Abstract
The objective of this study was to investigate the temporal effectiveness of repetitive peripheral magnetic stimulation (rPMS) on lower-limb motor skill performance in individuals with chronic stroke. In this sham-controlled crossover study, we hypothesized that individuals with stroke who received rPMS would demonstrate improved motor skill performance after the stimulation and maintain this enhanced performance at 30 and 60 min after the stimulation. Sixteen participants performed a visuomotor ankle-tracking task at multiple time points following either rPMS or sham stimulation. rPMS, delivered to the tibialis anterior muscle, did not result in statistically significant changes in spatiotemporal (p = 0.725) or spatial error (p = 0.566) metrics at any post-stimulation time point. These findings suggest that a single session of rPMS does not lead to measurable improvements in lower-limb motor skill performance in individuals with stroke, underscoring the need to refine stimulation parameters and target populations in future protocols.
1. Introduction
Stroke is a leading cause of long-term disability in the United States [1], often resulting in persistent motor impairments that compromise functional independence, especially during walking and balance tasks. These deficits are particularly pronounced in the lower limbs, where impairments in strength, motor control, and coordination can significantly limit mobility [2,3]. Although conventional rehabilitation strategies can yield modest improvements, many individuals experience a plateau in recovery, underscoring the need for adjunct interventions that promote neuroplasticity and target functional motor skills [4].
Neuroplasticity—the brain’s capacity to reorganize neural pathways in response to injury, experience, or training—is central to recovery after stroke [5]. Greater engagement of adaptive neuroplastic mechanisms has been linked to improved motor outcomes [6]. As a result, there is growing interest in interventions that might modulate the nervous system in ways that facilitate more effective motor learning. One area of exploration involves the use of “priming” strategies, which aim to transiently influence cortical or subcortical excitability before task-specific training [7,8]. These techniques are still under investigation, and while promising, their clinical utility and optimal parameters remain to be clearly defined.
Among various priming approaches, sensory-based priming has emerged as a promising strategy to enhance motor learning by modulating sensorimotor networks prior to task-specific training [9]. Across a range of sensory modalities, repetitive peripheral magnetic stimulation (rPMS) stands out for its non-invasive application and ability to stimulate deep neuromuscular structures. Previous studies have shown that a single session of rPMS can enhance cortical excitability and improve motor outcomes, particularly in the upper limbs [10,11,12,13]. While modalities such as electrical stimulation and vibration have been extensively explored in this context, the role of rPMS—particularly in lower-limb motor skill performance—remains underexamined. Most rPMS studies have focused on isolated motor outcomes such as strength, spasticity, or range of motion [14,15,16]. These outcomes, although clinically useful, do not reflect the demands of everyday motor behavior, which often requires visuomotor integration, sensorimotor coordination, and cognitive control.
Despite promising evidence that a single session of rPMS can modulate basic motor parameters such as strength and spasticity, its ability to enhance integrated, higher-order motor skills remains uncertain. Given the complexity of sensorimotor coordination required for functional tasks, the immediate effects observed in simpler paradigms may not translate to improvements in more demanding motor behaviors. Understanding whether rPMS can influence complex, functional performance—and whether such effects vary over time—is critical for determining its potential utility as a sensory-based priming tool. Insights into these temporal dynamics may help guide the timing and application of rPMS alongside functional training in stroke rehabilitation.
To address this gap, the present study investigated the temporal effectiveness of rPMS on lower-limb motor skill performance in individuals with chronic stroke. Using a visuomotor ankle-tracking task, we assessed motor performance at baseline and at 0, 30, and 60 min following stimulation. rPMS was delivered at 20 Hz and 10% above motor threshold for 15 min, consistent with protocols shown to enhance corticomotor excitability in prior studies [16]. These parameters were selected to allow comparison with previous findings while ensuring feasibility and tolerability in a clinical population. This approach enabled us to evaluate whether a single session of rPMS could acutely influence integrated motor performance and whether such effects vary across post-stimulation time points.
