Morphometrics of the Blue Crab Callinectes sapidus Rathbun, 1896 in a Northern Adriatic Saline Marsh Under Environmental Stress
Abstract
1. Introduction
2. Materials and Methods
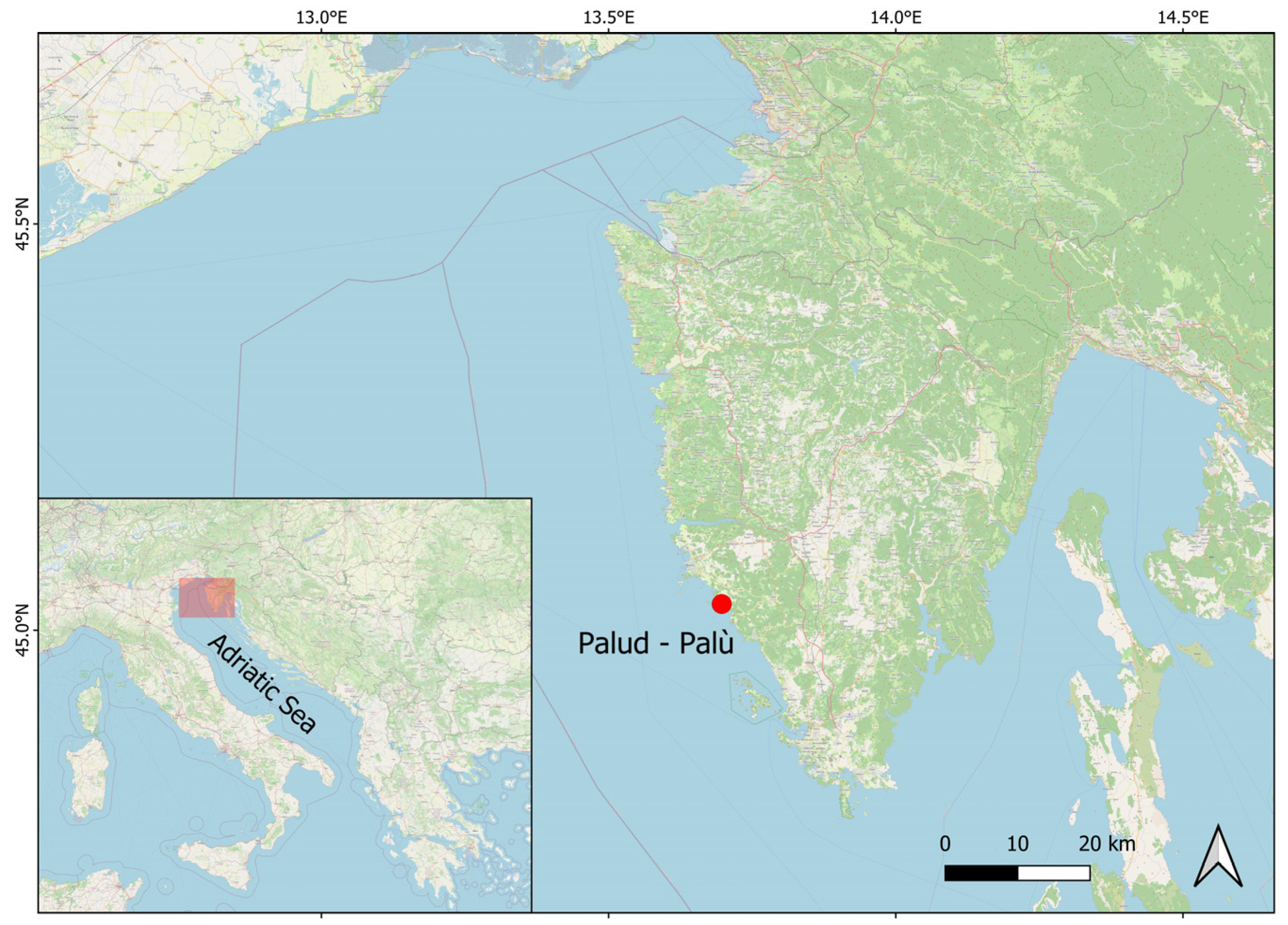
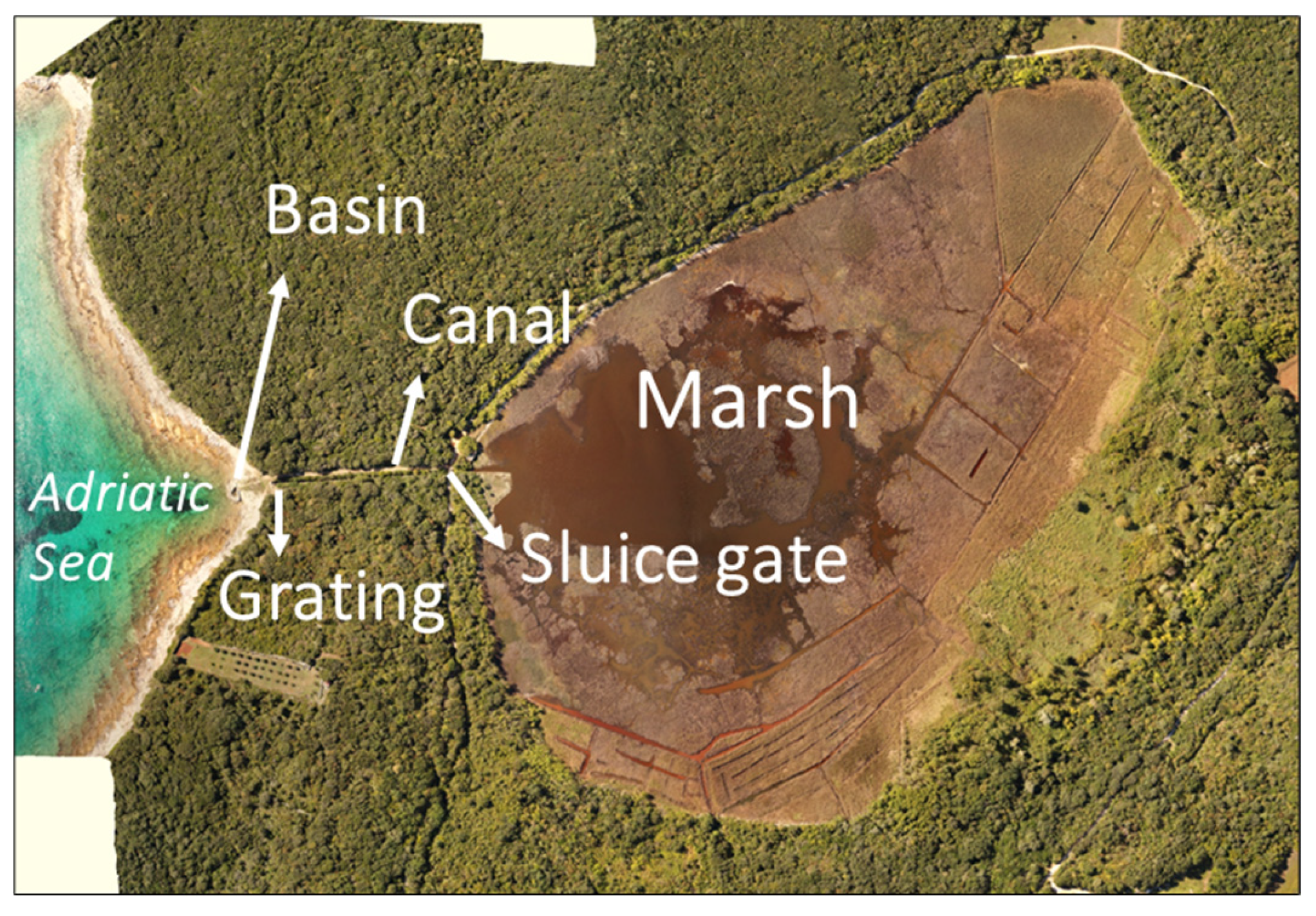
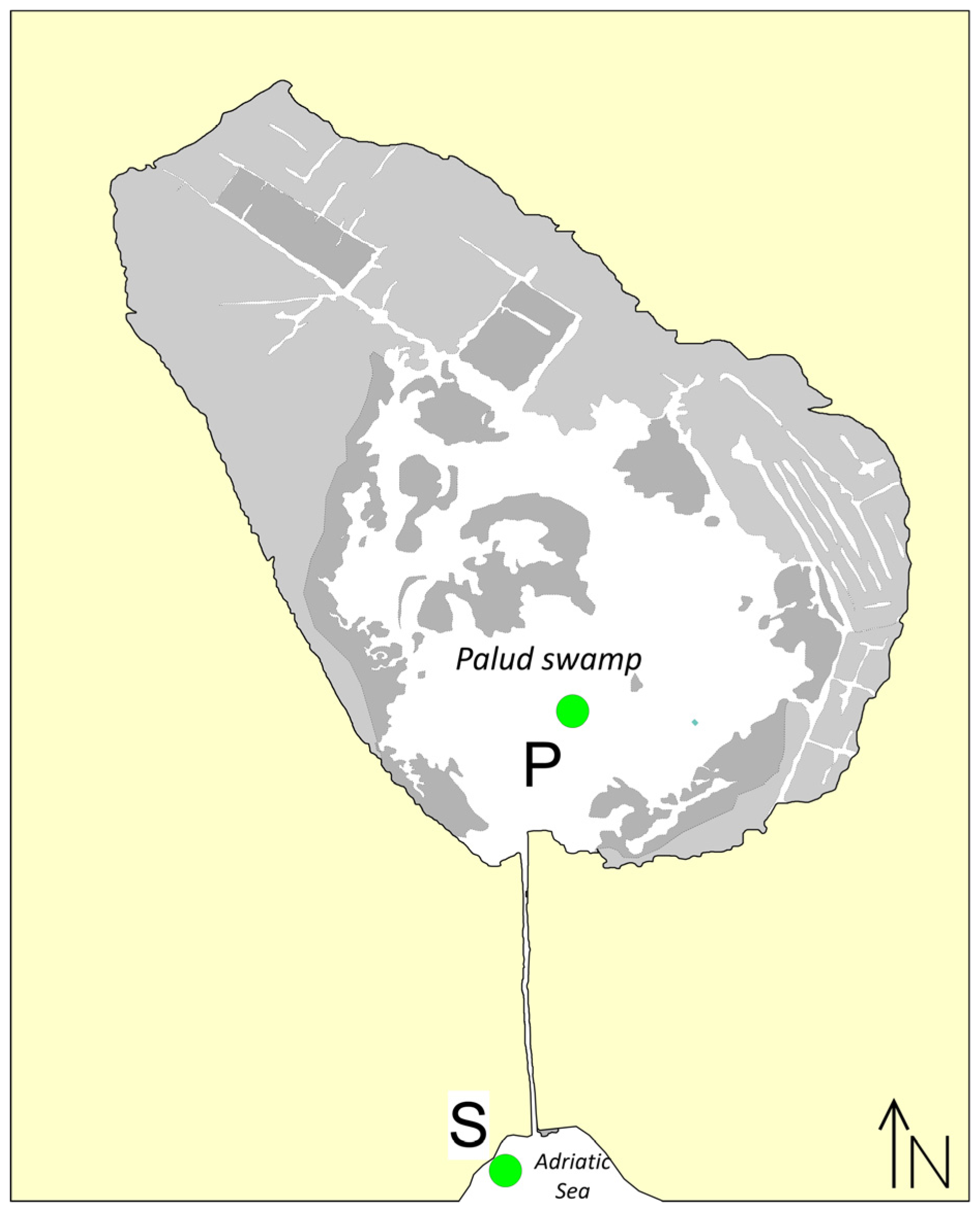
2.1. Study Area
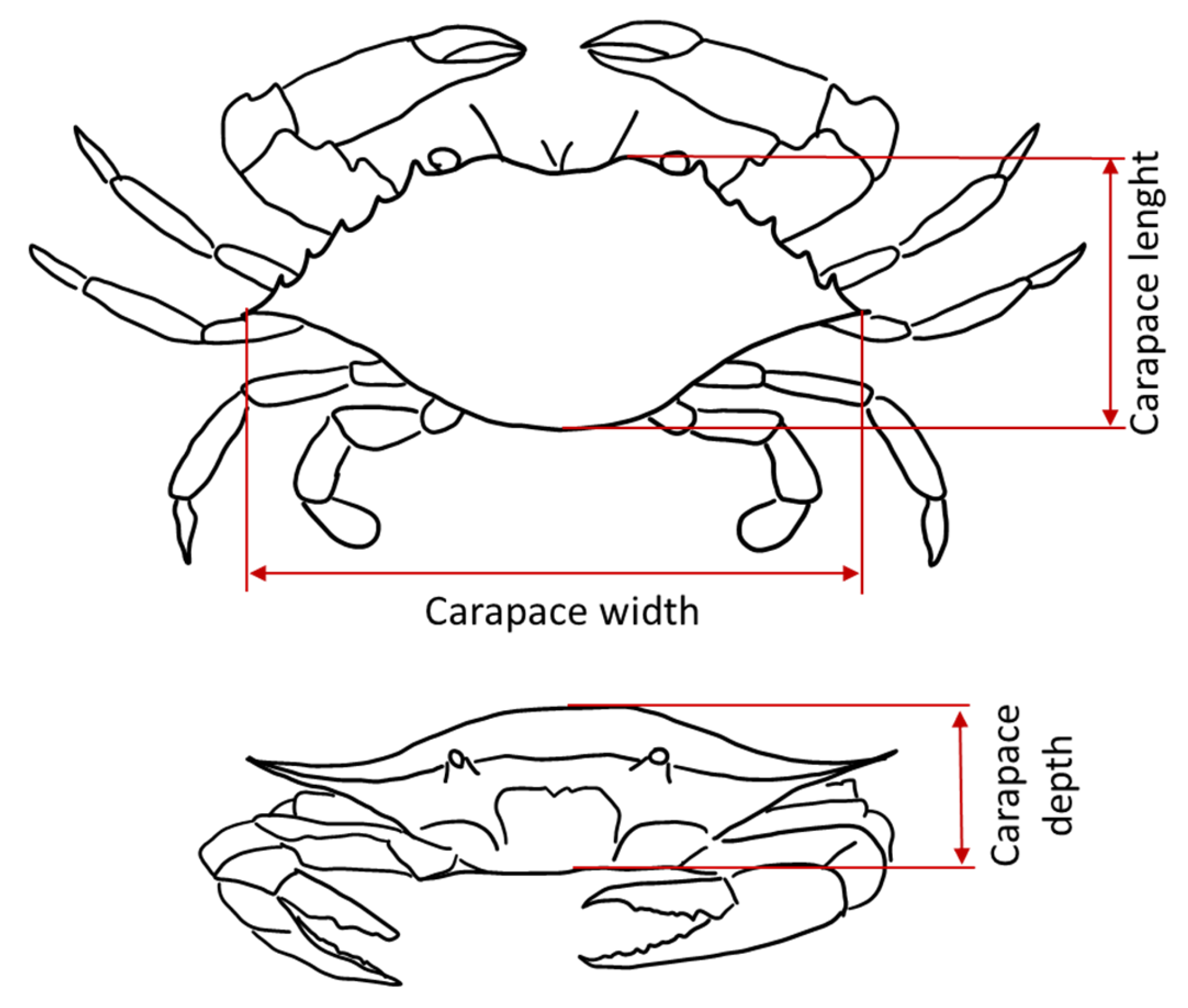
2.2. Sample Collection, and Abiotic and Morphometric Measurements
- K (%) = value of the condition factor
- W = blue crab weight (g)
- CW = carapace width (cm)
2.3. Statistical Analysis
3. Results
3.1. Abiotic Parameters
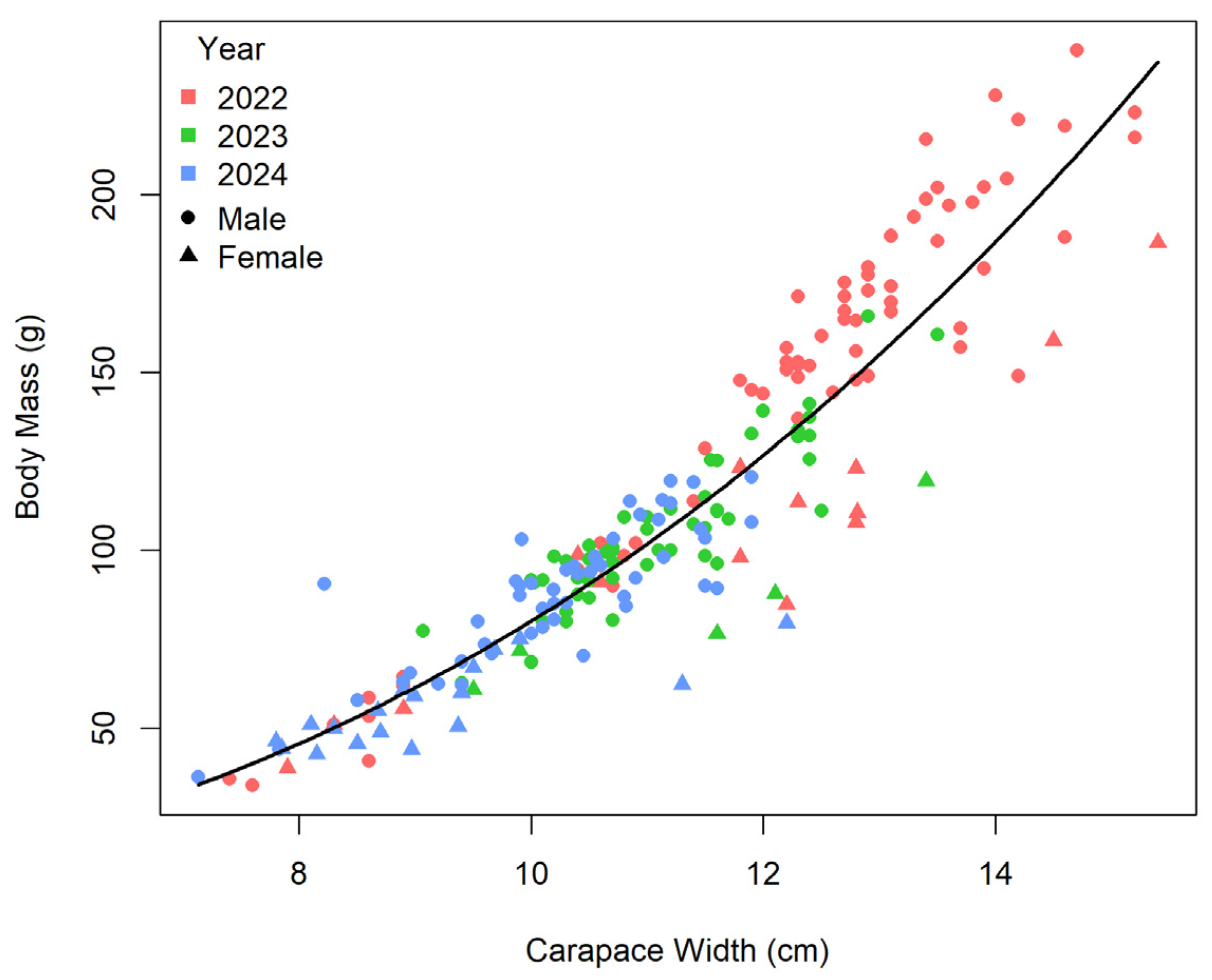
3.2. Morphometric Measurments
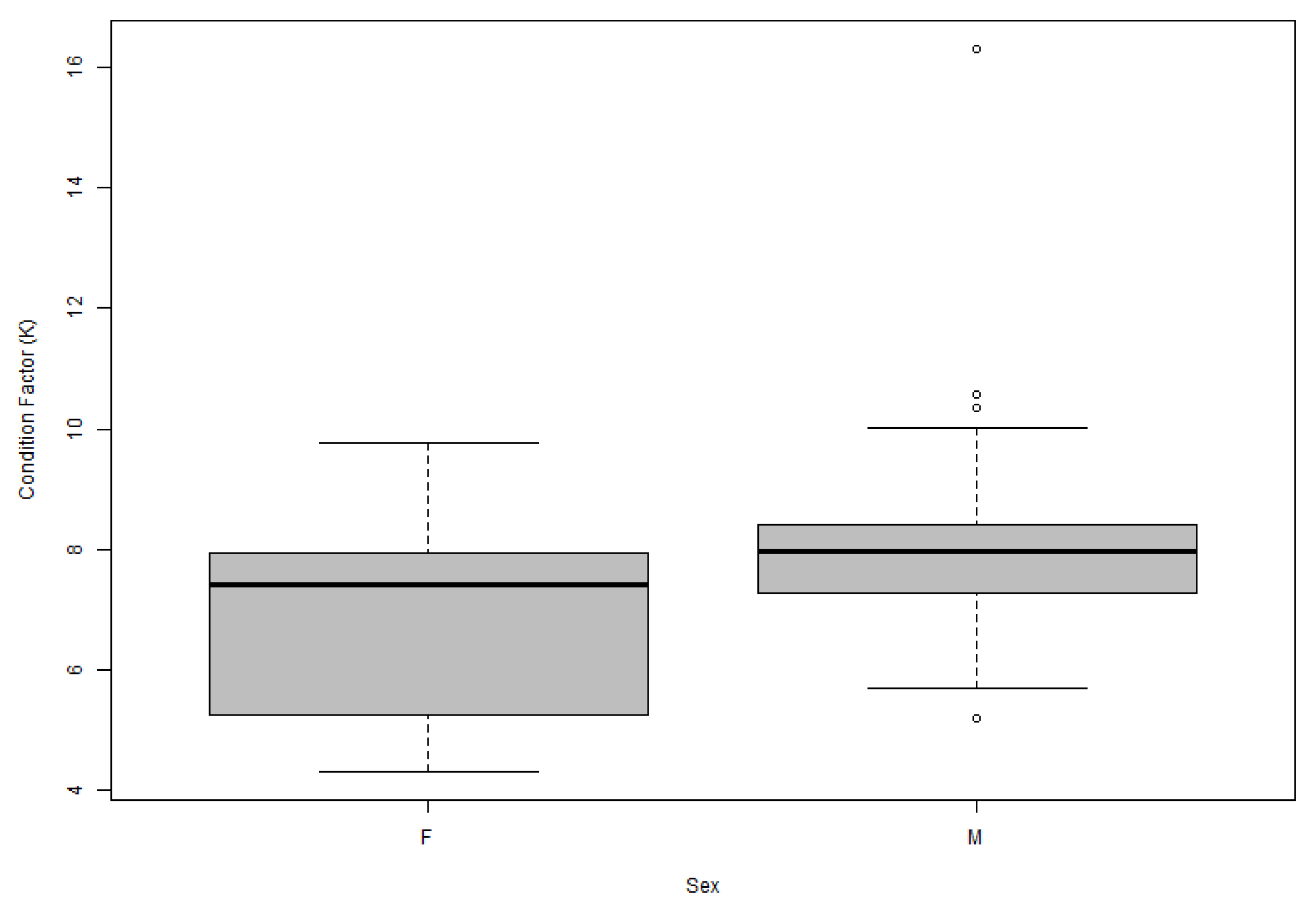
3.3. Sexual Dimorphism in Morphometric Traits
3.4. Interannual Variation in Morphometric Traits
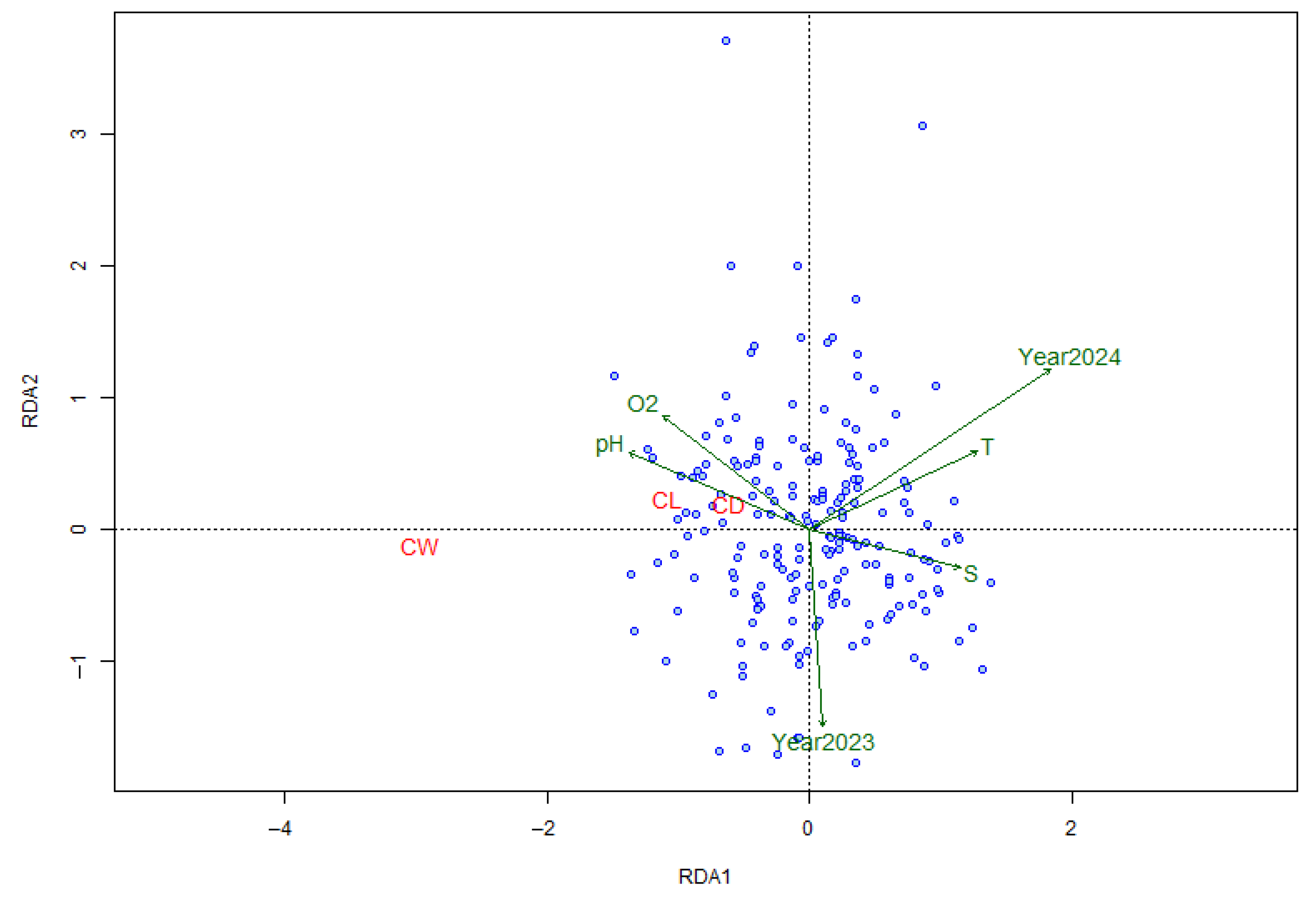
3.5. Multivariate Analysis of Morphometric Variation
3.6. Condition Factor Analysis
3.7. Environmental Influences on Morphometry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Rabaoui, L.; Arculeo, M.; Mansour, L.; Tlig-Zouari, S.; Fahd, K.; Arabia, S. Occurrence of the Lessepsian Species Portunus Segnis (Crustacea: Decapoda) in the Gulf of Gabes (Tunisia): First Record and New Information on Its Biology and Ecology. Cah. Biol. Mar. 2015, 56, 169–175. [Google Scholar]
- Falsone, F.; Scannella, D.; Geraci, M.L.; Vitale, S.; Sardo, G.; Fiorentino, F. Further Records of Callinectes sapidus (Rathbun, 1896) (Decapoda, Brachyura, Portunidae) in the Strait of Sicily. Mar. Biodivers. Rec. 2020, 13, 8. [Google Scholar] [CrossRef]
- Scalici, M.; Chiesa, S.; Mancinelli, G.; Rontani, P.M.; Voccia, A.; Nonnis Marzano, F. Euryhaline Aliens Invading Italian Inland Waters: The Case of the Atlantic Blue Crab Callinectes sapidus Rathbun, 1896. Appl. Sci. 2022, 12, 4666. [Google Scholar] [CrossRef]
- Castriota, L.; Falautano, M.; Perzia, P. When Nature Requires a Resource to Be Used—The Case of Callinectes sapidus: Distribution, Aggregation Patterns, and Spatial Structure in Northwest Europe, the Mediterranean Sea, and Adjacent Waters. Biology 2024, 13, 279. [Google Scholar] [CrossRef]
- Giordani Soika, A. Il Neptunus Pelagicus (L.) Nell’alto Adriatico. Natura 1951, 42, 18–20. [Google Scholar]
- Mizzan, L. Presence of Swimming Crabs of the Genus Callinectes (Stimpson)(Decapoda, Portunidae) in the Venice Lagoon (North Adriatic Sea-Italy): First Record of Callinectes Danae Smith in European Waters. Boll. Mus. Civ. Stor. Nat. Venezia 1993, 42, 31–43. [Google Scholar]
- Manfrin, C.; Bettoso, N.; Comisso, G.; dall’Asta, A.; Chung, J. The Return of the Blue Crab, Callinectes sapidus Rathbun, 1896, after 70 Years from Its First Appearance in the Gulf of Trieste, Northern Adriatic Sea, Italy (Decapoda: Portunidae). Check List 2016, 12, 1–7. [Google Scholar] [CrossRef]
