Assessment of Alveolar Bone Dimensions in Immediate Versus Staged Reconstruction in Sites with Implant Failure
Abstract
1. Introduction
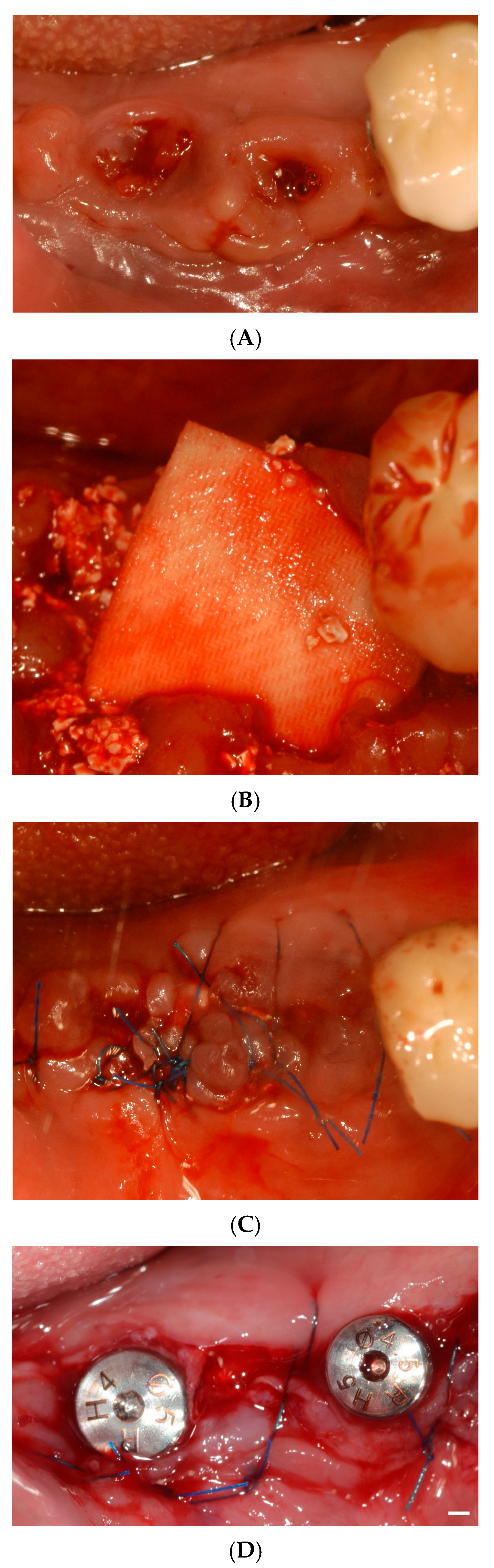
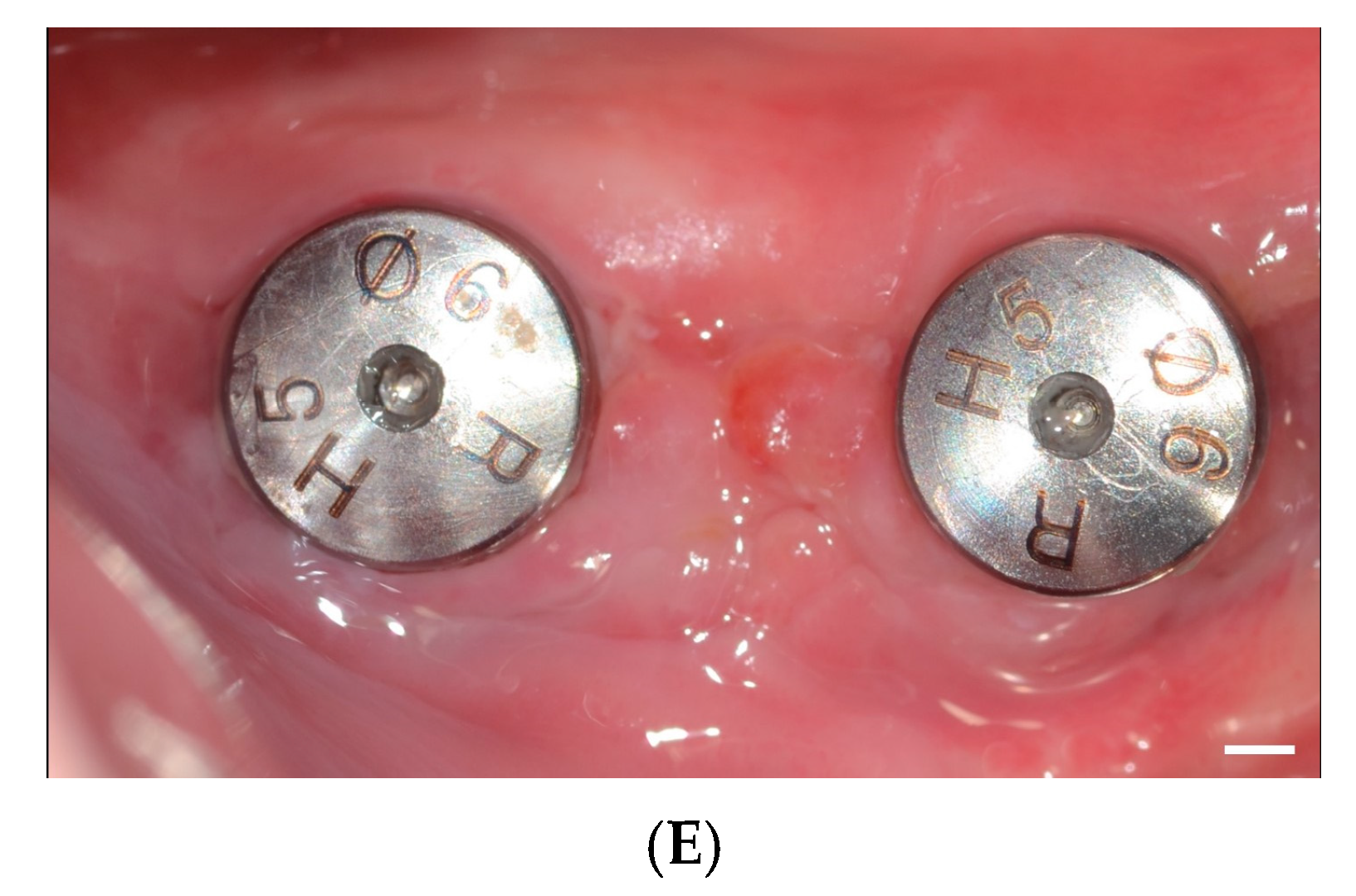
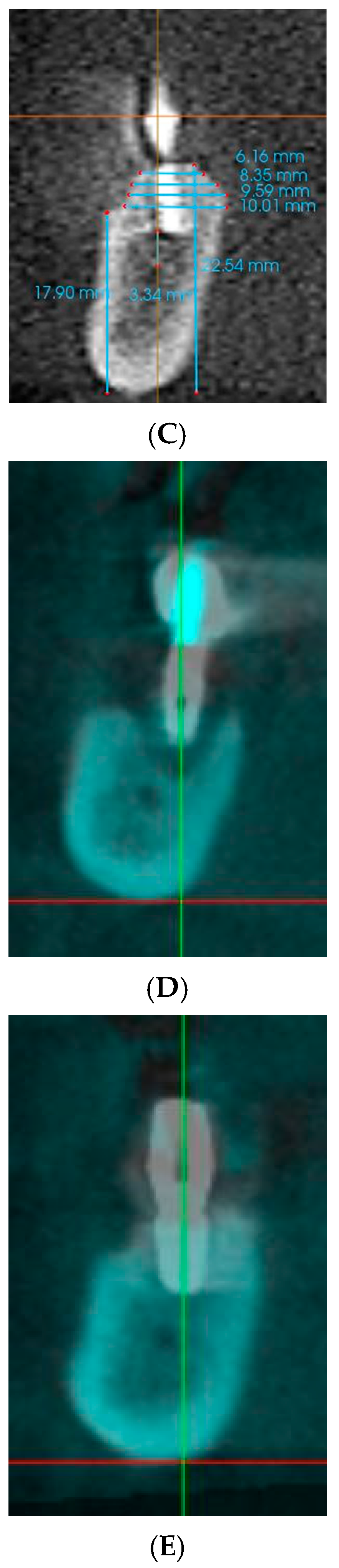
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
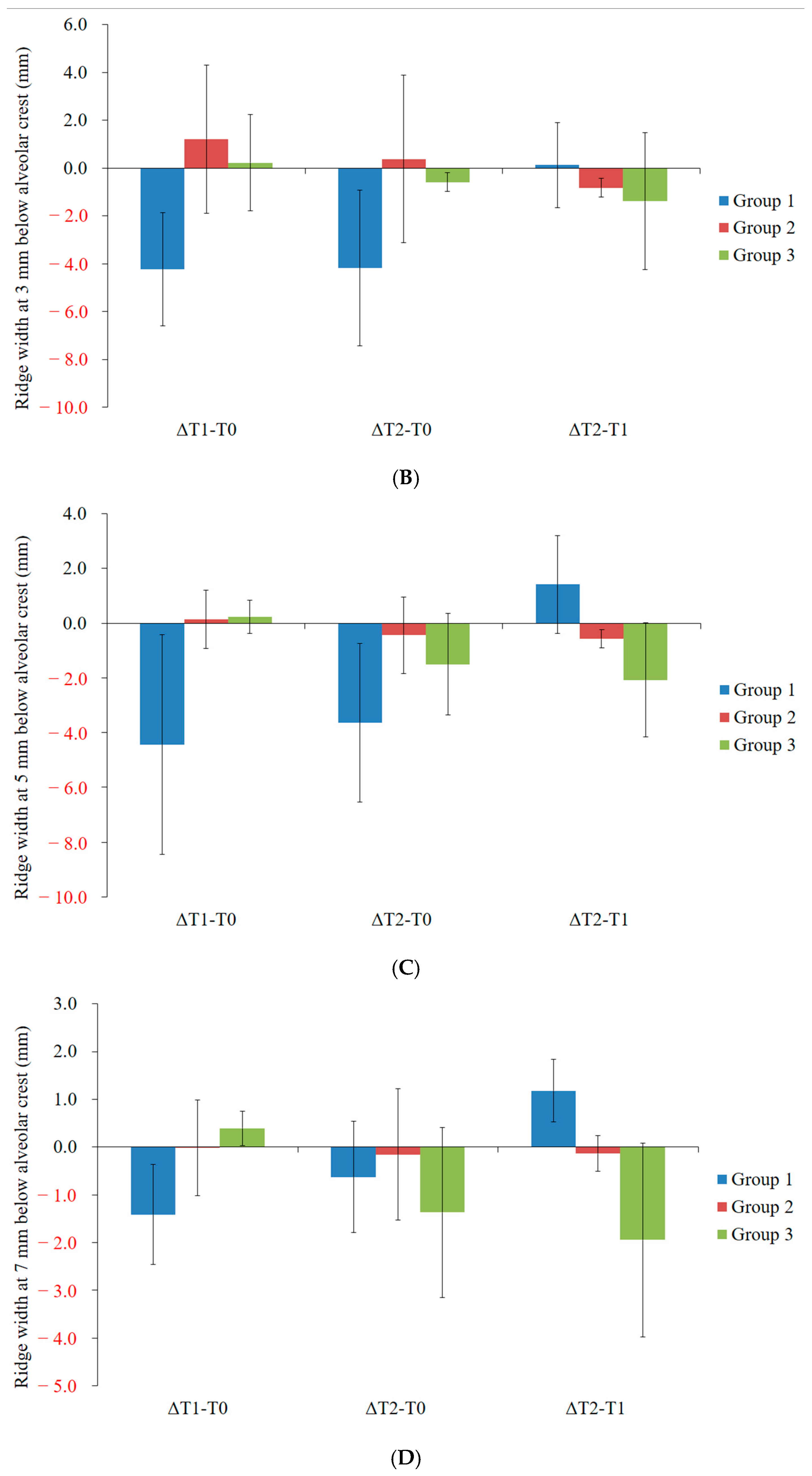
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Setzer, F.C.; Kim, S. Comparison of long-term survival of implants and endodontically treated teeth. J. Dent. Res. 2014, 93, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Nandini, N.; Kunusoth, R.; Alwala, A.M.; Prakash, R.; Sampreethi, S.; Katkuri, S. Cylindrical Implant Versus Tapered Implant: A Comparative Study. Cureus 2022, 14, e29675. [Google Scholar] [CrossRef] [PubMed]
- Aldhuwayhi, S. Zirconia in Dental Implantology: A Review of the Literature with Recent Updates. Bioengineering 2025, 12, 543. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Lee, S.H.; Kim, N.; Park, S.; Jin, S.H.; Choi, B.K.; Kim, K.K.; Ko, Y. Instrumentation With Ultrasonic Scalers Facilitates Cleaning of the Sandblasted and Acid-Etched Titanium Implants. J. Oral Implant. 2015, 41, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.C.P.; Khan, A.; Antunes, E.; Miller, C.M.; Sharma, D. The effects of physical decontamination methods on zirconia implant surfaces: A systematic review. J. Periodontal Implant. Sci. 2021, 51, 298–315. [Google Scholar] [CrossRef] [PubMed]
- Chopra, M.; Vermani, M.; Swarup, N.; Pal, A.; Chowdhary, Z. Surface Treatment and Implant Bone Interface: A Systematic Literature Review. J. Long Term Eff. Med. Implant. 2020, 30, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Kochar, S.P.; Reche, A.; Paul, P. The Etiology and Management of Dental Implant Failure: A Review. Cureus 2022, 14, e30455. [Google Scholar] [CrossRef] [PubMed]
