Reconstructive Therapy in Patients with Peri-Implantitis in a University Dental Hospital: A Preliminary Retrospective Case Series Focusing on Complications
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Peri-Implant Treatment and Maintenance Protocols
2.2.1. Diagnosis of Peri-Implantitis
2.2.2. Non-Surgical Treatment
2.2.3. Surgical Treatment
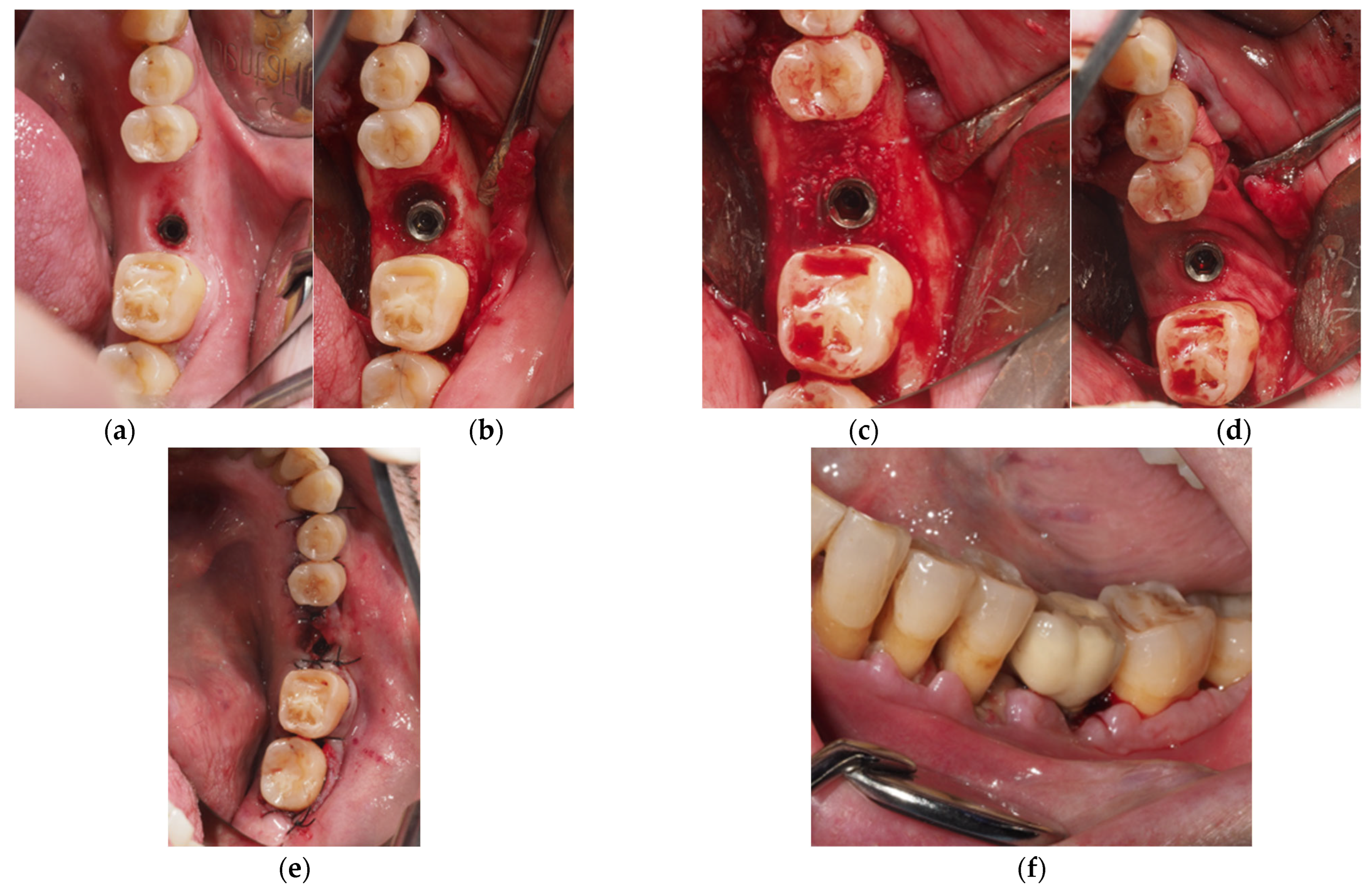
- Regenerative Approach: Deproteinized bovine bone mineral (DBBM, Geistlich Bio-Oss 0.25–1 mm, Geistlich Biomaterials, Wolhusen, Switzerland) was used to fill the defect, and a resorbable membrane (Geistlich BioGide, Geistlich Biomaterials, Wolhusen, Switzerland) was adapted around the implant neck. Non-resorbable polyamide (Supramid 4/0, St. With, Belgium) and PTFE (Cytoplast 4/0; Osteogenics Biomedical, Lubbock, TX, USA) sutures were used to close the wound, allowing for transmucosal healing (Figure 1).
