1. Introduction
Obesity has become a significant public health challenge worldwide, with its prevalence rising sharply over the past decades [
1,
2]. According to the Global Burden of Disease Initiative [
3,
4], obesity represents a leading risk factor for morbidity and mortality, contributing significantly to the burden of non-communicable disorders such as cardiovascular disease (CVD), type 2 diabetes mellitus (T2DM), and certain cancers.
Obesity is a complex, multifactorial disease characterized by an abnormal or excessive accumulation of adipose tissue to an extent that negatively impacts human health. It results from an imbalance between energy intake and expenditure, driven by genetic, behavioral, environmental, and socioeconomic factors [
5]. Various treatment strategies can be employed to prevent and manage obesity, including bariatric surgery [
6], structured exercise regimens [
7], dietary modifications [
8], behavioral therapy [
9], and pharmacological approaches [
10].
Exercise is widely regarded as a crucial therapeutic tool among the various options available due to its minimal harmful side effects and numerous health benefits [
11,
12]. Regular physical activity not only aids in weight reduction but also improves metabolic health and reduces the risk of obesity-related comorbidities. As a result, exercise remains a cornerstone of obesity management. Globally, numerous public health initiatives promote healthy dietary habits, active lifestyles, and the responsible use of weight management medications to counteract the adverse effects of obesity [
13].
Regular exercise enhances muscle strength and reduces visceral fat, improving insulin sensitivity and breaking insulin resistance. Additionally, it lowers the risk of metabolic disorders such as obesity, dyslipidemia, T2DM, hypertension, and CVD [
14]. Metformin is the most commonly prescribed medication for T2DM worldwide. Beyond its role in glycemic control, it is increasingly used, alone or in combination with other treatments, to manage obesity due to its ability to reduce insulin resistance and support weight loss [
15,
16]. Metformin improves glycemic control without causing hypoglycemia or weight gain [
16,
17,
18], unlike other antidiabetic medications, such as insulin and insulin secretagogues [
19]. It primarily exerts its effects through three key mechanisms: (i) reducing hepatic glucose production; (ii) enhancing peripheral glucose uptake and utilization in muscles, thereby improving insulin sensitivity; and (iii) delaying intestinal glucose absorption [
20]. These mechanisms contribute to its therapeutic role in weight management, with only a few rare but serious side effects [
21,
22].
Given the impact of metformin and exercise on energy metabolism in the liver, heart, and skeletal muscle, these tissues remain key research areas. Spexin, a recently discovered 14-amino acid peptide belonging to the galanin/kisspeptin/spexin family [
23], plays a crucial role in regulating appetite, nutrient intake, energy balance, weight reduction, carbohydrate metabolism, and lipid oxidation. It has been identified as a key factor in the pathophysiology of obesity [
24]. A recent systematic review suggests that spexin functions as an anorexigenic factor, suppressing appetite and influencing metabolic processes [
25]. One study classified spexin as an anorexigenic adipokine capable of modulating adipogenesis and glucose metabolism by reducing food intake and increasing energy expenditure [
26]. Other studies examining spexin levels in lean adults, children, and obese individuals found that obese individuals had the lowest circulating spexin levels, suggesting a potential role in obesity development [
27,
28,
29]. Consequently, low spexin levels may serve as a biomarker for obesity and, broadly, cardiometabolic health [
30].
In the present study, metformin was chosen for its well-established catabolic effects, weight-reducing properties, widespread use as the most commonly prescribed medication for T2DM, and minimal side effects. Similarly, aerobic exercise, which primarily utilizes fat as an energy source, is widely recommended as an effective intervention for obesity management [
31]. Despite the known benefits of these treatments, a gap exists in the literature regarding their individual and combined effects on spexin expression in key metabolic tissues.
This study aims to address this gap by investigating spexin expression in the liver, heart, and skeletal muscle following metformin administration, both alone and in combination with aerobic exercise, in rats subjected to a high-calorie diet. This study is particularly valuable given the limited research on spexin expression in these tissues. We hypothesized that metformin and aerobic exercise would enhance spexin levels, offering critical insights into their role in obesity management and providing a comprehensive perspective on how spexin expression can be influenced by both physiological and pharmacological factors. Since spexin is implicated in energy homeostasis and weight regulation, modulating its levels could contribute to advancements in obesity prevention and treatment, informing targeted therapeutic strategies and potentially optimizing treatment protocols.