- Tiralongo, F.; Mancini, E.; Gattelli, R.; di Pasquale, C.; Rossetti, E.; Martellucci, R.; Felici, A.; Mancinelli, G.; Mulder, C. The Devasting Economic Impact of Callinectes sapidus on the Clam Fishing in the Po Delta (Italy): Striking Evidence from Novel Field Data. arXiv 2025, arXiv:2502.07095. [Google Scholar]
- Gavioli, A.; Mancinelli, G.; Turolla, E.; Lanzoni, M.; Paesanti, V.; Soana, E.; Eggleston, D.B.; Christian, R.R.; Castaldelli, G. Impacts of the Invasive Blue Crab Callinectes Sapidus on Small-Scale Fisheries in a Mediterranean Lagoon Using Fishery Landing Data. Sci. Total Environ. 2025, 974, 179236. [Google Scholar] [CrossRef]
- Lipej, L.; Rogelja, M. Status of the Invasive Blue Crab Callinectes sapidus Rathbun, 1896 (Brachyura: Portunidae) in Slovenia. Acta Biol. Slov. 2021, 64, 24–33. [Google Scholar] [CrossRef]
- Glamuzina, L.; Pešić, A.; Marković, O.; Tomanić, J.; Pećarević, M.; Dobroslavić, T.; Brailo Šćepanović, M.; Conides, A.; Grđan, S. Population structure of the invasive Atlantic blue crab, Callinectes sapidus on the Eastern Adriatic coast (Croatia, Montenegro). Naše More 2023, 70, 153–159. [Google Scholar] [CrossRef]
- Tutman, P.; Iveša, N. Ichthyofauna of the Salt Marsh Palud—Palù (Istria, Croatia)—Summer Aspect. In Proceedings of the 2nd Southeast European Ichthyological Conference (SEEIC 2022): Book of Abstract; Institute of Oceanography and Fisheries: Supetar, Croatia, 2022; p. 39. [Google Scholar]
- Čief, M.; Iveša, N.; Buršić, M.; Turković, D.; Jelenović, R.; Kokorović, A.; Delcaro, N.; Paliaga, P. The Biological and Ecological Features of the Blue Crab (Callinectes sapidus Rathburn 1896) in the Palud—Palù Ornithological Reserve. In Proceedings of the Book of Abstracts (IV Scientific-Expert Conference Adaptation to Climate Change and Preservation of Marine Ecosystems of the Adriatic Sea with International Participation); University of Zadar: Krk, Croatia, 2023; pp. 52–53. [Google Scholar]
- Iveša, N.; Čief, M.; Buršić, M.; Lupret Obradović, S.; Meštrović, L.; Fiorentin, C.; Piria, M.; Paliaga, P.; Deklić, A. Preliminary Data on the Diet of the Invasive Atlantic Blue Crab (Callinectes sapidus Rathbun, 1896) in the Special Ornithological Reserve Palud—Palù in Istria. In Proceedings of the 5th Croatian Symposium on Invasive Species—With International Participation. Book of Abstracts; Croatian Ecological Society: Zagreb, Croatia, 2023; p. 63. [Google Scholar]