2. Materials and Methods
2.1. Study Design
This study utilized a sham-controlled, repeated-measure crossover design in individuals with chronic stroke [clinicaltrials.gov: NCT05833490]. Participants were randomized to receive either active rPMS or sham stimulation in two separate sessions, and were blinded to the stimulation conditions. To minimize potential carryover effects, a 5- to 7-day interval was maintained between visits. Motor skill performance of the paretic leg was evaluated at four time points: baseline, immediately after stimulation, 30 min post-stimulation, and 60 min post-stimulation.
2.2. Subjects
Participants were recruited through convenience sampling, with 16 meeting the inclusion criteria and completing the study. Inclusion criteria included age over 18 years, a single mono-hemispheric stroke occurring more than three months prior, and adequate cognitive function, indicated by a Mini-Mental State Examination (MMSE) score > 21. Exclusion criteria included brainstem or cerebellar lesions, other neurological disorders (e.g., Alzheimer’s disease, Parkinson’s disease), or complete paralysis that would preclude participation in the motor task. All participants provided written informed consent. Study procedures were approved by the Institutional Review Board at the University of Illinois at Chicago protocol #2022-0600 and conformed with the Declaration of Helsinki.
2.3. Experimental Setup
Participants visited the Brain Plasticity Laboratory on two occasions. Participants were randomized to receive either rPMS or sham stimulation in a counterbalanced order across two sessions. In both conditions, participants completed a visuomotor ankle-tracking task using a custom-built device. The task required them to follow a computer-generated sinusoidal waveform through ankle dorsiflexion and plantar flexion. Each trial lasted 60 s. To ensure familiarity, participants completed 10 practice trials, followed by 20 experimental trials (5 trials at each of the four assessment time points). Only participants were blinded to the stimulation condition. Outcome assessors were not blinded.
2.3.1. rPMS Application
For rPMS, stimulation was applied to the tibialis anterior (TA) muscle on the paretic side. A small support was placed under the foot to maintain slight TA contraction. After identifying and marking the midpoint of the TA muscle belly, the stimulation coil (figure-of-eight, air-cooled; Magstim Rapid2) was positioned parallel to the muscle fibers. Motor threshold (MT) was determined as the lowest stimulation intensity that elicited a visible muscle contraction [16,17]. Stimulation was then delivered at 10% above MT in 40 trains of 3-s stimulation, with a 19-s rest, totaling approximately 2400 pulses over 15 min.
2.3.2. Sham Application
Sham stimulation was administered using the same equipment and parameters, with two key modifications: the coil was positioned over the dorsal foot (head of the 1st metatarsal), and stimulation was set at 5% of the maximum output. This intensity level was verified to be below the threshold for afferent activation, producing no detectable vibration or muscle response. This site was chosen to avoid direct activation of the TA muscle, and the stimulation intensity (5% maximum output) was below the threshold for recruiting proprioceptive afferents [15]. Similar to the active condition, 40 trains were delivered over 15 min.
2.4. Baseline Assessments
We administered three standardized assessments on a separate day prior to the intervention sessions and included the Fugl-Meyer Lower Extremity (FMLE) sensory scale [18], the 10-m walk test (10MWT) for gait speed [19], and the timed up-and-go test (TUG) for functional mobility and balance [20]. These measures were used to characterize sensory integrity, walking capacity, and overall mobility.
2.5. Visuomotor Task
Participants completed a visuomotor ankle-tracking task using a custom-built ankle-tracking device previously developed and validated in our laboratory [21,22]. The apparatus consists of a hinged footplate connected to a rotary potentiometer that captures dorsiflexion and plantar flexion in real time, with voltage signals sampled at 100 Hz. Calibration was conducted prior to each session by mapping the participant’s full active range of motion to the visual output display to ensure consistent scaling. Participants were seated comfortably in front of a monitor. The participant’s paretic leg was secured to a custom-built ankle-tracking device and they were instructed to control the cursor presented in the screen by performing dorsiflexion and plantar flexion, attempting to match the trajectory of a moving sinusoidal target displayed on a screen (see Figure 1). The sinusoid had a random frequency (0.2–0.4 Hz) and an amplitude (60–80%) of each participant’s comfortable range of motion to allow an achievable target [21,22].