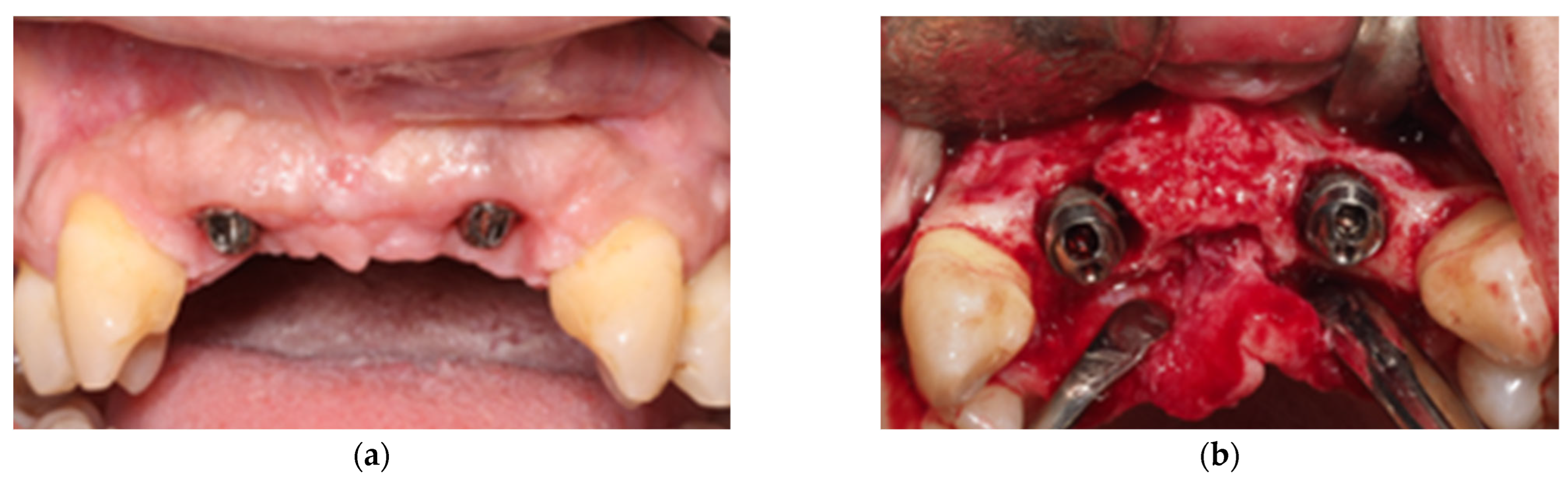
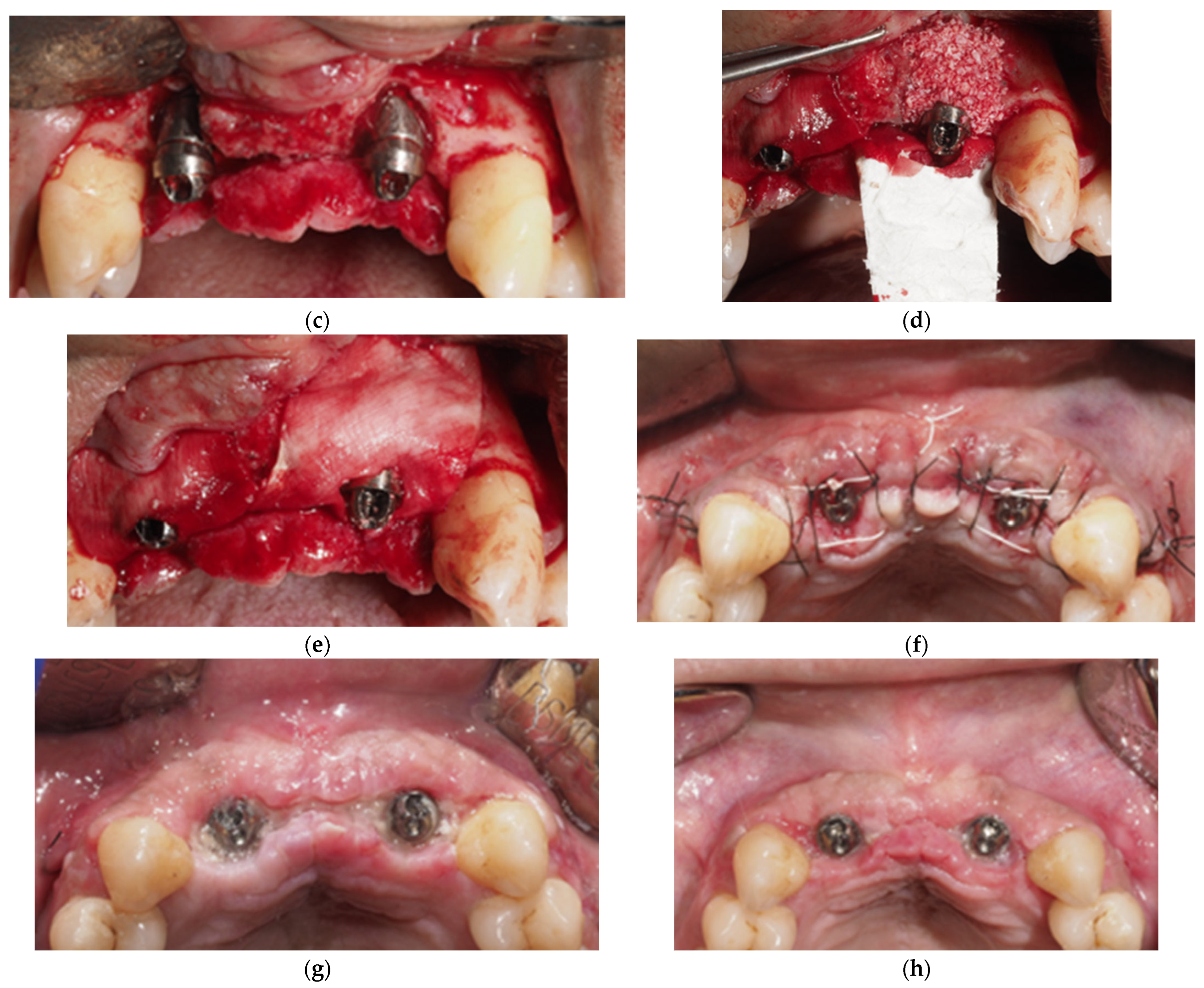
- Combined Approach: The implant area located in the suprabony compartment of the defect was treated with implantoplasty, and the implant surface located in the infrabony defect was debrided with curettes. Threads were removed and polished with specific burs: an oval-shape tungsten carbide bur (H379 314 023; Komet Dental, Lemgo, Germany) was used to remove the implant threads, and the surface was sequentially polished with two silicon carbide polishers (9618 314 030 and 9608 314 030, Komet Dental, Lemgo, Germany) [40]. In cases of inadequate keratinized tissue or thin biotype, a connective tissue graft was added. Deproteinized bovine bone mineral (DBBM, Geistlich Bio-Oss 0.25–1 mm, Geistlich Bio-materials, Wolhusen, Switzerland) was used to fill the defect, and a resorbable membrane (Geistlich BioGide, Geistlich Biomaterials, Wolhusen, Switzerland) was adapted around the implant neck. Non-resorbable polyamide (Supramid 4/0, St. With, Belgium) and PTFE (Cytoplast 4/0; Osteogenics Biomedical, Lubbock, TX, USA) sutures were used to close the wound, allowing for transmucosal healing (Figure 2).