- Solderer, A.; Al-Jazrawi, A.; Sahrmann, P.; Jung, R.; Attin, T.; Schmidlin, P.R. Removal of failed dental implants revisited: Questions and answers. Clin. Exp. Dent. Res. 2019, 5, 712–724. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Siow, L.; Zhang, X.; Wang, Y.; Wang, H.; Wang, B. Dental Reimplantation Treatment and Clinical Care for Patients with Previous Implant Failure-A Retrospective Study. Int. J. Environ. Res. Public Health 2022, 19, 15939. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Montalvillo, A.; Eguia, A.; Alkhraisat, M.H. Clinical outcomes of dental implants placed in the same region where previous implants failed due to peri-implantitis: A retrospective study. Int. J. Implant. Dent. 2021, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Machtei, E.E.; Mahler, D.; Oettinger-Barak, O.; Zuabi, O.; Horwitz, J. Dental implants placed in previously failed sites: Survival rate and factors affecting the outcome. Clin. Oral Implant. Res. 2008, 19, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Geraets, W.; Zhang, L.; Liu, Y.; Wismeijer, D. Annual bone loss and success rates of dental implants based on radiographic measurements. Dento Maxillo Facial Radiol. 2014, 43, 20140007. [Google Scholar] [CrossRef] [PubMed]
- Covani, U.; Marconcini, S.; Crespi, R.; Barone, A. Immediate implant placement after removal of a failed implant: A clinical and histological case report. J. Oral Implant. 2009, 35, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Soldini, M.C.; Rosen, P.S.; Tarnow, D.; Nart, J.; Pons, R. Alveolar Bone Reconstruction Simultaneous to Implant Removal due to Advanced Peri-Implantitis Defects: A Proof of Concept. J. Esthet. Restor. Dent. 2025, 37, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Lee, B.A.; Choi, S.H.; Kim, Y.T. Evaluation of failed implants and reimplantation at sites of previous dental implant failure: Survival rates and risk factors. J. Periodontal Implant. Sci. 2022, 52, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Pons, R.; Carreño, M.; Amerio, E.; Gargallo-Albiol, J.; Nart, J.; Monje, A. Hard tissue dimensional changes following implant removal due to peri-implantitis: A retrospective study. Clin. Implant. Dent. Relat. Res. 2021, 23, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.P. Bone Grafts in Dental Medicine: An Overview of Autografts, Allografts and Synthetic Materials. Materials 2023, 16, 4117. