Elevated Concentrations of Carbon Dioxide (CO2) on the Harbechy Plateau (Moravian Karst) Reveal a Gas-Rich Soil Layer (GRSL)
Abstract
1. Introduction
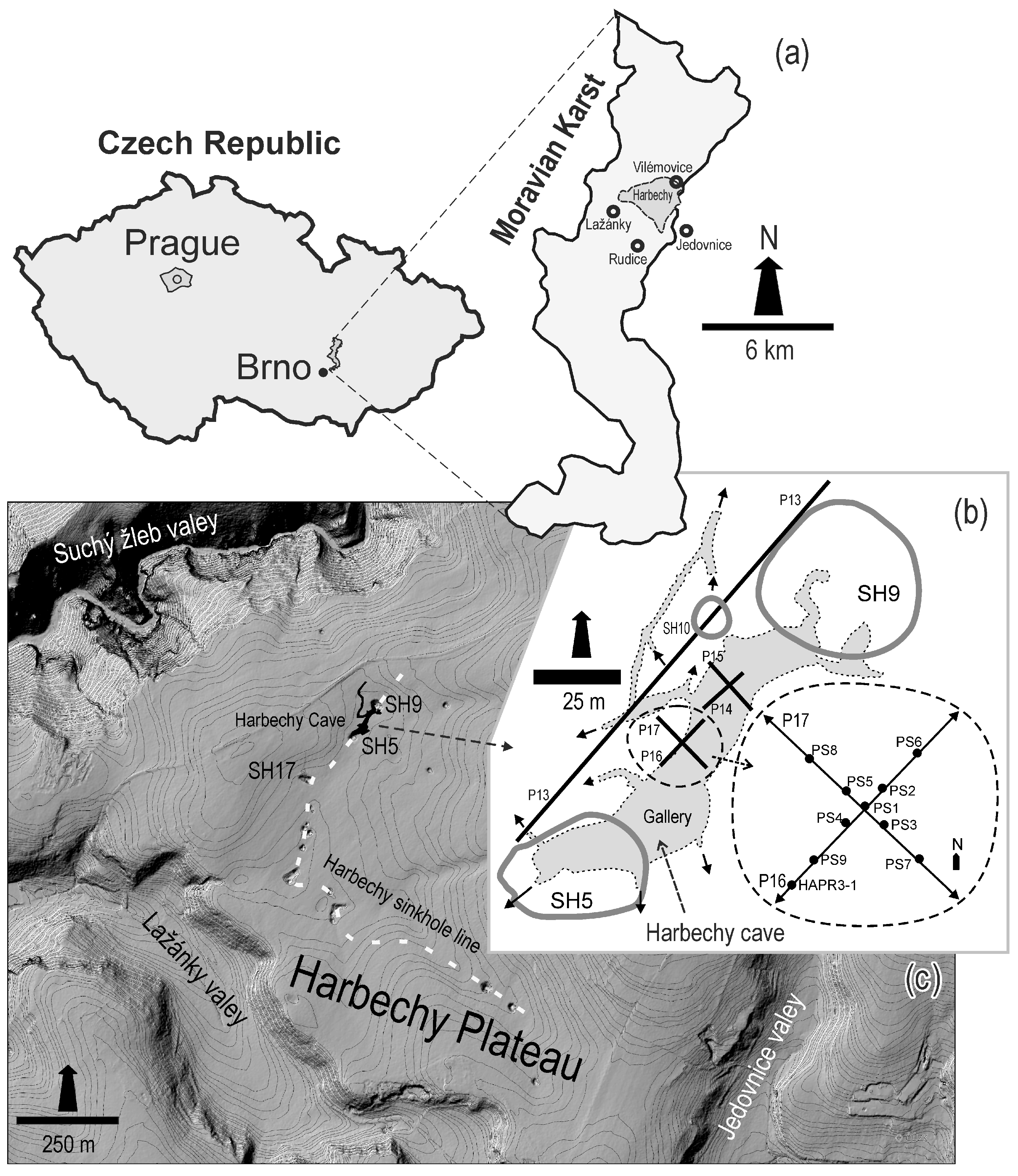
2. Site of Study
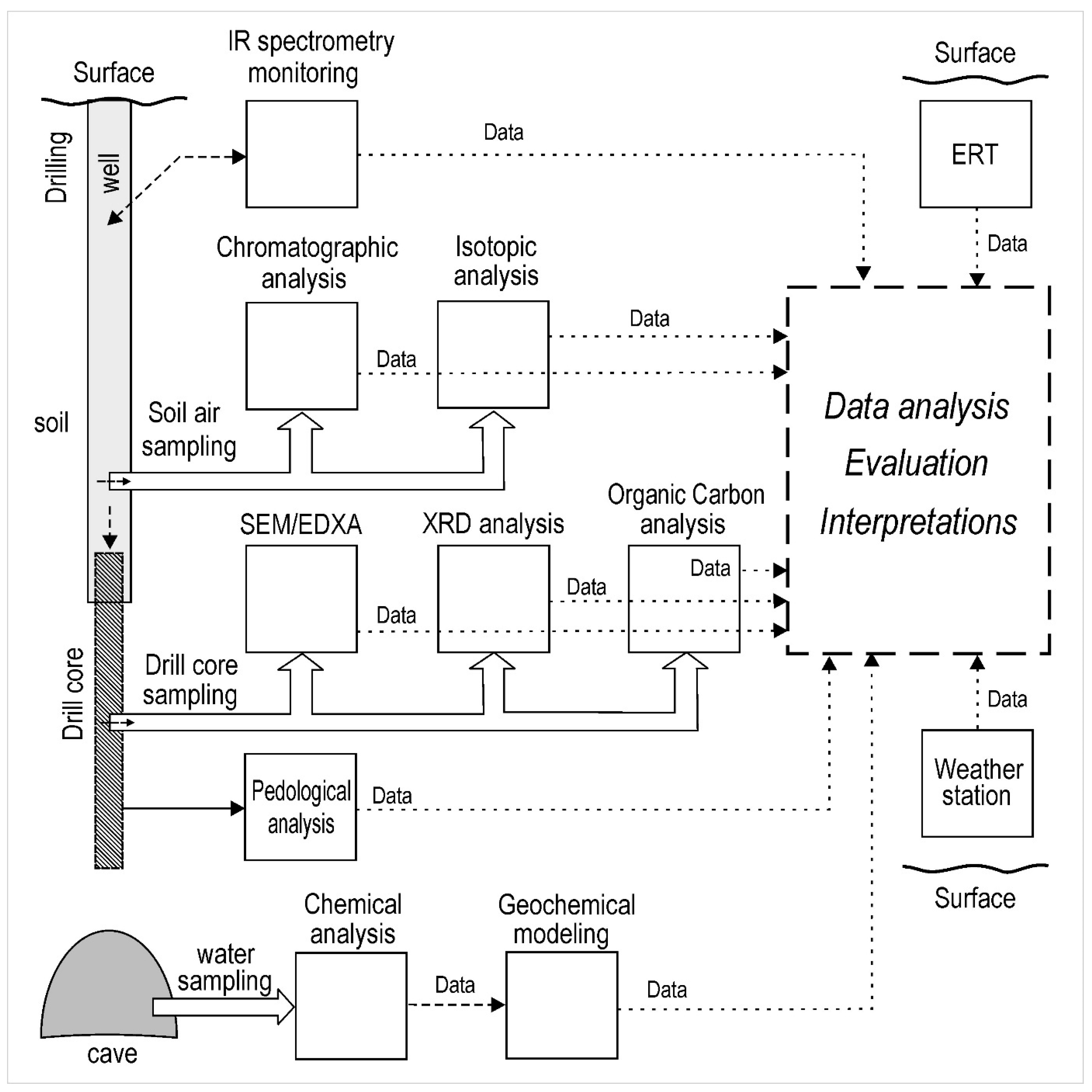
3. Methods
3.1. Electrical Resistivity Tomography
3.2. Soil Drilling
3.3. Gas Analysis
3.4. Organic Carbon in Drill Cores
3.5. Stable Isotopes
3.6. XRD Analysis
4. Results
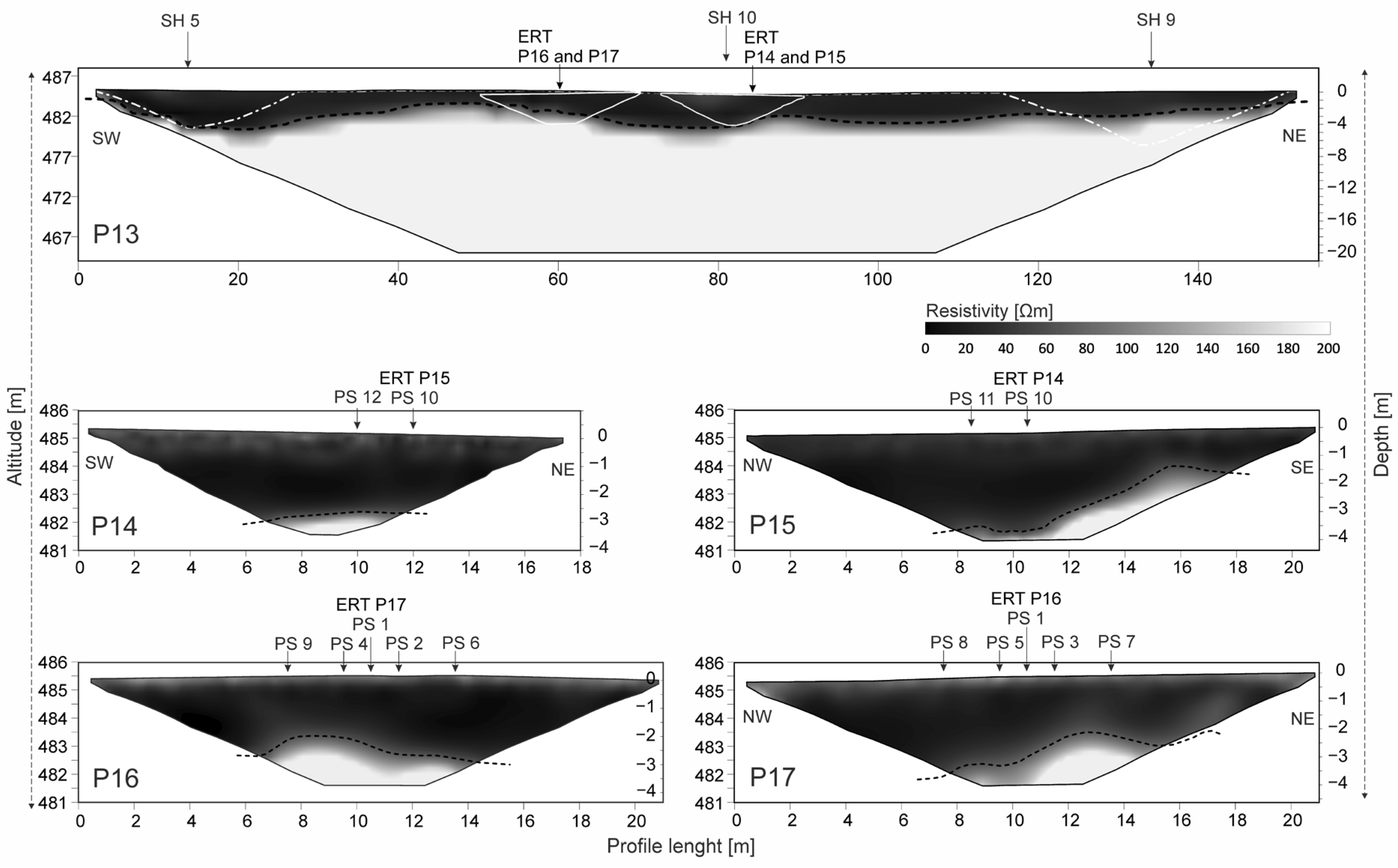
4.1. Electrical Resistivity Tomography (ERT)
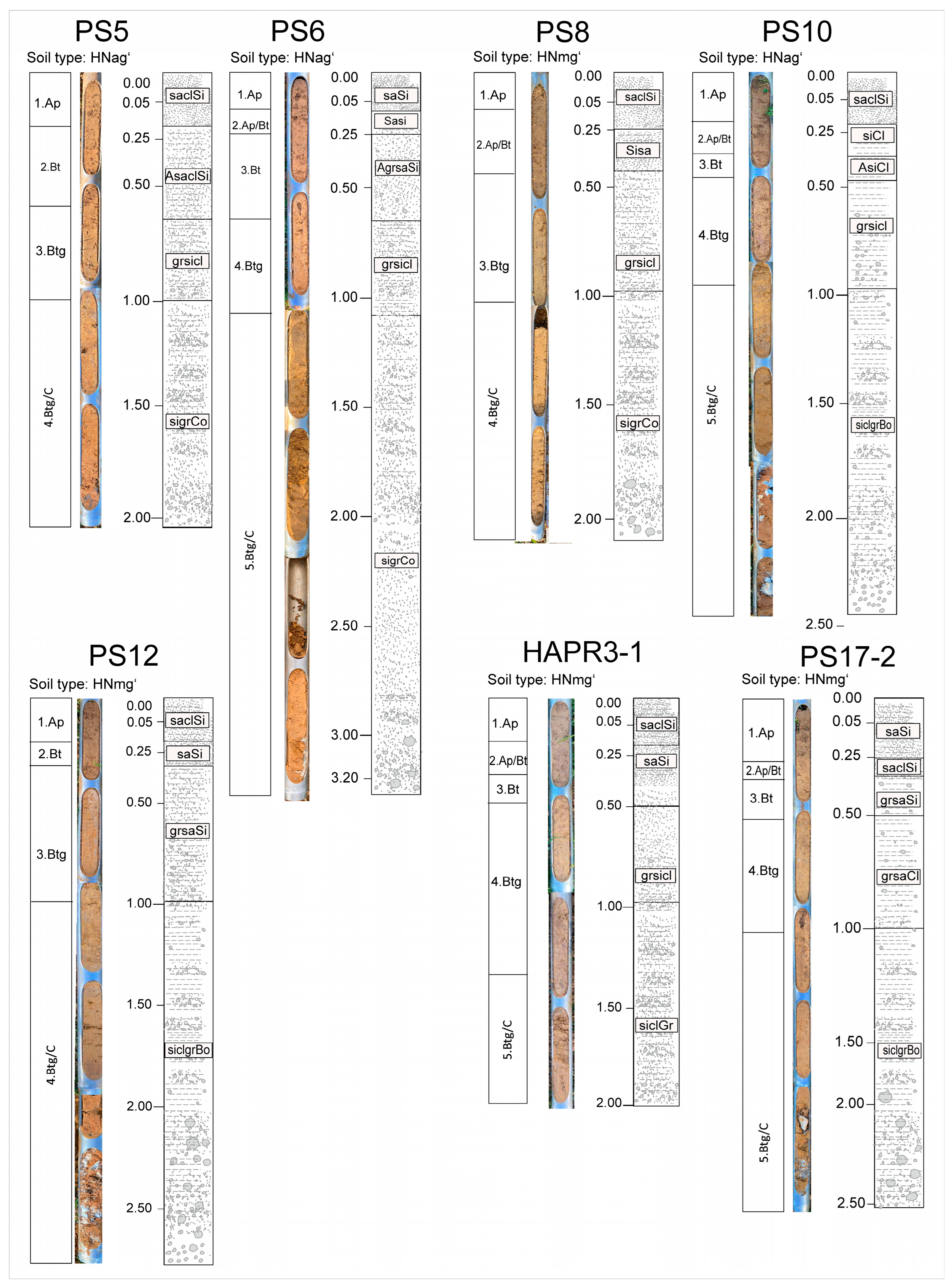
4.2. Soils
4.3. Drill Cores
4.4. Underground Water
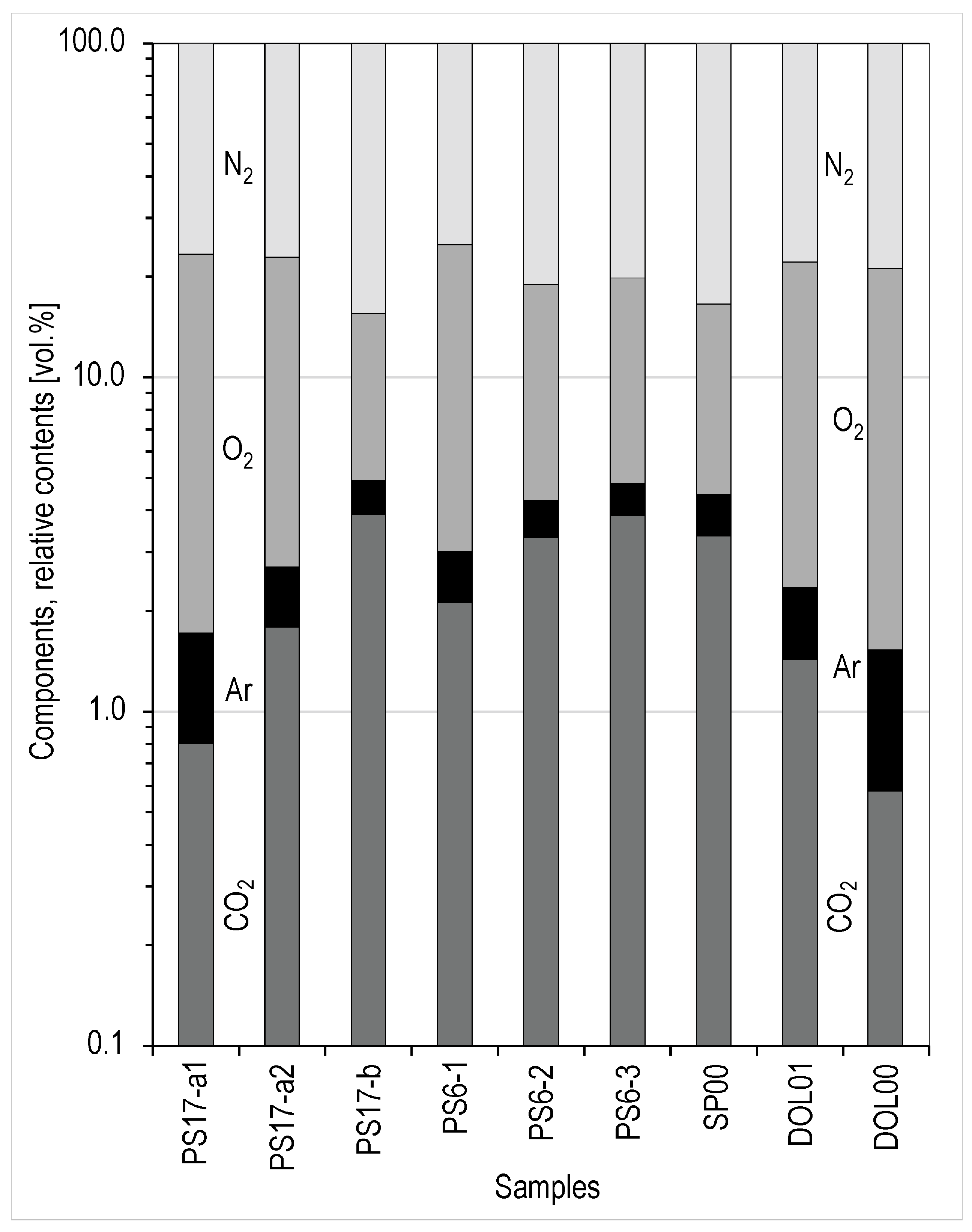
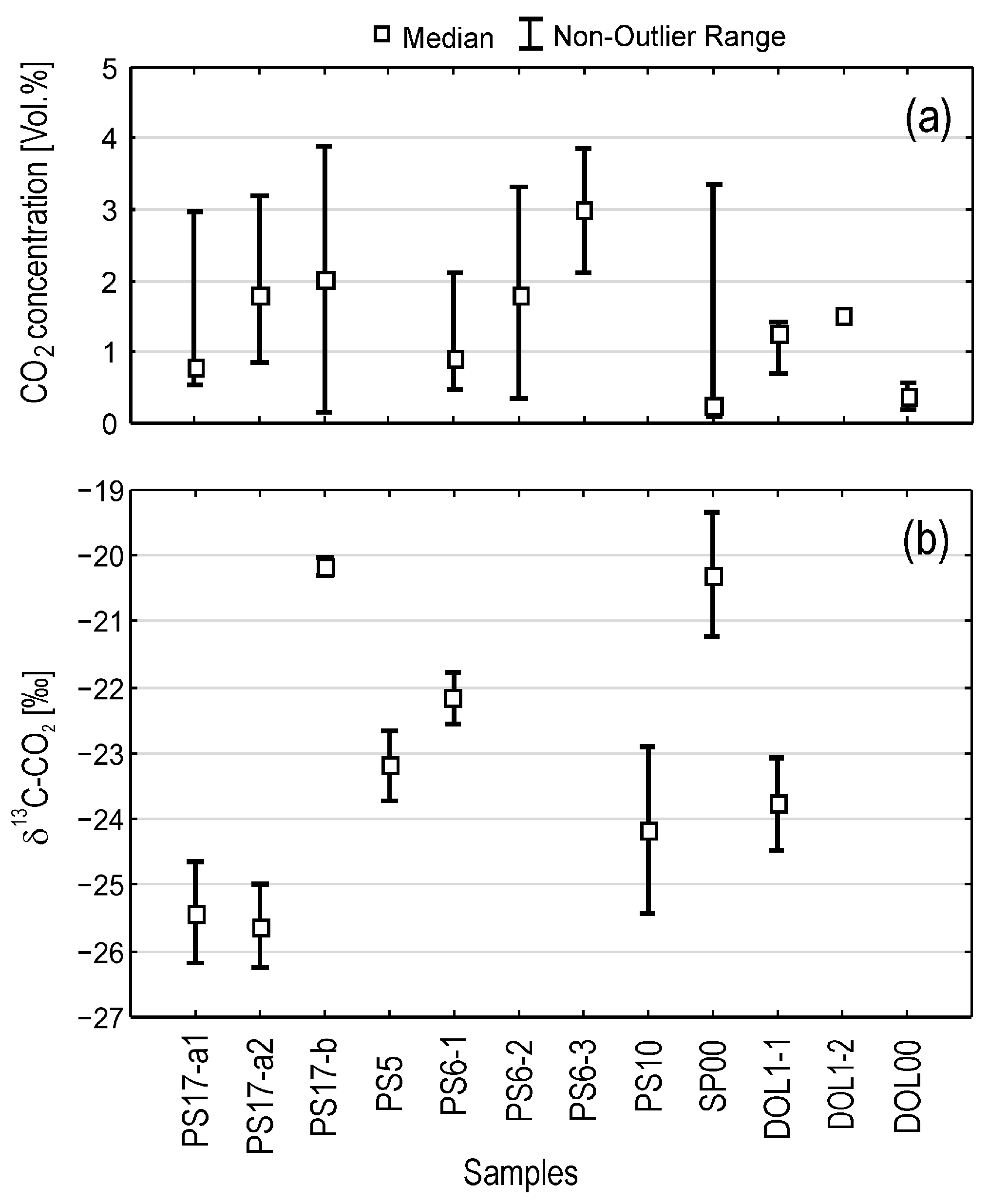
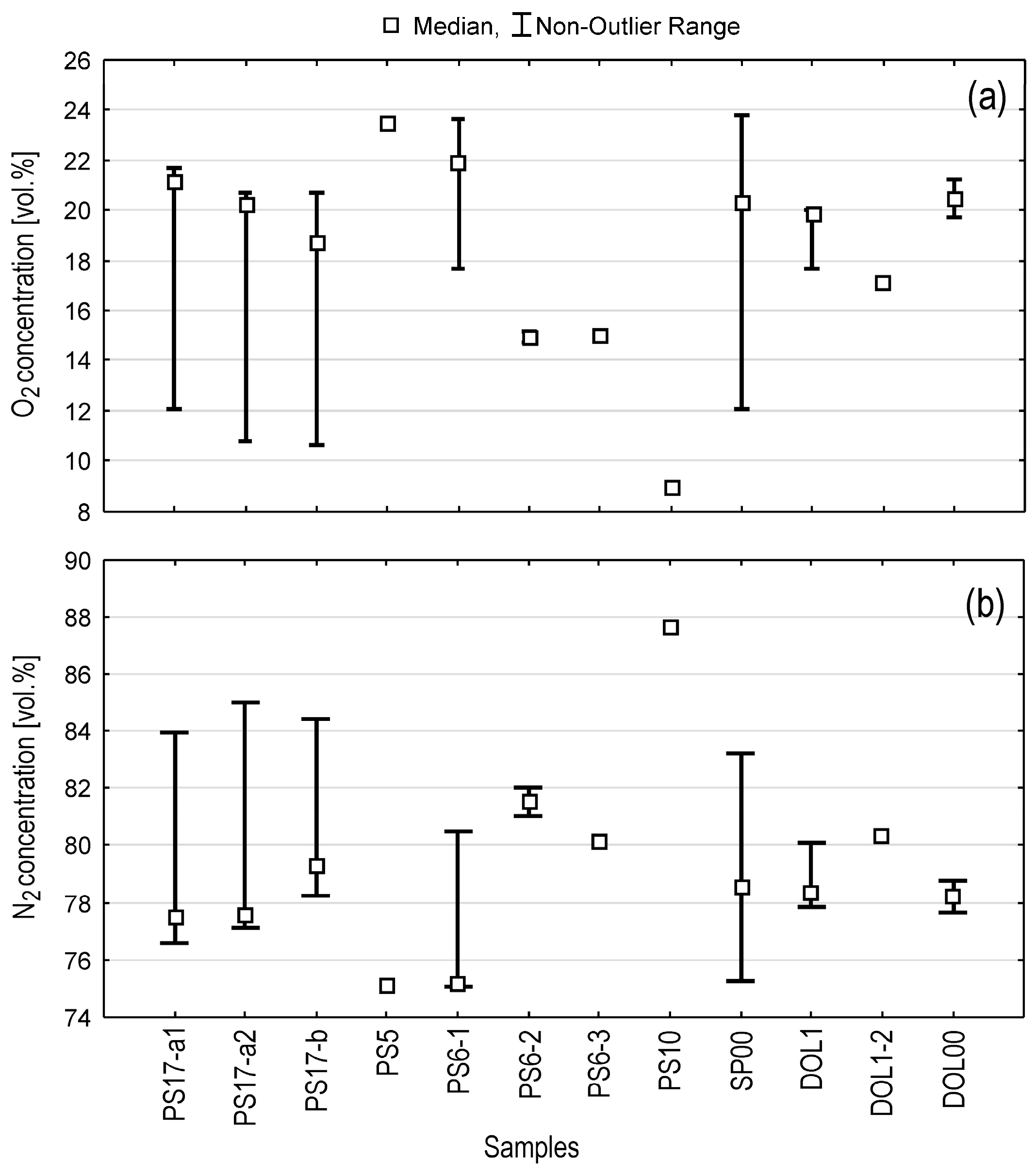
4.5. Composition of Soil Air in Wells
5. Discussion
5.1. Geophysical and Soil Characterization
- (1)
- Long-term agricultural use (potentially since the Neolithic);
- (2)
- A 0.4 m-thick dark Ap soil horizon formed by tillage and fertilization;
- (3)
5.2. Water Chemistry
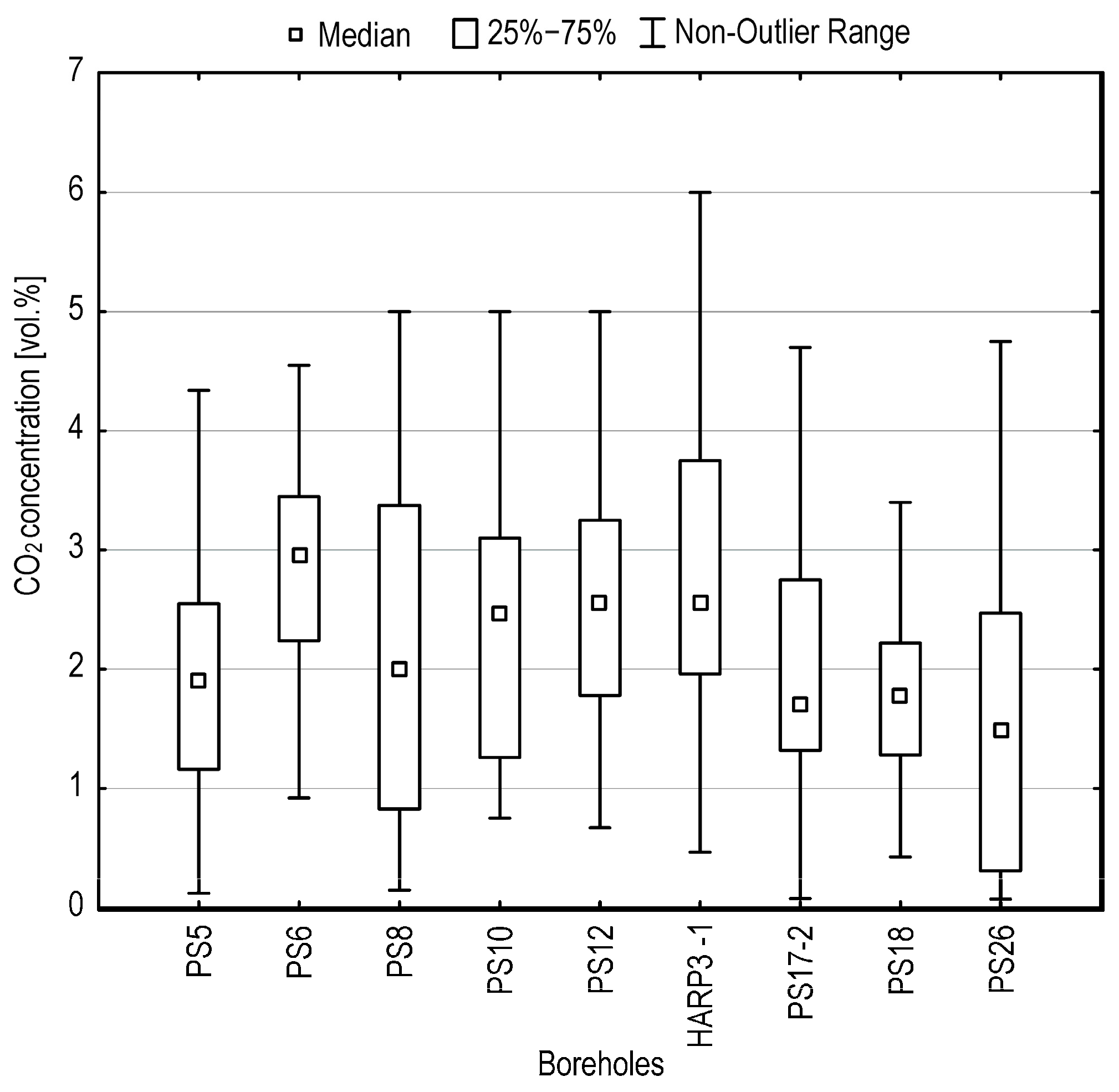
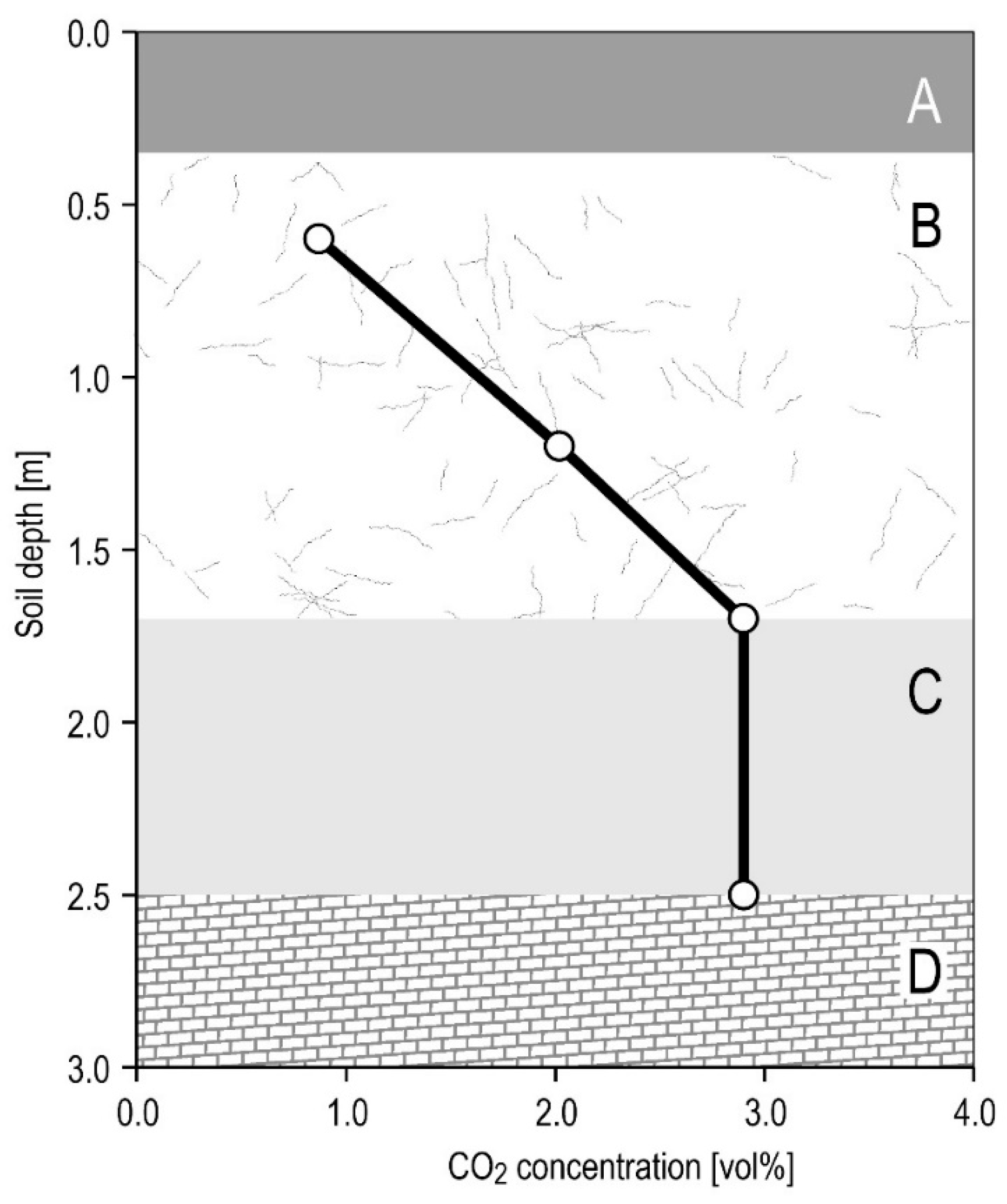
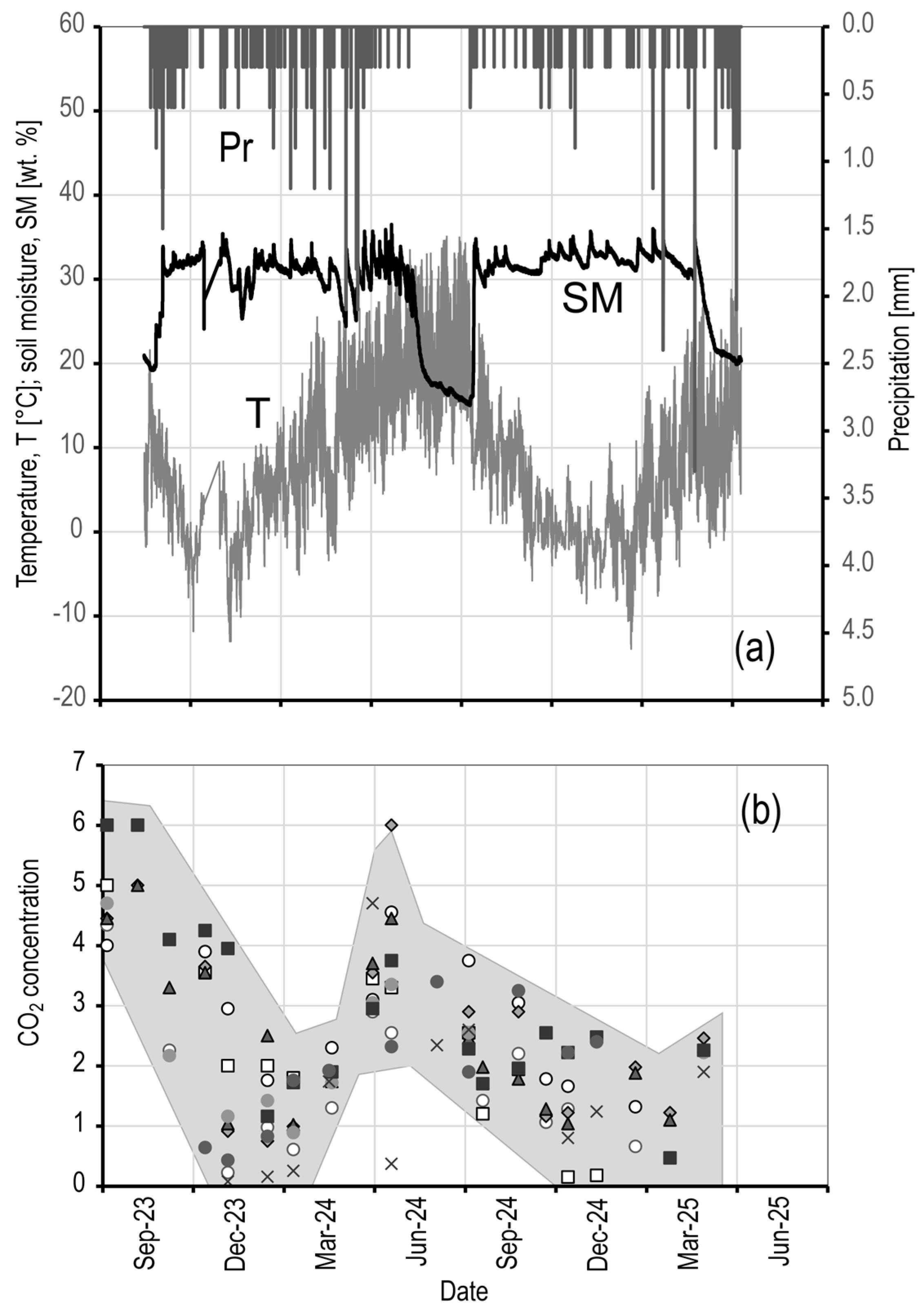
5.3. CO2 Dynamics
5.4. Isotopic Signatures
5.5. Climatic Influences
5.6. Conceptual Model
5.7. Implications
5.8. Environmental Notes
6. Conclusions
- Validation of the GRSL Hypothesis
- Over the past five decades, the hypothesis of a gas-rich soil layer (GRSL) that controls the composition of percolating water in the karst system has been developed. This study bridges the gap between drip-water-derived CO2 (PCO2(form) = 10–1.48, i.e., 3.8 vol. %) and topsoil measurements.
- Field Evidence from Harbechy Plateau
- The haplic anthrosol (loamic, protocalcaric) overlying Harbechy Cave. The GRSL was identified via: (a) drilling (direct detection at the soil–bedrock interface, 2–4 m depth), (b) ERT (confirmed undulating limestone bedrock topography), (c) CO2 gradients (average concentrations of 1.5–3 vol. %, peaking at 4–6 vol. % in a layer 0.8 m thick), and (d) leaking water composition.
- Isotopic Insights
- δ13C signatures reveal that GRSL CO2 is a mixture of (a) biogenic (δ13C = −25‰), (b) atmospheric (δ13C = −8‰), and (c) minor abiogenic sources (likely geogenic).
