Lactiplantibacillus sp. LH01 as an Adjuvant to Reduce Antibiotic Use in Recurrent Urinary Tract Infections in a Paediatric Patient with Hydronephrosis
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Description
2.2. Previous Interventions
2.3. Isolation and Identification of L. plantarum LH01
2.4. Cultivation and Safety Assessment of L. plantarum LH01
2.5. Application of L. plantarum LH01 as an Adjuvant Strategy
2.6. Statistical Analysis
3. Results and Discussion
3.1. In Vitro Evaluation of Antimicrobial Activity
3.2. In Vivo Evaluation
3.3. Statistical Analysis of the Inhibitory Response of the Probiotic and Antibiotics Used in the Patient
3.4. Nephrotoxicity Risk Assessment
3.5. Evaluation of Inhibitory Effects of Probiotics and Conventional Antibiotics Against ESBL E. coli
3.6. Phenotypic Modulation of Resistance in Gram-Negative Bacilli
3.7. Clinical Findings in the Patient
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ahumada Cota, R.E.; Olalde Ramírez, S.; Hernández Chiñas, U.; Acevedo Monroy, S.E.; Eslava Campos, C.A. Urinary Tract Infections in Mexico: A Public Health Problem. 2022. Available online: https://hdl.handle.net/11191/9630 (accessed on 16 June 2025).
- Lombardo-Aburto, E. Pediatric approach to urinary tract infections. Acta Pediátrica México 2018, 39, 85–90. [Google Scholar] [CrossRef]
- Durán-Pincay, Y.E.; Delgado-Vélez, K.D.; Sánchez-Ávila, C.L.; Baque-Mero, A.P. Epidemiology and clinical symptomatology of urinary tract infections in infants. MQRInvestigar 2022, 6, 1518–1536. [Google Scholar] [CrossRef]
- Barola, S.; Grossman, O.K.; Abdelhalim, A. Urinary Tract Infections in Children. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK599548/ (accessed on 16 June 2025).
- Meštrović Popovič, K.; Povalej Bržan, P.; Langerholc, T.; Marčun Varda, N. The impact of Lactobacillus plantarum PCS26 supplementation on the treatment and recurrence of urinary tract infections in children: A pilot study. J. Clin. Med. 2022, 11, 7008. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tian, L.; Zhao, R.; Xu, X.; Zhou, Z.; Xu, X.; Luo, D.; Zhou, Z.; Liu, Y.; Kushmaro, A.; Marks, R.S.; et al. Modulatory effects of Lactiplantibacillus plantarum on chronic metabolic diseases. Food Sci. Hum. Wellness 2023, 12, 959–974. [Google Scholar] [CrossRef]
- Lebowitz, R.L.; Olbing, H.; Parkkulainen, K.V.; Smellie, J.M.; Tamminen-Möbius, T.E. International radiographic grading system for vesicoureteral reflux. International Reflux Study in Children. Pediatr. Radiol. 1985, 15, 105–109. [Google Scholar] [CrossRef]
- Lutsar, I.; Chazallon, C.; Trafojer, U.; de Cabre, V.M.; Auriti, C.; Bertaina, C.; Carducci, F.I.C.; Canpolat, F.E.; Esposito, S.; Fournier, I.; et al. Meropenem vs standard of care for treatment of neonatal late onset sepsis (NeoMero1): A randomised controlled trial. PLoS ONE 2020, 15, e0229380. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, G.; Zhou, P.; Zhang, H.; Sun, B.; Tong, X.; Xing, Y. Extended infusion of meropenem in neonatal sepsis: A historical cohort study. Antibiotics 2022, 11, 341. [Google Scholar] [CrossRef] [PubMed]
- Alemán-Duarte, M.I.; Aguilar-Uscanga, B.R.; García-Robles, G.; Ramírez-Salazar, F.d.J.; Benítez-García, I.; Balcázar-López, E.; Solís-Pacheco, J.R. Improvement and validation of a genomic DNA extraction method for human breast milk. Methods Protoc. 2023, 6, 34. [Google Scholar] [CrossRef]
- Khehra, N.; Padda, I.S.; Swift, C.J. Polymerase Chain Reaction (PCR). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK589663/ (accessed on 16 June 2025).
