1. Introduction
In modern materials science and mechanical engineering, there is a persistent interest in developing effective methods for local hardening of the surfaces of structural steels. The quality and properties of the surface layer are key determinants of wear resistance, corrosion resistance, fatigue resistance, and the overall service life of a product. Electrolytic plasma treatment (EPT) is a promising area in the field of surface engineering, based on the interaction of an electric discharge with the surface of a conductive material immersed in an electrolyte.
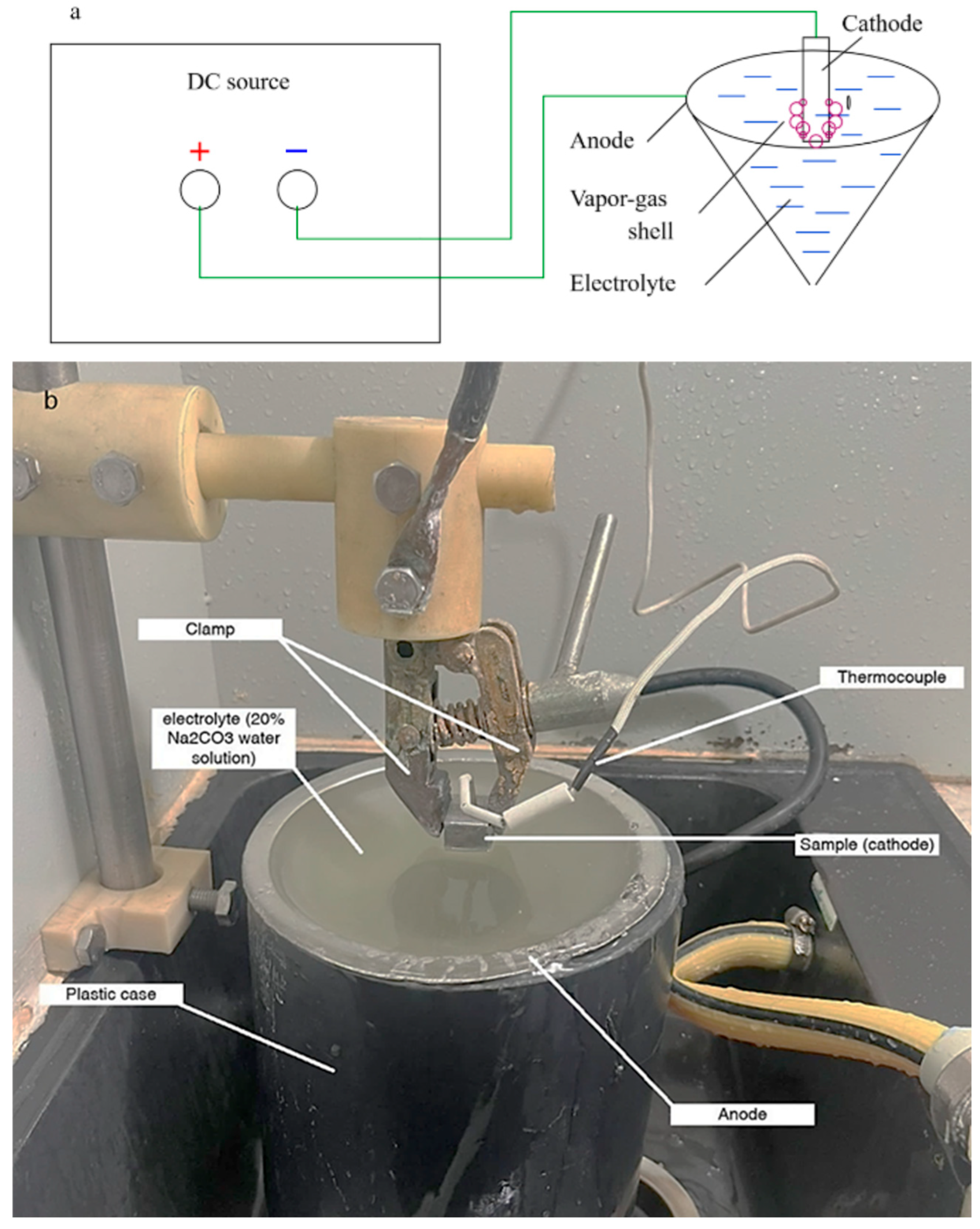
EPT relies on the creation of a plasma discharge between a metallic workpiece, acting as the cathode, and a liquid electrolyte. Upon exceeding a critical voltage (usually 120–400 V), a localized plasma sheath forms around the cathode, achieving temperatures in the range of 5000–8000 K [
1]. This discharge induces a significant thermal impact on the material’s surface, causing rapid heating, followed by immediate cooling in the electrolyte, leading to surface hardening and the formation of a hardened microstructure.
Of particular interest is the application of EPT to structural cast steel 20GL, prized for its high processability, strength, and availability. While 20GL steel is normally weakly susceptible to thermal hardening because of its low carbon content (~0.2%) and the prevalence of a ferrite–pearlite structure, the rapid heating and cooling rates in the EPT process can make it possible to achieve austenitization of the surface layer. This leads to the formation of a hardened structure after quenching, opening avenues for enhancing the wear and corrosion resistance of cast parts without significantly complicating their manufacturing technology.
In the work [
2], it was demonstrated that increasing the heating rate significantly expands the temperature range in which the phase transformation occurs, specifically the transition from ferrite to austenite. The author also indicated that even with the complete disappearance of ferrite, regions with a high carbon concentration may persist around former carbide particles. These carbon-rich areas can undergo hardening during rapid cooling, as demonstrated on 20GL steel.
Electrolytic plasma treatment, in all its variations, employs high-speed heating methodologies. Some works [
3,
4] indicate that cathode heating can yield heating rates ranging from 10 to 200 °C/s under constant voltage conditions. Furthermore, the cooling rate of the component following cathodic heating can be controlled via adjustments to the applied voltage, spanning from 12 to 80 °C/s within the zone of minimal austenite stability and from 20 to 140 °C/s in the martensitic transformation range [
5].
Continuing the development of research in the field of electrolytic plasma treatment, it is important to note that the thermal processes occurring in the near-surface layer of the metal play a key role in shaping the structure and properties of the processed material [
6]. The temperature mode of heating determines the depth and nature of phase transformations, while the cooling rate dictates the possibility of forming hardened structures, such as martensite. Therefore, a quantitative assessment of temperature, heat flow, as well as heating and cooling rates, holds significant practical importance, enabling the prediction of thermal hardening effectiveness across various processing modes.
Several studies [
7,
8,
9,
10] have explored the thermal and electrophysical phenomena that occur during anodic electrolytic plasma heating, including the modeling of temperature fields, heat flux density distribution, and electric potential within the electrolyte bath. These investigations have determined that the intensity of thermal exposure and the nature of plasma formation are influenced by factors such as the geometry of the treated component, the type of electrolyte used, the electrode material, and the applied voltage regime. However, the cathodic variant of electrolytic plasma treatment, specifically concerning the thermal state of the surface and its relationship to surface hardening, remains insufficiently studied [
11,
12]. One attempt to analyze the temperature field during cathodic EPT was conducted in [
13], where an analytical solution was put forth based on the assumption of constant thermal conductivity. Nevertheless, when considering the temperature dependence of thermal conductivity, the analytical formulation becomes considerably more complex, rendering the approach suggested previously inapplicable.
