Development of a Flexible and Conductive Heating Membrane via BSA-Assisted Electroless Plating on Electrospun PVDF-HFP Nanofibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning
2.3. Surface Pretreatment
2.4. Electroless Cu Plating
2.5. Morphological and Elemental Characterization
2.6. Contact Angle Measurement
2.7. Nanoscale Morphology and Crystallinity Analysis
2.8. Electrical Property Analysis
2.9. Adhesion Strength Test
2.10. Cu Particle Distribution
2.11. Thermal Performance Evaluation
2.12. Thermal Performance and Environmental Durability Evaluation
2.13. Statistical Analysis
3. Results
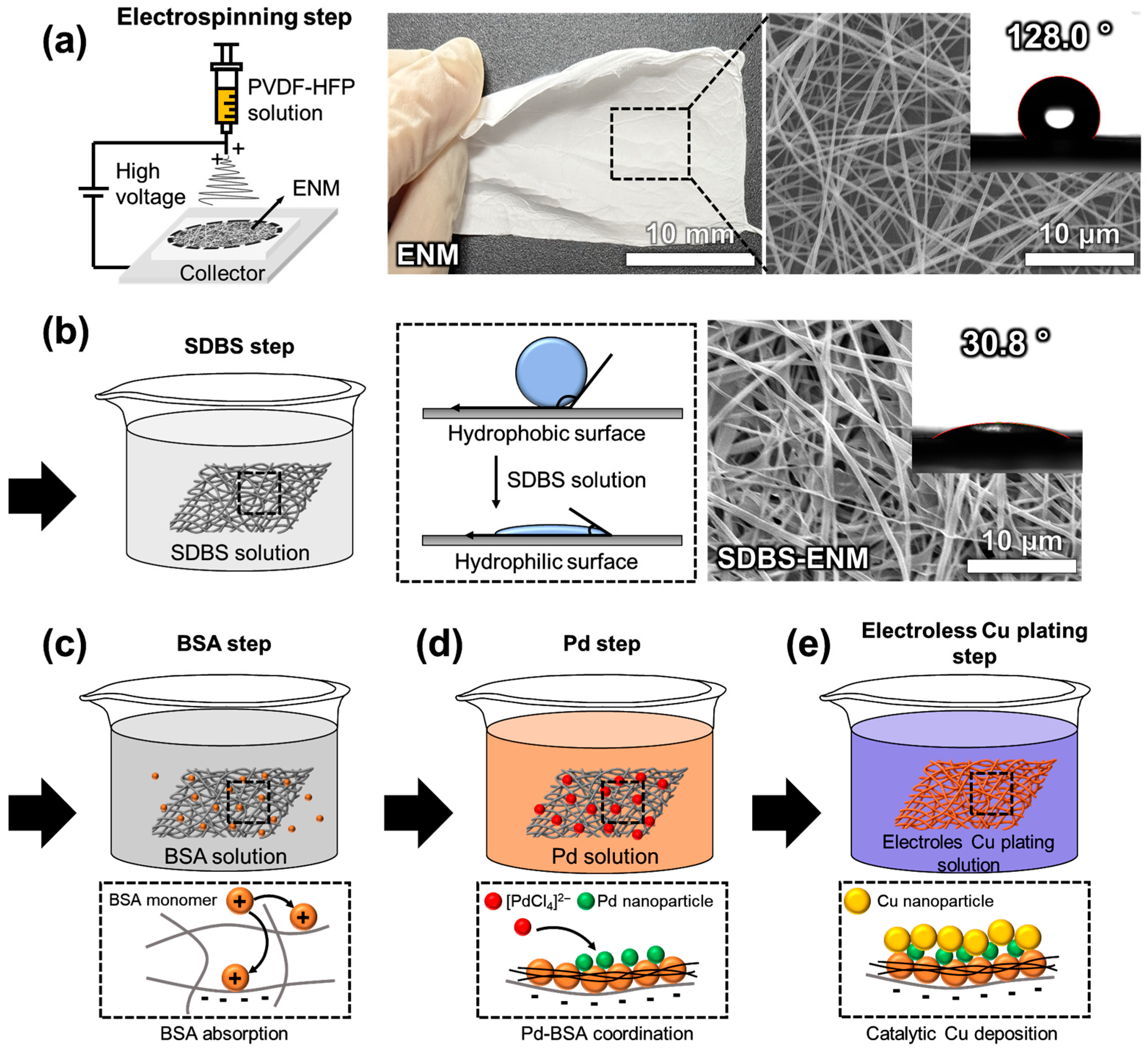
3.1. Fabrication of the ENMH
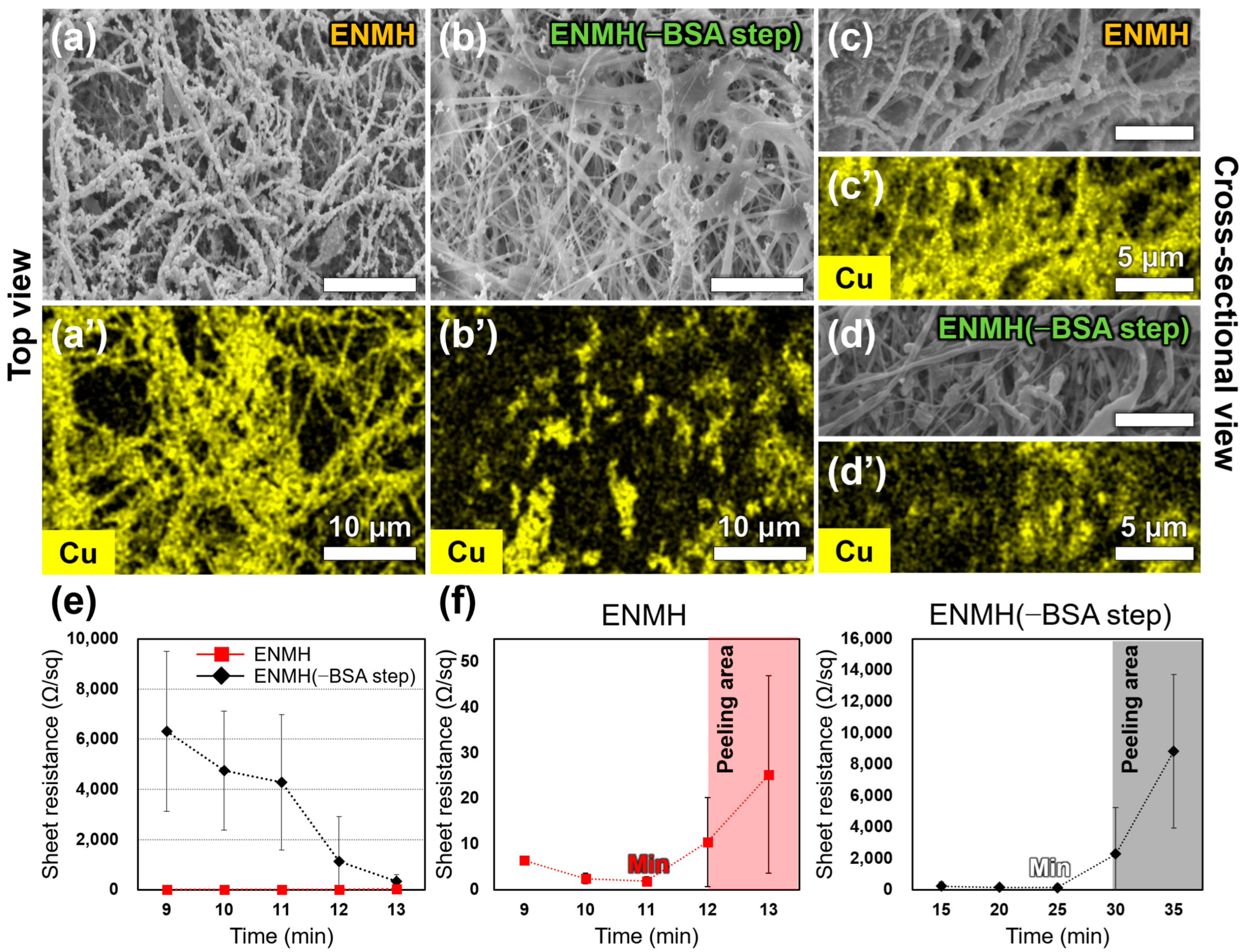
3.2. Effect of the BSA Step on the Morphology and Conductivity of ENMHs
3.3. Effect of BSA Step on Interfacial Adhesion Between the ENM and Cu Layer
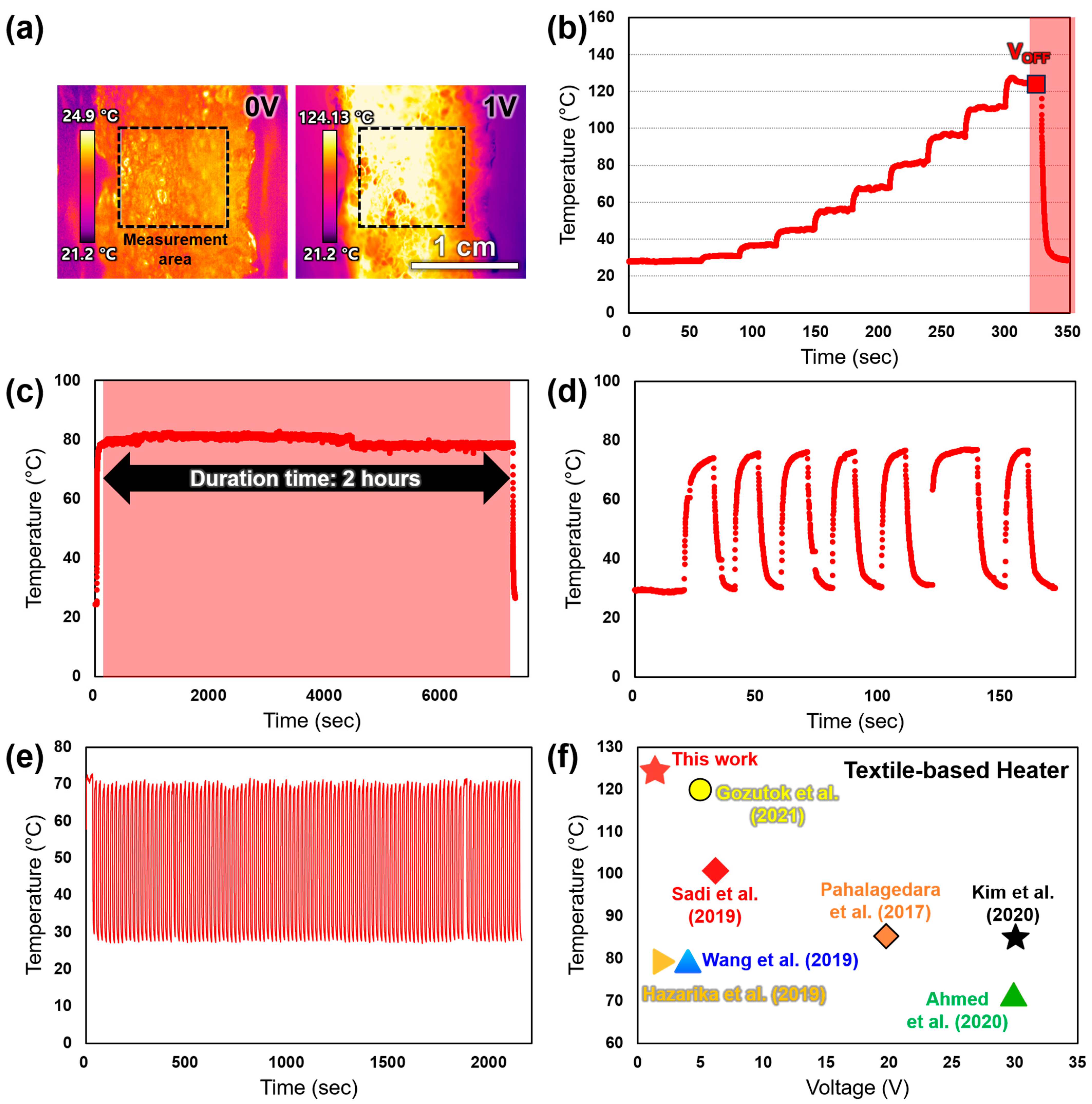
3.4. Thermal Performance Evaluation of the ENMH
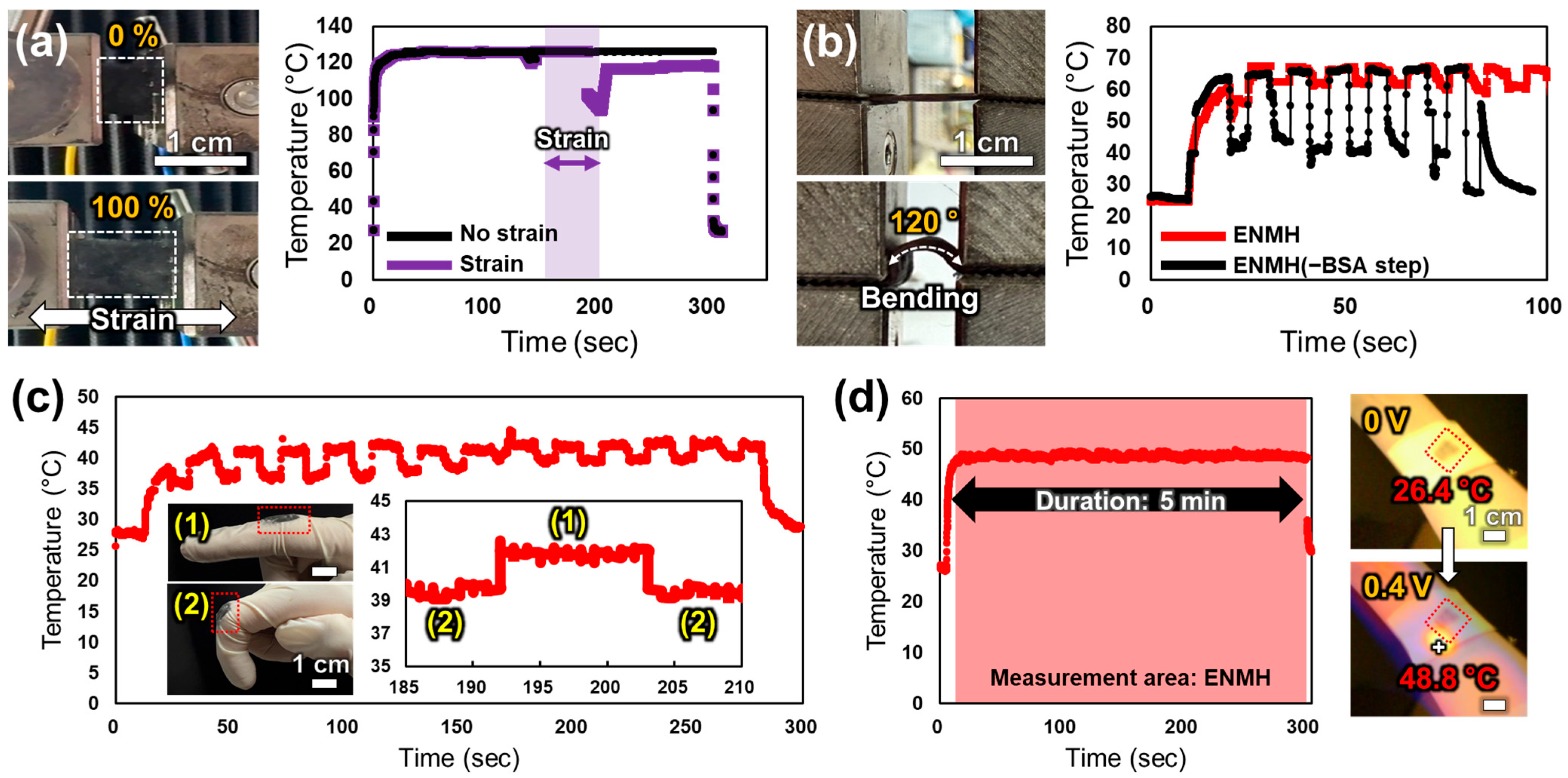
3.5. Evaluation of Mechanical Deformability and Practical Applicability of the ENMH
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nemomssa, H.D.; Bossuyt, F.; Vandecasteele, B.; De Pauw, H.; Gidi, N.W.; Bauwens, P. Revolutionizing Patient Care: A Comprehensive Review of Recent Advances in Flexible Printed Heaters for Wearable Medical Applications. Actuators 2025, 14, 1. [Google Scholar] [CrossRef]
- Chang, T.-B.; Sheu, J.-J.; Huang, J.-W. High-Efficiency HVAC System with Defog/Dehumidification Function for Electric Vehicles. Energies 2021, 14, 46. [Google Scholar] [CrossRef]
- Yang, J.S.; Min, J.K.; Yang, C.; Jung, K. Numerical Studies of Natural Convection Phenomena for a Vertical Cylinder with Multiple Lateral Baffles in Triangular and Hexagonal Enclosures. Case Stud. Therm. Eng. 2023, 45, 102971. [Google Scholar] [CrossRef]