- Britania Laboratories. M.R.S. Agar. Culture Medium for Lactobacillus spp. Technical Data Sheet; Technical Data Sheet Code B0220505/B0220506. Revision 02. March 2021. Available online: https://www.britanialab.com/back/public/upload/productos/upl_6092dd2543f1d.pdf (accessed on 28 June 2025).
- Ingredion Incorporated. NUTRAFLORA® P-95/L95-S. Soluble Prebiotic Fibre—Technical Data Sheet. Ingredion: Westchester, IL, USA, 2021; Available online: https://www.ingredion.com/content/dam/ingredion/pdf-downloads/cono-sur/NUTRAFLORA.pdf (accessed on 15 June 2025).
- Vélez Zea, J.; Gutiérrez, L.A.; Montoya Campuzano, O. Molecular identification and probiotic capacity evaluation of lactic acid bacteria isolated from sow colostrum. CES Med. Vet. Zootec. 2015, 10, 141–149. [Google Scholar]
- Amezcua López, J.A.; Aguilar Uscanga, B.R.; Pérez-Rulfo Ibarra, D.; Solís Pacheco, J.R.; Lange, V.; García Morales, E. Effectiveness of Powdered Human Milk in the Nutrition of a Premature Newborn with Prenatal Exposure to Toxic Substances: A Case Report. EC Clin. Med. Case Rep. 2024, 7, 1–10. Available online: https://www.researchgate.net/publication/384503371_EC_CLINICAL_AND_MEDICAL_CASE_REPORTS_EC_CLINICAL_AND_MEDICAL_CASE_REPORTS_Case_Report_Effectiveness_of_Powdered_Human_Milk_in_the_Nutrition_of_a_Premature_Newborn_with_Prenatal_Exposure_to_Toxic_Subst (accessed on 26 May 2025).
- Dunn, O.J. Multiple comparisons using rank sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- RStudio Team. Integrated Development for R. RStudio, PBC. 2022. Available online: https://www.rstudio.com/categories/integrated-development-environment/ (accessed on 28 May 2025).
- Statgraphics Technologies, Inc. Centurion 19 Product Details: Powerful Statistical Software Package. Statgraphics.com. 2020. Available online: https://www.statgraphics.com/centurion-19 (accessed on 26 May 2023).
- Kurniawati, E.M.; Hardianto, G.; Hadi, T.H.S.; Paraton, H.; Widyasari, A.; Rahmawati, N.A. The role of probiotics in urinary tract infections in women. Indones. J. Obstet. Gynecol. 2023, 11, 189–197. [Google Scholar] [CrossRef]
- Oliva Falcón, A. Bacterial Resistance and Detection of Β-Lactamases in Children Admitted for Urinary Tract Infection. Rev. Cubana Pediatr. 2024, 96. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0034-75312024000100025&lng=es (accessed on 11 June 2025).
- Kaufman, J.; Temple-Smith, M.; Sanci, L. Urinary tract infections in children: An overview of diagnosis and management. BMJ Paediatr. Open [Internet] 2019, 3, e000487. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, M.; Parolin, C.; Patrignani, S.; Sottile, G.; Antonazzo, P.; Vitali, B.; Lanciotti, R.; Patrignani, F. Human breast milk: A source of potential probiotic candidates. Microorganisms 2022, 10, 1279. [Google Scholar] [CrossRef] [PubMed]
- Soto, R.E.J.; Espinoza, J.I.V.; Chacha, K.G.C.; González, L.A.M.; Blacio, J.J.J. Implications of Lactobacillus plantarum in pathological disorders. Literature Review. Cienc. Lat. Rev. Cient. Multidiscip. 2024, 8, 4959–4978. [Google Scholar]
- Nordström, E.A.; Teixeira, C.; Montelius, C.; Jeppsson, B.; Larsson, N. Lactiplantibacillus plantarum 299v (LP299V®): Three decades of research. Benef. Microbes 2021, 12, 441–465. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Sanders, M.E.; Szajewska, H.; Cohen, H.; Eliakim, R.; Herrera, C.; Karakan, T.; Merenstein, D.; Piscoya, A.; Ramakrishna, B.; et al. World Gastroenterology Organisation Global Guidelines: Probiotics and prebiotics. J. Clin. Gastroenterol. 2024, 58, 533–553. (In Spanish) [Google Scholar] [CrossRef]
