Effects of Different Planting Environments on the Fragrance of Dalixiang (Oryza sativa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Cultivation
2.2. DNA Extraction, PCR Amplification, and Electrophoresis Detection
2.3. Testing of Gene Expression Levels
2.4. Determination of Enzyme Activity
2.5. Measurement of 2AP Content and Contents of Related Substances
2.6. Inspection of Soil Nutrients
2.7. Data Analysis
3. Results
3.1. Analysis of OsBadh2 Expression Level and 2AP Content
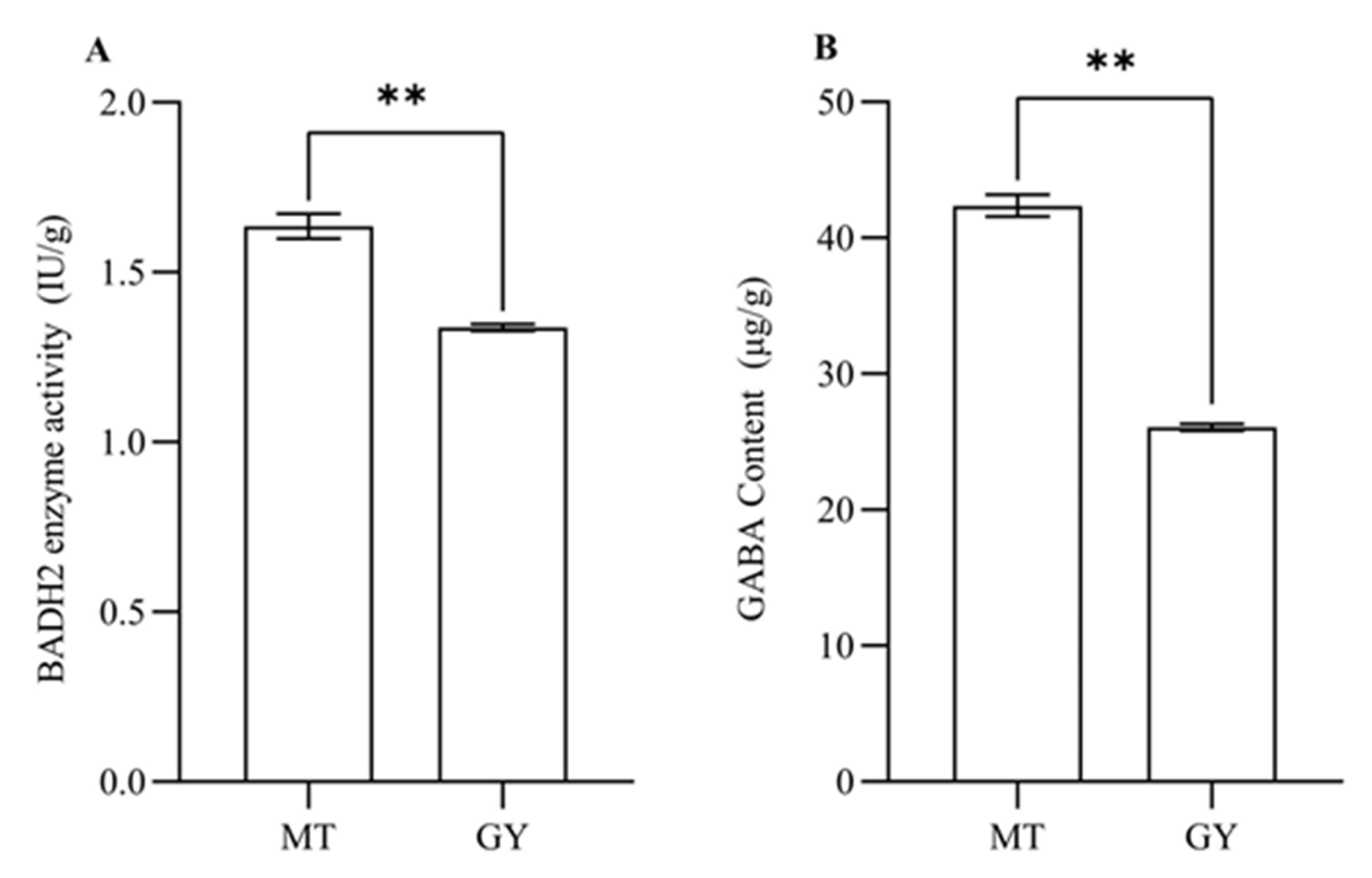
3.2. Analysis of BADH2 Enzyme Activity and GABA Content
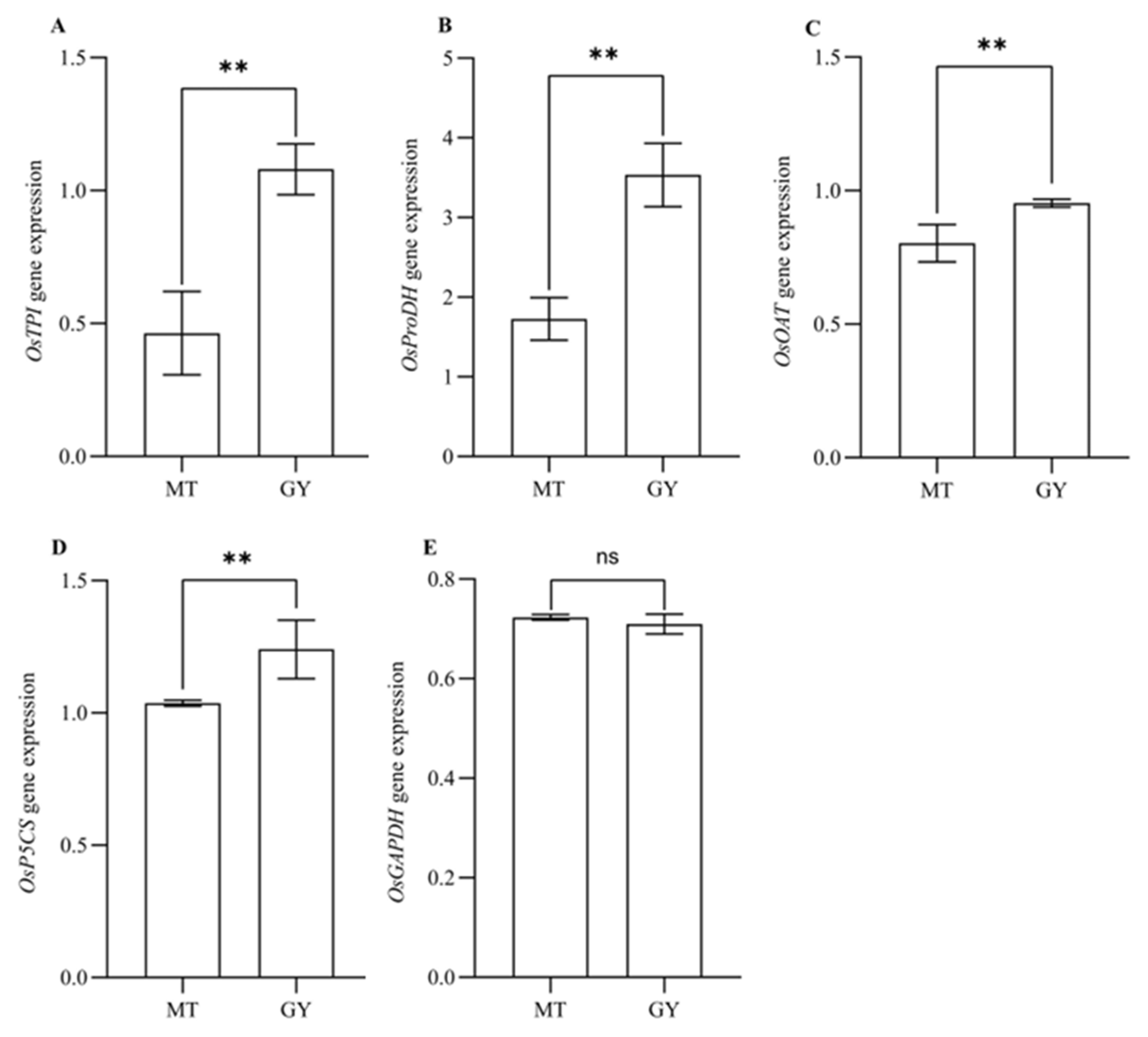
3.3. Analysis of the Expression Levels of Genes Involved in 2AP Biosynthesis
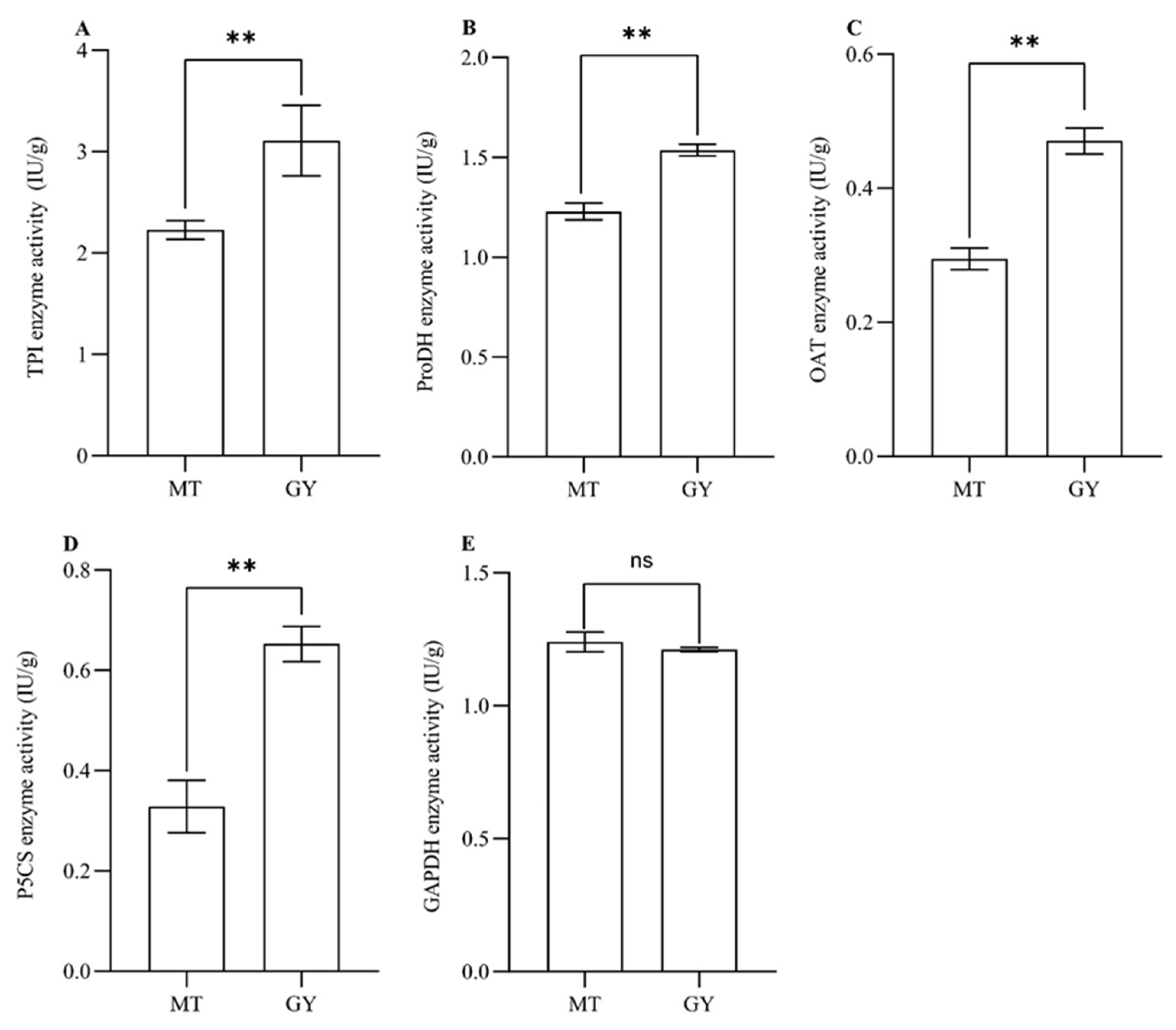
3.4. Analysis of the Enzyme Activities Involved in 2AP Biosynthesis
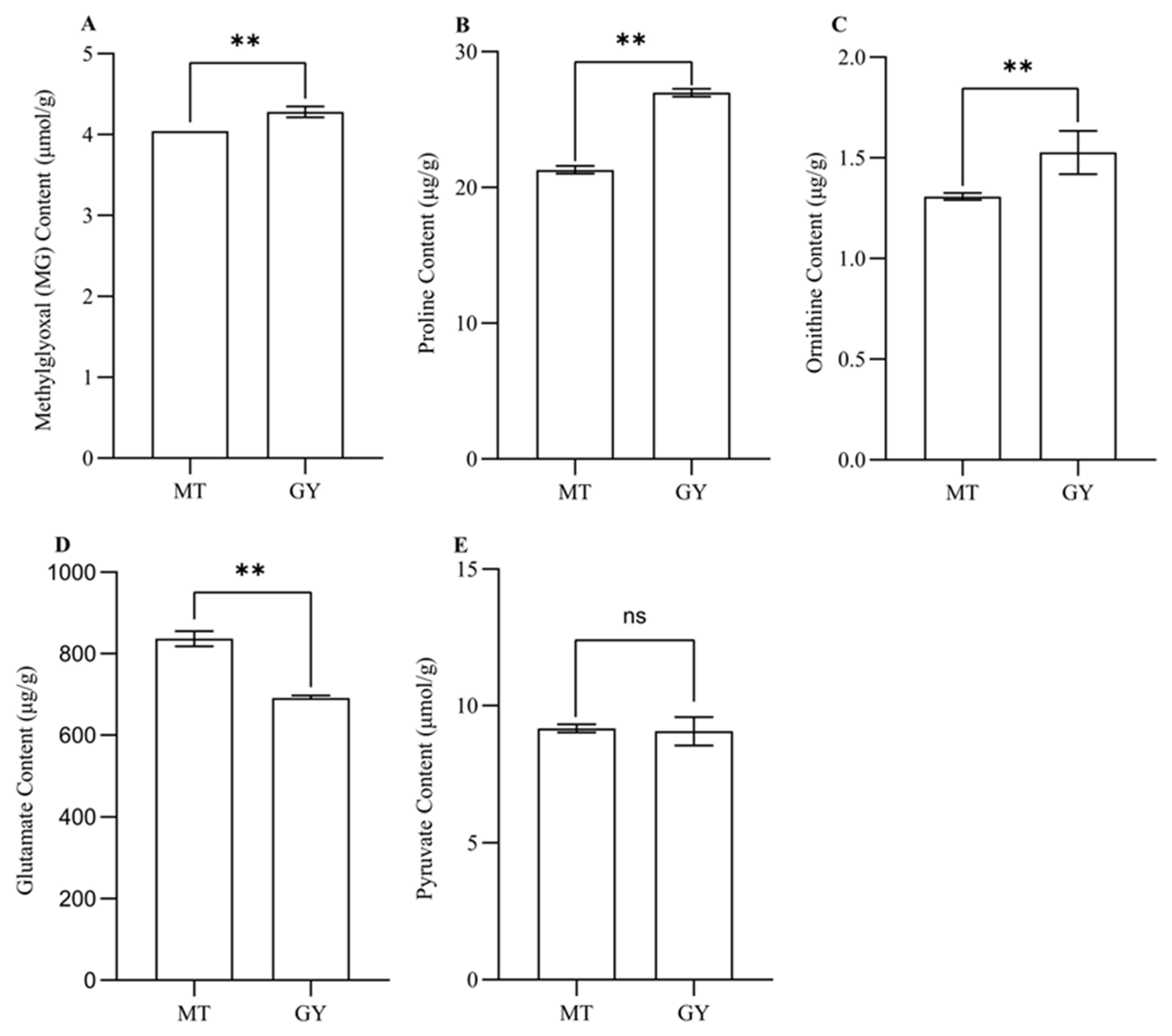
3.5. Analysis of the Contents of Substances Involved in 2AP Biosynthesis
3.6. Analysis of Soil Nutrients Under Different Ecological Environments
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Primer Name | Sequence (5′-3′) | References |
|---|---|---|
| OsBadh2-F | TGTGCTAAACATAGTGACTGGA | [6] |
| OsBadh2-F | CTTAACCATAGGAGCAGCT | |
| OsTPI-F | ATCAGATGAACTGAAAGTGCCGTT | [39] |
| OsTPI-R | GACTACGAAAACAAGTAATCAT | |
| OsP5CS-F | GCAATCTGAACCAAGGCATCAGG | [40] |
| OsP5CS-R | TTTAGCAGGACTGTTGGCACTGG | |
| OsProDH-F | TCATCAGACGAGCAGAGGAGAACAGG | [41] |
| OsProDH-R | CCCAGCATTGCAGCCTTGAACC | |
| OsGAPDH-F | ATGGCGAAGATTAAGATCGGGAT | [6] |
| OsGAPDH-R | CACAGTGTCATACTTGAACA | |
| OsOAT-F | CTGGAGCTGAAGGAGTGGAAACAGC | [40] |
| OsOAT-R | GATGGCCAGGAACCAATGGG | |
| EF1α-F | TTTCACTCTTGGTGTGAAGCAGAT | [3] |
| EF1α-R | GACTTCCTTCACGATTTCATCGTAA |