- Climate Decoupling
- No significant correlation was found between the GRSL CO2 fluctuations and the weather station data, suggesting that carbon dioxide dynamics are buffered by soil processes.
- Broader Implications
- The GRSL represents a previously overlooked carbon pool in karst agroecosystems, with potential impacts on (a) better understanding of karst hydrogeochemistry and karst process, (b) carbon cycling models (especially under land use change), and (c) speleothem formation (through altered seepage water chemistry).
- This study sheds light on the origin of high CO2 concentrations involved in calcite–CO2−water interactions. However, many questions still await clarification. We need to examine in detail other influences: climatic, local (nature of the soil, agricultural use), and others. It is necessary to examine in detail the influence of fertilization in relation to isotopic composition and concentration, including the establishment of limits.
- We believe that this work establishes a baseline for future detailed studies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1
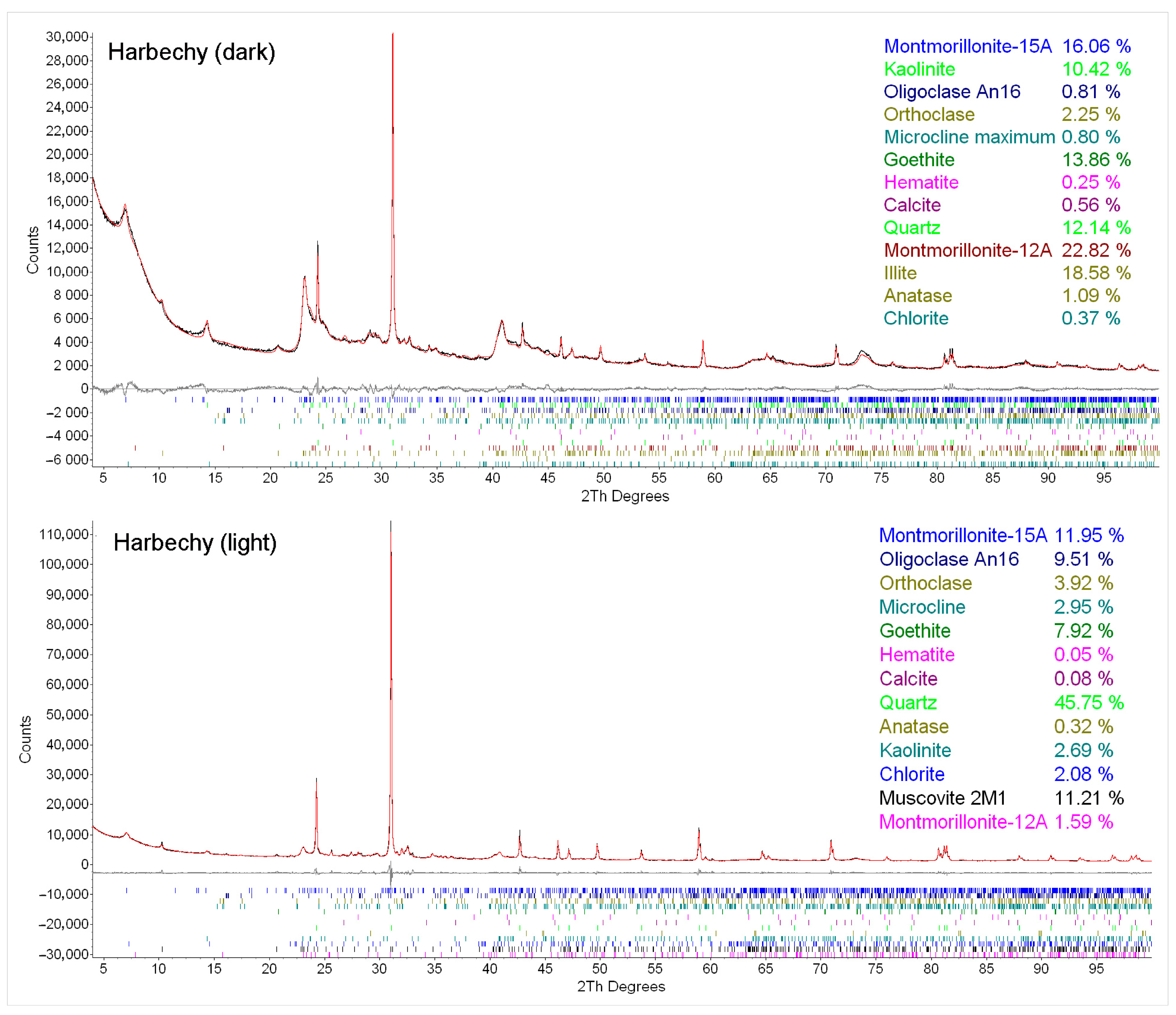
Appendix A.2
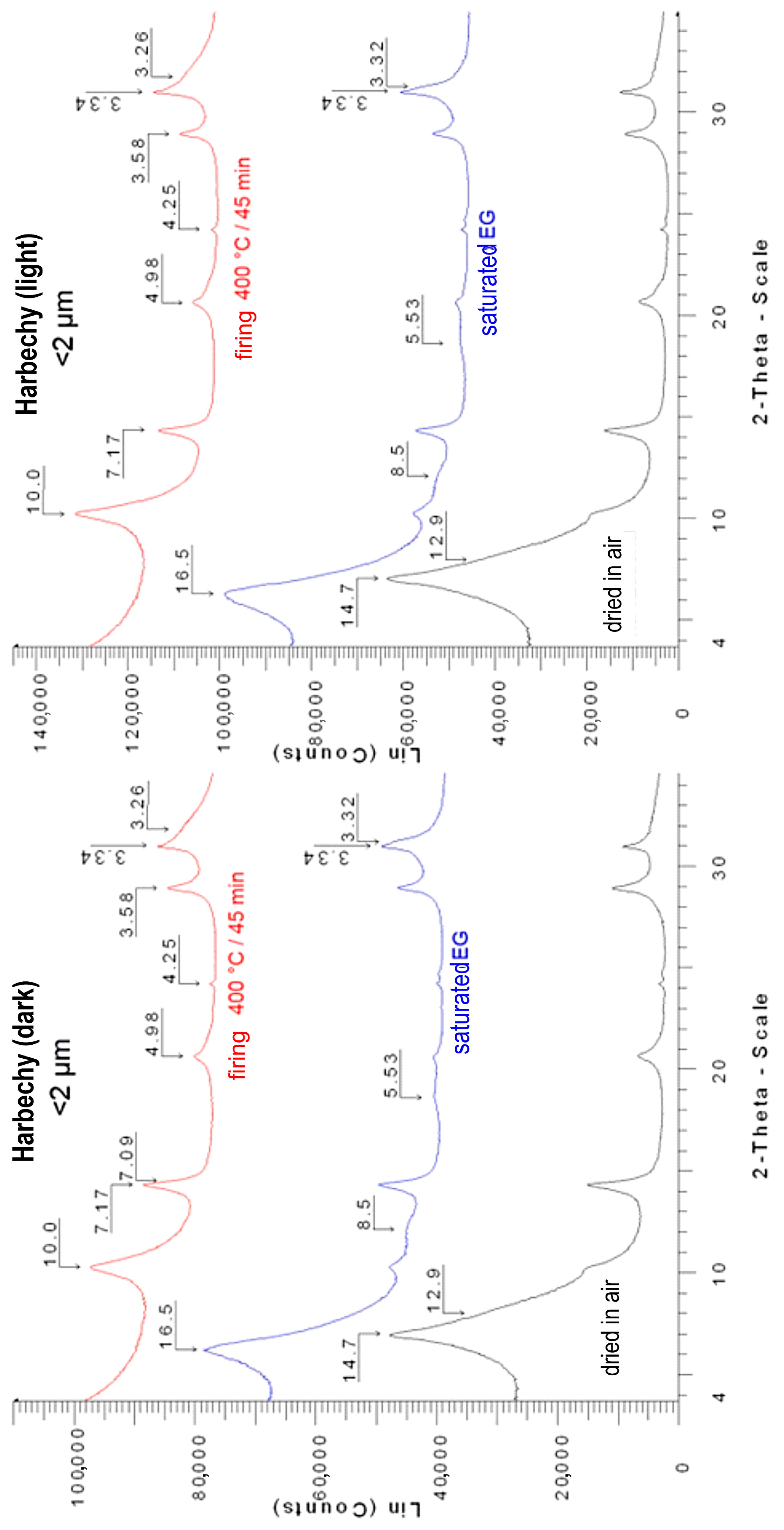
Appendix A.3
References
- Xu, M.; Shang, H. Contribution of soil respiration to the global carbon equation. J. Plant Physiol. 2016, 203, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Bai, X.; Zhao, C.; Tan, Q.; Luo, G.; Wang, J.; Li, Q.; Wu, L.; Chen, F.; Li, C.; et al. Global CO2 consumption by silicate rock chemical weathering: Its past and future. Earths Future 2021, 9, e2020EF001938. [Google Scholar] [CrossRef]
- Bertagni, M.B.; Porporato, A. The carbon-capture efficiency of natural water alkalinization: Implications for enhanced weathering. Sci. Total Environ. 2022, 838, 156524. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y.V.; Larionova, A.A. Contribution of rhizomicrobial and root respiration to the CO2 emission from soil (a review). Eurasian Soil Sci. 2006, 39, 753–764. [Google Scholar] [CrossRef]
- Holden, S.R.; Berhe, A.A.; Treseder, K.K. Decreases in soil moisture and organic matter quality suppress microbial decomposition following a boreal forest fire. Soil Biol. Biochem. 2015, 87, 1–9. [Google Scholar] [CrossRef]