2.2.4. Postoperative Care
2.3. Data Collection
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Block, M.S. Dental Implants: The Last 100 Years. J. Oral Maxillofac. Surg. 2018, 76, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Atieh, M.A.; AlAli, F.; Alsabeeha, N.H.M. Outcome of supportive peri-implant therapy on the rates of peri-implant diseases and marginal bone loss: A systematic review and meta-analysis. Quintessence Int. 2021, 52, 122–131. [Google Scholar] [PubMed]
- Atieh, M.A.; Almutairi, Z.; Amir-Rad, F.; Koleilat, M.; Tawse-Smith, A.; Ma, S.; Lin, L.; Alsabeeha, N.H. A retrospective analysis of biological complications of dental implants. Int. J. Dent. 2022, 2022, 1545748. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.A.; Salvi, G.E. Peri-implant mucositis. J. Periodontol. 2018, 89, S257–S266. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S237–S248. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H. Peri-implantitis. J. Periodontol. 2018, 89 (Suppl. S1), S267–S290. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Mombelli, A.; Schwarz, F.; Derks, J. Etiology, pathogenesis and treatment of peri-implantitis: A European perspective. Periodontol. 2000 2024, in press. [CrossRef] [PubMed]
- Derks, J.; Schaller, D.; Håkansson, J.; Wennström, J.L.; Tomasi, C.; Berglundh, T. Effectiveness of implant therapy analyzed in a swedish population. J. Dent. Res. 2016, 95, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Mir-Mari, J.; Mir-Orfila, P.; Figueiredo, R.; Valmaseda-Castellón, E.; Gay-Escoda, C. Prevalence of peri-implant diseases. A cross-sectional study based on a private practice environment. J. Clin. Periodontol. 2012, 39, 490–494. [Google Scholar] [CrossRef] [PubMed]
- García-García, M.; Mir-Mari, J.; Figueiredo, R.; Valmaseda-Castellón, E. Probing single-tooth dental implants with and without prostheses: A cross-sectional study comparing healthy and peri-implant mucositis sites. J. Clin. Periodontol. 2021, 48, 581–589. [Google Scholar] [CrossRef] [PubMed]
- García-García, M.; Mir-Mari, J.; Benic, G.I.; Figueiredo, R.; Valmaseda-Castellón, E. Accuracy of periapical radiography in assessing bone level in implants affected by peri-implantitis: A cross-sectional study. J. Clin. Periodontol. 2016, 43, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, R.; Reda, R.; Zanza, A.; Miccoli, G.; Di Nardo, D.; Testarelli, L. Can peri-implant marginal bone loss progression and a-MMP-8 be considered indicators of the subsequent onset of peri-implantitis? A 5-year study. Diagnostics 2022, 12, 2599. [Google Scholar] [CrossRef] [PubMed]
- Rebeiz, T.; Nasr, L.; Kassir, A.R.; Menassa, G.; Chakar, C. Assessment of the association between the Implant Disease Risk Assessment (IDRA) tool and peri-implantitis: A retrospective cohort study with up to 8 years of follow-up. Int. J. Oral Maxillofac. Surg. 2024, 53, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.A.; Heitz, F.; Lang, N.P. Implant Disease Risk Assessment IDRA–a tool for preventing peri-implant disease. Clin. Oral Implant. Res. 2020, 31, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, H.; Grischke, J.; Tiede, C.; Eberhard, J.; Schweitzer, A.; Toikkanen, S.E.; Glöckner, S.; Krause, G.; Stiesch, M. Epidemiology and risk factors of peri-implantitis: A systematic review. J. Periodontal Res. 2018, 53, 657–681. [Google Scholar] [CrossRef] [PubMed]
- Giok, K.C.; Veettil, S.K.; Menon, R.K. Risk factors for peri-implantitis: An umbrella review of meta-analyses of observational studies and assessment of biases. J. Dent. 2024, 146, 105065. [Google Scholar] [CrossRef] [PubMed]
- Romandini, M.; Laforí, A.; Pedrinaci, I.; Baima, G.; Ferrarotti, F.; Lima, C.; Holtzman, L.P.; Aimetti, M.; Cordaro, L.; Sanz, M. Effect of sub-marginal instrumentation before surgical treatment of peri-implantitis: A multi-centre randomized clinical trial. J. Clin. Periodontol. 2022, 49, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskaite, A.; Daugela, P.; Juodzbalys, G. Treatment of peri-implantitis: Meta-analysis of findings in a systematic literature review and novel protocol proposal. Quintessence Int. 2016, 47, 379–393. [Google Scholar] [PubMed]