2. Materials and Methods
2.1. Study Groups
The study protocol of the present investigation received ethical clearance from the Fırat University Local Ethics Committee for Animal Experiments (code: 2022/18-17, date: 16 November 2022). This study utilized 36 male Sprague–Dawley rats, aged 8 weeks, obtained from the Fırat University Experimental Animal Production and Research Center (FUDAM). The research followed a post-test-only control group experimental design.
At the start of this study, each rat was individually weighed using a precision scale while it was placed in an enclosed plastic enclosure, and the measurements were recorded. The rats were then randomly assigned into six experimental groups using a computer-generated randomization method (
https://random.org/lists) (accessed on 24 May 2025) by a staff member uninvolved in the measurements. The experimental groups were as follows: (i) decapitation group (n = 6): rats were decapitated at 8 weeks of age before the study to serve as a baseline reference; (ii) control group (n = 6): rats received no intervention and were maintained on a regular diet throughout this study; (iii) obese control group (n = 6): obesity was induced, but no metformin administration or exercise was administered; (iv) exercise group (n = 6): after obesity induction, rats participated in treadmill exercise sessions for 30 min per day, five days per week, over four weeks; (v) metformin group (n = 6): following obesity induction, rats received metformin administration (150 mg/kg/day, gavage) for four weeks; and, (vi) metformin + exercise group (n = 6): after obesity induction, rats received metformin administration (150 mg/kg/day, gavage) for four weeks and concurrently engaged in treadmill exercise (30 min per day, five days per week) over the same period.
The rats were housed and fed at FUDAM under controlled conditions. Each rat was kept in an individual cage, which was cleaned daily, and provided with a balanced diet. Food was supplied in specialized containers, while water was available ad libitum from glass bottles filled with tap water. Non-obese groups were fed pellet-form animal feed obtained from FÜDAM. The housing environment was maintained at a constant temperature of 21 ± 1 °C, with a 12 h light/12 h dark cycle. Rats in the control group received a standard diet ad libitum, composed of 51% carbohydrates, 4% fat, 21% protein, and 24% water.
2.2. Induction of Obesity
To achieve diet-induced obesity (DIO), rats were fed a high-calorie diet consisting of 33% standard rat food, 33% full-fat sweetened milk, 7% sucrose, and 27% water. This diet was specifically designed to promote obesity. As previously mentioned, at the start of this study, each rat was individually weighed using a sensitive scale while placed in a covered plastic container. Body mass index (BMI) was measured weekly to monitor the progression of DIO. BMI was calculated by measuring the body weight (g) divided by the square of the nose-to-anus length and expressed in g/cm
2 [
32]. The obesity induction process was completed after 12 weeks.
2.3. Metformin Administration
After inducing obesity in rats, the metformin and metformin + exercise groups received a daily gavage of 150 mg/kg body weight of metformin for four weeks. A previous study has shown that the optimal range for metformin binding to blood proteins and renal clearance is 100–200 mg/kg [
33]. Therefore, the dosage used in our study was selected based on these established pharmacokinetic parameters.
2.4. Exercise Program
In the present study, rats in the exercise and metformin + exercise groups underwent a five-day acclimatization phase on a treadmill (KN-73; Natsume Seisakusho Co., Ltd., Tokyo, Japan) to prepare for the exercise protocol. Following this, they participated in a four-week exercise program, conducted five days per week between 10:00 a.m. and 12:00 p.m. Each session consisted of 30 min of treadmill running at a constant speed of 20 m per minute with no incline. If needed, a 100-millivolt electric stimulus was applied to ensure compliance with the protocol on the specially designed treadmill.
2.5. Completion of the Experimental Protocols and Tissue Sampling
Twenty-four hours after completing the exercise protocol, the rats were decapitated following removal from their cages. Liver, whole heart, and skeletal muscle tissues from the gastrocnemius were immediately harvested. Tissues designated for immunohistochemical analyses were preserved in a formaldehyde solution. In contrast, those intended for Enzyme-Linked Immunosorbent Assay (ELISA) analysis were snap-frozen and stored at −80 °C until further processing.