- Sandoval, C.A.; Aravena, U.M.; Cofré, S.F.; Delpiano, M.L.; Hernández, M.R.; Hernández, E.M.; Izquierdo, C.G.; Labraña, C.Y.; Reyes, J.A. Antimicrobials in neonatology. Part I: Dosage recommendations based on the latest evidence in newborns. Advisory Committee on Neonatal Infections, Chilean Society of Infectology. Rev. Chil. Infectol. 2020, 37, 490–508. [Google Scholar]
- Alqahtani, S.; Abouelkheir, M.; Alsultan, A.; Elsharawy, Y.; Alkoraishi, A.; Osman, R.; Mansy, W. Optimizing amikacin dosage in pediatrics based on population pharmacokinetic/pharmacodynamic modeling. Paediatr. Drugs 2018, 20, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Laboratorios Normon S.A. Amikacin NORMON 500 mg/2 mL Solution for Injection and Infusion. Summary of Product Characteristics (SPC); Laboratorios Normon: Tres Cantos, Madrid, Spain, First Authorisation November 1986; Text Revised June 2023; Nº Autorización: 57 012. Via CIMA AEMPS. Available online: https://cima.aemps.es/cima/dochtml/p/57012/P_57012.html (accessed on 22 June 2025). (In Spanish)
- Association Española de Pediatría. Pediamecum: Amikacin—Dosage and Administration in Paediatrics; Asociación Española de Pediatría. 2021. Available online: https://www.aeped.es/comite-medicamentos/pediamecum/amikacina (accessed on 31 July 2025).
- González Rodríguez, J.D.; Fraga Rodríguez, G.M.; García Vera, C.J.; Gómez Fraile, A.; Martín Sánchez, J.I.; Mengual Gil, J.M.; Ochoa Sangrador, C.; Valenciano Fuentes, B.; Escribano Subías, J.; on behalf of the Working Group to update the Clinical Practice Guideline on Urinary Tract Infection in the Pediatric Population. Update of the Spanish clinical practice guideline on urinary tract infection in the paediatric population: Summary of recommendations on diagnosis, treatment and follow-up. An. Pediatr. (Engl. Ed.) 2024, 101, 132–144. [Google Scholar] [CrossRef]
- Lamponi Tappatá, L.; Tomás, A.L.; Hartstock, J.; Prieto, L.; Pfeiffer, C.; Gallardo, M.F.; Altube, A.J.; Sofio, M.P.; Dodero, R.; Maurizi, D. Acute kidney injury in patients treated with aminoglycosides. Updates AIDS Infectol. 2020, 28, 13–19. [Google Scholar]
- Ye, P.; Shi, J.; Guo, Z.; Yang, X.; Li, Q.; Chen, K.; Zhao, F.; Zhou, H.; Zhang, Y.; van den Anker, J.; et al. Piperacillin/tazobactam treatment in children: Evidence of subtherapeutic concentrations. Front. Pharmacol. 2024, 15, 1254005. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Northern California Pediatric Hospital Medicine Consortium. Consensus Guidelines for Management of Pediatric Urinary Tract Infection (UTI). UCSF Benioff Children’s Hospitals MedConnection. 11 February 2025. Available online: https://medconnection.ucsfbenioffchildrens.org/news/consensus-guidelines-for-management-of-pediatric-urinary-tract-infection-uti/ (accessed on 31 July 2025).
- Aguilar-Zapata, D. E. coli ESBL, the enterobacterium that has crossed borders. Médica Sur. 2016, 22, 57–63. [Google Scholar]
- Guastalegname, M.; Trecarichi, E.M.; Russo, A. Intravenous fosfomycin: The underdog player in the treatment of carbapenem-resistant Acinetobacter baumannii infections. Clin. Infect. Dis. 2023, 77, 1736–1737. [Google Scholar] [CrossRef]
- Laboratorios Normon S.A. Ciprofloxacin Normon 250 mg, 500 mg, and 750 mg Film-Coated Tablets: Summary of Product Characteristics (SPC); Laboratorios Normon: Tres Cantos, Madrid, Spain; Authorisation No. 62 300; Text Revised Most Recently as per CIMA Database. Available online: https://cima.aemps.es/cima/dochtml/ft/62301/FichaTecnica_62301.html. (accessed on 31 July 2025).