- Mancinelli, G.; Bardelli, R.; Zenetos, A. A Global Occurrence Database of the Atlantic Blue Crab Callinectes sapidus. Sci. Data 2021, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, G.; Chainho, P.; Cilenti, L.; Falco, S.; Kapiris, K.; Katselis, G.; Ribeiro, F. The Atlantic Blue Crab Callinectes sapidus in Southern European Coastal Waters: Distribution, Impact and Prospective Invasion Management Strategies. Mar. Pollut. Bull. 2017, 119, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Marchessaux, G.; Bosch-Belmar, M.; Cilenti, L.; Lago, N.; Mangano, M.C.; Marsiglia, N.; Sarà, G. The Invasive Blue Crab Callinectes sapidus Thermal Response: Predicting Metabolic Suitability Maps under Future Warming Mediterranean Scenarios. Front. Mar. Sci. 2022, 9, 1055404. [Google Scholar] [CrossRef]
- Haputhantri, S.S.K.; Weerasekera, S.J.W.W.M.M.P.; Bandaranayake, K.H.K. Morphometric Relationships in the Blue Swimming Crabs, (Portunus Pelagicus) (Linnaeus, 1758) from the Palk Bay, Sri Lanka. Asian J. Fish. Aquat. Res. 2021, 11, 29–38. [Google Scholar] [CrossRef]
- Padilla-Serrato, J.G.; Kuk-Dzul, J.G.; Flores-Rodríguez, P.; Flores-Garza, R.; Soriano-Reyes, N. Population Parameters and Size at Maturity of Callinectes Arcuatus Ordway, 1863 (Decapoda, Portunidae) in Apozahualco Lagoon, Guerrero, Mexico. Crustaceana 2019, 92, 397–414. [Google Scholar] [CrossRef]
- Diarte-Plata, G.; Escamilla-Montes, R.; Granados-Alcantar, S.; Luna-Gonzalez, A. Reproductive Cycle and Size at First Maturity in Females of Brown Crab Callinectes Bellicosus (Stimpson 1859) in the Southwestern Gulf of California, Mexico. Croat. J. Fish. 2021, 79, 125–135. [Google Scholar] [CrossRef]
- Milliken, M.R.; Williams, A.B. Synopsis of Biological Data on the Blue Crab, Callinectes sapidus, Rathbun; NOAA technical report NMFS 1, FAO fisheries synopsis no. 138; U.S. Department of Commerce; National Oceanic and Atmospheric Administration: Washington, DC, USA; National Marine Fisheries Service: Silver Spring, MD, USA, 1984; 39p. [Google Scholar]
- Williams, A.B. The Swimming Crabs of the Genus Callinectes (Decapoda: Portunidae). Fish. Bull. 1974, 72, 685–798. [Google Scholar]
- Dittel, A.I.; Epifanio, C.E. Growth and Development of the Portunid Crab Callinectes Arcuatus Ordway: Zoeae, Megalopae, and Juveniles. J. Crustac. Biol. 1984, 4, 491–494. [Google Scholar] [CrossRef]
- Moutopoulos, D.K.; Stergiou, K.I. Length–Weight and Length–Length Relationships of Fish Species from the Aegean Sea (Greece). J. Appl. Ichthyol. 2002, 18, 200–203. [Google Scholar] [CrossRef]
- Josileen, J. Morphometrics and Length-Weight Relationship in the Blue Swimmer Crab, Portunus Pelagicus (Linnaeus, 1758) (Decapoda, Brachyura) from the Mandapam Coast, India. Crustaceana 2011, 84, 1665–1681. [Google Scholar] [CrossRef]