- Karlsson, K.; Derks, J.; Håkansson, J.; Wennström, J.L.; Petzold, M.; Berglundh, T. Interventions for peri-implantitis and their effects on further bone loss: A retrospective analysis of a registry-based cohort. J. Clin. Periodontol. 2019, 46, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Herten, M.; Sager, M.; Bieling, K.; Sculean, A.; Becker, J. Comparison of naturally occurring and ligature-induced peri-implantitis bone defects in humans and dogs. Clin. Oral Implant. Res. 2007, 18, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Pons, R.; Insua, A.; Nart, J.; Wang, H.L.; Schwarz, F. Morphology and severity of peri-implantitis bone defects. Clin. Implant. Dent. Relat. Res. 2019, 21, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Jepsen, S.; Obreja, K.; Galarraga-Vinueza, M.E.; Ramanauskaite, A. Surgical therapy of peri-implantitis. Periodontol. 2000 2022, 88, 145–181. [Google Scholar] [CrossRef] [PubMed]
- Figuero, E.; Graziani, F.; Sanz, I.; Herrera, D.; Sanz, M. Management of peri-implant mucositis and peri-implantitis. Periodontol. 2000 2014, 66, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Serino, G.; Turri, A. Outcome of surgical treatment of peri-implantitis: Results from a 2-year prospective clinical study in humans. Clin. Oral Implant. Res. 2011, 22, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Romeo, E.; Lops, D.; Chiapasco, M.; Ghisolfi, M.; Vogel, G. Therapy of peri-implantitis with resective surgery. A 3-year clinical trial on rough screw-shaped oral implants. Part II: Radiographic outcome. Clin. Oral Implant. Res. 2007, 18, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Romeo, E.; Ghisolfi, M.; Murgolo, N.; Chiapasco, M.; Lops, D.; Vogel, G. Therapy of peri-implantitis with resective surgery: A 3-year clinical trial on rough screw-shaped oral implants. Part I: Clinical outcome. Clin. Oral Implant. Res. 2005, 16, 9–18. [Google Scholar]
- Lang, N.P.; Berglundh, T. Periimplant diseases: Where are we now? Consensus of the Seventh European Workshop on Periodontology. J. Clin. Periodontol. 2011, 38, 178–181. [Google Scholar] [PubMed]
- Carcuac, O.; Derks, J.; Abrahamsson, I.; Wennström, J.L.; Berglundh, T. Risk for recurrence of disease following surgical therapy of peri-implantitis—A prospective longitudinal study. Clin. Oral Implant. Res. 2020, 31, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Serino, G.; Turri, A.; Lang, N.P. Maintenance therapy in patients following the surgical treatment of peri-implantitis: A 5-year follow-up study. Clin. Oral Implant. Res. 2015, 26, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Romandini, M.; Bougas, K.; Alibegovic, L.; Hosseini, S.; Carcuac, O.; Berglundh, T.; Derks, J. Long-term outcomes and prognostic factors of surgical treatment of peri-implantitis—A retrospective study. Clin. Oral Implant. Res. 2023, 35, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, C.; Regidor, E.; Ortiz-Vigón, A.; Derks, J. Efficacy of reconstructive surgical therapy at peri-implantitis-related bone defects. A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 340–356. [Google Scholar] [CrossRef] [PubMed]
- González Regueiro, I.; Martínez Rodriguez, N.; Barona Dorado, C.; Sanz-Sánchez, I.; Montero, E.; Ata-Ali, J.; Duarte, F.; Martínez-González, J.M. Surgical approach combining implantoplasty and reconstructive therapy with locally delivered antibiotic in the treatment of peri-implantitis: A prospective clinical case series. Clin. Implant. Dent. Relat. Res. 2021, 23, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; John, G.; Schmucker, A.; Sahm, N.; Becker, J. Combined surgical therapy of advanced peri-implantitis evaluating two methods of surface decontamination: A 7-year follow-up observation. J. Clin. Periodontol. 2017, 44, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Berglundh, T.; Schwarz, F.; Chapple, I.; Jepsen, S.; Sculean, A.; Kebschull, M.; Papapanou, P.N.; Tonetti, M.S.; Sanz, M.; et al. Prevention and treatment of peri-implant diseases—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2023, 50, 4–76. [Google Scholar] [CrossRef] [PubMed]