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52 (Suppl. S2), S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Steijvers, E.; Ghei, A.; Xia, Z. Manufacturing artificial bone allografts: A perspective. Biomater. Transl. 2022, 3, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Yum, H.; Han, H.S.; Lee, J.T.; Cho, Y.D.; Kim, S. Bone regeneration using activin A/BMP2 chimera (AB204) with collagen membrane in rats with calvarial defects. J. Periodontal Implant. Sci. 2024, 54, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Jang, J.; Kim, B.Y.; Song, Y.S.; Lee, D.Y. Effect of Gelatin Content on Degradation Behavior of PLLA/Gelatin Hybrid Membranes. Tissue Eng. Regen. Med. 2024, 21, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of Collagen Membranes for Bone Regeneration: A Literature Review. Materials 2020, 13, 786. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Govila, V.; Anand, V.; Anand, B. Implant Maintenance: A Clinical Update. Int. Sch. Res. Not. 2014, 2014, 908534. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.S.; Kim, J.W.; Hwang, J.H.; Ahn, K.M. Frequency of bone graft in implant surgery. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 19. [Google Scholar] [CrossRef] [PubMed]
- Masaki, C.; Kondo, Y.; Tomoeda, K.; Nodai, T.; Munemasa, T.; Mukaibo, T.; Hosokawa, R. Treatment strategies for dental implant removal: A literature review. Jpn. Dent. Sci. Rev. 2024, 60, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Hartlev, J.; Kohberg, P.; Ahlmann, S.; Andersen, N.T.; Schou, S.; Isidor, F. Patient satisfaction and esthetic outcome after immediate placement and provisionalization of single-tooth implants involving a definitive individual abutment. Clin. Oral Implant. Res. 2014, 25, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Kouveliotis, G.; Papapmanoli, E.; Tasopoulos, T.; Tampakos, I.; Michas, D.; Tzanakakis, E.-G.; Zoidis, P. Fully Digital Workflow for Immediate Loading Using a Minimally Invasive Surgical Approach: A Case Report. Prosthesis 2025, 7, 25. [Google Scholar] [CrossRef]