- Lee, J.-G.; Lee, J.-H.; An, S.; Kim, D.-Y.; Kim, T.-G.; Al-Deyab, S.S.; Yarin, A.L.; Yoon, S.S. Highly Flexible, Stretchable, Wearable, Patternable and Transparent Heaters on Complex 3D Surfaces Formed from Supersonically Sprayed Silver Nanowires. J. Mater. Chem. A 2017, 5, 6677–6685. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, B.; Liang, J.; Wu, W. Recent Advances in Printed Flexible Heaters for Portable and Wearable Thermal Management. Mater. Horiz. 2021, 8, 1634–1656. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.-Y.; Kim, M.K.; Hudaya, C.; Park, J.H.; Byun, D.; Lim, J.-C.; Lee, J.K. Oxidation-Resistant Hybrid Metal Oxide/Metal Nanodots/Silver Nanowires for High Performance Flexible Transparent Heaters. Nanoscale 2016, 8, 3307–3313. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yokota, T.; Someya, T. Electrospun Nanofiber-Based Soft Electronics. NPG Asia Mater. 2021, 13, 22. [Google Scholar] [CrossRef]
- Shchegolkov, A.V.; Shchegolkov, A.V.; Kaminskii, V.V.; Chumak, M.A. Smart Polymer Composites for Electrical Heating: A Review. J. Compos. Sci. 2024, 8, 522. [Google Scholar] [CrossRef]
- Brito-Pereira, R.; Queirós, J.M.; Celaya-Azcoaga, L.; Fernández de Luiz, R.; Martins, P.; Lanceros-Mendez, S. Hexavalent Chromium Dual Water Remediation and Sensing Based on Hybrid Polymer/Metal-Organic Framework Composites. J. Environ. Chem. Eng. 2024, 12, 113839. [Google Scholar] [CrossRef]
- Tang, Y.; Xiong, Y.; Wu, L.; Xiong, X.; Me, T.; Wang, X. A Solid-State Lithium Battery with PVDF–HFP-Modified Fireproof Ionogel Polymer Electrolyte. ACS Appl. Energy Mater. 2023, 6, 4016–4026. [Google Scholar] [CrossRef]
- Sousa, R.E.; Nunes-Pereira, J.; Ferreira, J.C.C.; Costa, C.M.; Machado, A.V.; Silva, M.M.; Lanceros-Mendez, S. Microstructural Variations of Poly (Vinylidene Fluoride Co-Hexafluoropropylene) and Their Influence on the Thermal, Dielectric and Piezoelectric Properties. Polym. Test. 2014, 40, 245–255. [Google Scholar] [CrossRef]