Cathodic heating in aqueous electrolytes is distinguished by a high density of thermal action concentrated on a limited area, rendering it particularly advantageous for the localized thermal modification of steels, including those with low-carbon content. Despite the presence of individual experimental studies, a systematic analysis of thermal processes during cathodic electrolytic plasma treatment of 20GL steels is lacking. Consequently, quantitative models that correlate voltage modes and pulse duration with surface temperature, heat flow, and subsequent changes in hardness and microstructure have not been developed.
The aim of this work is to evaluate, both numerically and experimentally, the thermal processes occurring in the near-surface zone of 20GL steel during cathodic electrolytic plasma treatment. This assessment includes a study of the relationship between voltage modes, heating and cooling rates, hardness, and microstructural changes. Three voltage levels—150 V, 200 V, and 250 V—were selected as the modes to be studied, with a constant treatment duration applied. This will allow for an estimation of the heating and cooling rates during cathodic EPT using experimental data on the heat flux, and will also enable a comparison of the numerically calculated surface temperature with the characteristics of the hardened layer.
3. Results and Discussion
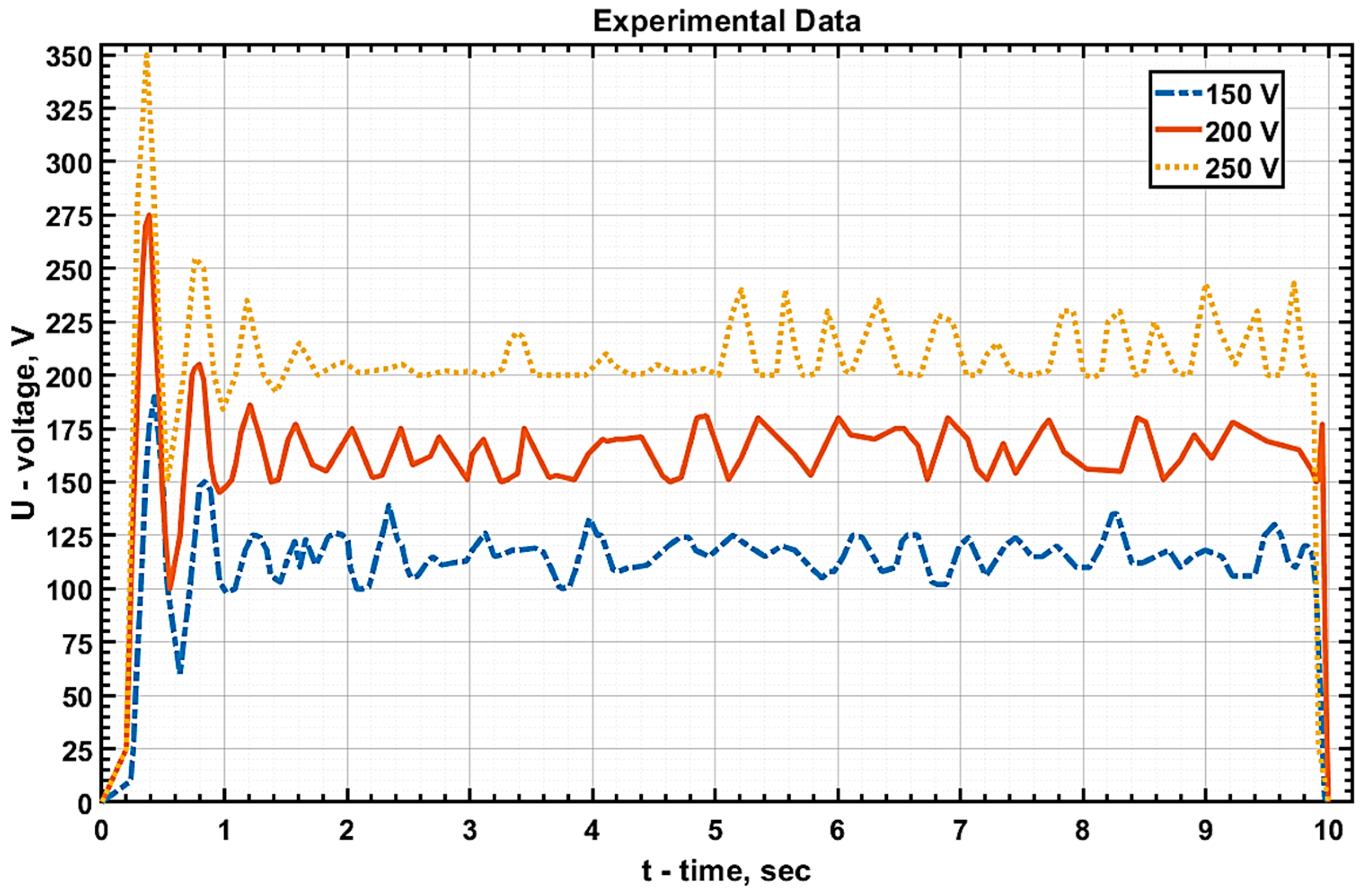
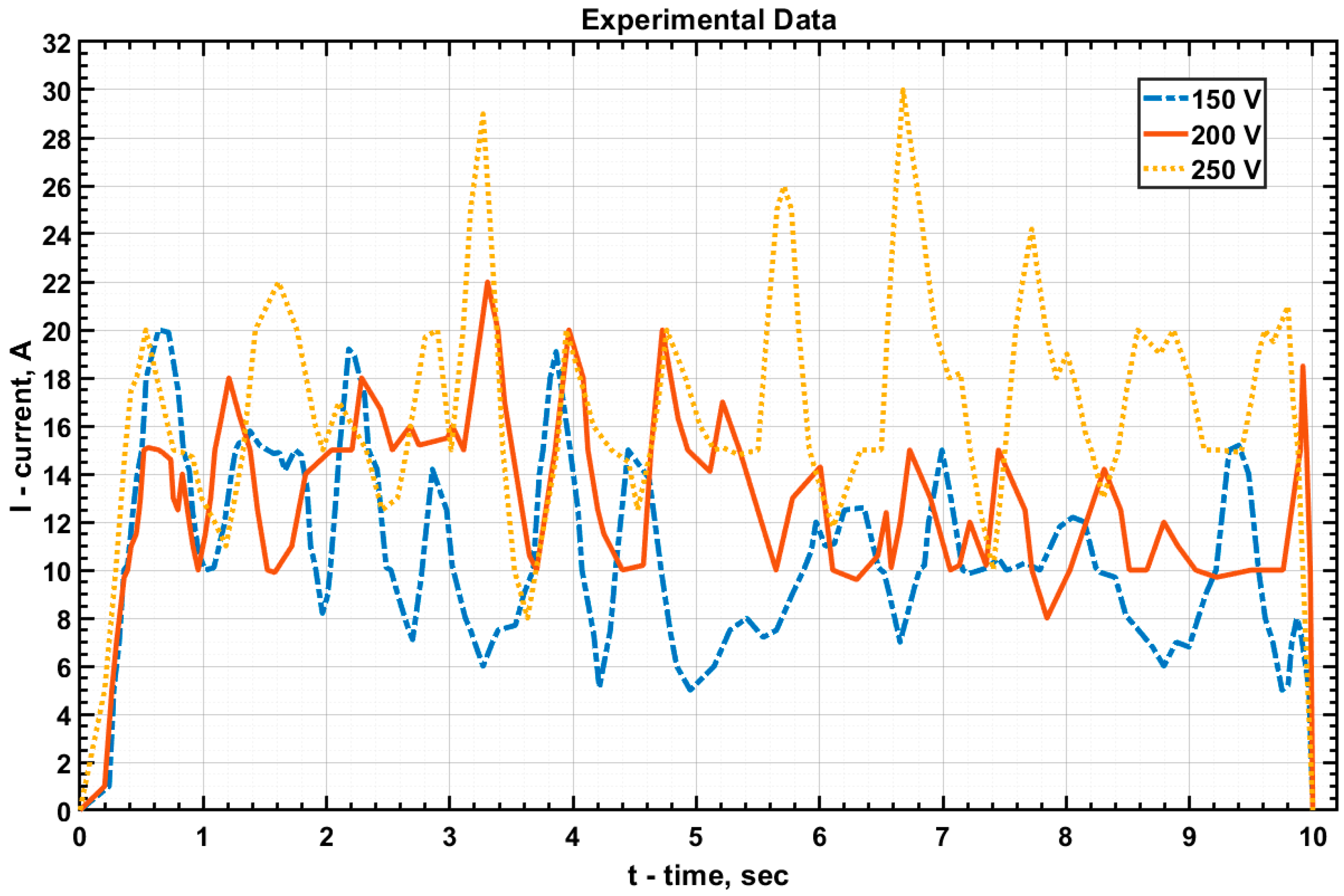
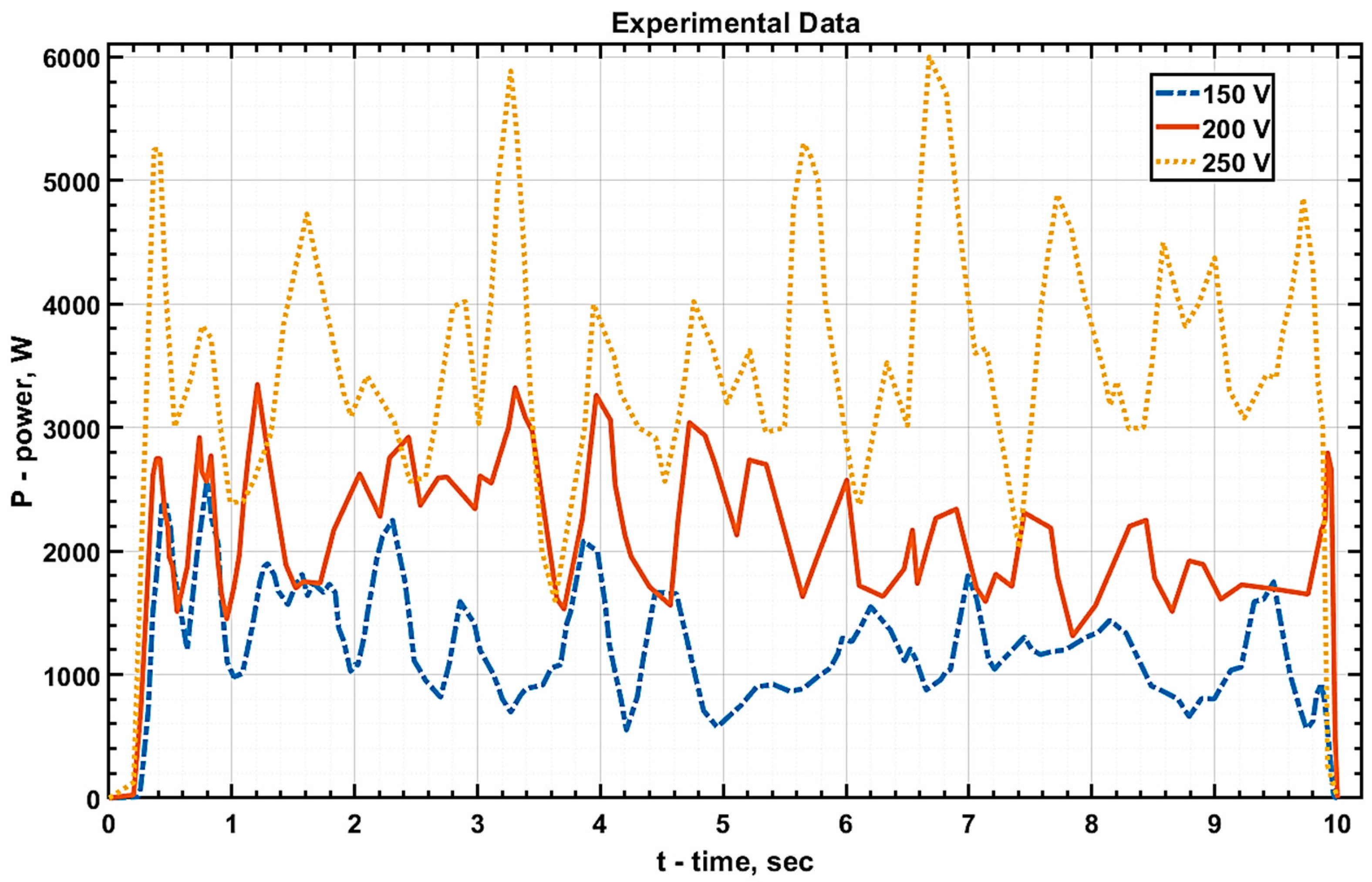
The values of current and voltage at the electrolytic cell were measured, and graphs of
U(
t),
I(
t), and
P(
t) were subsequently plotted based on these measurements (
Figure 4,
Figure 5 and
Figure 6, respectively).
Next, the amount of heat released as a result of the current flow was calculated using Formula (1). The calculation results are presented in
Table 1.
The electrolyte’s temperature was measured using an LT-300 thermometer (Termex, Tomsk, Russia) to evaluate its heating after processing. Initially, the electrolyte temperature was 20 °C. Following a 10 s hardening process at U = 150 V, the electrolyte temperature reached 20.02 °C. With voltages of 200 V and 250 V, the electrolyte temperature increased to 20.05 °C and 20.12 °C, respectively, after the same duration. The mass of the entire electrolyte solution in the tank was about 15 kg. The obtained values made it possible to calculate the amount of heat
Qh that went into heating the electrolyte using Formula (4) for each of the three hardening modes. The calculation results are shown in
Table 1.