2.6. Biochemical Analyses
Immunohistochemical analyses were conducted at the Department of Histology and Embryology, Faculty of Veterinary Medicine, Fırat University. Liver, heart, and skeletal muscle tissues stored at −80 °C from all groups were retrieved for processing. Each tissue sample was weighed using a precision scale and then washed three times with phosphate-buffered saline (PBS) to remove any blood residues before being dried with blotting paper. A 50 mg section was carefully excised using a sterile scalpel and weighed again on a sensitive scale. For homogenization, 50 mg of tissue was placed in a pre-labeled Eppendorf tube, along with four homogenizer beads (3.2 mm diameter, 500 g) and 450 μL of PBS. The tubes were securely sealed and processed in a Bullet Blender homogenizer (USA) at intensity level 8 for 5 min. Following homogenization, the samples were centrifuged at 4000 rpm at 4 °C (Beckman Coulter, Allegra X-30 model, Maharashtra, India). The resulting supernatant was carefully transferred to a new Eppendorf tube and stored at −80 °C for subsequent ELISA analysis.
2.7. Spexin Determination
Spexin levels in liver, heart, and skeletal muscle tissues were analyzed using the ELISA method, following the manufacturer’s instructions from the rat spexin ELISA kit (Catalog No. 201-11-3098, SunRed, Shanghai Shanghong Biotechnology Co., Ltd., Shanghai, China). The kit’s measurement range is 10 pg/mL to 3000 pg/mL, with a sensitivity of 8.627 pg/mL. The Intra-Assay variation is specified as Coefficient of Variation (CV) < 10%, while the Inter-Assay variation is CV < 12%. For analysis, the following laboratory equipment was used: a Bio-Tek ELX50 (BioTek Instruments, Winooski, VT, USA) for automatic plate washing, and a Chroma Microplate Reader P4300 (Awareness Technology Instruments, Palm City, FL, USA) for absorbance readings. Test results were expressed in pg/mL.
2.8. Histological Analyses
Heart, skeletal muscle, and liver tissues underwent immunohistochemical analysis using the immunoperoxidase method [
34]. Each analysis was performed in triplicate, and histoscores were calculated based on the extent and intensity of immunoreactivity in the stained sections. For immunohistochemical staining, 5–6 µm thick sections were obtained from paraffin blocks and mounted on polylysine-coated slides. The sections were deparaffinized and rehydrated through a graded alcohol series, followed by antigen retrieval via microwave heating (750 W) in citrate buffer (pH 6) for 15 min. After cooling at room temperature for 20 min, the sections were washed with PBS (P4417, Sigma-Aldrich, St. Louis, MO, USA) for 15 min. To block endogenous peroxidase activity, the sections were incubated with Hydrogen Peroxide Block (TA-125-HP, Lab Vision Corporation, Fremont, CA, USA) for 5 min, followed by Ultra V Block (TA-125-UB, Lab Vision Corporation, USA) for another 5 min to prevent nonspecific binding. The primary antibody (spexin, 1:200 dilution) was applied, and sections were incubated at room temperature in a humid environment for 60 min. After washing with PBS for 15 min, the sections were incubated with the secondary antibody (biotinylated Goat Anti-Polyvalent, TP-125-BN, Lab Vision Corporation, USA) for 30 min, rewashed, and subsequently treated with Streptavidin Peroxidase (TS-125-HR, Lab Vision Corporation, USA) for 30 min. Following a final PBS wash, sections were stained using the 3-Amino-9-Ethylcarbazole (AEC) Substrate + AEC Chromogen solution (TA-015 and TA-002-HAC, Lab Vision Corporation, USA), and the reaction was monitored under a light microscope. Counterstaining was performed with Mayer’s hematoxylin, and sections were rinsed with PBS and distilled water before being sealed with an appropriate mounting solution (Large Volume Vision Mount, TA-125-UG, Lab Vision Corporation, USA). The stained slides were examined using a Leica DM500 microscope, and images were captured with a Leica DFC295 camera. Immunoreactivity was assessed based on extent (0.1: <25%, 0.4: 26–50%, 0.6: 51–75%, 0.9: 76–100%) and intensity (0: none, +0.5: very low, +1: low, +2: moderate, +3: intense). Histoscores were then calculated as the product of extent and intensity, following a similar approach to previously published studies [
35,
36].
2.9. Statistical Analyses
Normality was assessed using the Shapiro–Wilk test to determine whether the data followed a normal distribution. Since the assumption of normality was not met, a Kruskal–Wallis test was applied to evaluate differences across the six distinct experimental groups. Post hoc comparisons were conducted using a Dunn-type test for multiple comparisons against a control group, with Bonferroni correction applied to adjust for multiple testing. Additionally, effect sizes (ESs) were computed using Cohen’s
d to quantify the magnitude of differences between groups [
37]. Cohen’s
d was chosen to facilitate interpretability and comparability with other studies, given its widespread use in biomedical research, despite the non-normal distribution of the data. All statistical analyses were performed using R version 4.2.3.