- Yuennan, J.; Muensit, N. Preparation and Characterization of Flexible PVDF-HFP Film for Piezoelectric Applications. IOP Conf. Ser. Mater. Sci. Eng. 2020, 715, 012107. [Google Scholar] [CrossRef]
- Feng, Y.; Yao, Y.; Wang, S.; Ma, X.; Han, Y.; Feng, J.; Wen, J.; Tian, R.; Sun, Q.; Tian, Y. Role of Hetero-Doped Reduced Graphene Oxide in Suppressing Elemental Dissolution in Manganese Selenide Cathode for Aqueous Zinc-Ion Batteries. ChemSusChem 2024, 18, e202402101. [Google Scholar] [CrossRef] [PubMed]
- Lelevic, A.; Walsh, F.C. Electrodeposition of Ni–P Alloy Coatings: A Review. Surf. Coat. Technol. 2019, 369, 198–220. [Google Scholar] [CrossRef]
- Wu, L.-P.; Zhao, J.-J.; Xie, Y.-P.; Yang, Z.-D. Progress of Electroplating and Electroless Plating on Magnesium Alloy. Trans. Nonferrous Met. Soc. China 2010, 20 (Suppl. S2), s630–s637. [Google Scholar] [CrossRef]
- Aminu, T.Q.; Juybari, H.F.; Warsinger, D.M.; Bahr, D.F. Electroless Deposition for Robust and Uniform Copper Nanoparticles on Electrospun Polyacrylonitrile (PAN) Microfiltration Membranes. Membranes 2024, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.K.; Kang, S.M.; Kim, T.; Kim, S.; Kim, G.H. Electroless Plating on Polymer Surfaces: Comprehensive Review of Mechanism, Process, Analysis, and Future Applications. Adv. Mater. Interfaces, 2025; in press. [Google Scholar] [CrossRef]
- Li, F.; Yeh, S.; Shi, Q.; Wang, P.; Wu, H.; Xin, J. A Novel Thermal-Driven Self-Assembly Method to Prepare Albumin Nanoparticles: Formation Kinetics, Degradation Behavior and Formation Mechanism. Int. J. Nanomed. 2022, 23, 250. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, N.; Moodi, M.; Kefayat, A.; Shokati, F.; Molaabasi, F. Challenges and Opportunities of Using Fluorescent Metal Nanocluster-Based Colorimetric Assays in Medicine. ACS Omega 2024, 9, 3143–3163. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Hwang, H.Y.; An, T. In Situ BSA (Bovine Serum Albumin) Assisted Electroless Plating Method with Superior Adhesion Property. Adv. Mater. Interfaces 2022, 9, 2101203. [Google Scholar] [CrossRef]