- Patel, K.; Goldman, J.L. Safety Concerns Surrounding Quinolone Use in Children. J. Clin. Pharmacol. 2016, 56, 1060–1075. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kunishima, H.; Ishibashi, N.; Wada, K.; Oka, K.; Takahashi, M.; Yamasaki, Y.; Aoyagi, T.; Takemura, H.; Kitagawa, M.; Kaku, M. The effect of gut microbiota and probiotic organisms on the properties of extended spectrum beta-lactamase producing and carbapenem resistant Enterobacteriaceae, including growth, beta-lactamase activity and gene transmissibility. J. Infect. Chemother. 2019, 25, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, Y.; Liu, L.; Jin, Y.; Li, Y.; Li, P.; Feng, S.; Gu, Q. Effect of Lactiplantibacillus plantarum on the multidrug-resistant Klebsiella pneumoniae in the neonatal distal colon in vitro. J. Funct. Foods 2025, 129, 106823. [Google Scholar] [CrossRef]
- Savinova, O.S.; Glazunova, O.A.; Moiseenko, K.V.; Begunova, A.V.; Rozhkova, I.V.; Fedorova, T.V. Exoproteome analysis of antagonistic interactions between probiotic bacteria Limosilactobacillus reuteri LR1 and Lacticaseibacillus rhamnosus F and multidrug-resistant strain of Klebsiella pneumoniae. Int. J. Mol. Sci. 2021, 22, 10999. [Google Scholar] [CrossRef]
- Chamber of Deputies of the Honourable Congress of the Union. General Health Law. Latest Amendment Published in the Official Gazette of the Federation on 7 June 2024. Available online: https://mexico.justia.com/federales/leyes/ley-general-de-salud/ (accessed on 6 August 2025).
- United Nations. Convention on the Rights of the Child. United Nations General Assembly. 1989. Available online: https://www.un.org/es/events/childrenday/pdf/derechos.pdf (accessed on 22 June 2025).