Furthermore, a hierarchical biclustering analysis was performed to explore spexin expression and its tissue-specific modulation, allowing for the identification of clusters of tissues with similar expression patterns.
3. Results
No statistically significant differences in body weight were observed among the groups at the initial measurement (
p > 0.05), confirming the homogeneity of the groups before the intervention period. After DIO, body weights differed significantly across the groups compared to controls (
p < 0.001). Significant reductions in body weight were observed after the intervention period, which included aerobic exercise, metformin administration, or their combination (
p < 0.001). Aerobic exercise alone resulted in a 13.7% reduction in body weight. In comparison, metformin administration led to a 14.6% decrease, and the combination of metformin and exercise produced the most marked decrease, with a 21.1% reduction, highlighting the effects of combining metformin with exercise in mitigating DIO (
Table 1).
In
Figure 1, the control group exhibited a mean spexin expression of 628 ± 160.5 pg/mL in skeletal muscle. The decapitated group showed a slightly lower mean of 514 ± 111.4 pg/mL, while the exercise group had a mean of 489 ± 102.7 pg/mL. Metformin administration resulted in a mean expression of 505 ± 63.9 pg/mL, whereas the combination of metformin and exercise led to an expression of 349 ± 84.7 pg/mL. The obese control group demonstrated a mean spexin expression of 399 ± 100.9 pg/mL.
The highest mean spexin expression in the liver was observed in the control group, with a value of 443 ± 240.8 pg/mL. The decapitated group had a lower mean of 375 ± 124.6 pg/mL, followed by the exercise group, which exhibited a mean of 322 ± 57.0 pg/mL. The administration of metformin resulted in a mean spexin expression of 358 ± 126.0 pg/mL, while the combination of metformin and exercise produced the lowest mean expression in the liver, measured at 254 ± 20.4 pg/mL. The obese control group had a mean of 275 ± 26.5 pg/mL.
Spexin expression was highest in the heart in the control group, with a mean of 618 ± 53.2 pg/mL. The decapitated group showed a mean expression of 532 ± 82.7 pg/mL, while the exercise group had a mean of 599 ± 91.8 pg/mL. The metformin group demonstrated a mean of 609 ± 100.9 pg/mL, whereas the combination of metformin and exercise resulted in a mean expression of 617 ± 25.2 pg/mL. The obese control group exhibited a mean spexin expression of 518 ± 83.1 pg/mL.
In the Kruskal–Wallis analysis, the group variable significantly impacted spexin expression, particularly in the muscle (p = 0.010) and, to a lesser extent, in the liver (p = 0.105) and the heart (p = 0.109).
In the muscle, post hoc analyses revealed significant pairwise differences only between control and metformin + exercise (