- Regidor, E.; Ortiz-Vigón, A.; Romandini, M.; Dionigi, C.; Derks, J.; Sanz, M. The adjunctive effect of a resorbable membrane to a xenogeneic bone replacement graft in the reconstructive surgical therapy of peri-implantitis: A randomized clinical trial. J. Clin. Periodontol. 2023, 50, 765–783. [Google Scholar] [CrossRef] [PubMed]
- Roos-Jansåker, A.M.; Lindahl, C.; Persson, G.R.; Renvert, S. Long-term stability of surgical bone regenerative procedures of peri-implantitis lesions in a prospective case-control study over 3 years. J. Clin. Periodontol. 2011, 38, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Roos-Jansåker, A.; Persson, G.R.; Lindahl, C.; Renvert, S. Surgical treatment of peri-implantitis using a bone substitute with or without a resorbable membrane: A 5-year follow-up. J. Clin. Periodontol. 2014, 41, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, S.; Berglundh, T.; Genco, R.; Aass, A.M.; Demirel, K.; Derks, J.; Figuero, E.; Giovannoli, J.L.; Goldstein, M.; Lambert, F.; et al. Primary prevention of peri-implantitis: Managing peri-implant mucositis. J. Clin. Periodontol. 2015, 42, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Polyzois, I.N. Clinical approaches to treat peri-implant mucositis and peri-implantitis. Periodontol. 2000 2015, 68, 369–404. [Google Scholar] [CrossRef] [PubMed]
- Costa-Berenguer, X.; García-García, M.; Sánchez-Torres, A.; Sanz-Alonso, M.; Figueiredo, R.; Valmaseda-Castellón, E. Effect of implantoplasty on fracture resistance and surface roughness of standard diameter dental implants. Clin. Oral Implant. Res. 2018, 29, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Sahm, N.; Schwarz, K.; Becker, J. Impact of defect configuration on the clinical outcome following surgical regenerative therapy of peri-implantitis. J. Clin. Periodontol. 2010, 37, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.; Sanz-Sánchez, I.; Monje, A.; Montero, E. Complications and treatment errors in peri-implant hard tissue management. Periodontol. 2000 2023, 92, 278–298. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.A.; Marini, L.; Pilloni, A.; Sahrmann, P. Early wound healing outcomes after regenerative periodontal surgery with enamel matrix derivatives or guided tissue regeneration: A systematic review. BMC Oral Health 2019, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.; Lin, G.-H.; Monje, A.; Chan, H.-L.; Wang, H.-L. Wound healing complications following guided bone regeneration for ridge augmentation: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Schmucker, A.; Becker, J. Efficacy of alternative or adjunctive measures to conventional treatment of peri-implant mucositis and peri-implantitis: A systematic review and meta-analysis. Int. J. Implant. Dent. 2015, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Roccuzzo, M.; Gaudioso, L.; Lungo, M.; Dalmasso, P. Surgical therapy of single peri-implantitis intrabony defects, by means of deproteinized bovine bone mineral with 10% collagen. J. Clin. Periodontol. 2016, 43, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Chapple, I.L.C.; Jepsen, S.; Sanz, M. Primary and secondary prevention of periodontal and peri-implant diseases. J. Clin. Periodontol. 2015, 42, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Roos-Jansåker, A.M.; Renvert, H.; Lindahl, C.; Renvert, S. Submerged healing following surgical treatment of peri-implantitis: A case series. J. Clin. Periodontol. 