- Chamber of Deputies of the Honourable Congress of the Union. Federal Law on the Protection of Personal Data Held by Private Parties. New Law Published in the Official Gazette of the Federation on 20 March 2025. Available online: https://www.gob.mx/indesol/documentos/ley-federal-de-proteccion-de-datos-personales-en-posesion-de-los-particulares (accessed on 6 August 2025).
- World Medical Association. Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Participants. 8th Revision, Adopted by the General Assembly in October 2024. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki/ (accessed on 30 July 2025).

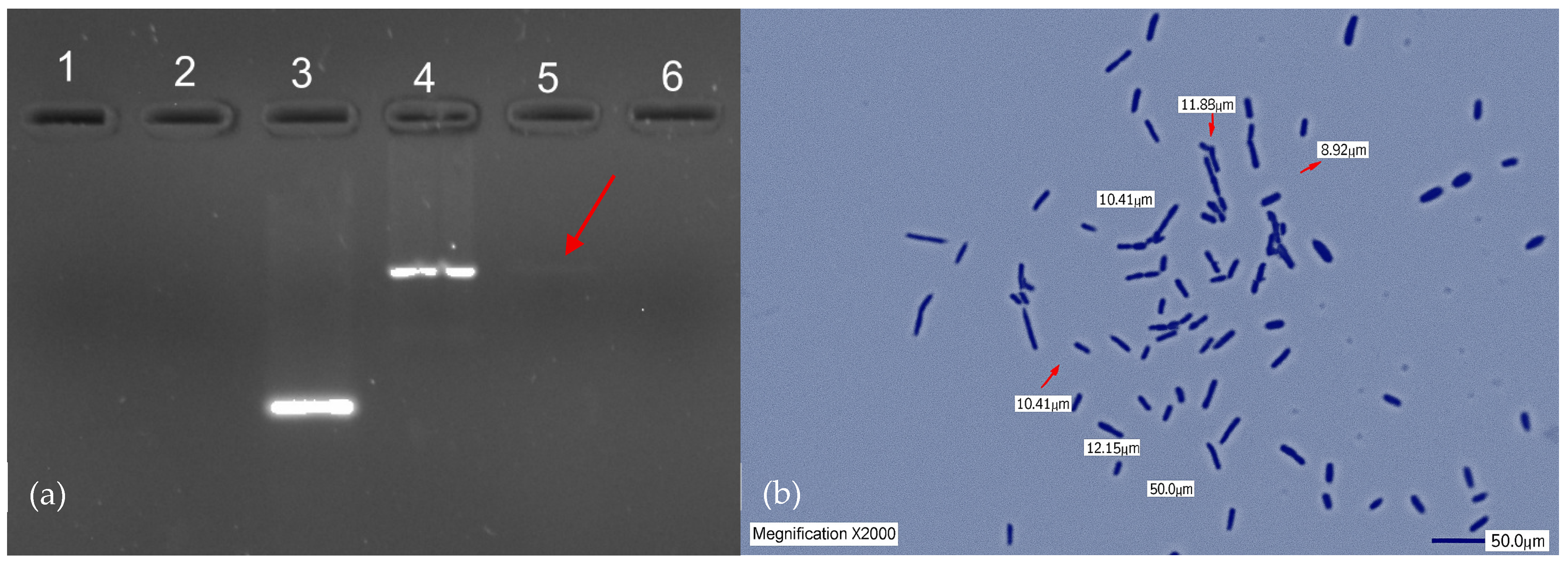
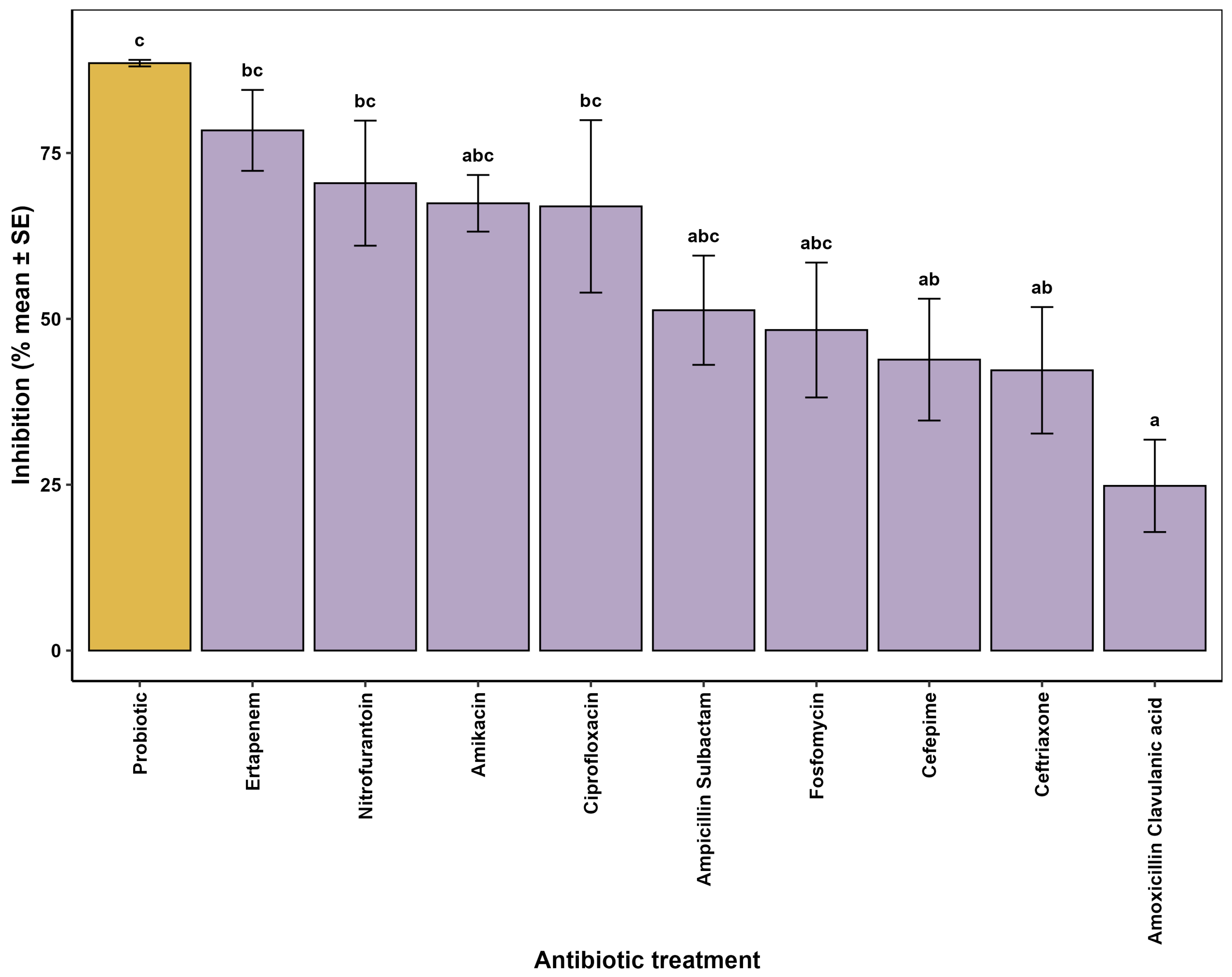
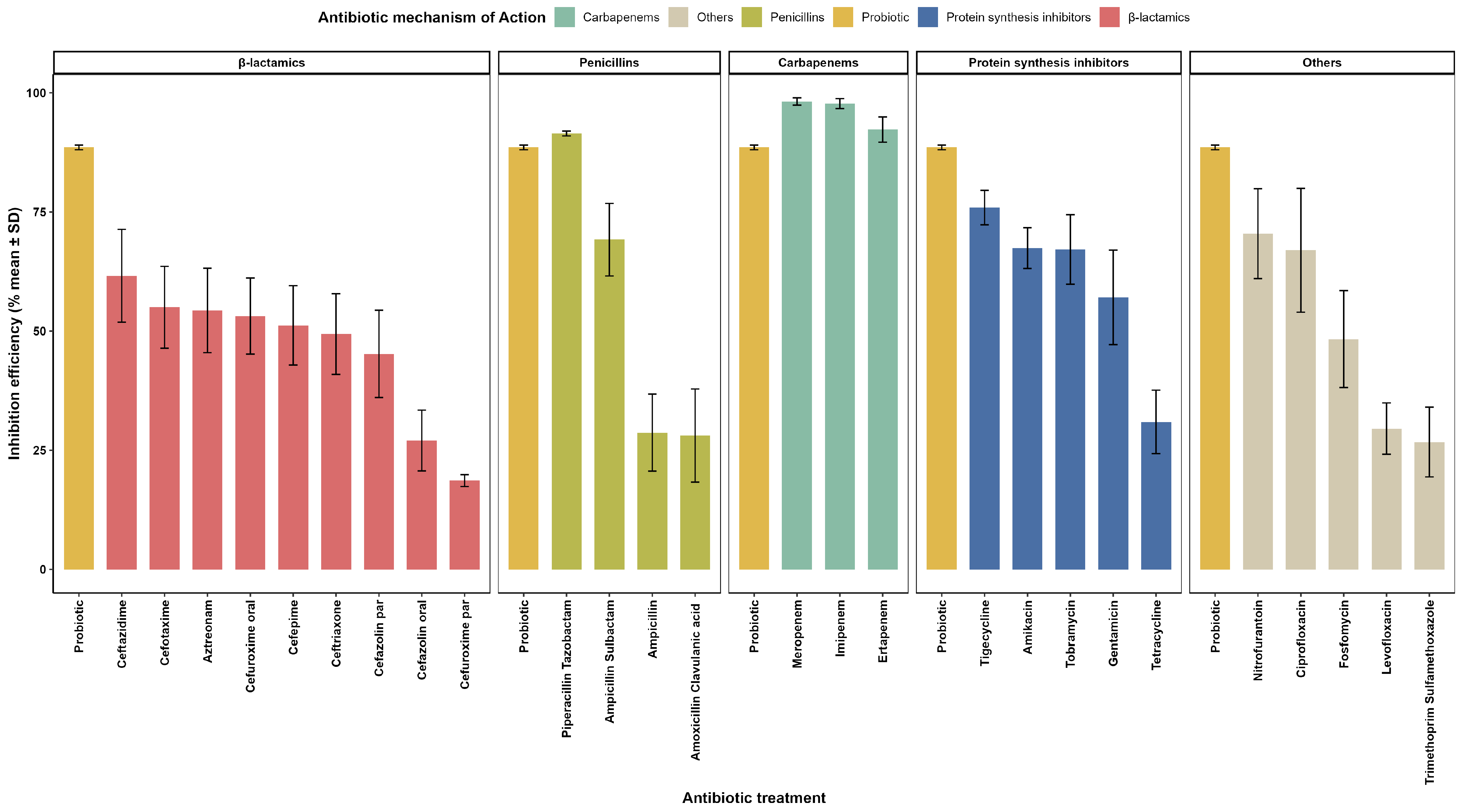
| Date | Antibiotic(s) | Duration (Days) | Indication | Clinical Evolution and Observations |
|---|---|---|---|---|
| 30 January 2021 | Meropenem | 10 | Prenatal origin urosepsis caused by ESBL E. coli. | Clinical control of infection; good drug tolerance. |
| 22 February 2021 | Ampicillin + Amikacin | 7 | Neonatal fever, suspected sepsis. | Favourable evolution; negative blood cultures. |
| 4 March 2021 | Cefotaxime + Vancomycin | 6 | Persistent fever, thrombocytopenia, elevated inflammatory markers. | No bacterial growth in cultures; regimen discontinued. |
| 10 March 2021 | Meropenem + Amikacin | 8 | Suspected severe sepsis. | Clinical improvement and negative microbiological results. |
| Species | Target Gene | Primer | Sequence (5′–3′) | Product Size (bp) |
|---|---|---|---|---|