p = 0.003; Cohen’s
d = 2.17), while the difference between control and obese control was nominal (
p = 0.050; Cohen’s
d = 1.71) (
Figure 1).
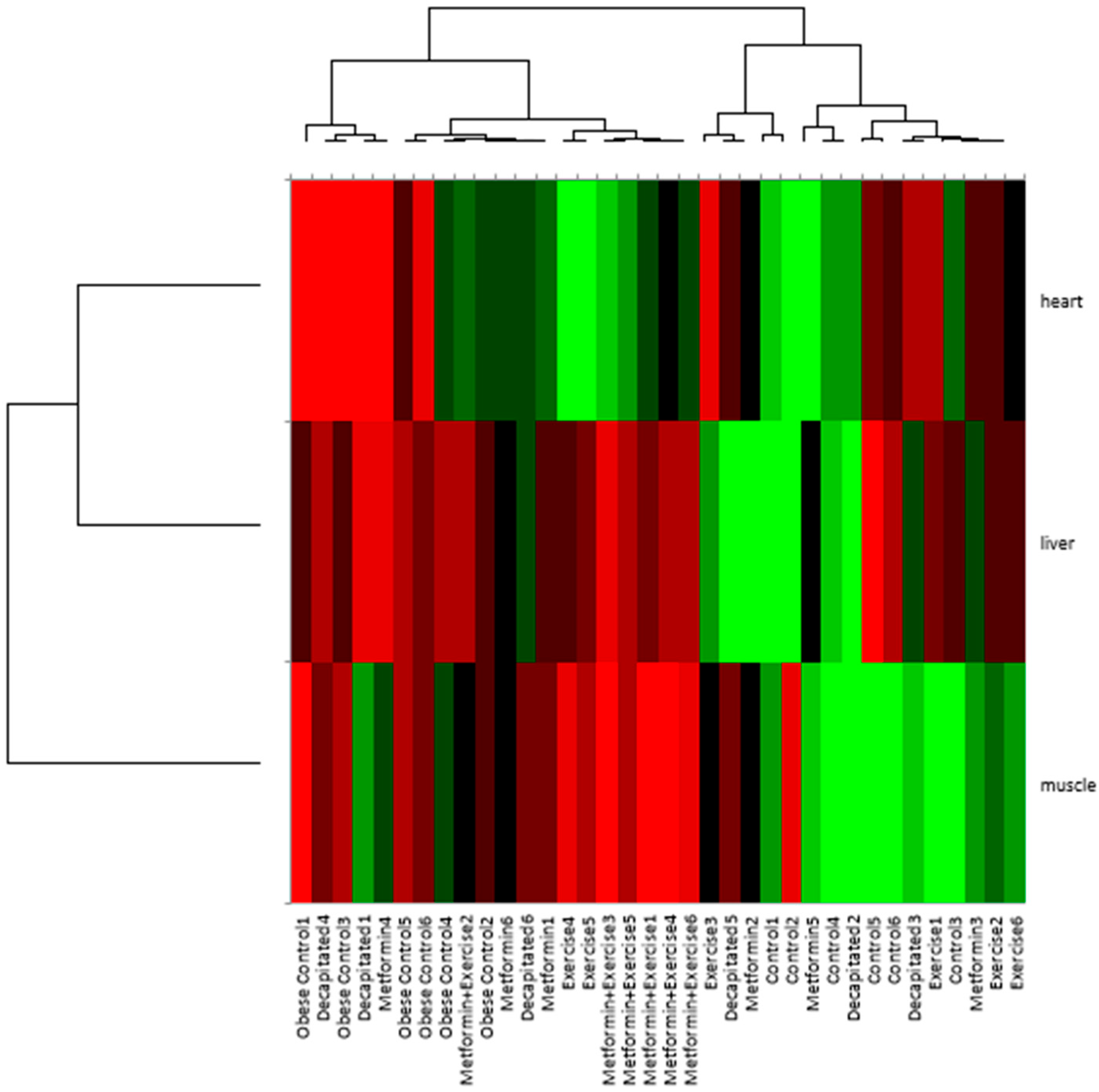
In
Figure 2, the biclustering analysis underscored the complex interplay between metabolic interventions and tissue-specific responses in regulating spexin expression.
At the sample level, the obese control group consistently formed a separate cluster, indicating that obesity significantly alters spexin expression patterns compared to the control and intervention groups. Conversely, the exercise, metformin, and metformin + exercise groups tended to cluster together, suggesting that these interventions shared similar regulatory effects on spexin expression.
At the tissue level, a biclustering pattern emerged, wherein liver and heart samples consistently co-clustered, indicating that spexin expression in these tissues follows a similar regulatory trajectory across conditions. This suggests shared physiological or metabolic mechanisms influencing spexin regulation in the liver and heart, likely reflecting their interconnected roles in systemic metabolism. In contrast, muscle samples formed a distinct and separate cluster, highlighting a fundamentally different pattern of spexin expression in skeletal muscle compared to heart and liver. This distinct separation suggests that muscle tissue exhibits an independent regulatory mechanism of spexin, possibly due to its unique metabolic demands and response to obesity and therapeutic interventions. Specifically, the exercise + metformin group displayed an intermediate clustering pattern, aligning more closely with the individual exercise and metformin groups rather than forming a wholly distinct cluster. This suggests that while the combined intervention exhibited an additive or partially synergistic effect, it did not create an entirely new regulatory state. Instead, its impact on spexin expression appears to integrate characteristics of both exercise and metformin treatments, particularly in heart tissue, where its expression was comparable to that of controls, unlike in muscle and liver tissues. More specifically, in the muscle, the combined intervention followed a more distinct trajectory, suggesting that skeletal muscle may respond differently to metabolic modulation.
Immunohistochemical Results of Skeletal Muscle, Heart, and Liver Tissues
In all images in
Figure 3, the scale bar is set at 20 micrometers (μm).
Figure 3 illustrates the spexin immunopositivity in the skeletal muscle tissue across the different experimental groups. The regions marked with black arrows indicate spexin immunopositivity, with signals concentrated in the A bands of the muscle sarcomeres.