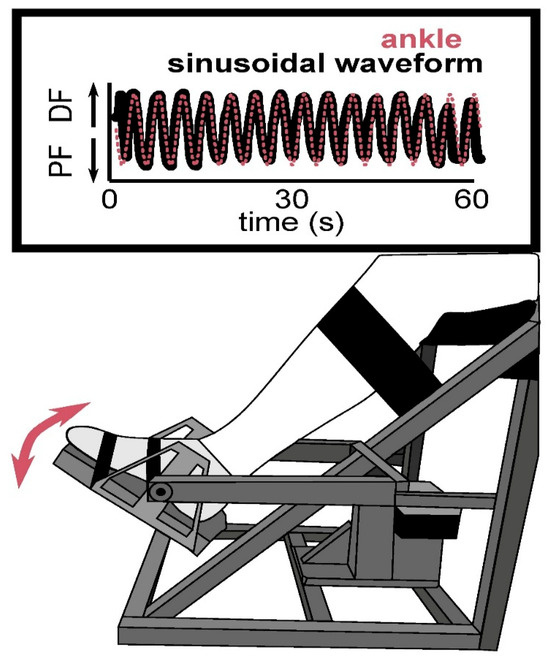
Figure 1.
Experimental setup for the visuomotor ankle-tracking task. The illustration shows a participant seated with their leg secured in a custom-built apparatus that allows for controlled dorsiflexion (DF) and plantar flexion (PF) of the ankle. The task required participants to track a sinusoidal target waveform (pink line) by moving their ankle to match the pattern in real time, as reflected by their ankle trajectory (black line) over a 60-s trial.
2.6. Data Analyses
Ankle motor performance data were processed using Spike2 software (Cambridge Electronic Design, Cambridge, UK) and MATLAB (Version R2022b; MathWorks, Natick, MA, USA). Two primary performance metrics were computed to evaluate tracking accuracy during the visuomotor task:
- Spatiotemporal Error (NRMSE): This metric quantified the overall deviation between the participant’s ankle movement and the sinusoidal target waveform over time. It was calculated as the root mean square error (RMSE) between the ankle position and target trajectory, then normalized to each participant’s ankle range of motion (ROM). The error was computed over 6000 time points per 60-s trial and averaged across five trials for each time point.
- Spatial Error (SE): This measure assessed the positional alignment between the participant’s movement and the target waveform independent of timing. It was derived from a cross-correlation analysis of the ankle position time series with the target waveform. The maximum correlation coefficient (R2) was identified, and spatial error was calculated as 1—R2. This value was then normalized to ankle ROM to ensure comparability across participants [23].
Both NRMSE and SE values ranged from 0 (perfect accuracy) to 1 (maximum error), with lower values indicating better performance. For each participant, data were averaged across five trials per time point. Percentage changes from baseline were also calculated to capture relative improvements or declines in performance. Outliers were defined as values exceeding three standard deviations from the group mean for each condition and time point. These outliers were excluded from both statistical analyses and data visualization.
2.7. Sample Size Estimation
A prior power analysis using G*Power software (Version 3.1; University of California, Los Angeles, CA, USA), based on pilot data, indicated that a sample of 14 participants was required to detect a 20% change in NRMSE (α = 0.05, power = 0.80). To account for attrition, 16 individuals were enrolled. While SE was also considered a primary outcome, a separate power analysis for this variable was not conducted, and this is acknowledged as a limitation.
2.8. Statistical Analyses
Normality and homogeneity assumptions were tested using Shapiro–Wilk and Levene’s tests, respectively. The Shapiro–Wilk test indicated no significant deviations from normality (p > 0.05), and Levene’s test confirmed homogeneity of variances across groups (p > 0.05). Therefore, separate two-way repeated-measure ANOVAs were performed for NRMSE and SE, with condition (rPMS vs. sham) and time (baseline, post-0, post-30, post-60 min) as within-subject factors. Mauchly’s test assessed sphericity, and Greenhouse–Geisser correction was applied when necessary.