- Wangkam, T.; Yodmongkol, S.; Disrattakit, J.; Sutapun, B.; Amarit, R.; Somboonkaew, A.; Srikhirin, T. Adsorption of Bovine Serum Albumin (BSA) on Polystyrene (PS) and Its Acid Copolymer. Curr. Appl. Phys. 2012, 12, 44–52. [Google Scholar] [CrossRef]
- Nawade, A.; Busi, K.B.; Dalapati, G.K.; Chakrabortty, S.; Mukhopadhyyay, S. Hybrid Inorganic-Biomolecular Materials for Bioelectronics Applications. J. Electron. Mater. 2025, 54, 3509–3519. [Google Scholar] [CrossRef]
- Sivaselvam, S.; Mohankumar, A.; Thiruppathi, G.; Sundararaj, P.; Viswanathan, C.; Ponpandian, N. Engineering the Surface of Graphene Oxide with Bovine Serum Albumin for Improved Biocompatibility in Caenorhabditis elegans. Nanoscale Adv. 2020, 2, 5219. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.H.; Chen, G.X.; Wang, S.; Teng, C.H.; Huang, W.C.; Cheng, H.L.; Chou, W.Y. Biocompatible OFETs for Selective and Real-Time Bacterial Detection Using BSA and Lysozyme Layers. ACS Appl. Bio Mater. 2025, 8, 2867–2874. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, J.; Matsui, H.; Lee, S.; Wang, W.; Guo, S.; Chen, H.; Fang, K.; Ito, Y.; Inoue, D.; et al. All-Solution-Processed Ultraflexible Wearable Sensor Enabled with Universal Trilayer Structure for Organic Optoelectronic Devices. Sci. Adv. 2024, 10, eadk9460. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Fukuda, K.; Someya, T. Recent Progress in Solution-Processed Flexible Organic Photovoltaics. NPJ Flex. Electron. 2022, 6, 89. [Google Scholar] [CrossRef]
- van Meerakker, J.E.A.M.; Scholtens, E. Side Reactions in Electroless Copper Solutions with Formaldehyde as Reducing Agent. Ber. Bunsenges. Phys. Chem. 1989, 93, 786–791. [Google Scholar] [CrossRef]