No spexin immunopositivity was observed in the negative control group, confirming the specificity of the staining protocol (
Figure 3A). In the control group (
Figure 3B), the histoscore was determined to be 2.14 ± 0.44. The decapitated group (
Figure 3C) exhibited a slightly higher histoscore of 2.48 ± 0.60. In the obese control group (
Figure 3D), the histoscore was measured at 1.48 ± 0.37, showing lower spexin immunopositivity than the control group. In the exercise group (
Figure 3E), the histoscore was computed at 1.98 ± 0.41, indicating that physical activity strongly enhances spexin expression. However, the increase was lower than in the decapitated group, suggesting that exercise may establish a certain balance in spexin regulation. In the metformin group (
Figure 3F), the histoscore was determined to be 2.08 ± 0.24, indicating a moderate increase in spexin levels following metformin administration. In the metformin + exercise group (
Figure 3G), the histoscore was measured at 1.22 ± 0.29, reflecting the combined effect of the interventions on spexin expression.
In all images in
Figure 4, the scale bar is set at 20 micrometers (μm).
Figure 4 illustrates the distribution of spexin immunopositivity in the cardiac muscle tissue across the different experimental groups. Microscopic observations revealed that spexin immunopositivity was organized in a specific pattern within the sarcomeric structure of cardiac muscle cells. Signals were particularly concentrated in the A bands, suggesting a particular role for spexin in areas rich in myosin filaments.
No spexin immunopositivity was detected in the negative control group (
Figure 4A). In the control group (
Figure 4B), the histoscore for spexin immunopositivity was 1.33 ± 0.27. The decapitated group (
Figure 4C) exhibited a histoscore of 2.36 ± 0.21. In the obese control group (
Figure 4D), the histoscore was computed at 1.67 ± 0.26. In the exercise group (
Figure 4E), a notable increase in spexin immunopositivity was observed, with a histoscore of 2.05 ± 0.30, suggesting that physical activity may enhance spexin expression in the cardiac muscle. In the metformin group (
Figure 4F), the histoscore was 2.20 ± 0.35. In contrast, in the metformin + exercise group (
Figure 4G), spexin immunopositivity was found to be the highest, with a histoscore of 2.34 ± 0.09, suggesting that spexin may be jointly regulated by both pharmacological treatment and physical activity, thus playing a significant role in cardiac muscle metabolism.
In all images in
Figure 5, the scale bar is set at 50 micrometers (μm).
Figure 5 shows the distribution of spexin immunopositivity in the liver tissue across the different experimental groups. Spexin was concentrated around hepatocytes, particularly surrounding the central vein located at the center of the classic hepatic lobule.
No spexin immunopositivity was observed in the negative control group (
Figure 5A). In the control group (
Figure 5B), the histoscore for spexin immunopositivity was measured at 2.00 ± 0.60. In the decapitated group (
Figure 5C), the histoscore for spexin immunopositivity was 2.08 ± 0.88, similar to that of the control group. In the obese control group (
Figure 5D), the histoscore was measured at 1.75 ± 0.17. In the exercise group (
Figure 5E), the histoscore was 1.75 ± 0.35, similar to that of the obese control group, suggesting that physical activity may have a limited effect on increasing spexin levels in the liver or that exercise might primarily influence liver metabolism through mechanisms other than spexin regulation. In the metformin group (
Figure 5F), the histoscore was measured at 2.00 ± 0.46. Finally, in the metformin + exercise group (
Figure 5G), the histoscore was the lowest, at 1.23 ± 0.09, suggesting that the combination of metformin and physical activity may not directly enhance spexin expression in the liver, but could instead prioritize other metabolic pathways.
4. Discussion
The findings of this study highlight the complex interplay between metabolic interventions, tissue-specific responses, and the regulation of spexin expression in the management of obesity. By integrating biochemical quantification through ELISA with histological visualization via immunohistochemistry, this investigation provides a nuanced understanding of how metformin, exercise, and their combination influence spexin expression in key tissues, namely, skeletal muscle, liver, and heart.