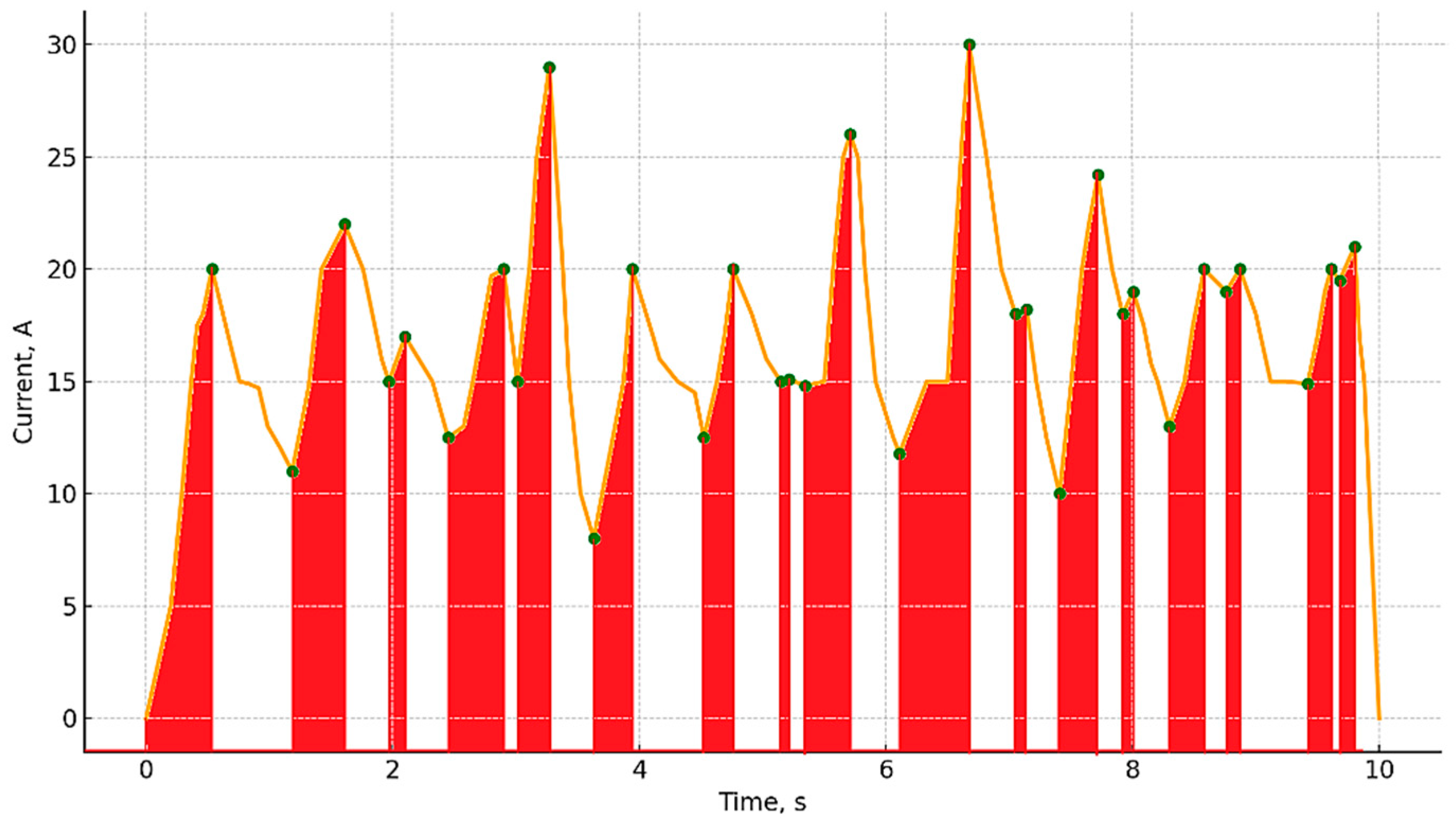
When analyzing the dynamics of the current
I(
t) in the system, it was found that each single discharge corresponds to a local peak on the experimental curve (
Figure 7). Therefore, the average number of pulses in the process can be approximately estimated by the number of pronounced local current maxima within the time interval of processing. Between two adjacent peaks, there are characteristic sections that reflect the vapor–gas shell accumulation stage. This stage is visualized as an interval from a local minimum to the subsequent maximum and can be interpreted as a gradual increase in resistance due to the formation and growth of the shell (red area on the graph). The discharge itself occurs when this shell is broken through and manifests itself as a local current maximum. This is followed by the recovery phase—an area on the graph from a local maximum to a subsequent minimum, reflecting the redistribution of the electric field, stabilization of the system and repeated growth of the shell. Accordingly, for each processing mode, it is possible to estimate the number N of such shell recoveries, which is subsequently used to estimate the amount of heat
Qv that went to electrolyte evaporation. The calculation results are reflected in
Table 1.
In
Table 1, the calculated values of vaporization heat
Qv for both the 150 V and 250 V regimes appear identical. This was due to the use of the same number of vapor–gas envelope pulsations (N = 17), as observed from current oscillograms, along with identical assumptions for vaporization volume, electrolyte density, and latent heat of vaporization, which were considered approximately constant between the two regimes. Although the input power was different, the frequency and number of pulsation cycles remained similar during the 10 s treatment interval, resulting in comparable cumulative energy expenditure for vapor generation in both cases.
As a first approximation, the holder was considered more massive than the part, and it was assumed that heat dissipation to the holder was more significant than to the surrounding air. It was also assumed that the pressure in the contact zone was moderate, with small oxides and roughness. Under these conditions, the contact conductivity typically ranges from 500 to 2000 W/m·K in the literature [
28]. To provide a more physically grounded estimation, we employed the solid-spot contact model proposed by C.V. Madhusudana [
29]. This model considers “rough–rough” metal contact, where both surfaces undergo plastic deformation.
In our case, the surface roughness was estimated as Ra ≈ 1 μm for the 20GL steel sample (sides roughly grounded but not polished), and Ra ≈ 3–5 μm for the stainless steel holder (as-manufactured). The contact pressure was estimated to lie between 0.4 and 1.2 MPa, based on the tightening torque (1–1.5 N·m) applied during assembly, assuming a thread friction coefficient of μ ≈ 0.2 and a contact area of 125 mm
2. According to Madhusudana’s empirical formulation for plastically deformable rough surfaces, the thermal contact conductance can be approximated by
where
P—contact pressure, MPa. Substituting the estimated pressure values yields
, which is consistent with the empirical range found in prior experimental and theoretical studies for similar materials and conditions. To validate the robustness of the model, a sensitivity analysis for the U = 200 V regime was performed by varying
within this range (
Table 2). The results indicated that surface temperatures changed to 12.8 °C, and internal temperatures also remained moderately affected. These findings suggest that the simulation is only weakly sensitive to this parameter, and the selected range is both physically justified and numerically stable. In further numerical calculations, the value of contact conductivity was taken as
.
To enhance the accuracy of the thermal model in the high-temperature regime (U = 250 V), radiative heat losses from the exposed surfaces of the sample were incorporated into the boundary conditions. The radiation condition was written as follows
where
ε—emissivity coefficient;
—Stefan–Boltzmann constant;
T—current surface temperature;
—the temperature of the environment where the radiation goes.
This modification addresses the increasing significance of radiative dissipation above 1000 °C, where the discrepancy between simulated and experimental temperatures becomes more pronounced if radiation is neglected.
Radiation boundary conditions were applied to both the heated surface in contact with the electrolyte and the lateral surfaces exposed to air. The emissivity values were selected based on the surface finish:
ε ≈ 0.4–0.5 for the polished working surface and
ε ≈ 0.7–0.8 for rougher lateral sides. These values were taken from [
30]. The ambient temperature was set to 293 K (20 °C), consistent with the temperature of the circulating electrolyte.
Numerical results demonstrated that accounting for radiation led to a measurable reduction in the calculated surface temperature at U = 250 V, from 1518.5 °C to 1490.6 °C—a difference of approximately 28 °C (1.84%). In contrast, the effect was negligible for lower voltages: 5.9 °C (0.67%) for 200 V, and 1.2 °C (0.25%) for 150 V.
Given these results, radiative losses were included in the model only for the 250 V mode, where their impact on accuracy is most significant. For lower-voltage modes, radiation was omitted to avoid unnecessary model complexity, as its influence on the results was within the numerical error margin.
After calculating and summarizing all energy terms in
Table 1, these values were used to define the third kind of boundary conditions in the Elcut 6.6 program.