- Kampouris, T.E.; Kouroupakis, E.; Batjakas, I.E. Morphometric Relationships of the Global Invader Callinectes sapidus Rathbun, 1896 (Decapoda, Brachyura, Portunidae) from Papapouli Lagoon, NW Aegean Sea, Greece. with Notes on Its Ecological Preferences. Fishes 2020, 5, 5. [Google Scholar] [CrossRef]
- Zairion; Fauziyah; Riani, E.; Hakim, A.A.; Mashar, A.; Madduppa, H.; Wardiatno, Y. Morphometric Character Variation of the Blue Swimming Crab (Portunus Pelagicus Linnaeus, 1758) Population in Western and Eastern Part of Java Sea. IOP Conf. Ser. Earth Environ. Sci. 2020, 420, 012034. [Google Scholar] [CrossRef]
- González-Gurriarán, E.; Freire, J. Movement Patterns and Habitat Utilization in the Spider Crab Maja Squinado (Herbst) (Decapoda, Majidae) Measured by Ultrasonic Telemetry. J. Exp. Mar. Biol. Ecol. 1994, 184, 269–291. [Google Scholar] [CrossRef]
- Block, J.D.; Rebach, S. Correlates of Claw Strength in the Rock Crab, Cancer Irroratus (Decapoda, Brachyura). Crustaceana 1998, 71, 468–473. [Google Scholar] [CrossRef]
- Rufino, M.; Abelló, P.; Yule, A.B. Male and Female Carapace Shape Differences in Liocarcinus Depurator (Decapoda, Brachyura): An Application of Geometric Morphometric Analysis to Crustaceans. Ital. J. Zool. 2004, 71, 79–83. [Google Scholar] [CrossRef]
- Torres, M.V.; Collins, P.A.; Giri, F. Morphological Variation of Freshwater Crabs Zilchiopsis Collastinensis and Trichodactylus Borellianus (Decapoda, Trichodactylidae) among Localities from the Middle Paraná River Basin during Different Hydrological Periods. Zookeys 2014, 457, 171–186. [Google Scholar] [CrossRef]
- Zelditch, M.; Swiderski, D.; Sheets, H.D. Geometric Morphometrics for Biologists: A Primer; Academic Press: Amsterdam, The Netherlands, 2012; ISBN 978-0-12-386903-6. [Google Scholar]
- Nehring, S. Invasion History and Success of the American Blue Crab Callinectes Sapidus in European and Adjacent Waters. In In the Wrong Place—Alien Marine Crustaceans: Distribution, Biology and Impacts; Galil, B.S., Clark, P.F., Carlton, J.T., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 607–624. ISBN 978-94-007-0591-3. [Google Scholar]
- Ragonese, S.; Bertolino, F.; Bianchini, M.L. Biometric Relationships of the Red Shrimp, Aristaeomorpha Foliacea Risso 1827, in the Strait of Sicily (Mediterranean Sea). Sci. Mar. 1997, 61, 367–377. [Google Scholar]
- Taylor, L.T.; Hadžalić, S.; Horvat, K.; Lelas, L. The Breeding Birds of Palud, Istria. Larus—Godišnjak Zavoda Za Ornitol. Hrvat. Akad. Znan. I Umjet. 2020, 55, 34–41. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Romano, N.; Wu, X.; Zeng, C.; Genodepa, J.; Elliman, J. Growth, Osmoregulatory Responses and Changes to the Lipid and Fatty Acid Composition of Organs from the Mud Crab, Scylla Serrata, over a Broad Salinity Range. Mar. Biol. Res. 2014, 10, 460–471. [Google Scholar] [CrossRef]