- Fang, C.; Smith, P.; Smith, J.U.; Moncrieff, J.B. Incorporating microorganisms as decomposers into models to simulate soil organic matter decomposition. Geoderma 2025, 129, 139–146. [Google Scholar] [CrossRef]
- Tipping, E.; Cayman, C.J.; Luster, J. The C:N:P:S stoichiometry of soil organic matter. Biogeochemistry 2016, 130, 117–131. [Google Scholar] [CrossRef]
- Dehghani, F.; Reitz, T.; Schlüter, S.; Kastner, M.; Blagodatskaya, E. Decoupling of heat and CO2 release during decomposition of cellulose and its building blocks in soil. Soil Biol. Biochem. 2025, 206, 109801. [Google Scholar] [CrossRef]
- Yuan, X.; Cai, W.; Liang, X.-F.; Su, H.; Yuan, Y.; Li, A.; Tao, Y.-X. Lignocellulose degradation mechanisms across the Tree of Life. Curr. Opin. Chem. Biol. 2015, 29, 108–119. [Google Scholar] [CrossRef]
- Hall, S.J.; Huang, W.; Hammel, K.E. Lignin lags, leads, or limits the decomposition of litter and soil organic carbon. Ecology 2020, 101, e03113. [Google Scholar] [CrossRef]
- Faimon, J.; Lang, M. What actually controls the minute to hour changes in soil carbon dioxide concentrations? Geoderma 2018, 323, 52–64. [Google Scholar] [CrossRef]
- Lang, M.; Faimon, J. Effect of water excess on soil carbon dioxide, seepage water chemistry, and calcite speleothem growth: An experimental and modelling approach. Hydrol. Processes 2020, 34, 4334–4349. [Google Scholar] [CrossRef]
- Faimon, J.; Baldík, V.; Štelcl, J.; Všianský, D.; Rez, J.; Pracný, P.; Novotný, R.; Lang, M.; Roubal, Z.; Szabó, Z.; et al. Corrosion of calcite speleothems in epigenic caves of Moravian Karst (Czech Republic). Environ. Earth Sci. 2024, 83, 184. [Google Scholar] [CrossRef]
- Woessner, W.W.; Poeter, E.P. Hydrogeologic Properties of Earth Materials and Principles of Groundwater Flow; The Groundwater Project: Guelph, ON, Canada, 2020. [Google Scholar] [CrossRef]
- Přibyl, J. Harbešská jeskyně v Moravském krasu [Harbešská Cave in the Moravian Karst]. Čs. Kras 1972, 23, 55–67. (In Czech) [Google Scholar]
- Faimon, J.; Ličbinská, M.; Zajíček, P.; Sracek, O. Partial pressures of CO2 in epikarstic zone deduced from hydrogeochemistry of permanent drips, the Moravian Karst, Czech Republic. Acta Carsol. 2012, 41, 47–57. [Google Scholar] [CrossRef]