| L. plantarum | Unique gene | plantarum-F | GCT GGC AAT GCC ATC GTG CT | 147 |
| plantarum-R | TCT CAA CGG TTG CTG TAT CG | |||
| L. fermentum | Unique gene | fermentum-F | GAC CAG CGC ACC AAG TGA TA | 129 |
| fermentum-R | AGC GTA GCG TTC GTG GTA AT | |||
| L. reuteri | 16S–23S Unique region | reuteri-F | GAT TGA CGA TGG ATC ACC AGT | 161 |
| reuteri-R | CAT CCC AGA GTG ATA GCC AA |
| Dose (µL) L. plantarum LH01 | UFC/mL | ESBL E. coli Inhibition Zone (mm) | ESBL K. pneumonae Inhibition Zone (mm) |
|---|---|---|---|
| Control (carbenicillin) | 20 mg/mL | 0 | 0 |
| 50 | 104 | 8 | 7.5 |
| 100 | 106 | 12 | 7.8 |
| 200 | 109 | 16 | 8.5 |
| 300 | 1012 | 18 | 9.2 |
| Date | Event/Infection | Isolated Bacteria | Antibiotic Therapy | Duration of Treatment (Days) |
|---|---|---|---|---|
| January 2021 | Pyelonephritis | ESBL E. coli | Meropenem | 10 |
| February 2021 | UTIs | Multidrug-resistant K. pneumoniae | Prophylactic nitrofurantoin | 7 |
| March 2021 | UTIs | Resistant K. aerogenes | Nitrofurantoin | 7 |
| April 2021 | Complicated recurrent UTIs | Serratia marcescens | Cefotaxime + Amikacin | 6 |
| June 2021 | UTIs | ESBL E. coli | L. plantarum LH01 | 30 |
| July 2021 | UTIs | ESBL E. coli | Amikacin, Ciprofloxacin, prophylaxis | 6 |
| March 2022 | Complicated acute pyelonephritis | ESBL E. coli | L. plantarum LH01 | 30 |
| April 2022 | Complicated acute pyelonephritis | ESBL E. coli | Ceftriaxone + Amikacin, de-escalation to Ciprofloxacin | 7 |
| January 2023 | UTIs | ESBL E. coli | L. plantarum LH01 | 30 |
| February 2023 | UTIs | Multidrug-resistant K. pneumoniae | Ertapenem | 7 |
| February 2023 | UTIs | E. coli | L. plantarum LH01 | 30 |