- Tomasetti, S.J.; Morrell, B.K.; Merlo, L.R.; Gobler, C.J. Individual and Combined Effects of Low Dissolved Oxygen and Low pH on Survival of Early Stage Larval Blue Crabs, Callinectes sapidus. PLoS ONE 2018, 13, e0208629. [Google Scholar] [CrossRef] [PubMed]
- Rome, M.S.; Young-Williams, A.C.; Davis, G.R.; Hines, A.H. Linking Temperature and Salinity Tolerance to Winter Mortality of Chesapeake Bay Blue Crabs (Callinectes sapidus). J. Exp. Mar. Biol. Ecol. 2005, 319, 129–145. [Google Scholar] [CrossRef]
- Marchessaux, G.; Barré, N.; Mauclert, V.; Lombardini, K.; Durieux, E.D.H.; Veyssiere, D.; Filippi, J.-J.; Bracconi, J.; Aiello, A.; Garrido, M. Salinity Tolerance of the Invasive Blue Crab Callinectes sapidus: From Global to Local, a New Tool for Implementing Management Strategy. Sci. Total Environ. 2024, 954, 176291. [Google Scholar] [CrossRef] [PubMed]
- Galil, B. Callinectes sapidus (Blue Crab). CABI Compend. 2024, 90126. [Google Scholar] [CrossRef]
- Waldbusser, G.G.; Salisbury, J.E. Ocean Acidification in the Coastal Zone from an Organism’s Perspective: Multiple System Parameters, Frequency Domains, and Habitats. Annu. Rev. Mar. Sci. 2014, 6, 221–247. [Google Scholar] [CrossRef]
- Baumann, H.; Wallace, R.B.; Tagliaferri, T.; Gobler, C.J. Large Natural pH, CO2 and O2 Fluctuations in a Temperate Tidal Salt Marsh on Diel, Seasonal, and Interannual Time Scales. Estuaries Coasts 2015, 38, 220–231. [Google Scholar] [CrossRef]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.; Hendriks, I.E.; Ramajo, L.; Singh, G.S.; Duarte, C.M.; Gattuso, J.-P. Impacts of Ocean Acidification on Marine Organisms: Quantifying Sensitivities and Interaction with Warming. Glob. Change Biol. 2013, 19, 1884–1896. [Google Scholar] [CrossRef]
- Whiteley, N.M. Physiological and Ecological Responses of Crustaceans to Ocean Acidification. Mar. Ecol. Prog. Ser. 2011, 430, 257–271. [Google Scholar] [CrossRef]
- Byrne, M.; Przeslawski, R. Multistressor Impacts of Warming and Acidification of the Ocean on Marine Invertebrates’ Life Histories. Integr. Comp. Biol. 2013, 53, 582–596. [Google Scholar] [CrossRef]
- Herrera, I.; de Carvalho-Souza, G.F.; González-Ortegón, E. Physiological Responses of the Invasive Blue Crabs Callinectes sapidus to Salinity Variations: Implications for Adaptability and Invasive Success. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2024, 297, 111709. [Google Scholar] [CrossRef]
- Tiralongo, F.; Nota, A.; Pasquale, C.D.; Muccio, E.; Felici, A. Trophic Interactions of Callinectes sapidus (Blue Crab) in Vendicari Nature Reserve (Central Mediterranean, Ionian Sea) and First Record of Penaeus Aztecus (Brown Shrimp). Diversity 2024, 16, 724. [Google Scholar] [CrossRef]