- Peyraube, N.; Lastennet, R.; Denis, A. Geochemical evolution of groundwater in the unsaturated zone of a karstic massif, using the PCO2-SIc relationship. J. Hydrol. 2012, 430–431, 13–24. [Google Scholar] [CrossRef]
- Peyraube, N.; Lastennet, R.; Denis, A.; Malaurent, P. Estimation of epikarst air PCO2 using measurements of water δ13CTDIC, cave air PCO2 and δ13CCO2. Geochim. Cosmochim. Acta 2013, 118, 1–17. [Google Scholar] [CrossRef]
- Blecha, M.; Faimon, J. Spatial and temporal variations in carbon dioxide (CO2) concentrations in selected soils of the Moravian Karst (Czech Republic). Carbonates Evaporites 2014, 29, 395–408. [Google Scholar] [CrossRef]
- Absolon, K. Moravský Kras [Moravian Karst]; Academia: Praha, Czech Republic, 1970. (In Czech) [Google Scholar]
- Köppen, W. Das geographische System der Klimate. In Handbuch der Klimatologie; Köppen, W., Geiger, R., Eds.; Gebrüder Borntraeger: Berlin, Germany, 1936; Volume 1, Part C. [Google Scholar]
- Quitt, E. Klimatické Oblasti Československa; Geografický ústav ČSAV: Brno, Czech Republic, 1971. (In Czech) [Google Scholar]
- Agentura Ochrany Přírody a Krajiny ČR. Podnebí, Charakteristika Oblasti [Climate, Area Characteristics]. 2025. Available online: https://moravskykras.aopk.gov.cz/podnebi (accessed on 10 June 2025). (In Czech)
- Redhaounia, B.; Ilondo, B.O.; Gabtni, H.; Sami, K.; Bédir, M. Electrical Resistivity Tomography (ERT) applied to karst carbonate aquifers: Case study from Amdoun, Northwestern Tunisia. Pure Appl. Geophys. 2016, 173, 1289–1303. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C., Jr. X-Ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Milanolo, S.; Gabrovšek, F. Estimation of carbon dioxide flux degassing from percolating waters in a karst cave: Case study from Bijambare cave, Bosnia and Herzegovina. Geochemistry 2015, 75, 465–474. [Google Scholar] [CrossRef]