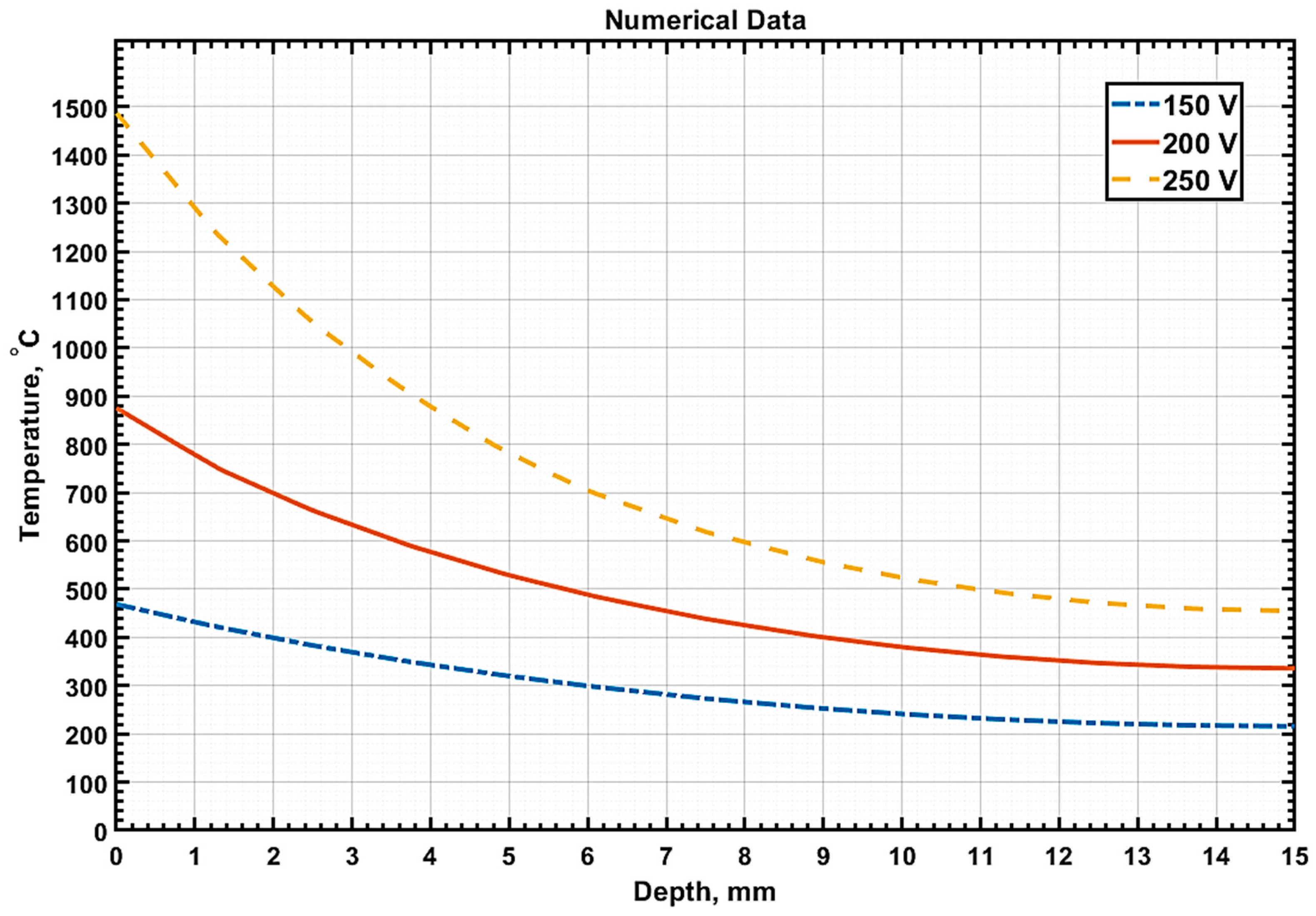
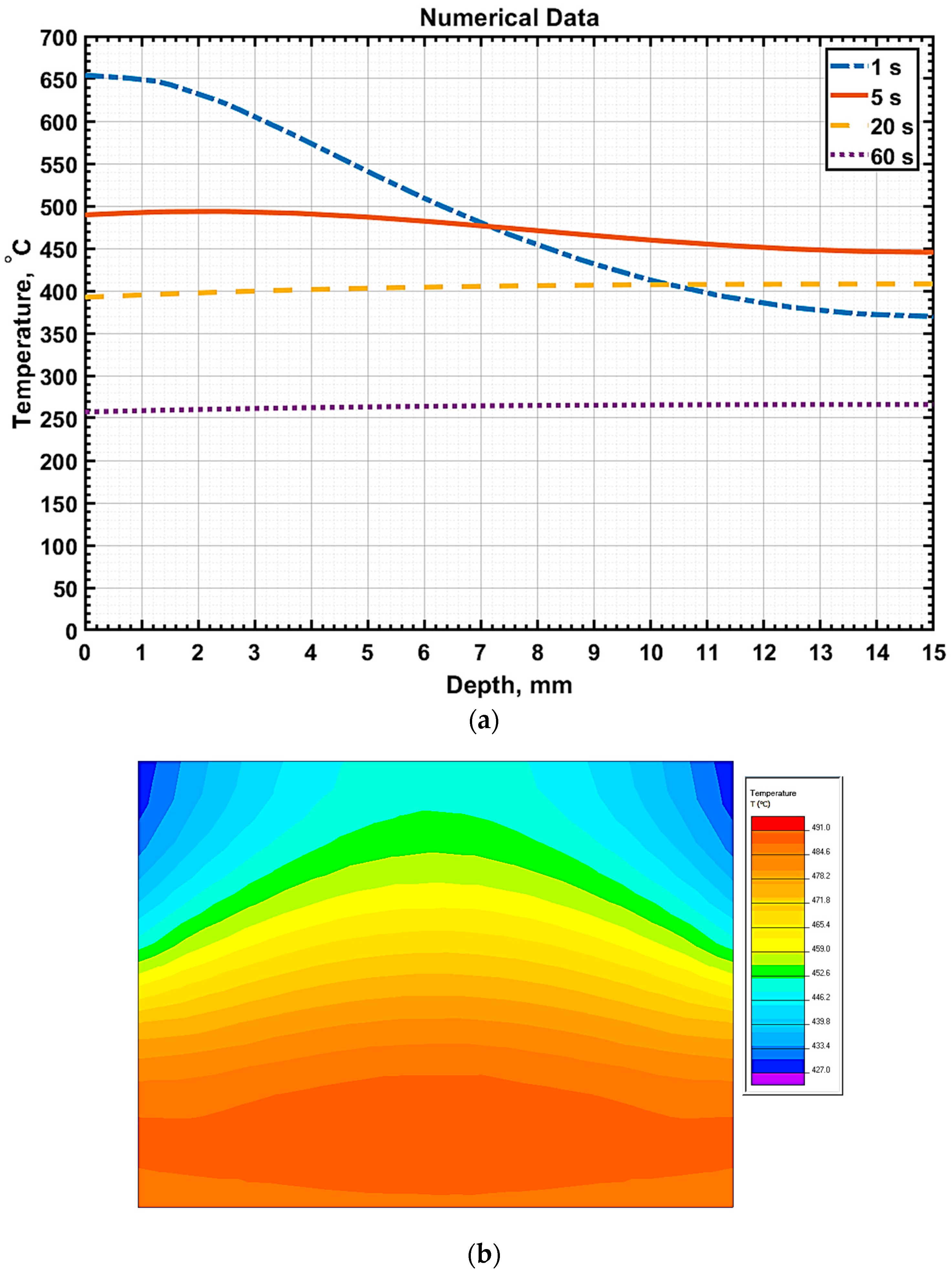
Figure 8 illustrates the temperature distribution graphs along the sample depth at
t = 10 s for three processing modes of 20GL steel, measured precisely along the line
x =
a/2, which is at the sample’s midpoint.