- Marchessaux, G.; Sibella, B.; Garrido, M.; Abbruzzo, A.; Sarà, G. Can We Control Marine Invasive Alien Species by Eating Them? The Case of Callinectes sapidus. Ecol. Soc. 2024, 29, 19. [Google Scholar] [CrossRef]
- Olmi, E.J., III; Bishop, J.M. Variations in Total Width-Weight Relationships of Blue Crabs, Callinectes sapidus, in Relation to Sex, Maturity, Molt Stage, and Carapace Form. J. Crustac. Biol. 1983, 3, 575–581. [Google Scholar] [CrossRef]
- Hines, A.H. Ecology of Juvenile and Adult Blue Crabs. In The Blue Crab: Callinectes Sapidus; Maryland Sea Grant College, University of Maryland: College Park, MD, USA, 2007; pp. 565–654. ISBN 978-0-943676-67-8. [Google Scholar]
- Jivoff, P. Sexual Competition Among Male Blue Crab, Callinectes sapidus. Biol. Bull. 1997, 193, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Kevrekidis, K. Relative Growth of the Blue Crab Callinectes sapidus in Thermaikos Gulf (Methoni Bay), Northern Aegean Sea. Cah. Biol. Mar. 2019, 60, 395–397. [Google Scholar] [CrossRef]
- Green, B.S.; Gardner, C.; Hochmuth, J.D.; Linnane, A. Environmental Effects on Fished Lobsters and Crabs. Rev. Fish Biol. Fish. 2014, 24, 613–638. [Google Scholar] [CrossRef]
- Hines, A.H.; Johnson, E.G.; Darnell, M.Z.; Rittschof, D.; Miller, T.J.; Bauer, L.J.; Rodgers, P.; Aguilar, R. Predicting Effects of Climate Change on Blue Crabs in Chesapeake Bay. In Proceedings of the Symposium Biology and Management of Exploited Crab Populations Under Climate Change, Anchorage, AK, USA, 10–13 March 2009; Alaska Sea Grant, University of Alaska Fairbanks: Fairbanks, AK, USA, 2010; pp. 109–127. [Google Scholar]
- Bauer, L.J.; Miller, T.J. Spatial and Interannual Variability in Winter Mortality of the Blue Crab (Callinectes sapidus) in the Chesapeake Bay. Estuaries Coasts 2010, 33, 678–687. [Google Scholar] [CrossRef]
- Mancinelli, G.; Lago, N.; Scirocco, T.; Lillo, O.A.; De Giorgi, R.; Doria, L.; Mancini, E.; Mancini, F.; Potenza, L.; Cilenti, L. Abundance, Size Structure, and Growth of the Invasive Blue Crab Callinectes sapidus in the Lesina Lagoon, Southern Adriatic Sea. Biology 2024, 13, 1051. [Google Scholar] [CrossRef]
- Tiralongo, F.; Marcelli, M.; Anselmi, G.; Gattelli, R.; Felici, A. Invasion of Freshwater Systems by the Atlantic Blue Crab Callinectes sapidus Rathbun, 1896—New Insights from Italian Regions. Acta Adriat. 2024, 65, 193–204. [Google Scholar] [CrossRef]