2007, 34, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, R.; Lang, N.P. Role of flap tension in primary wound closure of mucoperiosteal flaps: A prospective cohort study. Clin. Oral Implant. Res. 2010, 21, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Kim, C.S.; Choi, S.H.; Cho, K.S.; Chai, J.K.; Jung, U.W. Flap extension attained by vertical and periosteal-releasing incisions: A prospective cohort study. Clin. Oral Implant. Res. 2012, 23, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Roos-Jansåker, A.M.; Renvert, H.; Lindahl, C.; Renvert, S. Surgical treatment of peri-implantitis using a bone substitute with or without a resorbable membrane: A prospective cohort study. J. Clin. Periodontol. 2007, 34, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Roos-Jansåker, A.M.; Lindahl, C.; Renvert, H.; Renvert, S. Nine- to fourteen-year follow-up of implant treatment. Part I: Implant loss and associations to various factors. J. Clin. Periodontol. 2006, 33, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, R.; Lang, N.P. Influence of suturing on wound healing. Periodontol. 2000 2015, 68, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.C.; Sabri, H.; Dastouri, E.; Huang, W.X.; Barootchi, S.; Wang, H.L. Submerged vs nonsubmerged reconstructive approach for surgical treatment of peri-implantitis: Reanalysis of two prospective clinical studies. Int. J. Oral Maxillofac. Implant. 2024, 39, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Camps-Font, O.; Pérez-Beltrán, I.; Fornés-Nieto, V.; González-Barnadas, A.; Costa-Berenguer, X.; García-García, M.; Figueiredo, R.; Valmaseda-Castellón, E. Patient-centered outcomes after surgical treatment of peri-implantitis: A prospective clinical study. Med. Oral Patol. Oral Cir. Bucal 2023, 28, e72–e80. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.C.; Barootchi, S.; Wang, H.L.; Huang, W.X. Non-submerged reconstructive approach for peri-implantitis osseous defect with removal of implant crowns: One-year outcomes of a prospective case series study. J. Periodontol. 2022, 93, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.C.; Barootchi, S.; Huang, W.X.; Wang, H.L. Surgical reconstructive treatment for infraosseous peri-implantitis defects with a submerged healing approach: A prospective controlled study. J. Periodontol. 2022, 93, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Pons, R.; Roccuzzo, A.; Salvi, G.E.; Nart, J. Reconstructive therapy for the management of peri-implantitis via submerged guided bone regeneration: A prospective case series. Clin. Implant. Dent. Relat. Res. 2020, 22, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Martín, I.; Regidor, E.; Cosyn, J.; Wiedemeier, D.B.; Thoma, D.S. Buccal soft tissue dehiscence defects at dental implants—Associated factors and frequency of occurrence: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2022, 33, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.; Lim, L.P. An Overview of Different Interdental Cleaning Aids and Their Effectiveness. Dent. J. 2019, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.; Mombelli, A. The therapy of peri-implantitis: A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29, 325–345. [Google Scholar] [CrossRef] [PubMed]
- Eun, S.-M.; Son, K.; Hwang, S.-M.; Son, Y.-T.; Kim, Y.-G.; Suh, J.-Y.; Hwang, J.H.; Kwon, S.-M.; Lee, J.H.; Kim, H.D.; et al. The Impact of Mechanical Debridement Techniques on Titanium Implant Surfaces: A Comparison of Sandblasted, Acid-Etched, and Femtosecond Laser-Treated Surfaces. J. Funct. Biomater. 2023, 14, 502. [Google Scholar] [CrossRef] [PubMed]
- Sahrmann, P.; Winkler, S.; Gubler, A.; Attin, T. Assessment of implant surface and instrument insert changes due to instrumentation with different tips for ultrasonic-driven debridement. BMC Oral Health 2021, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Yewale, M.; Parthasarathi, N.L.; Balasundaram, R.; Gopalkrishna, P.; Bhat, S.G. Evaluation of the Loss of Surface Roughness Following the Use of Four Different Instruments for Mechanical Debridement of Dental Implants: An In-vitro Pilot Study. J. Bio-Tribo-Corros. 2024, 10, 77. [Google Scholar] [CrossRef]