References
- Withana, W.V.E.; Kularathna, R.M.R.E.; Kottearachchi, N.S.; Kekulandara, D.S.; Weerasena, J.; Steele, K.A. In silico analysis of the fragrance gene (BADH2) in Asian rice (Oryza sativa L.) germplasm and validation of allele specific markers. Plant Genet. Resour. Charact. Util. 2020, 18, 71–80. [Google Scholar] [CrossRef]
- Yang, S. Effects of Nitrogen Application and Combined Application of Nitrogen and Zinc Fertilizers on 2AP Content, Yield and Quality of Japonica Fragrant Rice. Master’s Thesis, Yangzhou University, Yangzhou, China, 2023. [Google Scholar]
- Khandagale, K.S.; Zanan, R.L.; Mathure, S.V.; Nadaf, A.B. Haplotype variation of Badh2 gene, unearthing of a new fragrance allele and marker development for non-basmati fragrant rice ‘Velchi’ (Oryza sativa L.). Agri. Gene 2017, 6, 40–46. [Google Scholar] [CrossRef]
- Chen, S.H.; Yang, Y.; Shi, W.W.; Ji, Q.; He, F.; Zhang, Z.D.; Cheng, Z.K.; Liu, X.N.; Xu, M.L. Badh2, encoding betaine aldehyde dehydrogenase, inhibits the biosynthesis of 2-acetyl-1-pyrroline, a major component in rice fragrance. Plant Cell 2008, 20, 1850–1861. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Schieberle, P. 2-Oxopropanal, hydroxy-2-propanone, and 1-pyrroline- important intermediates in the generation of the roast-smelling food flavor compounds 2-acetyl-1-pyrroline and 2-acetyltetrahydropyridine. J. Agric. Food Chem. 1998, 46, 2270–2277. [Google Scholar] [CrossRef]
- Hinge, V.R.; Patil, H.B.; Nadaf, A.B. Aroma volatile analyses and 2AP characterization at various developmental stages in Basmati and Non-Basmati scented rice (Oryza sativa L.) cultivars. Rice 2016, 9, 38. [Google Scholar] [CrossRef]