- ISO 105-C06:2010; Textiles—Tests for Colour Fastness—Colour Fastness to Domestic and Commercial Laundering. International Organization for Standardization: Geneva, Switzerland, 2010.
- Abdulhussain, R.; Adebisi, A.; Conway, B.R.; Asare-Addo, K. Electrospun nanofibers: Exploring process parameters, polymer selection, and recent applications in pharmaceuticals and drug delivery. J. Drug Deliv. Sci. Technol. 2023, 90, 105156. [Google Scholar] [CrossRef]
- Yarin, A.L.; Koombhongse, S.; Reneker, D.H. Bending instability in electrospinning of nanofibers. J. Appl. Phys. 2001, 89, 3018–3026. [Google Scholar] [CrossRef]
- Shenoy, S.L.; Bates, W.D.; Frisch, H.L.; Wnek, G.E. Role of chain entanglements on fiber formation during electrospinning of polymer solutions: Good solvent, non-specific polymer–polymer interaction limit. Polymer 2005, 46, 3372–3384. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, X.; Wu, L.; Han, Y.; Sheng, J. Study on morphology of electrospun poly(vinyl alcohol) mats. Eur. Polym. J. 2005, 41, 423–432. [Google Scholar] [CrossRef]
- Nugraha, A.S.; Chou, C.C.; Yu, P.H.; Lin, K.L. Effects of applied voltage on the morphology and phases of electrospun poly(vinylidene difluoride) nanofibers. Polym. Int. 2022, 71, 1176–1183. [Google Scholar] [CrossRef]
- He, W.; Ding, H.; Chen, X.; Yang, W. Three-dimensional LLZO/PVDF-HFP fiber network-enhanced ultrathin composite solid electrolyte membrane for dendrite-free solid-state lithium metal batteries. J. Membr. Sci. 2023, 665, 121095. [Google Scholar] [CrossRef]
- Sha, Z.; Boyer, C.; Li, G.; Yu, Y.; Allioux, F.M.; Kalantar-Zadeh, K.; Wang, C.H.; Zhang, J. Electrospun liquid metal/PVDF-HFP nanofiber membranes with exceptional triboelectric performance. Nano Energy 2022, 92, 106713. [Google Scholar] [CrossRef]
- Lin, M.-F.; Chang, K.-W.; Lee, C.-H.; Wu, X.-X.; Huang, Y.-C. Electrospun P3HT/PVDF-HFP semiconductive nanofibers for triboelectric nanogenerators. Sci. Rep. 2022, 12, 14842. [Google Scholar] [CrossRef] [PubMed]