The present study revealed that aerobic exercise alone reduced body weight by 13.7%, metformin alone by 14.6%, and combined by 21.1% in diet-induced obese rats. These findings are well supported by recent research demonstrating the joint effects of metformin and exercise on obesity and metabolic health. A study on rats found that long-term moderate exercise reduced body fat by over 20%. When combined with metformin, body fat decreased even more significantly, up to 34.21%, compared to sedentary controls, indicating a combined effect on reducing adiposity [
38]. Another investigation found that metformin and swimming exercise improved lipid profiles, insulin sensitivity, and reduced body weight in high-fat diet-induced obese rats [
39]. The combination therapy showed greater benefits than either treatment alone. Research also highlights that combining metformin with exercise enhances antioxidant defenses and reduces inflammation markers more effectively than either intervention alone, which may contribute to improved metabolic health and weight regulation [
40]. Both interventions activate AMP-activated protein kinase (AMPK), a critical energy sensor that regulates glucose and lipid metabolism. Metformin primarily activates AMPK in the liver and adipose tissue, suppressing hepatic glucose production, reducing extracellular matrix deposition in white adipose tissue, and improving insulin sensitivity by inhibiting pro-fibrotic and inflammatory signaling pathways such as TGF-β1/Smad3 [
41,
42]. Exercise primarily activates AMPK in skeletal muscle, enhancing glucose uptake by translocating GLUT4, increasing fatty acid oxidation, promoting mitochondrial biogenesis, and improving insulin sensitivity [
43,
44]. The combination of metformin and exercise leads to complementary activation of AMPK across tissues, resulting in greater reductions in lipid accumulation, inflammation, and improved metabolic function compared to either treatment alone [
44,
45]. Metformin also modulates appetite regulation and gut microbiota composition, contributing to weight loss [
41,
42]. Together, these mechanisms explain the superior efficacy of combined metformin and aerobic exercise in mitigating DIO through enhanced energy expenditure, improved glucose homeostasis, and reduced chronic inflammation.
While the overall metabolic benefits of these interventions are well established in terms of weight loss [
45], the differential expression of spexin observed across tissues underscores the need for a more refined perspective on its role in obesity and metabolic homeostasis. In skeletal muscle, the ELISA results indicated a reduction in spexin levels in the metformin and exercise group compared to the control, suggesting that the combination of pharmacological and physiological interventions did not enhance spexin expression at the whole-tissue level. However, the immunohistochemical analysis painted a more complex picture, revealing increased spexin localization within the A-bands of sarcomeres in the same group. This apparent discrepancy between ELISA and IHC findings may not be contradictory but rather indicative of a redistribution of spexin within the muscle rather than an actual reduction in its presence. Since skeletal muscle plays a central role in glucose metabolism and energy expenditure [
46], spexin may be selectively upregulated in functionally relevant regions, thereby supporting enhanced insulin sensitivity and contractile efficiency [
47] without a concomitant increase in total tissue levels. These findings suggest that spexin may participate in a tissue remodeling process in response to metabolic interventions, a hypothesis that warrants further investigation through functional studies and downstream analyses.
In the liver, both ELISA and IHC findings consistently showed that the combination of metformin and exercise led to the lowest spexin expression levels among all groups. While metformin and exercise alone exhibited moderate effects on liver spexin, their combined action did not appear to potentiate its expression. This result suggests that the metabolic benefits of metformin and exercise in the liver may be mediated through pathways other than spexin regulation. Given that the liver is a central organ in energy homeostasis and gluconeogenesis [
48], these interventions may drive metabolic improvements through enhanced lipid oxidation, mitochondrial efficiency, or alternative hormonal signaling mechanisms rather than through direct modulation of spexin. The suppression of spexin in this context may reflect a compensatory downregulation to prioritize other metabolic pathways in response to systemic metabolic demands. Additionally, the liver’s role in regulating systemic insulin sensitivity and lipid metabolism [
49] might involve feedback mechanisms that differentially regulate spexin compared to other tissues.
In contrast to the skeletal muscle and liver, the heart exhibited a strong alignment between ELISA and IHC findings, with both methods demonstrating that metformin and exercise maintained or even enhanced spexin levels relative to the control. This consistency suggests that spexin plays a direct and potentially beneficial role in cardiac metabolism under metabolic stress and intervention conditions. Given the established cardioprotective effects of both metformin and exercise [
50], the increased spexin expression observed in the heart may reflect an adaptive response to improve myocardial energy efficiency, reduce oxidative stress, or enhance glucose utilization. The localization of spexin within the sarcomeric structures further supports the notion that it may contribute to cardiac muscle integrity and contractile function. This finding aligns with previous research suggesting that spexin is involved in cardiovascular regulation and energy balance, positioning it as a potential biomarker or therapeutic target for obesity-related cardiometabolic disorders [
51].