| March 2024 | Febrile episode, probable pyelonephritis | E. coli | Fosfomycin | 10 |
| July 2024 | UTIs | E. coli | L. plantarum LH01 | 30 |
| March 2025 | UTIs | E. coli | Amikacin | 10 |
| March 2025 | Maintenance therapy | No growth of pathogenic bacteria | L. plantarum LH01 | 30 |
| Antibiogram | ||||||||
|---|---|---|---|---|---|---|---|---|
| Antibiotic | E. coli | K. pneumoniae | ||||||
| 2021 | 2022 | 2023 | 2024 | 2025 | 2021 | 2022 | 2023 | |
| Amikacin | S | S | S | I | I | S | S | S |
| Amoxicillin/Clavulanic Acid | R | R | R | I | S | R | R | S |
| Ampicillin/Sulbactam | S | I | S | I | S | S | R | S |
| Ampicillin | R | R | S | R | S | R | R | R |
| Cefepime | R | R | S | S | S | S | R | S |
| Cefotaxime | R | R | S | I | I | S | R | S |
| Ceftazidime | R | R | S | S | S | S | R | S |
| Ceftriaxone | R | R | S | S | S | S | R | S |
| Oral Cefuroxime | R | R | S | S | S | S | S | S |
| Cefuroxime | R | R | S | S | S | S | R | S |
| Ciprofloxacin | S | S | R | I | S | S | S | S |
| Ertapenem | S | S | S | S | S | S | S | S |
| Gentamicin | S | R | S | S | R | S | R | S |
| Imipenem | S | S | S | S | S | S | S | S |
| Levofloxacin | R | R | R | I | I | S | S | S |
| Meropenem | S | S | S | S | S | S | S | S |
| Piperacillin/Tazobactam | S | S | S | S | S | I | I | S |
| Tetracycline | R | R | R | S | S | R | R | S |
| Tigecycline | S | I | I | S | S | S | S | S |
| Tobramycin | S | I | I | S | R | S | S | S |
| Trimethoprim/Sulfamethoxazole | R | R | S | R | R | S | R | S |
| Fosfomycin | R | R | S | S | S | R | R | R |
| Aztreonam | R | R | R | S | S | -- | -- | -- |
| Cefoxitin | R | R | R | S | S | R | R | R |