Statistical significance was set at p < 0.05. Post hoc analyses using paired t-tests comparisons were planned in the event of significant main effects or interactions. Effect sizes were reported as partial eta squared (ηp2), with thresholds of 0.01 (small), 0.06 (medium), and 0.14 (large) [24], informed by prior studies using similar visuomotor tracking tasks with neurostimulation protocols [23]. Outliers exceeding three standard deviations above or below the mean were excluded prior to statistical analysis, and no imputation methods were applied. All analyses were performed in SPSS Statistics 31 (IBM, Armonk, NY, USA).
3. Results
Sixteen participants completed the study (mean age: 59.9 ± 9.1 years; 4 females). A summary of participant characteristics is presented in Table 1. All participants completed baseline assessments and both experimental sessions without adverse events.

Table 1.
Participant demographics and clinical characteristics at baseline (n = 16). Abbreviations: MMSE = Mini-Mental State Examination; FMLE = Fugl-Meyer Lower Extremity; 10MWT = 10-m walk test; TUG = timed up and go.
Baseline cognitive function was assessed using the Mini-Mental State Examination (MMSE), with participants scoring an average of 29.3 ± 0.9, indicating intact cognitive status. Gait performance, measured via the 10-m walk test (10MWT), showed mean walking speeds of 0.81 ± 0.2 m/s (comfortable pace) and 1.14 ± 0.3 m/s (fast pace). Functional mobility, measured by the timed up and go (TUG) test, yielded a mean completion time of 16.9 ± 5.2 s. Sensory function of the affected lower limb, assessed using the Fugl-Meyer Lower Extremity (FMLE) sensory subscale, resulted in an average score of 10.7 ± 2.3. Detailed baseline data are provided in Table 1.
3.1. Spatiotemporal Error (NRMSE)
Repeated-measure ANOVA revealed no significant main effect of: condition F(1, 26) = 0.02, p = 0.892, ηp2 = 0.001; time, F(2, 52) = 0.324, p = 0.725, ηp2 = 0.012; or condition × time interaction, F(2, 52) = 1.92, p = 0.157, ηp2 = 0.069. All observed effect sizes ranged from small to medium, indicating limited practical significance. Since the ANOVA did not yield any statistically significant effects, no follow up t-tests were performed.
In the rPMS condition, spatiotemporal error increased by 2.1% immediately post-stimulation (Post 0), decreased by 0.8% at 30 min (Post 30), and increased by 3.6% at 60 min (Post 60) relative to baseline. In the sham condition, errors decreased by 1.4% at Post 0, increased by 6.7% at Post 30, and 0.7% at Post 60 (see Figure 2). Although descriptive trends indicated a slight increase in error following rPMS, this change did not reach statistical significance and should therefore be interpreted with caution (Figure 2).
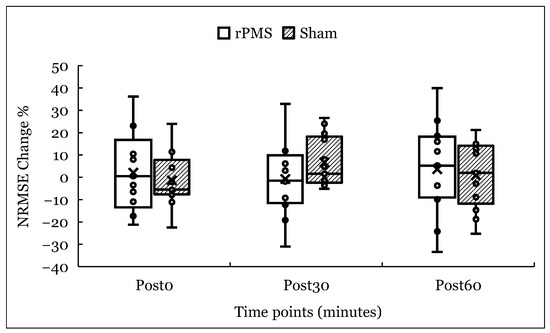
Figure 2.
Percentage change in spatiotemporal error (NRMSE) from baseline at 0, 30, and 60 min post-stimulation for rPMS and sham conditions. Bars represent group means; individual participant data are overlaid. “X” markers denote group means. No significant main effects or interactions were observed.
3.2. Spatial Error (SE)
No significant effects were observed for spatial error. The main effect of condition was nonsignificant, F(1, 24) = 0.042, p = 0.839, ηp2 = 0.002, as were the effects of time, F(2, 48) = 0.576, p = 0.566, ηp2 = 0.023, and the condition × time interaction, F(2, 48) = 0.919, p = 0.406, ηp2 = 0.037. All observed effect sizes ranged from small to medium, indicating limited practical significance. Since the repeated-measure ANOVA did not yield any statistically significant effects, no follow up t-tests were performed.