The clustering analysis further supported the tissue-specific regulation of spexin, with the liver and heart showing similar expression patterns across the experimental conditions, while skeletal muscle formed a distinct cluster. This separation highlights the distinct metabolic functions of these tissues and suggests that spexin is regulated context-dependently. The clustering of the metformin and exercise groups indicates that these interventions share overlapping mechanisms in modulating metabolic responses; however, their combined effect did not produce an entirely novel regulatory pattern. Instead, the combination of metformin and exercise appeared to integrate characteristics of both individual treatments, particularly in the heart, where spexin expression remained comparable to control levels. Together, these findings illustrate that the effects of metabolic interventions on spexin expression are highly tissue-specific. The discrepancies between ELISA and IHC in skeletal muscle suggest that total protein levels may not always reflect functionally relevant protein localization and activity changes. Furthermore, the downregulation of spexin in the liver following combined treatment suggests that other compensatory pathways are at play in hepatic metabolism. In contrast, the heart maintains spexin expression in response to metabolic interventions. These insights highlight the importance of integrating multiple analytical approaches to comprehend the complex regulatory mechanisms that govern metabolic homeostasis.
Future studies should aim to elucidate the functional consequences of these changes in spexin expression, particularly regarding metabolic flexibility, insulin sensitivity, and energy partitioning across different tissues. Understanding these dynamics may help refine therapeutic strategies for obesity management, optimizing pharmacological and lifestyle-based interventions to achieve the most effective metabolic outcomes.
The current study has several limitations. First, the duration of the interventions (aerobic exercise and metformin) in this study was relatively short, at four weeks. Long-term effects and sustained changes in spexin expression may not be fully captured within the specified timeframe. Future studies with extended intervention periods could provide insights into the persistence of the observed effects. Second, this study focused on the liver, heart, and musculoskeletal tissues. While these tissues are relevant to the metabolic and musculoskeletal aspects of obesity, other tissues expressing spexin, such as adipose tissue, were not included in the analysis. A more comprehensive examination of spexin expression across various tissues could offer a more nuanced understanding. Third, other parameters, such as fasting blood sugar, glucose tolerance, and insulin levels, should also be investigated. Examining the relationship between spexin levels and variables associated with glucose metabolism in future studies could contribute more significantly to the elucidation of physiological mechanisms.
Finally, spexin, a peptide with emerging roles in energy homeostasis and metabolism, is downregulated in individuals with obesity [
24,
52], suggesting its potential involvement in the pathophysiology of this condition [
52,
53]. While the current study demonstrates associations between interventions, such as aerobic exercise and metformin, and altered spexin expression, the precise molecular mechanisms underlying these effects remain poorly understood. Future research should focus on elucidating the mechanistic pathways through which these interventions modulate spexin levels, which may offer valuable insights into novel therapeutic strategies for obesity. Additionally, this study primarily utilized an obese rat model to investigate the modulation of spexin expression. While rodent models offer valuable insights, caution should be exercised in directly translating these findings to human physiology. Species differences may impact the generalizability of the results to obese individuals.
5. Conclusions
The present study offers novel insights into the tissue-specific regulation of spexin in response to aerobic exercise and metformin administration in the DIO rat model. While both interventions effectively reduced body weight, their effects on spexin expression varied markedly across tissues. In skeletal muscle, combined intervention significantly reduced overall spexin concentration; however, immunohistochemical analysis revealed enhanced localization within sarcomeric A-bands, suggesting a functional redistribution rather than downregulation. Conversely, metformin plus exercise in the liver resulted in the lowest spexin levels, indicating that its hepatic benefits may be mediated through alternative metabolic pathways. Notably, in the heart, spexin expression was preserved or even enhanced across interventions, with the combination group exhibiting the highest histoscore, highlighting the heart’s adaptive responsiveness to metabolic stimuli. Hierarchical biclustering further supported the hypothesis of tissue-dependent regulation, with skeletal muscle forming a distinct expression cluster, while liver and heart co-clustered. These findings underscore that spexin does not follow a uniform expression pattern and may serve different physiological roles depending on tissue type and intervention modality. Overall, spexin emerges as a promising, yet complex, biomarker of metabolic adaptation. The differential responses observed suggest that future anti-obesity therapies might benefit from targeting spexin in a tissue-specific manner. Further research is warranted to elucidate the mechanistic underpinnings of spexin regulation and its functional consequences, especially regarding glucose metabolism, insulin sensitivity, and energy homeostasis. Expanding this work to include longer intervention durations, additional tissues (e.g., adipose tissue), and translational human studies will be essential to fully harness the therapeutic potential of spexin in treating obesity and metabolic syndrome.