- Narkhede, N.; Uttam, B.; Rao, C.P. Calixarene-Assisted Pd Nanoparticles in Organic Transformations: Synthesis, Characterization, and Catalytic Applications in Water for C–C Coupling and for the Reduction of Nitroaromatics and Organic Dyes. ACS Omega 2019, 4, 4908–4917. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.B.; Carter, C.B. Transmission Electron Microscopy: A Textbook for Materials Science, 2nd ed.; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Coppage, R.; Slocik, J.M.; Briggs, B.D.; Frenkel, A.I.; Naik, R.R.; Knecht, M.R. Determining Peptide Sequence Effects That Control the Size, Structure, and Function of Nanoparticles. ACS Nano 2012, 6, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-W.; Zhang, H.-B.; Liu, J.; Zhao, S.; Xie, X.; Liu, L.; Yang, R.; Koratkar, N.; Yu, Z.-Z. Multifunctional and water-resistant MXene-decorated polyester textiles with outstanding electromagnetic interference shielding and Joule heating performances. Adv. Funct. Mater. 2019, 29, 1806819. [Google Scholar] [CrossRef]
- Hazarika, A.; Deka, B.K.; Jeong, C.; Park, Y.B.; Park, H.W. Biomechanical Energy-Harvesting Wearable Textile-Based Personal Thermal Management Device Containing Epitaxially Grown Aligned Ag-Tipped-NixCo1−xSe Nanowires/Reduced Graphene Oxide. Adv. Funct. Mater. 2019, 29, 1903114. [Google Scholar] [CrossRef]
- Sadi, M.S.; Yang, M.; Luo, L.; Cheng, D.; Cai, G.; Wang, X. Direct screen printing of single-faced conductive cotton fabrics for strain sensing, electrical heating and color changing. Cellulose 2019, 26, 6179–6188. [Google Scholar] [CrossRef]
- Pahalagedara, L.R.; Siriwardane, I.W.; Tissera, N.D.; Wijesena, R.N.; de Silva, K.M.N. Carbon black functionalized stretchable conductive fabrics for wearable heating applications. RSC Adv. 2017, 7, 19174–19180. [Google Scholar] [CrossRef]