In the rPMS condition, spatial error increased by 0.7% immediately post-stimulation (Post 0) and decreased by 6% at 30 min (Post 30), but increased by 5% at 60 min (Post 60). In the sham condition, spatial error decreased by 3.7% at Post 0, increased by 4.6% at Post 30, and rose by 3.8% at Post 60. These fluctuations were not statistically significant (Figure 3).
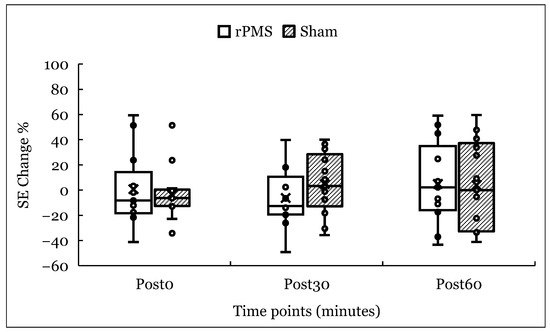
Figure 3.
Percentage change in spatial error (SE) from baseline at 0, 30, and 60 min post-stimulation for rPMS and sham conditions. Bars represent group means; individual participant data points are overlaid. “X” markers denote group means. No significant effects of time, condition, or their interaction were observed.
Descriptive statistics for mean and standard deviation values of both NRMSE and SE across all time points are provided in Table 2. As detailed in Table 2 and Figure 2 and Figure 3, changes in both spatiotemporal and spatial errors were generally small and followed similar trajectories in both rPMS and sham conditions. While rPMS showed modest fluctuations over time, these did not differ meaningfully from the sham condition. These trends align with the nonsignificant statistical findings, suggesting no clear time- or condition-dependent effects of a single rPMS session on motor skill performance.

Table 2.
Motor skill performance parameters (means ± SD). NRMSE = normalized root mean square error; SE = spatial error.
4. Discussion
The primary aim of this study was to examine the temporal effectiveness of repetitive peripheral magnetic stimulation (rPMS) on lower-limb motor skill performance in individuals with chronic stroke. Contrary to our hypothesis, rPMS did not produce significant improvements in motor skill performance at any of the post-stimulation time points (immediate, 30 min, or 60 min). These findings contrast with previous studies that reported benefits of rPMS on motor outcomes such as reduced spasticity, improved range of motion, and increased muscle strength [10,14,15].
For example, two studies on upper-limb function showed that a single rPMS session enhanced finger extension speed, improved movement efficiency, and reduced spasticity [6,10]. Similarly, one study observed improvements in ankle range of motion and dorsiflexor strength following rPMS applied to the lower limb. While those studies focused primarily on isolated motor responses, the present study investigated performance on a visuomotor tracking task, a higher-order behavior that integrates motor, sensory, and cognitive components [21,25].
Although our results did not reach statistical significance, descriptive data revealed minor variations in spatiotemporal error (NRMSE) across time points. In the rPMS condition, a slight increase in error was noted at Post 0 and Post 60, with a modest decrease at Post 30. In the sham condition, error appeared to increase at Post 30 and Post 60, following a small decrease at Post 0. Similarly, spatial error (SE) demonstrated minor inconsistencies across time points. In the rPMS condition, error slightly decreased at Post 0 and Post 30, followed by a small increase at Post 60. The sham condition showed a minimal decrease in error at Post 0, returning to baseline levels by Post 30, and Post 60. While these trends were not statistically or clinically significant, they may reflect minor variations that were insufficient in magnitude or consistency to produce measurable behavioral effects.
Although previous studies have reported that rPMS can modulate cortical excitability, the current study did not include neurophysiological measures to directly assess such mechanisms. Given the null results, any observed patterns should be interpreted with caution and viewed as hypothesis-generating rather than indicative of physiological effect. While these minor variations may hint at a differential responsiveness to rPMS, the absence of statistically significant findings likely reflects methodological and population-level factors that constrained the sensitivity of our measures or the responsiveness of our sample.