Following the heating phase, the cooling of the part after voltage removal was simulated using the Elcut 6.6 program, with
q = 0 while maintaining the established boundary conditions.
Figure 9a depicts the temperature distribution pattern relative to the sample’s depth (on the line
x =
a/2) during cooling at different time intervals, as well as the temperature distribution pattern across the sample’s cross-section 5 s after the voltage was disconnected (
Figure 9b).
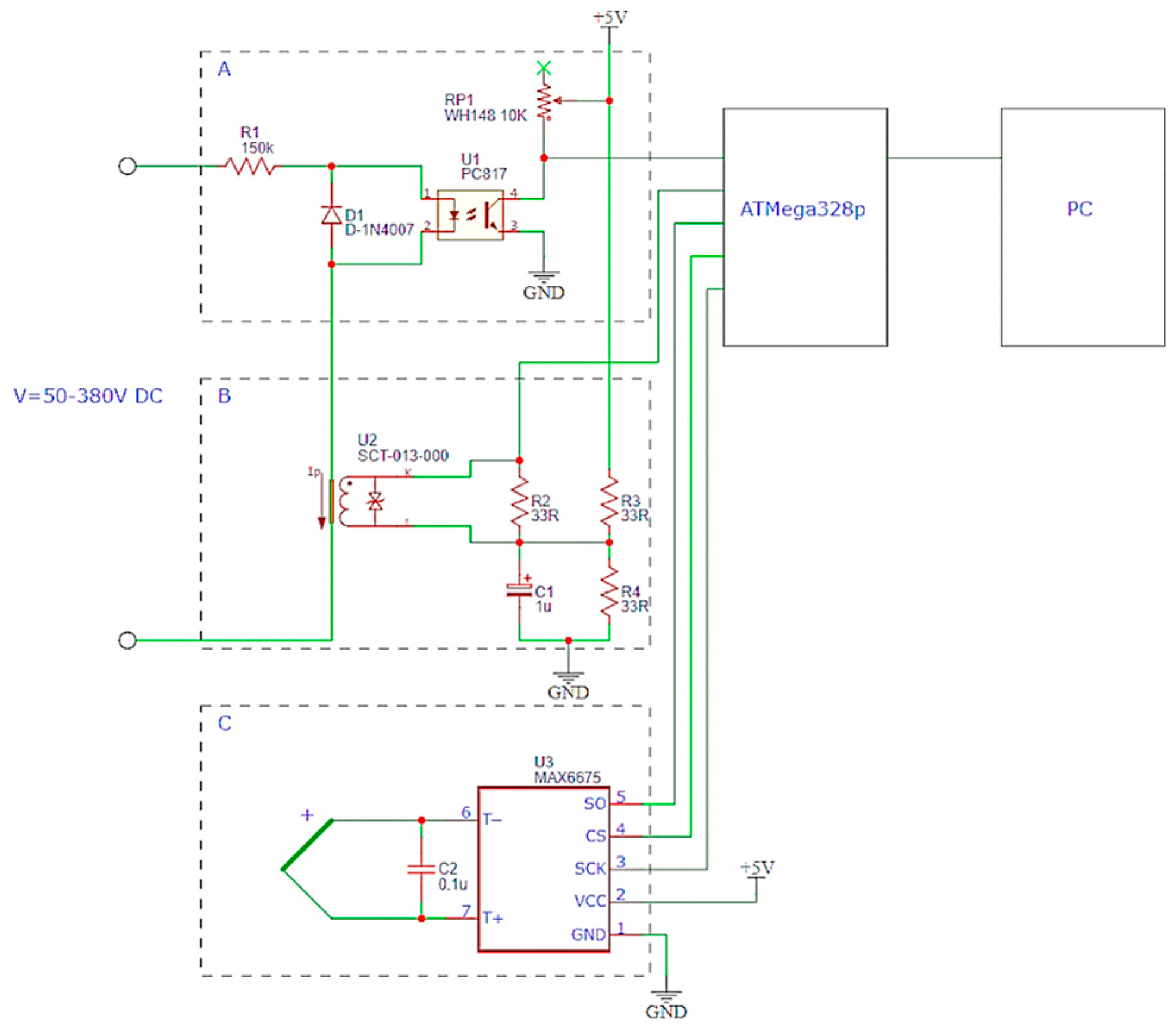
To ensure a valid comparison, the numerical calculation results were analyzed at a depth of 2 mm, consistent with the experimental temperature measurements obtained using a thermocouple. However, the interpretation of experimental data must consider the inherent measurement inertia; the MEGEON26003 thermocouple’s response time is approximately 4 s, according to its technical specifications. Consequently, the thermocouple readings may lag behind the actual temperature of the sample, particularly under the rapid heating conditions characteristic of electrolytic plasma hardening.
To estimate this delay, a classical model, described by a linear differential equation of the first order, was used to represent the response of the inertial measuring device. The solution to such an equation is an exponential approximation:
where
Ttc represents the thermocouple reading,
Treal represents the true temperature within the sample,
t is the current time, and
τ is the thermocouple time constant. This expression was detailed in several authoritative sources, including standard manuals on measurement systems and heat transfer [
31].
For the 200 V processing mode at 10 s, corresponding to the end of heating, the numerical calculation predicted a steel temperature of approximately 698.6 °C at a depth of 2 mm. Concurrently, the thermocouple recorded 592 °C. When accounting for the exponential delay, a recalculation using the formula suggests the actual temperature in the part at that time could be around 645 °C, indicating a relative deviation of approximately 7.7% from the numerical prediction. Similarly, for the 150 V mode, the thermocouple measured 343.6 °C, which, when adjusted for inertia, yields 374.3 °C. This value corresponds to a deviation of about 6.2% when compared to the numerical value of 398.9 °C.