| Cefazolin | R | R | R | I | S | -- | -- | -- |
| Nitrofurantoin | S | S | I | I | S | R | R | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguirre Hernández, N.; Pérez-Rulfo Ibarra, D.; Aguilar Uscanga, B.R.; García Morales, E.; Flores Fong, I.; Amezcua López, J.A. Lactiplantibacillus sp. LH01 as an Adjuvant to Reduce Antibiotic Use in Recurrent Urinary Tract Infections in a Paediatric Patient with Hydronephrosis. Appl. Sci. 2025, 15, 8805. https://doi.org/10.3390/app15168805
Aguirre Hernández N, Pérez-Rulfo Ibarra D, Aguilar Uscanga BR, García Morales E, Flores Fong I, Amezcua López JA. Lactiplantibacillus sp. LH01 as an Adjuvant to Reduce Antibiotic Use in Recurrent Urinary Tract Infections in a Paediatric Patient with Hydronephrosis. Applied Sciences. 2025; 15(16):8805. https://doi.org/10.3390/app15168805
Chicago/Turabian StyleAguirre Hernández, Naomi, Daniel Pérez-Rulfo Ibarra, Blanca Rosa Aguilar Uscanga, Elisa García Morales, Ixtlilxochitl Flores Fong, and Jesús Alonso Amezcua López. 2025. "Lactiplantibacillus sp. LH01 as an Adjuvant to Reduce Antibiotic Use in Recurrent Urinary Tract Infections in a Paediatric Patient with Hydronephrosis" Applied Sciences 15, no. 16: 8805. https://doi.org/10.3390/app15168805
APA StyleAguirre Hernández, N., Pérez-Rulfo Ibarra, D., Aguilar Uscanga, B. R., García Morales, E., Flores Fong, I., & Amezcua López, J. A. (2025). Lactiplantibacillus sp. LH01 as an Adjuvant to Reduce Antibiotic Use in Recurrent Urinary Tract Infections in a Paediatric Patient with Hydronephrosis. Applied Sciences, 15(16), 8805. https://doi.org/10.3390/app15168805