4.1. Potential Methodological Factors
One likely explanation lies in the type of outcomes assessed. Prior studies primarily focused on isolated motor responses—such as strength, joint mobility, or spasticity—which may be more directly affected by peripheral stimulation. In contrast, our study evaluated motor skill performance, a more complex construct that integrates sensory, motor, and cognitive processes [23,26]. This added complexity may reduce sensitivity to short-term neuromodulatory effects from a single rPMS session.
While rPMS has been shown to increase corticomotor excitability, this physiological response does not always result in improved behavioral outcomes, especially in tasks requiring multi-joint coordination, visuomotor processing, and cognitive engagement [27,28]. The translation of increased excitability into functional performance may depend on the intensity, frequency, and repetition of stimulation sessions, as well as the timing of subsequent task practice [29,30]. It is also possible that isolated gains in neural excitability are insufficient to drive measurable behavioral change without concurrent task-specific training or consolidation over time. This distinction may explain why protocols that produce immediate changes in simple motor outputs (e.g., muscle activation or strength) may not be adequate for enhancing more complex motor behaviors in a single session.
Stimulation parameters may also have played a role. Although we followed established protocols [15,16], using 20 Hz frequency and 10% above MT, other studies have reported stronger effects with higher intensities. For instance, stimulation at 20–50% above MT has been shown to increase corticomotor excitability in neurotypical adults [17,31]. The relatively conservative stimulation dose used here may have been insufficient to elicit measurable effects. Likewise, a 15-min session may not be adequate to influence integrated motor behaviors. Future studies should explore whether increased intensity, longer duration, or patterned protocols yield more robust outcomes.
4.2. Population-Related Factors
Participant heterogeneity may have also contributed to the null results. Approximately one-third of our participants exhibited sensory impairments on FMLE assessment. Sensory deficits are known to impair skilled motor execution, as effective motor control depends on sensorimotor integration. It is possible that individuals with impaired sensory function were less responsive to rPMS, which could have masked potential benefits in those with intact sensation. However, our small sample precluded subgroup analysis to explore this further.
Other sources of variability, such as differences in stroke chronicity, lesion location, and baseline function, may also have influenced responsiveness. Larger, stratified studies will be needed to identify the patient subgroups most likely to benefit from rPMS. Ethnic and racial variability in physiological features such as skin thickness, proprioception, or muscle strength could also influence stimulation conduction and motor response. Future studies with larger, stratified samples are needed to investigate potential group-specific effects.
4.3. Limitations
This study has several limitations. First, the small sample (n = 16) limited statistical power and prevented subgroup analysis. Future studies with larger cohorts will be needed to confirm and extend these findings. Second, although a 5- to 7-day interval was implemented to minimize carryover effects, we cannot entirely rule out residual stimulation effects influencing sham responses. A longer washout period or counterbalanced design may be more appropriate.
Third, we did not include a standardized fatigue assessment, which may have influenced task performance—particularly during later time points. Including such measures in future studies would improve interpretability. Fourth, knee stabilization in the ankle apparatus may have restricted natural movement, potentially influencing tracking performance. More flexible apparatus designs could increase ecological validity. Finally, we did not assess functional outcomes of post-intervention, limiting our ability to evaluate real-world benefits. Incorporating functional mobility measures in future protocols would offer a more comprehensive view of rPMS efficacy. Moreover, this was a single-blinded study in which participants were blinded to the stimulation conditions; however, we did not assess participants’ subjective perception of stimulation, which may have influenced motor performance or engagement. Future studies should include post-session questionnaires to assess perceived stimulation and verify the effectiveness of blinding procedures. Lack of blinding among assessors may have introduced bias, and future studies should consider double-blind protocols
5. Conclusions
In this study, we examined the short-term effects of a single session of repetitive peripheral magnetic stimulation (rPMS) on lower-limb motor skill performance in individuals with chronic stroke. Using a visuomotor ankle-tracking task, we found no significant improvements in spatiotemporal or spatial error at 0, 30, or 60 min post-stimulation. These findings suggest that under the parameters tested, rPMS may not elicit immediate changes in complex motor skill performance.