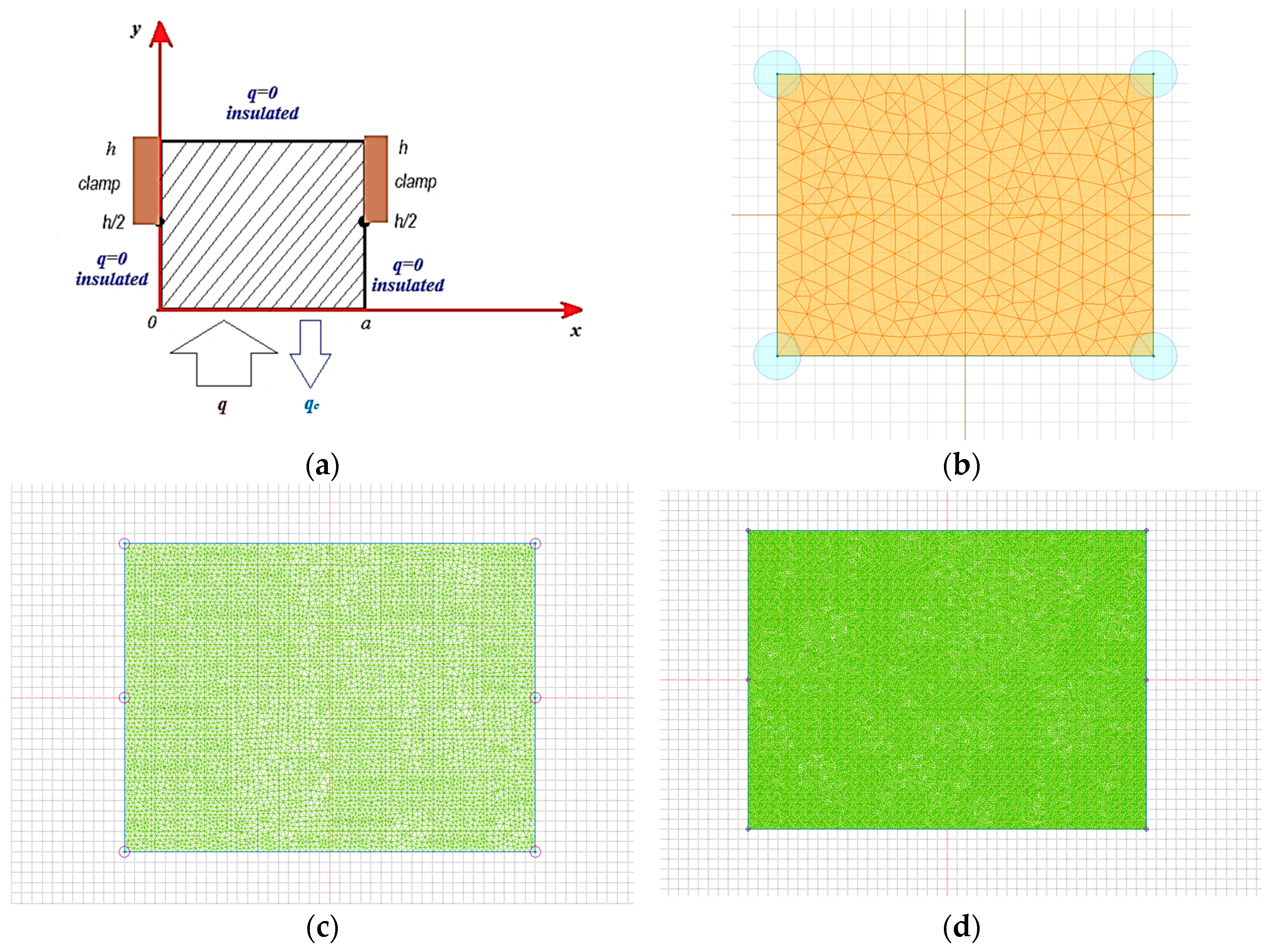
To further verify the numerical reliability of the thermal model, a mesh convergence test was conducted at the 200 V processing mode. The simulation was repeated using two progressively refined grids with approximately 6000 and 40,000 nodes.
The calculated maximum surface temperatures for these cases were 878.9 °C and 878.903 °C, respectively. Compared to the base mesh with 254 nodes (875.45 °C), the deviation between the coarsest and finest mesh solutions was only 3.45 °C, corresponding to a relative error of approximately 0.39%. These results demonstrate that the thermal field distribution stabilizes with increasing mesh resolution, and the base mesh provides sufficiently accurate results for engineering-level simulations while ensuring acceptable computational efficiency.
The data reveal a strong correlation between measured and calculated values for the 150 V and 200 V modes. However, a notable discrepancy arises at 250 V, where the numerical calculation estimates a temperature of 1127.1 °C, while the thermocouple records approximately 870 °C. This variance is primarily attributed to a technical issue involving compromised thermal contact between the thermocouple and the sample. The LOK-59 solder, used to affix the thermocouple at a 2 mm depth, has a melting point of around 900 °C. As temperatures surpass this threshold, partial melting of the solder can occur, impeding effective heat transfer between the sample and the thermocouple’s sensing element, thereby causing an underestimation of actual temperature values. Furthermore, radiative heat exchange between the sample’s heated surface and the surrounding environment becomes more significant above 1000 °C. This can induce heat redistribution and deviations from the idealized conditions assumed in the model. These factors clarify the divergence between computed and empirical data at 250 V, underscoring the necessity for meticulous interpretation of thermometry results in high-temperature electrolytic plasma hardening scenarios.
For future studies involving elevated-temperature EPH regimes, it is recommended to employ thermocouples with higher thermal stability and reduced response time, such as tungsten–rhenium or miniature fast-response sensors. Additionally, the use of high-melting-point solders (e.g., VPR-1 or VPR-11, with melting points exceeding 1000 °C) is advisable to ensure reliable thermal contact throughout the entire heating cycle. These modifications would enhance the accuracy of temperature measurements and support more robust validation of thermal models under extreme processing conditions.
To assess the thermal action efficiency across various EPT modes, the average heating rates at a 2 mm depth from the sample surface were calculated over the entire heating period. Experimentally, these rates were found to be 35.4 °C/s at 150 V, 62.5 °C/s at 200 V, and 92.9 °C/s at 250 V. Numerical estimations produced similar values of 37.9 °C/s, 67.9 °C/s, and 112.4 °C/s, respectively. These findings indicate a strong correlation between experimental results and calculations, accounting for errors introduced by the measuring system’s inertia. Notably, the local heating rates near the surface during the initial processing seconds significantly surpass these averages, potentially reaching hundreds of degrees per second, particularly at 250 V. This underscores the thermal effect’s intensity and elucidates the rapid attainment of critical temperatures in the material’s surface layers.
The average cooling rates at a depth of 2 mm following the EPD, as determined numerically, were 16.7 °C/s (150 V), 34.6 °C/s (200 V), and 59.8 °C/s (250 V). Experimentally, slightly lower values were observed at 15.0 °C/s, 30.4 °C/s, and 47.8 °C/s, respectively. This difference can be attributed to the thermocouple’s inertia and potential degradation of thermal contact at elevated temperatures. Notably, during the initial seconds after voltage termination, instantaneous cooling rates can reach hundreds of degrees per second. Such rapid thermal unloading fosters conditions conducive to martensite and bainite formation, even in low-carbon steel 20GL, particularly within the surface layers.
The elevated values obtained from the numerical calculations can be primarily attributed to inherent simplifications within the model. These simplifications pertain to the boundary conditions, specifically the assumption of a heat-insulated surface, given the substantial size of the holder. Additionally, the model does not account for the oscillatory nature of heating as a boundary condition with heat flow. For preliminary engineering assessments, such assumptions are deemed reasonable to avoid unnecessary complexity. The primary objective of this study was to investigate the feasibility of hardening low-carbon steel 20GL through electrolytic plasma treatment and to evaluate the depth of thermal influence on the samples. Future research endeavors will aim to incorporate a more comprehensive analysis of all conditions involved in the electrolytic plasma hardening of components.