- Schropp, L.; Isidor, F.; Kostopoulos, L.; Wenzel, A. Patient experience of, and satisfaction with, delayed-immediate vs. delayed single-tooth implant placement. Clin. Oral Implant. Res. 2004, 15, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Park, J.Y.; Kim, S.G.; Lee, H.J. Prognosis of the implants replaced after removal of failed dental implants. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 110, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.V.; Puleo, D.A. Infection, inflammation, and bone regeneration: A paradoxical relationship. J. Dent. Res. 2011, 90, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Juncar, M.; Bran, S.; Juncar, R.I.; Baciut, M.F.; Baciut, G.; Onisor-Gligor, F. Odontogenic cervical necrotizing fasciitis, etiological aspects. Niger. J. Clin. Pr. 2016, 19, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Covani, U.; Barone, A.; Cornelini, R.; Crespi, R. Clinical outcome of implants placed immediately after implant removal. J. Periodontol. 2006, 77, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ortiz, G.; Elangovan, S.; Kramer, K.W.; Blanchette, D.; Dawson, D.V. Effect of alveolar ridge preservation after tooth extraction: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, S.; Romanos, G.; Testori, T.; Del Fabbro, M. Management of Advanced Peri-Implantitis by Guided Bone Regeneration in Combination with Trabecular Metal Fixtures, Two Months after Removal of the Failed Implants: Two-Year Results of a Single-Cohort Clinical Study. J. Clin. Med. 2024, 13, 713. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Loutan, L.; Garavaglia, G.; Hashim, D. Removal of osseointegrated dental implants: A systematic review of explantation techniques. Clin. Oral Investig. 