- Alabbad, M.; Silikas, N.; Thomas, A. Effect of mechanical instrumentation on titanium implant surface properties. Dent. Mater. 2025, 41, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.P. Soft tissue grafting with the tunnel technique in the mandibular anterior: Myths and realities. J. Esthet. Restor. Dent. 2021, 33, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Hadzik, J.; Błaszczyszyn, A.; Gedrange, T.; Dominiak, M. Soft-Tissue Augmentation around Dental Implants with a Connective Tissue Graft (CTG) and Xenogeneic Collagen Matrix (CMX)—5-Year Follow-Up. J. Clin. Med. 2023, 12, 924. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Polyzois, I. Risk indicators for peri-implant mucositis: A systematic literature review. J. Clin. Periodontol. 2015, 42, S172–S186. [Google Scholar] [CrossRef] [PubMed]
- Romanos, G.E.; Javed, F.; Delgado-Ruiz, R.A.; Calvo-Guirado, J.L. Peri-implant Diseases. Dent. Clin. N. Am. 2015, 59, 157–178. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]

| Variable | Total N (%) or Mean (SD) | With Dehiscence N (%) | Without Dehiscence N (%) | p-Value |
|---|---|---|---|---|
| Age (years) | 62.9 (10.7) | 63.4 (10.3) | 64.1 (9.6) | 0.868 |
| Sex | ||||
| Female | 6 (42.9) | 3 (21.4) | 3 (21.4) | 0.640 |
| Male | 8 (57.1) | 5 (35.7) | 3 (21.4) | |
| Tobacco use (yes) | 6 (42.9) | 4 (28.6) | 2 (14.3) | 0.533 |
| Periodontitis (yes) | 13 (92.9) | 8 (57.2) | 5 (35.7) | 0.231 |
| Interdental brushes (yes) | 6 (42.9) | 1 (7.1) | 5 (35.7) | 0.008 |
| Mouthwash use (yes) | 2 (14.3) | 1 (7.1) | 1 (7.1) | 0.825 |
| Restoration (multiple) | 10 (71.4) | 6 (42.9) | 4 (28.6) | 0.733 |
| Implant position (anterior) | 13 (61.9) | 7 (33.3) | 6 (28.6) | 0.608 |
| Location (maxilla) | 14 (66.7) | 4 (19.0) | 10 (47.6) | 0.003 |
| Variable | Total N (%) or Mean (SD) | With Dehiscence N (%) | Without Dehiscence N (%) | p-Value |
|---|---|---|---|---|
| Implantoplasty (Yes) | 13 (61.9) | 8 (38.1) | 5 (23.8) | 0.284 |
| CTG (Yes) | 5 (23.8) | 2 (9.5) | 3 (14.3) | 0.525 |
| Membrane Exposure (Yes) | 4 (19.1) | 4 (19.1) | 0 (0) | 0.034 |
| Biofilm in Sutures (Yes) | 12 (57.1) | 8 (38.1) | 4 (19.1) | 0.130 |
| Incision Length (mm) | 17.7 (5.2) | 19.2 (4.8) | 16.0 (5.4) | 0.160 |
| Type of Surgery | Incision | Dehiscence | |||
|---|---|---|---|---|---|
| N (%) | (mm) | N (%) | (mm) | (%) | |
| Mean (SD) | Mean (SD) | ||||
| Combined | 13 (61.9) | 18.7 (5.1) | 8 (61.5) | 7.9 (2.2) | 25.7 (22.5) |
| Regenerative | 8 (38.1) | 15.9 (5.2) | 3 (37.5) | 6.8 (0.5) | 14.5 (21.9) |
| p-value | 0.237 | 0.220 | 0.274 | ||
| Total | 21 (100) | 17.7 (5.2) | 11 (52.4) | 7.6 (2.0) | 21.4 (22.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alahmari, A.; Costa-Berenguer, X.; Figueiredo, R.; Valmaseda-Castellón, E.; Sánchez-Torres, A.; García-García, M. Reconstructive Therapy in Patients with Peri-Implantitis in a University Dental Hospital: A Preliminary Retrospective Case Series Focusing on Complications. Appl. Sci. 2025, 15, 8040. https://doi.org/10.3390/app15148040
Alahmari A, Costa-Berenguer X, Figueiredo R, Valmaseda-Castellón E, Sánchez-Torres A, García-García M. Reconstructive Therapy in Patients with Peri-Implantitis in a University Dental Hospital: A Preliminary Retrospective Case Series Focusing on Complications. Applied Sciences. 2025; 15(14):8040. https://doi.org/10.3390/app15148040
Chicago/Turabian StyleAlahmari, Ahmad, Xavier Costa-Berenguer, Rui Figueiredo, Eduard Valmaseda-Castellón, Alba Sánchez-Torres, and Marta García-García. 2025. "Reconstructive Therapy in Patients with Peri-Implantitis in a University Dental Hospital: A Preliminary Retrospective Case Series Focusing on Complications" Applied Sciences 15, no. 14: 8040. https://doi.org/10.3390/app15148040
APA StyleAlahmari, A., Costa-Berenguer, X., Figueiredo, R., Valmaseda-Castellón, E., Sánchez-Torres, A., & García-García, M. (2025). Reconstructive Therapy in Patients with Peri-Implantitis in a University Dental Hospital: A Preliminary Retrospective Case Series Focusing on Complications. Applied Sciences, 15(14), 8040. https://doi.org/10.3390/app15148040