- Pracný, P.; Faimon, J.; Kabelka, L.; Hebelka, J. Variations of carbon dioxide in the air and droplets of Punkva Caves (Moravian Karst, Czech Republic). Carbonates Evaporites 2016, 31, 375–386. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources, 4th ed.; International Union of Soil Sciences: Vienna, Austria, 2022. [Google Scholar]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3-A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey Techniques and Methods: Reston, VA, USA, 2013; Book 6, Chapter A43. [Google Scholar]
- Pataki, D.E.; Ehleringer, J.R.; Flanagan, L.B.; Yakir, D.; Bowling, D.R.; Still, C.J.; Buchmann, N.; Kaplan, J.O.; Berry, J.A. The application and interpretation of Keeling plots in terrestrial carbon cycle research. Global Biogeochem. Cycles 2003, 17, 1022. [Google Scholar] [CrossRef]
- Bowling, D.R.; Pataki, D.E.; Randerson, J.T. Carbon isotopes in terrestrial ecosystem pools and CO2 fluxes. New Phytol. 2007, 174, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Keeling, C.D. The concentration and isotopic abundance of atmospheric carbon dioxide in rural areas. Geochim. Cosmochim. Acta 1958, 13, 322–334. [Google Scholar] [CrossRef]
- Gillon, M.; Barbecot, F.; Gibert, E.; Plain, C.; Corcho-Alvarado, J.-A.; Massault, M. Controls on 13C and 14C variability in soil CO2. Geoderma 2012, 189–190, 431–441. [Google Scholar] [CrossRef]
- Cerling, T.E.; Solomon, D.K.; Quade, J.; Bowman, J.R. On the isotopic composition of carbon in soil carbon dioxide. Geochim. Cosmochim. Acta 1991, 55, 3403–3405. [Google Scholar] [CrossRef]
- Gao, W.; Chen, M.; Xu, X. Tracing controls of autotrophic and heterotrophic nitrification in terrestrial soils. Eur. J. Soil Biol. 2022, 110, 103409. [Google Scholar] [CrossRef]
- Whiticar, M.J. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem. Geol. 1999, 161, 291–314. [Google Scholar] [CrossRef]
- Hatté, C.; Balesdent, J.; Pizol, L. δ13C of CO2 from cellulose decomposition in soils ranges from −27‰ to −32‰. Soil Biol. Biochem. 2008, 40, 409–412. [Google Scholar] [CrossRef]
- Halešová, T.; Kotyzová, M. Zatravnění I. zóny CHKO Moravský kras [Grassland restoration in Zone I of the CHKO Moravian Karst. Ochrana Přírody 2021, 76, 15–18. (In Czech) [Google Scholar]
- Benavente, J.; Vadillo, I.; Carrasco, F.; Soler, A.; Liñán, C.; Moral, F. Air Carbon Dioxide Contents in the Vadose Zone of a Mediterranean Karst. Vadose Zone J. 2010, 9, 126–136. [Google Scholar] [CrossRef]