- Romanczyk, L.J.; Mcclelland, C.A.; Post, L.S.; Aitken, W.M. Formation of 2-acetyl-1-pyrroline by several bacillus cereus strains isolated from cocoa fermentation boxes. J. Agric. Food Chem. 1995, 43, 469–475. [Google Scholar] [CrossRef]
- Schieberle, P. The role of free amino acids presents in yeast as precursors of the odorants 2-acetyl-1-pyrroline and 2-acetyltetrahydropyridine in wheat bread crust. Z. Für Lebensm.-Unters. Und-Forsch. 1990, 191, 206–209. [Google Scholar] [CrossRef]
- Sansenya, S.; Wechakorn, K. Effect of rainfall and altitude on the 2-acetyl-1-pyrroline and volatile compounds profile of black glutinous rice (Thai upland rice). J. Sci. Food Agric. 2021, 101, 5784–5791. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, Z.N.; Li, Z.J.; Xu, H.F.; Gong, J.Y.; Pu, X.C.; Zheng, G.Y.; Zhang, Y.S.; Zhu, S.S. Study on the correlation between aroma components and soil properties of fragrant rice “Goudang 3”. Jiangsu Agric. Sci. 2023, 51, 168–173. [Google Scholar] [CrossRef]
- Sun, S.X.; Liu, S.C. Study of the effects of N and Zn fertilizers on the aroma of rice. Acta Agron. Sin. 1991, 6, 430–435. [Google Scholar]
- Hu, S.L.; Huang, Q.W.; Xu, Q.G. Relationship of sweet rice quality with the contents of microelements. Acta Agron. Sin. 2001, 4, 12–15. [Google Scholar] [CrossRef]
- Zheng, C.G.; Cai, G.Z.; Dai, H.Y. A report on the breeding improvement and farming of scented rice. Tillage Cultiv. 2005, 4, 7–9. [Google Scholar]