- Hines, A.H.; Johnson, E.G.; Young, A.C.; Aguilar, R.; Kramer, M.A.; Goodison, M.; Zmora, O.; Zohar, Y. Release Strategies for Estuarine Species with Complex Migratory Life Cycles: Stock Enhancement of Chesapeake Blue Crabs (Callinectes sapidus). Rev. Fish. Sci. 2008, 16, 175–185. [Google Scholar] [CrossRef]
- Jennings, S.; Kaiser, M.J. The Effects of Fishing on Marine Ecosystems. In Advances in Marine Biology; Blaxter, J.H.S., Southward, A.J., Tyler, P.A., Eds.; Academic Press: Amsterdam, The Netherlands, 1998; Volume 34, pp. 201–352. [Google Scholar]
- Lipcius, R.N.; Van Engel, W.A. Blue Crab Population Dynamics in Chesapeake Bay: Variation in Abundance (York River, 1972–1988) and Stock-Recruit Functions. Bull. Mar. Sci. 1990, 46, 180–194. [Google Scholar]
- Marchessaux, G.; Gjoni, V.; Sarà, G. Environmental Drivers of Size-Based Population Structure, Sexual Maturity and Fecundity: A Study of the Invasive Blue Crab Callinectes sapidus (Rathbun, 1896) in the Mediterranean Sea. PLoS ONE 2023, 18, e0289611. [Google Scholar] [CrossRef]
- Noori, A.; Moghaddam, P.; Kamrani, E.; Akbarzadeh, A.; Neitali, B.K.; Pinheiro, M.A.A. Condition Factor and Carapace Width versus Wet Weight Relationship in the Blue Swimming Crab Portunus Segnis. Anim. Biol. 2015, 65, 87–99. [Google Scholar] [CrossRef]
- Vermeiren, P.; Lennard, C.; Trave, C. Habitat, Sexual and Allometric Influences on Morphological Traits of Intertidal Crabs. Estuaries Coasts 2021, 44, 1344–1362. [Google Scholar] [CrossRef]
- De Carvalho-Souza, G.F.; Medeiros, D.V.; de A. Silva, R.; González-Ortegón, E. Width/Length–Weight Relationships and Condition Factor of Seven Decapod Crustaceans in a Brazilian Tropical Estuary. Reg. Stud. Mar. Sci. 2023, 60, 102880. [Google Scholar] [CrossRef]
- Costa, E.F.S.; Encarnação, J.; Teodósio, M.A.; Morais, P. Aquatic Species Shows Asymmetric Distribution Range Shifts in Native and Non-Native Areas. Front. Mar. Sci. 2023, 10, 1158206. [Google Scholar] [CrossRef]
- Farella, G.; Tassetti, A.N.; Menegon, S.; Bocci, M.; Ferrà, C.; Grati, F.; Fadini, A.; Giovanardi, O.; Fabi, G.; Raicevich, S.; et al. Ecosystem-Based MSP for Enhanced Fisheries Sustainability: An Example from the Northern Adriatic (Chioggia—Venice and Rovigo, Italy). Sustainability 2021, 13, 1211. [Google Scholar] [CrossRef]
- Nardelli, L.; Fucilli, V.; Pinto, H.; Elston, J.N.; Carignani, A.; Petrontino, A.; Bozzo, F.; Frem, M. Socio-Economic Impacts of the Recent Bio-Invasion of Callinectus Sapidus on Small-Scale Artisanal Fishing in Southern Italy and Portugal. Front. Mar. Sci. 2024, 11, 1466132. [Google Scholar] [CrossRef]
- Kuhlbrodt, T.; Swaminathan, R.; Ceppi, P.; Wilder, T. A Glimpse into the Future: The 2023 Ocean Temperature and Sea Ice Extremes in the Context of Longer-Term Climate Change. Bull. Am. Meteorol. Soc. 2024, 105, E474–E485. [Google Scholar] [CrossRef]