- Vadillo, I.; Benavente, J.; Carrasco, F.; Soler, A.; Liñán, C. Isotopic (13C) Signature of CO2 Sources in the Vadose Zone of a Mediterranean Karst (Nerja Cave Site, Southern Spain). In Advances in Research in Karst Media; Andreo, B., Carrasco, F., Durán, J., LaMoreaux, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 71–76. [Google Scholar] [CrossRef]
- Atkinson, T.C. Carbon dioxide in the atmosphere of the unsaturated zone: An important control of groundwater hardness in limestones. J. Hydrol. 1977, 35, 111–123. [Google Scholar] [CrossRef]

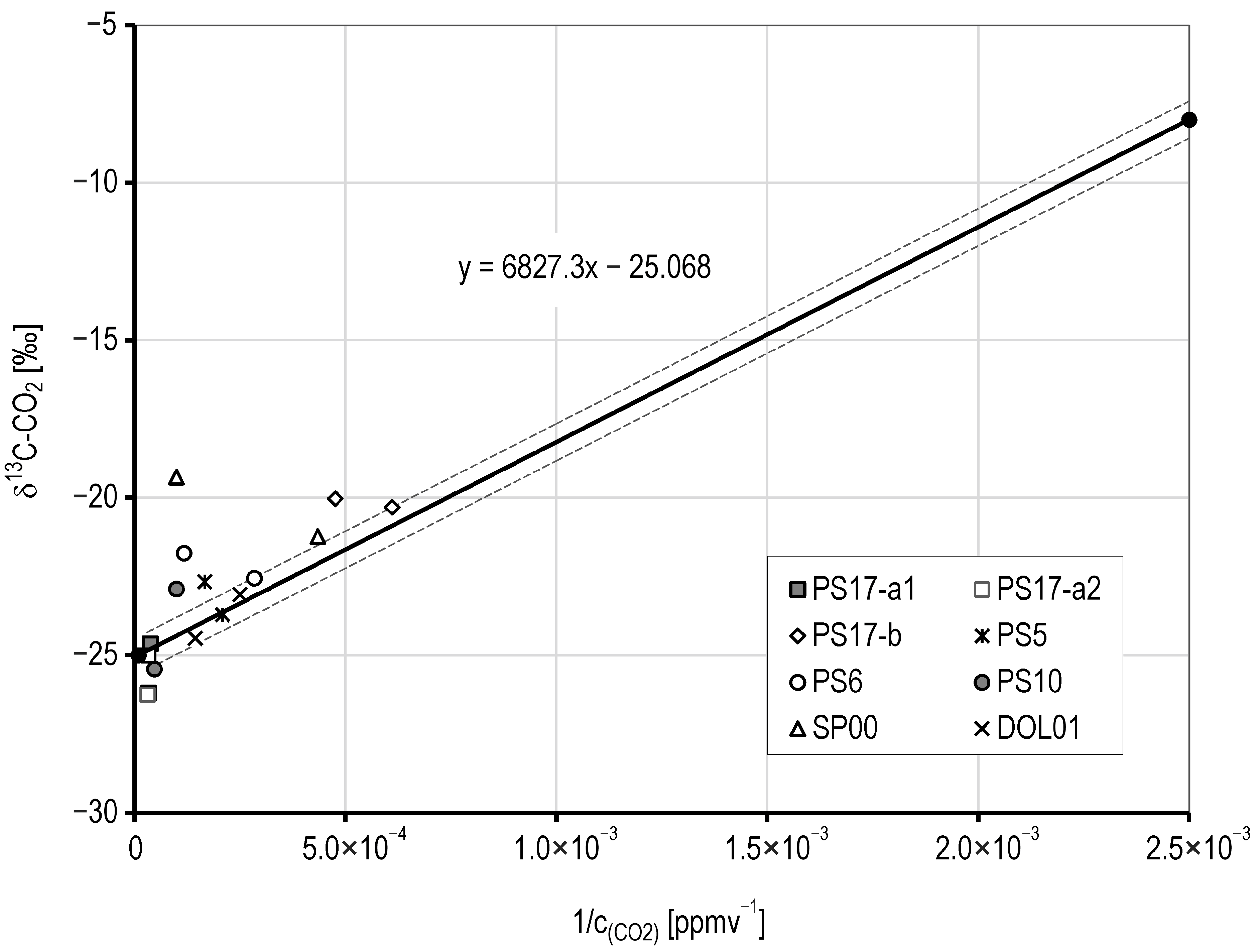

| X | Y | Z | WGS | ||
|---|---|---|---|---|---|
| P14 | −587,451.50 | −1,143,616.89 | 485.08 | 49.35860111 | 16.72924 |
| P14 | −587,455.96 | −1,143,620.85 | 485.23 | 49.35856146 | 16.72918 |
| P15 | −587,463.04 | −1,143,613.18 | 485.17 | 49.3586233 | 16.72907 |
| P15 | −587,455.96 | −1,143,620.85 | 485.23 | 49.35856146 | 16.72918 |
| P15 | −587,448.61 | −1,143,628.76 | 485.38 | 49.35849773 | 16.72929 |
| P16 | −587,464.15 | −1,143,632.08 | 485.59 | 49.35845324 | 16.72909 |
| P16 | −587,478.85 | −1,143,647.42 | 485.50 | 49.35830208 | 16.72891 |
| P17 | −587,479.08 | −1,143,632.27 | 485.51 | 49.35843732 | 16.72888 |
| P17 | −587,463.58 | −1,143,646.88 | 485.80 | 49.35832145 | 16.72912 |
| PS1 | −587,471.31 | −1,143,639.42 | 485.61 | 49.35838079 | 16.72900 |
| PS2 | −587,470.62 | −1,143,638.71 | 485.59 | 49.5838780 | 16.72901 |
| PS3 | −587,470.55 | −1,143,640.16 | 485.62 | 49.3583749 | 16.72901 |
| PS4 | −587,472.08 | −1,143,640.08 | 485.60 | 49.5837416 | 16.72899 |
| PS5 | −587,472.05 | −1,143,638.82 | 485.60 | 49.35838545 | 16.72899 |
| PS6 | −587,469.23 | −1,143,637.31 | 485.63 | 49.35840164 | 16.72902 |
| PS7 | −587,469.15 | −1,143,641.52 | 485.68 | 49.35836407 | 16.72903 |
| PS8 | −587,473.52 | −1,143,637.53 | 485.53 | 49.35839559 | 16.72897 |
| PS9 | −587,473.34 | −1,143,641.56 | 485.61 | 49.35835972 | 16.72897 |
| PS10 | −587,455.97 | −1,143,620.86 | 485.26 | 49.35856136 | 16.72918 |
| PS11 | −587,457.36 | −1,143,619.42 | 485.24 | 49.35857291 | 16.72916 |
| PS12 | −587,457.39 | −1,143,622.22 | 485.36 | 49.35854784 | 16.72916 |