2020, 24, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Min, E.J.; Hwa, S.; Lee, H.; Ko, Y.; Park, J.B. Crestal approach for maxillary sinus augmentation in individuals with limited alveolar bone height: An observational study. Medicine 2024, 103, e40331. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, Y.K.; Park, J.Y.; Kim, S.G.; Kim, M.J.; Yun, P.Y. Short-term clinical retrospective study of implants in geriatric patients older than 70 years. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 110, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.S.; Jo, I. Bone Morphogenic Protein-2-Conjugated Three-Dimensional-Printed Poly (L-Lactic Acid) (PLLA) Scaffold is likely Promising as an Effective Bone Substitute. Tissue Eng. Regen. Med. 2023, 20, 155–156. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jung, N.; Lee, D.J.; Oh, S.; Kim, S.; Cho, S.W.; Kim, J.E.; Moon, H.S.; Park, Y.B. Enhanced Bone Formation by Rapidly Formed Bony Wall over the Bone Defect Using Dual Growth Factors. Tissue Eng. Regen. Med. 2023, 20, 767–778. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Hwa, S.; Ko, Y.; Park, J.-B. Assessment of Alveolar Bone Dimensions in Immediate Versus Staged Reconstruction in Sites with Implant Failure. Appl. Sci. 2025, 15, 7934. https://doi.org/10.3390/app15147934
Lee H, Hwa S, Ko Y, Park J-B. Assessment of Alveolar Bone Dimensions in Immediate Versus Staged Reconstruction in Sites with Implant Failure. Applied Sciences. 2025; 15(14):7934. https://doi.org/10.3390/app15147934
Chicago/Turabian StyleLee, Heera, Somyeong Hwa, Youngkyung Ko, and Jun-Beom Park. 2025. "Assessment of Alveolar Bone Dimensions in Immediate Versus Staged Reconstruction in Sites with Implant Failure" Applied Sciences 15, no. 14: 7934. https://doi.org/10.3390/app15147934
APA StyleLee, H., Hwa, S., Ko, Y., & Park, J.-B. (2025). Assessment of Alveolar Bone Dimensions in Immediate Versus Staged Reconstruction in Sites with Implant Failure. Applied Sciences, 15(14), 7934. https://doi.org/10.3390/app15147934