- Galappaththi, E.K.; Susarla, V.B.; Loutet, S.J.T.; Ichien, S.T.; Hyman, A.A.; Ford, J.D. Climate Change Adaptation in Fisheries. Fish Fish. 2022, 23, 4–21. [Google Scholar] [CrossRef]
- Garcia-Rueda, A.L.; Mascaro, M.; Rodriguez-Fuentes, G.; Caamal-Monsreal, C.P.; Diaz, F.; Paschke, K.; Rosas, C. Moderate Hypoxia Mitigates the Physiological Effects of High Temperature on the Tropical Blue Crab Callinectes sapidus. Front. Physiol. 2023, 13, 1089164. [Google Scholar] [CrossRef]
- Vasconcelos, P.; Carvalho, A.N.; Piló, D.; Pereira, F.; Encarnação, J.; Gaspar, M.B.; Teodósio, M.A. Recent and Consecutive Records of the Atlantic Blue Crab (Callinectes sapidus Rathbun, 1896): Rapid Westward Expansion and Confirmed Establishment along the Southern Coast of Portugal. Thalassas 2019, 35, 485–494. [Google Scholar] [CrossRef]

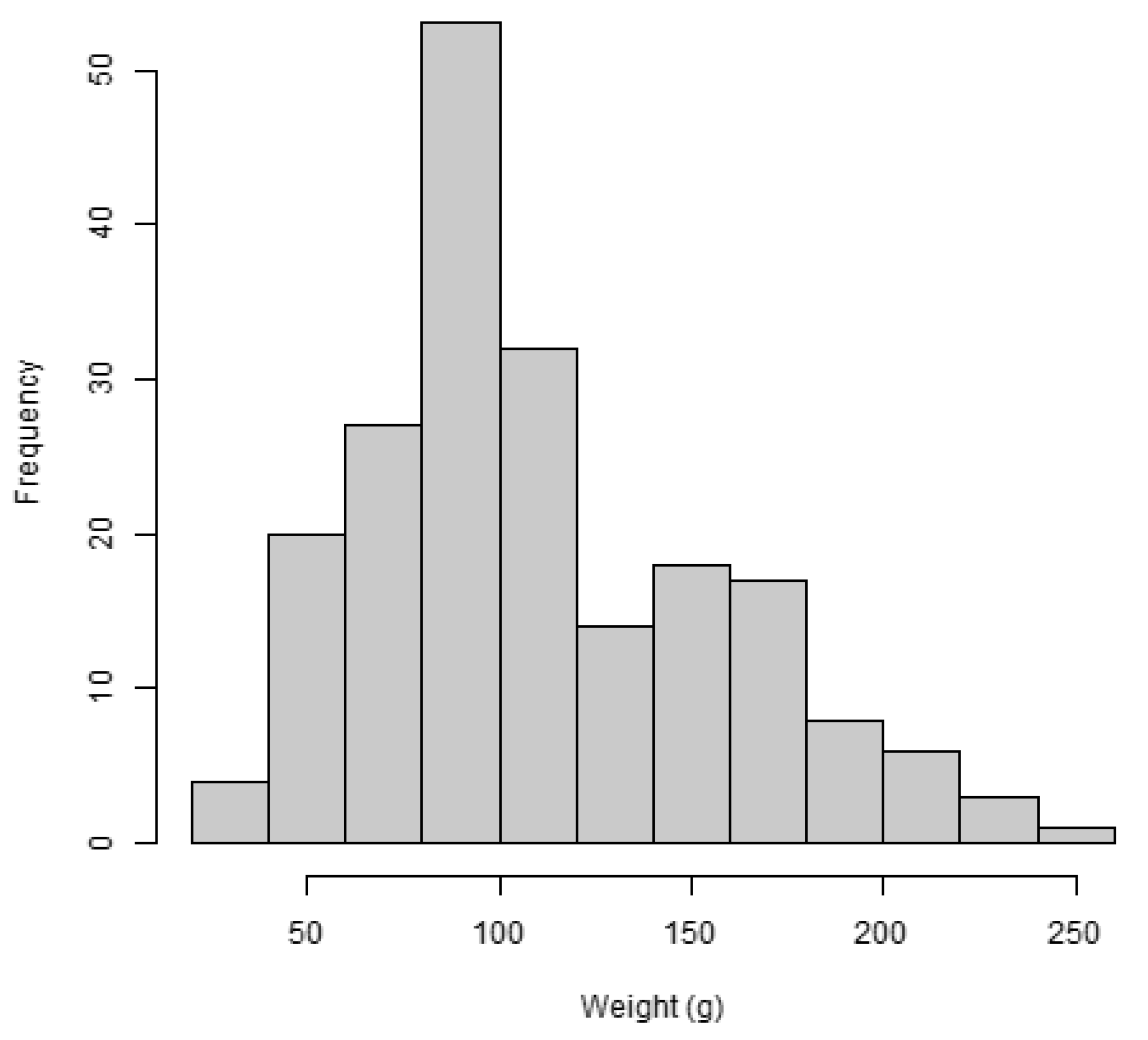
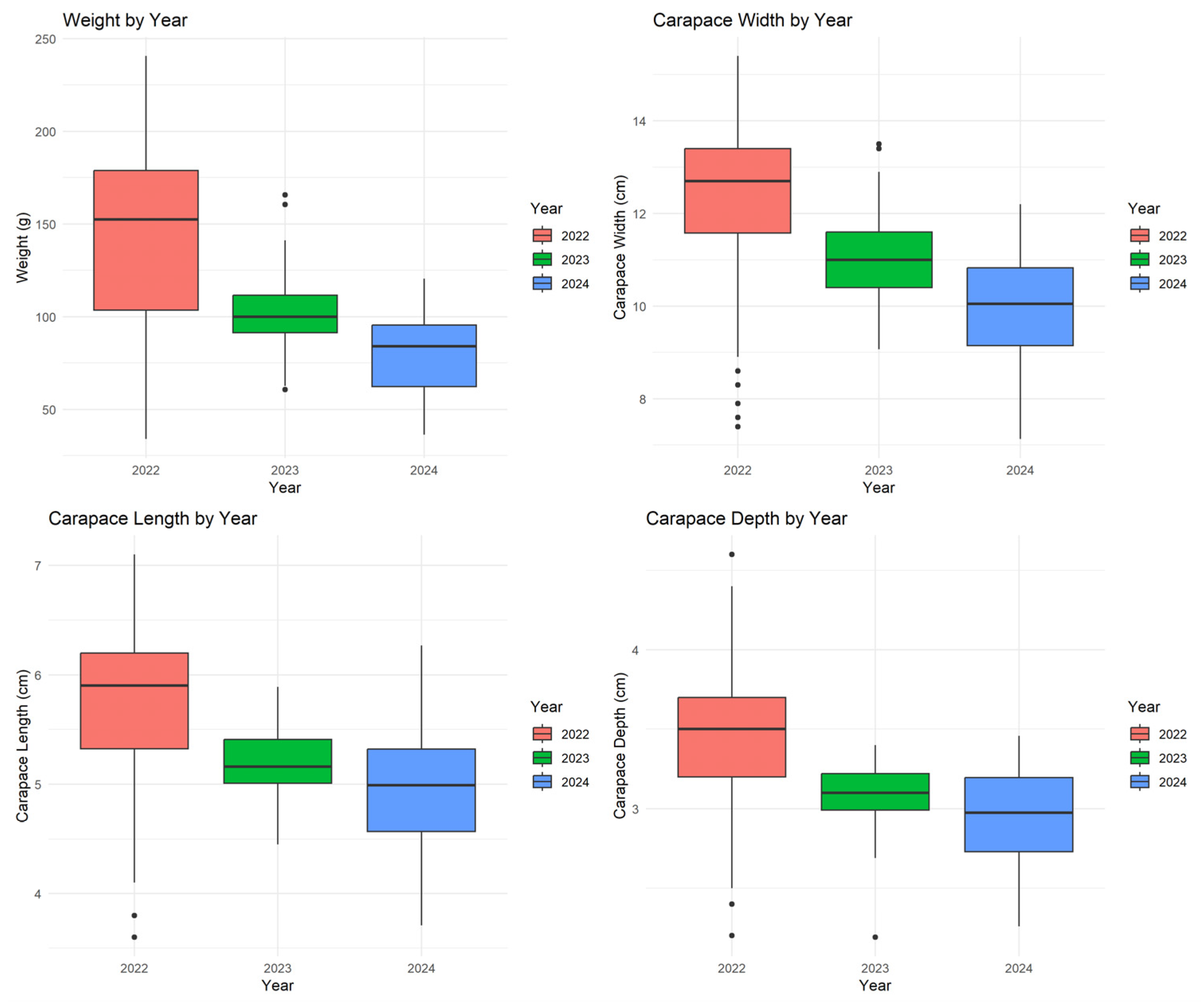
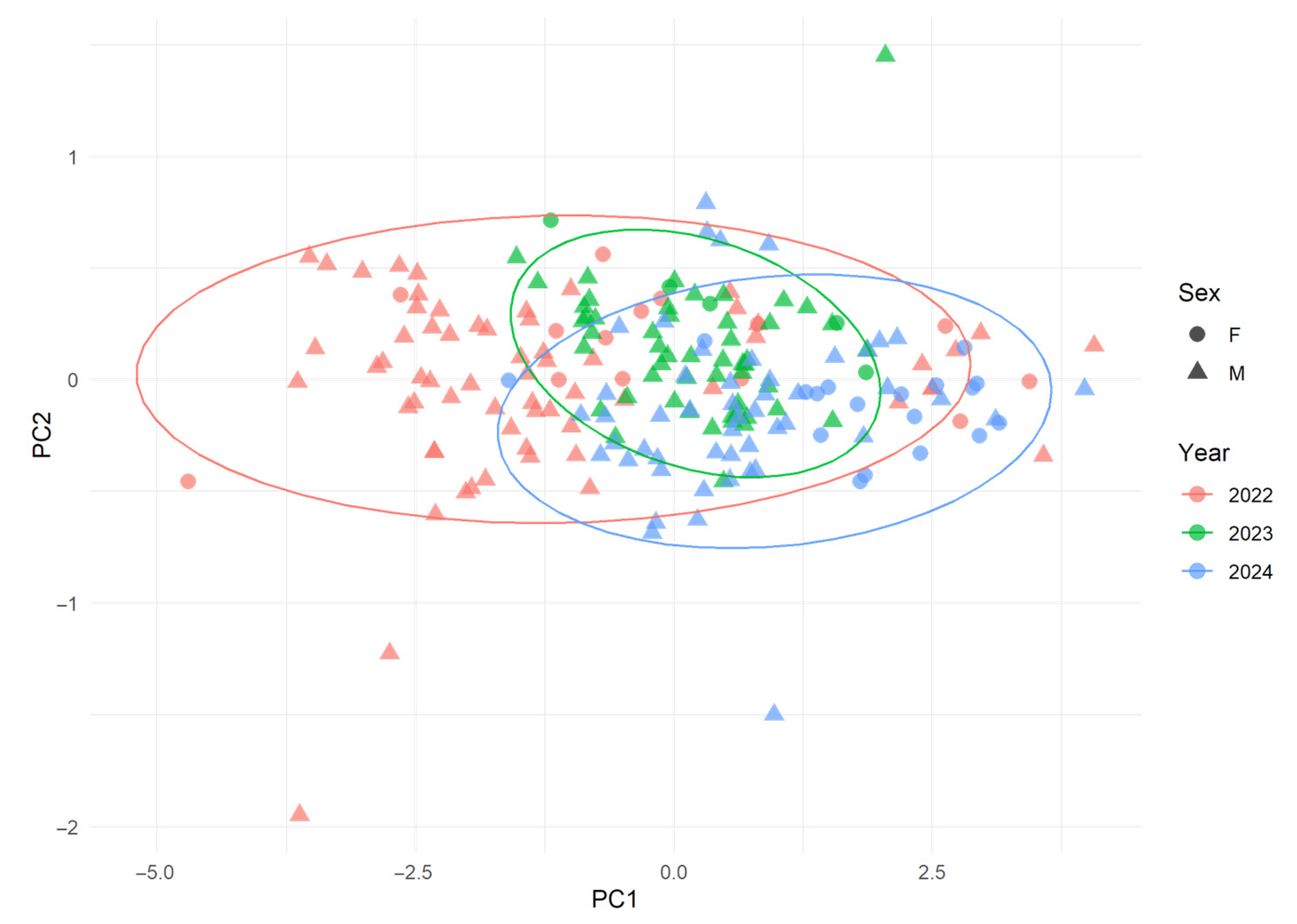
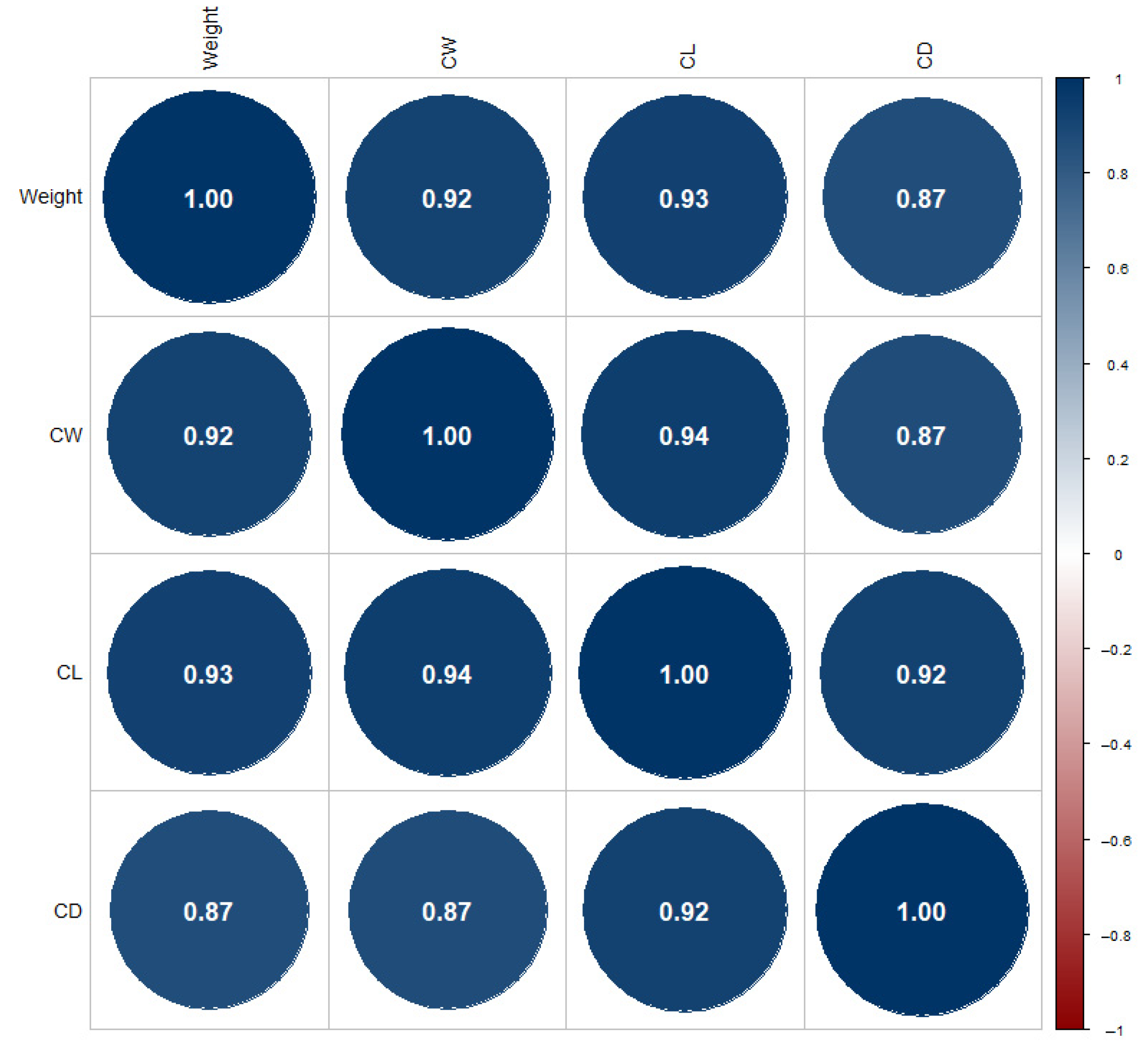
| Variable | Count (n) | Average | Median | Min | Max | SD |
|---|---|---|---|---|---|---|
| Weight (g) | 203 | 111.08 | 100.00 | 34.00 | 240.60 | 45.79 |
| CW (cm) | 203 | 11.14 | 11.00 | 7.13 | 15.40 | 1.73 |
| CL (cm) | 203 | 5.30 | 5.26 | 3.60 | 7.10 | 0.68 |
| CD (cm) | 203 | 3.16 | 3.13 | 2.19 | 4.60 | 0.40 |
| Source | df | Sum of Squares | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Sex | 1 | 27.21 | 27.209 | 18.29 | <0.001 |
| Residuals | 201 | 298.94 | 1.487 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iveša, N.; Paliaga, P.; Čief, M.; Burić, P.; Pitacco, V.; Buršić, M. Morphometrics of the Blue Crab Callinectes sapidus Rathbun, 1896 in a Northern Adriatic Saline Marsh Under Environmental Stress. Appl. Sci. 2025, 15, 7990. https://doi.org/10.3390/app15147990
Iveša N, Paliaga P, Čief M, Burić P, Pitacco V, Buršić M. Morphometrics of the Blue Crab Callinectes sapidus Rathbun, 1896 in a Northern Adriatic Saline Marsh Under Environmental Stress. Applied Sciences. 2025; 15(14):7990. https://doi.org/10.3390/app15147990
Chicago/Turabian StyleIveša, Neven, Paolo Paliaga, Matej Čief, Petra Burić, Valentina Pitacco, and Moira Buršić. 2025. "Morphometrics of the Blue Crab Callinectes sapidus Rathbun, 1896 in a Northern Adriatic Saline Marsh Under Environmental Stress" Applied Sciences 15, no. 14: 7990. https://doi.org/10.3390/app15147990
APA StyleIveša, N., Paliaga, P., Čief, M., Burić, P., Pitacco, V., & Buršić, M. (2025). Morphometrics of the Blue Crab Callinectes sapidus Rathbun, 1896 in a Northern Adriatic Saline Marsh Under Environmental Stress. Applied Sciences, 15(14), 7990. https://doi.org/10.3390/app15147990