- Gozutok, Z.; Agırbas, O.; Bahtiyari, M.I.; Ozdemir, A.T. Low-voltage textile-based wearable heater systems fabricated by printing reactive silver inks. Sens. Actuators A Phys. 2021, 322, 112610. [Google Scholar] [CrossRef]
- Ahmed, A.; Jalil, M.A.; Hossain, M.; Moniruzzaman; Adak, B.; Islam, M.T.; Parvez, S.; Mukhopadhyay, S. A PEDOT:PSS and graphene-clad smart textile-based wearable electronic Joule heater with high thermal stability. J. Mater. Chem. C 2020, 8, 16204–16215. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.S.; Lee, S. Thermal insulation property of graphene/polymer-coated textile based multi-layer fabric heating element with aramid fabric. Sci. Rep. 2020, 10, 17586. [Google Scholar] [CrossRef] [PubMed]
- Ahn, T.-C.; Hong, H.-S.; Kim, S.-S.; Hwang, H.-Y. Improvement of Adhesion Characteristics of Copper Electrodes on Polyvinylidene Fluoride Films Using Bovine Serum Albumin. Int. J. Adhes. Adhes. 2022, 112, 103025. [Google Scholar] [CrossRef]
- Ha, J.W.; Choi, J.Y.; Boo, Y.C. Differential Effects of Histidine and Histidinamide versus Cysteine and Cysteinamide on Copper Ion-Induced Oxidative Stress and Cytotoxicity in HaCaT Keratinocytes. Antioxidants 2023, 12, 801. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jang, D.; Chung, Y.S.; Lee, S. Cost-Effective and Highly Efficient Surface Heating Elements Using High Thermal Conductive Carbon Fibers. Compos. Part A Appl. Sci. Manuf. 2020, 137, 105992. [Google Scholar] [CrossRef]
- Claypole, A.; Claypole, J.; Leeder, J.; Stevens, G.; Johnson, F.; Bezodis, N.; Parker, M.; Claypole, T.; Gethin, D.; Kilduff, L. Large Area, Stretchable, Wearable, Screen-Printed Carbon Heaters for Use in Elite Sport. J. Coat. Technol. Res. 2023, 20, 261–273. [Google Scholar] [CrossRef]
- NASA Office of the Chief Health & Medical Officer. Physiological Effects of Touch Temperature (OCHMO-TB-009 Rev A). Available online: https://www.nasa.gov/wp-content/uploads/2023/12/ochmo-tb-009-touch-temperature.pdf (accessed on 5 June 2025).