| PS17 | −587,655.67 | −1,143,761.03 | 486.12 | 49.35711779 | 16.72665 |
| PS18 | −586,072.57 | −1,139,866.72 | 504.42 | 49.39344700 | 16.74265 |
| PS25 | −586,349.49 | −1,140,723.66 | 484.62 | 49.38552100 | 16.74010 |
| HAPR3-1 | −587,477.42 | −1,143,645.52 | 485.45 | 49.35832100 | 16.72892 |
| Na | Mg | Al | Si | K | Ca | Ti | Mn | Fe | O | |
|---|---|---|---|---|---|---|---|---|---|---|
| Spot Analysis | n | 0.89 | 6.56 | 32.49 | 0.79 | 0.49 | 0.26 | n | 11.32 | 47.21 |
| 0.29 | 2.78 | 38.37 | 0.31 | 0.23 | n | n | n | 8.92 | 49.1 | |
| 0.25 | 0.64 | 7.13 | 34.46 | 0.92 | 0.64 | n | 0.72 | 6.58 | 48.65 | |
| 2.5 | 0.82 | 11.63 | 28.93 | 2.44 | 0.7 | n | n | 5.83 | 47.16 | |
| n | 1.05 | 13.88 | 24.91 | 5.1 | 0.91 | 0.46 | n | 8.21 | 45.48 | |
| n | 0.55 | 7.67 | 35.67 | 1.08 | 0.56 | n | n | 4.82 | 49.65 | |
| n | 1.22 | 14.97 | 25.12 | 4.94 | 0.55 | 0.35 | n | 6.73 | 46.12 | |
| Area 50 × 50 µm | n | 1.06 | 10.74 | 28.39 | 2.07 | 0.64 | 0.53 | n | 10.05 | 46.51 |
| Minerals/Samples | Harbechy Loesses—Dark | Harbechy Loesses—Light | |
|---|---|---|---|
| Clay minerals | Smectite | 38.9 | 13.5 |
| IIllite and mica structures | 18.6 | 11.2 | |
| Kaolinite | 10.4 | 2.7 | |
| Chlorite | 0.4 | 2.1 | |
| Oxides | Anatase | 1.1 | 0.3 |
| Goethite | 13.9 | 7.9 | |
| Hematite | 0.3 | 0.1 | |
| Quartz | 12.2 | 45.8 | |
| Carbonates | Calcite | 0.6 | 0.1 |
| Silicates | K-feldspar | 3.1 | 6.9 |
| Plagioclase | 0.8 | 9.5 | |
| Sum | 100.3 | 100.1 | |
| Concentrations | Method | ||
|---|---|---|---|
| pH | 6.8 | Potentiometry | |
| Sodium | Na | 3.97 × 10−4 | ICP-OES |
| Potassium | K | <1 × 10−5 | ICP-OES |
| Ammonium ions | NH4+ | 7.22 × 10−6 | Spectrophotometry |
| Calcium | Ca | 6.74 × 10−3 | ICP-OES |
| Magnesium | Mg | 1.65 × 10−4 | ICP-OES |
| Sulfates | S | 4.69 × 10−4 | ICP-OES |
| Chlorides | Cl | 8.47 × 10−5 | Spectrophotometry |
| Nitrites | NO2− | 7.15 × 10−7 | Spectrophotometry |
| Nitrates | NO3− | 8.57 × 10−3 | Spectrophotometry |
| Phosphates | P | 3.23 × 10−6 | Spectrophotometry |
| Alkalinity | 5.88 × 10−3 | Volumetric |
| Mineral Phase | SI | log IAP | log KT | |
|---|---|---|---|---|
| Anhydrite | CaSO4 | −1.79 | −6.13 | −4.34 |