- Udovic, M.; Mcbride, M. Influence of compost addition on lead and arsenic bioavailability in reclaimed orchard soil assessed using Porcellio scaber bioaccumulation test. J. Hazard. Mater. 2012, 205–206, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.W.; Li, Y.H.; Nie, J.; He, L.X.; Pan, S.G.; Duan, M.Y.; Tian, H.; Xiao, L.Z.; Zhong, K.Y.; Tang, X.R. Nitrogen application and different water regimes at booting stage improved yield and 2-acetyl-1-pyroline (2AP) formation in fragrant rice. Rice 2019, 12, 77. [Google Scholar] [CrossRef]
- Tian, S.C.; Ma, J.J. Effect of soil fertility on yield of rice and establishment of fertilization index system. Chin. Agric. Sci. Bull. 2015, 31, 1–4. [Google Scholar] [CrossRef]
- Hang, Y.J.; Wang, Q.S.; Xu, G.C.; Liu, X.; Yang, B.; Jin, M. Ecological response of nutrient properties of paddy field to different tillage practices. Chin. Agric. Sci. Bull. 2017, 33, 106–112. [Google Scholar] [CrossRef]
- Yan, C. Studies on Decomposition Regularity of Returning Rice Straw and Soil Nutrient Properties. Ph.D. Thesis, Northeast Agricultural University, Harbin, China, 2015. [Google Scholar] [CrossRef]
- Zhan, M.; Cao, C.G.; Jiang, Y.; Wang, J.P.; Le, L.X.; Cai, M.L. Dynamics of active organic carbon in a paddy soil under different rice farming modes. Chin. J. Appl. Ecol. 2010, 21, 2010–2016. [Google Scholar] [CrossRef]
- Luo, R.F.; Luo, J.; Mei, Y.X.; Li, T.H.; Chen, Y.X.; Lv, C.Z.; Tan, B. The Purification, Rejuvenation and Application of Good Quality Rice Dalixiang of Maogong Brand. Seed 2012, 31, 131–132. [Google Scholar] [CrossRef]
- Wang, L.L.; Peng, Q.; Li, J.L.; Zhang, D.S.; Wu, J.Q.; Jiang, X.; Zhu, S.S. The CDS variance analysis of OsBADH2 gene and aroma appraisal of 7 Guizhou HE (Oryza sativa). Mol. Plant Breed. 2018, 16, 4138–4142. [Google Scholar] [CrossRef]
- Deng, L.X.; Liu, D.; Chen, Y.H.; Feng, Z.L.; Long, Q.H. Development of molecular marker for fragrance gene of high quality rice ‘Dalixiang’ in Guizhou province. Mol. Plant Breed. 2021, 19, 6059–6063. [Google Scholar] [CrossRef]