- Lee, S.-Y.; Park, K.R.; Kang, S.-G.; Lee, J.-H.; Jeon, E.-C.; Shim, C.-H.; Ahn, J.-P.; Kim, D.-I.; Han, H.N.; Joo, Y.-C.; et al. Selective Crack Suppression during Deformation in Metal Films on Polymer Substrates Using Electron Beam Irradiation. Nat. Commun. 2019, 10, 4454. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lian, H.; Li, Z.; Yin, L.; Ji, Q.; Li, K.; Qi, F.; Huang, Y. Crack Engineering Boosts the Performance of Flexible Sensors. VIEW 2022, 3, 20220025. [Google Scholar] [CrossRef]
- Mei, H.; Pang, Y.; Im, S.H.; Huang, R. Fracture, Delamination, and Buckling of Elastic Thin Films on Compliant Substrates. In Proceedings of the Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems (ITherm), Orlando, FL, USA, 28–31 May 2008; IEEE: Piscataway, NJ, USA, 2008; pp. 762–767. [Google Scholar] [CrossRef]
- Jang, D.-W.; Lee, J.-H.; Kim, A.; Lee, S.-B.; Hong, S.-G. Self-heating-induced deterioration of electromechanical performance in polymer-supported metal films for flexible electronics. Sci. Rep. 2017, 7, 12506. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi, A.; Gonzalez, M.; van den Brand, J.; Bossuyt, F.; Vervust, T.; Verplancke, R.; Vanfleteren, J.; De Baets, J. Stretchable Circuits with Horseshoe Shaped Conductors Embedded in Elastic Polymers. Jpn. J. Appl. Phys. 2013, 52, 05DA18. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, G.; Sun, Q.; Feng, Y.; Wang, X.; Ma, X.; Li, J.; Zhang, H.; Wen, J.; Feng, J.; et al. Flexible Electronic Systems via Electrohydrodynamic Jet Printing: A MnSe@rGO Cathode for Aqueous Zinc-Ion Batteries. ACS Nano 2023, 17, 13256–13268. [Google Scholar] [CrossRef] [PubMed]
- Veeramuthu, L.; Chen, B.-Y.; Tsai, C.-Y.; Liang, F.-C.; Venkatesan, M.; Jiang, D.-H.; Chen, C.-W.; Cai, X.; Kuo, C.-C. Novel stretchable thermochromic transparent heaters designed for smart window defroster applications by spray coating silver nanowire. RSC Adv. 2019, 9, 35786–35796. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shi, Y.; Ying, B.; Hu, Y.; Gao, Y.; Luo, S.; Liu, X. Kirigami-enabled stretchable laser-induced graphene heaters for wearable thermotherapy. Mater. Horiz. 2024, 11, 2010–2020. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.-Y.; Lee, D.-K.; Kim, M.-G.; Kim, W.-J.; Park, S.-H. Temperature-Responsive Hybrid Composite with Zero Temperature Coefficient of Resistance for Wearable Thermotherapy Pads. Micromachines 2025, 16, 108. [Google Scholar] [CrossRef] [PubMed]
- Salihoglu, O.; Uzlu, H.B.; Yakar, O.; Aas, S.; Balci, O.; Kakenov, N.; Balci, S.; Olcum, S.; Süzer, S.; Kocabas, C. Graphene-Based Adaptive Thermal Camouflage. Nano Lett. 2018, 18, 4541–4548. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Wang, R.; Ni, H.; Liu, H.; Liu, L. A Review of Flexible Electric Heating Element and Electric Heating Garments. J. Ind. Text. 2020, 51, 101S–136S. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.J.; Yoon, D.H.; Park, Y.S.; Nam, H.; Kim, G.H. Development of a Flexible and Conductive Heating Membrane via BSA-Assisted Electroless Plating on Electrospun PVDF-HFP Nanofibers. Appl. Sci. 2025, 15, 8023. https://doi.org/10.3390/app15148023
Choi MJ, Yoon DH, Park YS, Nam H, Kim GH. Development of a Flexible and Conductive Heating Membrane via BSA-Assisted Electroless Plating on Electrospun PVDF-HFP Nanofibers. Applied Sciences. 2025; 15(14):8023. https://doi.org/10.3390/app15148023
Chicago/Turabian StyleChoi, Mun Jeong, Dae Hyeob Yoon, Yoo Sei Park, Hyoryung Nam, and Geon Hwee Kim. 2025. "Development of a Flexible and Conductive Heating Membrane via BSA-Assisted Electroless Plating on Electrospun PVDF-HFP Nanofibers" Applied Sciences 15, no. 14: 8023. https://doi.org/10.3390/app15148023
APA StyleChoi, M. J., Yoon, D. H., Park, Y. S., Nam, H., & Kim, G. H. (2025). Development of a Flexible and Conductive Heating Membrane via BSA-Assisted Electroless Plating on Electrospun PVDF-HFP Nanofibers. Applied Sciences, 15(14), 8023. https://doi.org/10.3390/app15148023