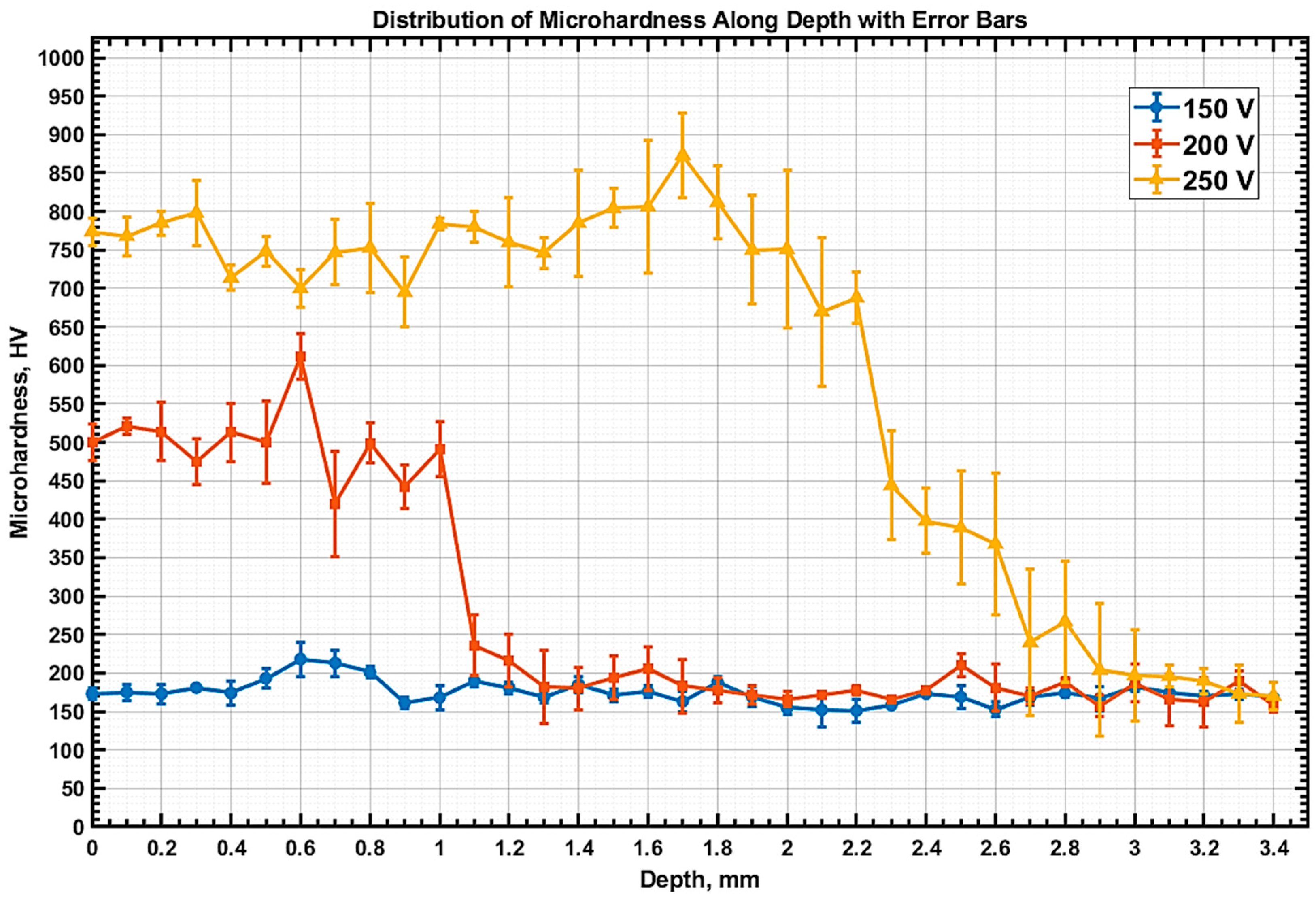
The distribution of microhardness by depth (
Figure 10) clearly shows how the hardening of 20GL steel depends on the electrolytic plasma treatment mode. Initially, the samples had a microhardness of approximately 150–160 HV. The cross-section for measurement was made at a distance of approximately 5 mm from the edge of the sample. At 150 V, hardening is minimal, with a maximum hardness of about 200 HV. This aligns with the calculated temperature of less than 400 °C at a depth of 2 mm, indicating an inability to reach the critical temperature required for phase transformations. At 200 V, a notable increase in microhardness to 500 HV occurs in the surface zone, followed by a sharp decline after 1 mm of depth. Although the numerical temperature distribution predicts heating to 700–800 °C at a depth of 1–2 mm, the reduction in hardness suggests an inadequate cooling rate in these layers, which restricted the formation of bainite or martensite structures. The most substantial strengthening is observed at 250 V, where high microhardness (750–850 HV) is sustained in the zone up to approximately 2.2–2.4 mm. This aligns with calculations showing that the temperature at this depth exceeded 750 °C, and the cooling rate (>50 °C/s) was sufficient to develop a martensitic structure. Therefore, for 20GL steel, which has limited hardenability, the effective strengthening depth depends not only on the heating temperature but also on achieving critical cooling rates, especially during high-voltage EPH.
The sharp drop in hardness observed at 200 V can be explained as follows. At the end of the heating stage (t = 10 s), the surface and subsurface layers (up to ~1.5 mm) had reached temperatures in the range of 723–875 °C, sufficient for austenitization. During the subsequent 5 s cooling phase in a vigorously circulated electrolyte (at ~20 °C), a rapid temperature drop occurred. The computed cooling rate at the sample surface reached approximately 77 °C/s, gradually decreasing with depth. At depths of 1.25 mm and 1.5 mm, the cooling rate fell to around 52 °C/s and 48 °C/s, respectively—close to the critical threshold for martensitic transformation in low-carbon steels. Beyond these depths, the cooling rate continued to decline, becoming insufficient to support the formation of martensite.
This behavior aligns well with the experimentally observed hardness profile: a hardened layer of ~1.5 mm with high hardness (>300 HV), followed by a steep decrease in hardness to base values (~150 HV) corresponding to the ferrite–pearlite microstructure in the unaffected core. In this way, the interplay between two critical conditions—(1) exceeding the austenitization temperature and (2) ensuring a high enough cooling rate—explains the formation of a relatively shallow hardened layer. This confirms that the sharp drop in hardness is not an anomaly but a direct consequence of the thermal field and transformation kinetics, further validating the numerical model.
Microstructural analysis of 20GL steel samples following EPH (
Figure 11) enables a visual evaluation of how different treatment modes impact surface alterations in the material. Initially, the microstructure exhibits characteristics typical of as-cast low-carbon steel, featuring a coarse-grained ferrite–pearlite morphology (
Figure 11a) and a corresponding low microhardness of approximately 150 HV. After treatment at 150 V (
Figure 11b), the microstructure remains largely unchanged, with only isolated regions of dispersed pearlite observed, leading to a modest increase in microhardness up to 180 HV. This suggests minimal phase transformation, consistent with the temperature profile, which indicates that heating in the surface region was insufficient to induce austenitization.
At 200 V (
Figure 11c), a more noticeable alteration in the microstructure occurs, where the surface takes on a finer grain morphology. Zones of transitional bainite or incomplete martensitic transformation may also be present, correlating with a sharp increase in microhardness to 500 HV. The most substantial changes are observed at 250 V (
Figure 11d), where the structure exhibits a typical martensitic morphology with an acicular texture and a maximum microhardness of approximately 800 HV. This indicates that at high voltage, the conditions necessary for complete austenitization and subsequent martensitic transformation are achieved in the surface layers, including a high temperature above 1100 °C and intensive cooling.
Therefore, the presented micrographs corroborate that the effectiveness of EPH for 20GL steel is directly contingent on achieving critical temperatures and cooling rates. The latter factor, particularly for cast steel, is decisive in the formation of a hardened structure.