While the current findings did not demonstrate functional improvements, they do not entirely rule out the potential utility of rPMS in rehabilitation. Rather, they underscore the limitations of the present study—such as the small sample and single-session design—highlighting the need for further investigation. Future research should explore optimized stimulation parameters (e.g., intensity, frequency, and duration), evaluate the potential benefit of multi-session protocols, and consider stratifying participants by sensory integrity or motor impairment level. Incorporating robust and clinically meaningful outcome measures will be critical to better assess the translational utility of rPMS as a neuromodulatory intervention in rehabilitation.
While the present study yielded null findings, it contributes to the growing body of literature aimed at refining neuromodulatory strategies for post-stroke recovery. It underscores the complexity of modulating integrated motor behavior and the importance of aligning stimulation approaches with the demands of functional, task-specific training.
Author Contributions
All authors contributed substantially to the work, specifically for conceptualization—R.A. and S.M.; methodology—R.A. and S.M.; data analyses—R.A., S.M., S.K. and C.S.; original draft preparation—R.A. and S.M.; review and editing—R.A., S.M., S.K. and C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All procedures performed in studies involving human participants were in accordance with the guidelines of the declaration of Helsinki and approved by the Institutional Review Board of the University of Illinois at Chicago, protocol #2022-0600, from 07.08.2022 to 07.08.2023.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Hosokawa, T.; Tsuji, I. Relationship of Muscle Strength for Knee Extension to Walking Capacity in Patients with Spastic Hemiparesis. Tohoku J. Exp. Med. 1985, 145, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Lodha, N.; Patel, P.; Casamento-Moran, A.; Hays, E.; Poisson, S.N.; Christou, E.A. Strength or Motor Control: What Matters in High-Functioning Stroke? Front. Neurol. 2019, 9, 1160. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.M.; Madhavan, S.; Roth, H.; Stinear, J.W. Transforming Neurorehabilitation of Walking Following Stroke: The Promise of Non-Invasive Brain Stimulation. Restor. Neurol. Neurosci. 2011, 29, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Boroojerdi, B.; Ziemann, U.; Chen, R.; Bütefisch, C.M.; Cohen, L.G. Mechanisms Underlying Human Motor System Plasticity. Muscle Nerve 2001, 24, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Classen, J.; Cohen, L.G. Neural Plasticity and Its Contribution to Functional Recovery. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 110, pp. 3–12. [Google Scholar] [CrossRef]
- Takeuchi, N.; Izumi, S. Maladaptive Plasticity for Motor Recovery after Stroke: Mechanisms and Approaches. Neural Plast. 2012, 2012, 359728. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Xu, W. Enhancing Brain Plasticity to Promote Stroke Recovery. Front. Neurol. 2020, 11, 554089. [Google Scholar] [CrossRef] [PubMed]
- Stoykov, M.E.; Madhavan, S. Motor Priming in Neurorehabilitation. J. Neurol. Phys. Ther. 2015, 39, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Struppler, A.; Havel, P.; Müller-Barna, P. Facilitation of Skilled Finger Movements by Repetitive Peripheral Magnetic Stimulation (RPMS): A New Approach in Central Paresis. NeuroRehabilitation 2003, 18, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L.D.; Schneider, C. Repetitive Peripheral Magnetic Stimulation to Reduce Pain or Improve Sensorimotor Impairments: A Literature Review on Parameters of Application and Afferents Recruitment. Neurophysiol. Clin. 2015, 45, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L.D.; Schneider, C. Effects of Repetitive Peripheral Magnetic Stimulation on Normal or Impaired Motor Control: A Review. Neurophysiol. Clin. 2013, 43, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Yasufuku, Y.; Kamo, T.; Ota, E.; Momosaki, R. Repetitive Peripheral Magnetic Stimulation for Patients After Stroke. Stroke 2020, 51, e105–e106. [Google Scholar] [CrossRef] [PubMed]
