/factsheets/general-public (accessed on 3 March 2025).
- Frye, J.; Galgano, M.; Pellegrini, F.; Catalano, E.; Capozzi, L.; Del Sambro, L.; Sposato, A.; Stella Lucente, M.; Iris Vasinioti, V.; Catella, C.; et al. Acquired Bacterial Resistance to Antibiotics and Resistance Genes: From Past to Future. Antibiotics 2025, 14, 222. [Google Scholar] [CrossRef]
- Gajic, I.; Tomic, N.; Lukovic, B.; Jovicevic, M.; Kekic, D.; Petrovic, M.; Jankovic, M.; Trudic, A.; Culafic, D.M.; Milenkovic, M.; et al. A Comprehensive Overview of Antibacterial Agents for Combating Multidrug-Resistant Bacteria: The Current Landscape, Development, Future Opportunities, and Challenges. Antibiotics 2025, 14, 221. [Google Scholar] [CrossRef]
- Nguyen, B.A.T.; Chen, Q.L.; He, J.Z.; Hu, H.W. Microbial Regulation of Natural Antibiotic Resistance: Understanding the Protist-Bacteria Interactions for Evolution of Soil Resistome. Sci. Total Environ. 2020, 705, 135882. [Google Scholar] [CrossRef]
- Agyepong, N.; Govinden, U.; Owusu-Ofori, A.; Essack, S.Y. Multidrug-Resistant Gram-Negative Bacterial Infections in a Teaching Hospital in Ghana. Antimicrob. Resist. Infect. Control 2018, 7, 1–8. [Google Scholar] [CrossRef]
- Vaithiyam, V.S.; Rastogi, N.; Ranjan, P.; Mahishi, N.; Kapil, A.; Dwivedi, S.N.; Soneja, M.; Wig, N.; Biswas, A. Antimicrobial Resistance Patterns in Clinically Significant Isolates from Medical Wards of a Tertiary Care Hospital in North India. J. Lab. Physicians 2020, 12, 196–202. [Google Scholar] [CrossRef]
- Merk, H.; Diaz Högberg, L.; Plachouras, D.; Suetens, C.; Monnet, D.L. Assessing the Health Burden of Infections with Antibiotic-Resistant Bacteria in the EU/EEA, 2016–2020; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2022.
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global Burden of Bacterial Antimicrobial Resistance 1990–2021: A Systematic Analysis with Forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Poletajew, S.; Pawlik, K.; Bonder-Nowicka, A.; Pakuszewski, A.; Nyk, Ł.; Kryst, P. Multi-Drug Resistant Bacteria as Aetiological Factors of Infections in a Tertiary Multidisciplinary Hospital in Poland. Antibiotics 2021, 10, 1232. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Zhang, Z.; Sun, Z. Antimicrobial Resistance Trends in Bloodstream Infections at a Large Teaching Hospital in China: A 20-Year Surveillance Study (1998–2017). Antimicrob. Resist. Infect. Control 2019, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Daneman, N.; Tan, C.; Brownstein, J.S.; MacFadden, D.R. Evaluating the Relationship Between Hospital Antibiotic Use and Antibiotic Resistance in Common Nosocomial Pathogens. Infect. Control Hosp. Epidemiol. 2017, 38, 1457–1463. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).