- Yang, X.J.; Tang, X.R.; Wen, X.C.; Li, Y.H.; Zhou, X.J. Effects of sowing date on aroma formation, quality and yield of early season aromatic rice. Acta Agric. Boreali-Occident. Sin. 2014, 29, 128–135. [Google Scholar] [CrossRef]
- Okpala, N.E.; Potcho, M.P.; An, T.; Ahator, S.D.; Duan, L.X.; Tang, X.R. Low temperature increased the biosynthesis of 2-AP, cooked rice elongation percentage and amylose content percentage in rice. J. Cereal Sci. 2020, 93, 102980. [Google Scholar] [CrossRef]
- Itani, T.; Tamaki, M.; Hayata, Y.; Fushimi, T.; Hashizume, K. Variation of 2-acetyl-1-pyrroline concentration in aromatic rice grains collected in the same region in Japan and factors affecting its concentration. Plant Prod. Sci. 2004, 7, 178–183. [Google Scholar] [CrossRef]
- Wu, Y.F. Study on Suitability Evaluation of Ecological Geological Environment of Tea Planting Based on RS and GIS. Master’s Thesis, Guizhou University, Guiyang, China, 2019. [Google Scholar]
- Zhong, W.M.; Zhang, W.B.; Tang, D.M.; Yang, J.T.; Li, Z.C.; Qin, J. Climatic adaptability regionalization of actinidia deliciosain Guiyang area based on GIS. Guizhou Agric. Sci. 2022, 50, 125–131. [Google Scholar] [CrossRef]
- Huang, S.Z. Relationship between soil characteristics and rice quality in Hunan fragrant rice producing areas. Hunan Agric. Sci. 1990, 4, 37–40. [Google Scholar] [CrossRef]