- Struppler, A.; Binkofski, F.; Angerer, B.; Bernhardt, M.; Spiegel, S.; Drzezga, A.; Bartenstein, P. A Fronto-Parietal Network Is Mediating Improvement of Motor Function Related to Repetitive Peripheral Magnetic Stimulation: A PET-H2O15 Study. NeuroImage 2007, 36 (Suppl. 2), T174–T186. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L.D.; Massé-Alarie, H.; Brouwer, B.; Schneider, C. Noninvasive Neurostimulation in Chronic Stroke: A Double-Blind Randomized Sham-Controlled Testing of Clinical and Corticomotor Effects. Top. Stroke Rehabil. 2015, 22, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L.D.; Massé-Alarie, H.; Camiré-Bernier, S.; Ribot-Ciscar, É.; Schneider, C. After-Effects of Peripheral Neurostimulation on Brain Plasticity and Ankle Function in Chronic Stroke: The Role of Afferents Recruited. Neurophysiol. Clin. 2017, 47, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Gallasch, E.; Christova, M.; Kunz, A.; Rafolt, D.; Golaszewski, S. Modulation of Sensorimotor Cortex by Repetitive Peripheral Magnetic Stimulation. Front. Hum. Neurosci. 2015, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.J.; Tilson, J.K.; Cen, S.Y.; Rose, D.K.; Hershberg, J.; Correa, A.; Gallichio, J.; McLeod, M.; Moore, C.; Wu, S.S.; et al. Fugl-Meyer Assessment of Sensorimotor Function After Stroke: Standardized Training Procedure for Clinical Practice and Clinical Trials. Stroke 2011, 42, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Collen, F.M.; Wade, D.T.; Bradshaw, C.M. Mobility After Stroke: Reliability of Measures of Impairment and Disability. Int. Disabil. Stud. 1990, 12, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Flansbjer, U.B.; Holmbäck, A.M.; Downham, D.; Patten, C.; Lexell, J. Reliability of Gait Performance Tests in Men and Women with Hemiparesis After Stroke. J. Rehabil. Med. 2005, 37, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Madhavan, S. Non-Paretic Leg Movements Can Facilitate Cortical Drive to the Paretic Leg in Individuals Post Stroke with Severe Motor Impairment: Implications for Motor Priming. Eur. J. Neurosci. 2023, 58, 2853–2867. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, S.; Cleland, B.T.; Sivaramakrishnan, A.; Freels, S.; Lim, H.; Testai, F.D.; Corcos, D.M. Cortical Priming Strategies for Gait Training after Stroke: A Controlled, Stratified Trial. J. Neuroeng. Rehabil. 2020, 17, 111. [Google Scholar] [CrossRef]
- Cummings, M.; Doshi, A.; Madhavan, S. Understanding the Interaction of Transcranial Direct Current Stimulation and Visual Feedback During an Ankle Movement Task. Mot. Control 2023, 27, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Poulton, E.C. Tracking Skill and Manual Control; Academic Press: New York, NY, USA, 1974. [Google Scholar]
- Wong, A.L.; Haith, A.M.; Krakauer, J.W. Motor Planning. Neuroscientist 2015, 21, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, K.; Kacar, A.; Rothwell, J.C. Differential Modulation of Motor Cortical Plasticity and Excitability in Early and Late Phases of Human Motor Learning. J. Neurosci. 2007, 27, 12058–12066. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, S.A.; Farina, D.; Falla, D. The Role of Motor Learning and Neuroplasticity in Designing Rehabilitation Approaches for Musculoskeletal Pain Disorders. Man. Ther. 2010, 15, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Classen, J.; Liepert, J.; Wise, S.P.; Hallett, M.; Cohen, L.G. Rapid Plasticity of Human Cortical Movement Representation Induced by Practice. J. Neurophysiol. 1998, 79, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Schambra, H.M.; Cohen, L.G.; Buch, E.R.; Fritsch, B.; Zarahn, E.; Krakauer, J.W. Noninvasive Cortical Stimulation Enhances Motor Skill Acquisition over Multiple Days through an Effect on Consolidation. Proc. Natl. Acad. Sci. USA 2009, 106, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Liu, X.; Wei, J.; Li, D.; Wang, C.; Wang, X.; Liu, H. Modulation of the Corticomotor Excitability by Repetitive Peripheral Magnetic Stimulation on the Median Nerve in Healthy Subjects. Front. Neural Circuits 2021, 15, 616084. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).