| Aragonite | CaCO3 | −0.19 | −8.44 | −8.25 |
| Calcite | CaCO3 | −0.04 | −8.44 | −8.4 |
| CO2(g) | CO2 | −1.38 | −2.62 | −1.24 |
| Dolomite | CaMg(CO3)2 | −1.82 | −18.49 | −16.67 |
| Gypsum | CaSO4.2H2O | −1.53 | −6.13 | −4.6 |
| Halite | NaCl | −9.14 | −7.6 | 1.54 |
| Hydroxyapatite | Ca5(PO4)3OH | −2.06 | −3.87 | −1.82 |
| Mineral Phase | SI | log IAP | log KT | |
|---|---|---|---|---|
| Anhydrite | CaSO4 | −1.79 | −6.13 | −4.34 |
| Aragonite | CaCO3 | −0.16 | −8.4 | −8.25 |
| Calcite | CaCO3 | 0 | −8.4 | −8.4 |
| CO2(g)form | CO2 | −1.42 | −2.66 | −1.24 |
| Dolomite | CaMg(CO3)2 | −1.74 | −18.42 | −16.67 |
| Gypsum | CaSO4:2H2O | −1.53 | −6.13 | −4.6 |
| Halite | NaCl | −9.14 | −7.6 | 1.54 |
| Hydroxyapatite | Ca5(PO4)3OH | −1.85 | −3.67 | −1.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faimon, J.; Baldík, V.; Rez, J.; Hadacz, R.; Novotný, R.; Ocásková, D.; Dostalík, M.; Všianský, D.; Nečas, J.; Štelcl, J.; et al. Elevated Concentrations of Carbon Dioxide (CO2) on the Harbechy Plateau (Moravian Karst) Reveal a Gas-Rich Soil Layer (GRSL). Appl. Sci. 2025, 15, 8907. https://doi.org/10.3390/app15168907
Faimon J, Baldík V, Rez J, Hadacz R, Novotný R, Ocásková D, Dostalík M, Všianský D, Nečas J, Štelcl J, et al. Elevated Concentrations of Carbon Dioxide (CO2) on the Harbechy Plateau (Moravian Karst) Reveal a Gas-Rich Soil Layer (GRSL). Applied Sciences. 2025; 15(16):8907. https://doi.org/10.3390/app15168907
Chicago/Turabian StyleFaimon, Jiří, Vít Baldík, Jiří Rez, Roman Hadacz, Roman Novotný, Daniela Ocásková, Martin Dostalík, Dalibor Všianský, Jiří Nečas, Jindřich Štelcl, and et al. 2025. "Elevated Concentrations of Carbon Dioxide (CO2) on the Harbechy Plateau (Moravian Karst) Reveal a Gas-Rich Soil Layer (GRSL)" Applied Sciences 15, no. 16: 8907. https://doi.org/10.3390/app15168907
APA StyleFaimon, J., Baldík, V., Rez, J., Hadacz, R., Novotný, R., Ocásková, D., Dostalík, M., Všianský, D., Nečas, J., Štelcl, J., Kuda, F., Křenovská, I., & Chalupka, F. (2025). Elevated Concentrations of Carbon Dioxide (CO2) on the Harbechy Plateau (Moravian Karst) Reveal a Gas-Rich Soil Layer (GRSL). Applied Sciences, 15(16), 8907. https://doi.org/10.3390/app15168907