- Bradbury, L.M.T.; Fitzgerald, T.L.; Henry, R.J.; Jin, Q.S.; Waters, D.L. The gene for fragrance in rice. Plant Biotechnol. J. 2005, 3, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Shafiq, S.; Ashraf, U.; Qi, J.Y.; Mo, Z.W.; Tang, X.R. Biosynthesis of 2-acetyl-1-pyrroline in fragrant rice: Recent insights into agro-management, environmental factors, and functional genomics. J. Agric. Food Chem. 2023, 71, 4201–4215. [Google Scholar] [CrossRef] [PubMed]
- Schieberle, P. Quantitation of important roast-smelling odorants in popcorn by stable isotope dilution assays and model studies on flavor formation during popping. J. Agric. Food Chem. 1995, 43, 2442–2448. [Google Scholar] [CrossRef]
- Huang, T.C.; Huang, Y.W.; Hung, H.J.; Ho, C.T.; Wu, M.L. Delta1-pyrroline-5-carboxylic acid formed by proline dehydrogenase from the Bacillus subtilis ssp. natto expressed in Escherichia coli as a precursor for 2-acetyl-1-pyrroline. J. Agric. Food Chem. 2007, 55, 5097–5102. [Google Scholar] [CrossRef]
- Huang, Z.L.; Tang, X.R.; Wang, Y.L.; Chen, M.J.; Zhao, Z.K.; Duan, M.Y.; Pan, S.G. Effects of increasing aroma cultivation on aroma and grain yield of aromatic rice and their mechanism. Sci. Agric. Sin. 2012, 45, 1054–1065. [Google Scholar]
- Li, M.J.; Ashraf, U.; Tian, H.; Mo, Z.W.; Pan, S.G.; Anjum, S.A.; Duan, M.Y.; Tang, X.R. Manganese-induced regulations in growth, yield formation, quality characters, rice aroma and enzyme involved in 2-acetyl-1-pyrroline biosynthesis in fragrant rice. Plant Physiol. Biochem. 2016, 103, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Renuka, N.; Barvkar, V.T.; Ansari, Z.; Zhao, C.F.; Wang, C.L.; Zhang, Y.D.; Nadaf, A.B. Co-functioning of 2AP precursor amino acids enhances 2-acetyl-1-pyrroline under salt stress in aromatic rice (Oryza sativa L.) cultivars. Sci. Rep. 2022, 12, 3911. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.T.; Li, M.Y.; Ling, X.T.; Guo, D.S.; Yang, Y.W.; Liu, Q.; Zhang, B.L.; Wang, J.Y. Discovery of OsODC as a key enhancer of aroma and development of highly fragrant rice. Plant Commun. 2025, 6, 101141. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Roychoudhury, A. Differential levels of metabolites and enzymes related to aroma formation in aromatic indica rice varieties: Comparison with non-aromatic varieties. 3 Biotech 2018, 8, 25. [Google Scholar] [CrossRef]
- Banerjee, A.; Ghosh, P.; Roychoudhury, A. Differential regulation of genes co-involved in aroma production and stress amelioration during salt acclimation in indica rice cultivars. Biologia 2020, 75, 495–506. [Google Scholar] [CrossRef]
- Sharma, S.; Mustafiz, A.; Singla-Pareek, S.L.; Srivastava, P.S.; Sopry, S.K. Characterization of stress and methylglyoxal inducible triose phosphate isomerase (OscTPI) from rice. Plant Signal. Behav. 2012, 7, 1337–1345. [Google Scholar] [CrossRef]
- Huang, T.C.; Teng, C.S.; Chang, J.L.; Chang, H.S.; Ho, T.C.; Wu, M.L. Biosynthetic mechanism of 2-acetyl-1-pyrroline and its relationship with delta1-pyrroline-5-carboxylic acid and methylglyoxal in aromatic rice (Oryza sativa L.) callus. J. Agric. Food Chem. 2008, 56, 7399–7404. [Google Scholar] [CrossRef]
- Luo, H.W.; Zhang, Q.Q.; Lai, R.F.; Zhang, S.M.; Yi, W.T.; Tang, X.R. Regulation of 2-Acetyl-1-pyrroline Content in Fragrant Rice under Different Temperatures at the Grain-Filling Stage. J. Agric. Food Chem. 2024, 72, 10521–10530. [Google Scholar] [CrossRef]

| 2AP Content in Leaves at the Heading Stage | 2AP Content in Grain at Maturity | |
|---|---|---|
| 2AP content in leaves at the heading stage | 1.000 | 0.667 ** |
| Soil Nutrient | Planting Point | |
|---|---|---|
| MT | GY | |
| Ammonium nitrogen (mg/kg) | 9.42 ± 0.072 b | 10.73 ± 0.122 a |
| Nitrate nitrogen (mg/kg) | 0.118 ± 0.006 a | 0.125 ± 0.003 a |
| pH | 6.73 ± 0.017 b | 6.88 ± 0.039 a |
| Organic matter (g/kg) | 26.38 ± 0.167 b | 41.89 ± 0.163 a |
| Total nitrogen (g/kg) | 2.54 ± 0.109 b | 3.45 ± 0.062 a |
| Total phosphorus (g/kg) | 0.645 ± 0.011 a | 0.644 ± 0.029 a |
| Total potassium (g/kg) | 11.49 ± 0.129 a | 9.57 ± 0.052 b |
| Available phosphorus (mg/kg) | 31.75 ± 0.296 a | 15.42 ± 0.225 b |
| Fast-acting potassium (mg/kg) | 124.43 ± 3.766 a | 58.82 ± 0.513 b |
| Alkaline hydrolyzable nitrogen (mg/kg) | 128.19 ± 0.386 b | 212.52 ± 0.696 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Que, T.; Gong, Y.; Wang, Q.; Wang, Z.; Long, W.; Wu, X.; Zhu, S. Effects of Different Planting Environments on the Fragrance of Dalixiang (Oryza sativa L.). Appl. Sci. 2025, 15, 8781. https://doi.org/10.3390/app15168781
Que T, Gong Y, Wang Q, Wang Z, Long W, Wu X, Zhu S. Effects of Different Planting Environments on the Fragrance of Dalixiang (Oryza sativa L.). Applied Sciences. 2025; 15(16):8781. https://doi.org/10.3390/app15168781
Chicago/Turabian StyleQue, Tao, Yanlong Gong, Qian Wang, Zhongni Wang, Wuhua Long, Xian Wu, and Susong Zhu. 2025. "Effects of Different Planting Environments on the Fragrance of Dalixiang (Oryza sativa L.)" Applied Sciences 15, no. 16: 8781. https://doi.org/10.3390/app15168781
APA StyleQue, T., Gong, Y., Wang, Q., Wang, Z., Long, W., Wu, X., & Zhu, S. (2025). Effects of Different Planting Environments on the Fragrance of Dalixiang (Oryza sativa L.). Applied Sciences, 15(16), 8781. https://doi.org/10.3390/app15168781




