Homogeneous and Heterogeneous Photo-Fenton-Based Photocatalytic Techniques for the Degradation of Nile Blue Dye
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
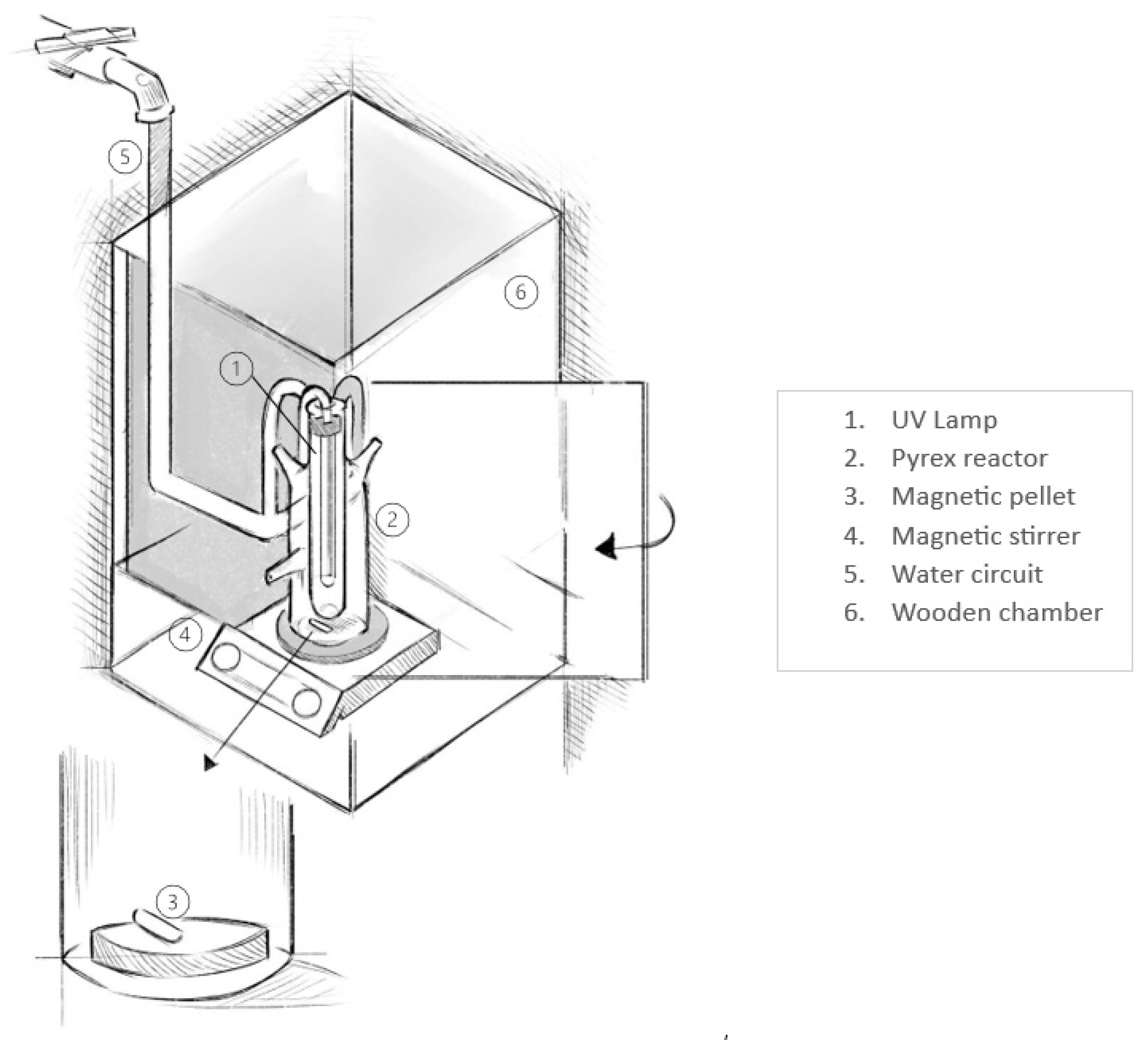
2.2. Experimental Setup
2.3. Experimental Procedures
2.4. Analytical Processes
3. Results
3.1. Preliminary/Comparative Experiments
3.1.1. Photolysis
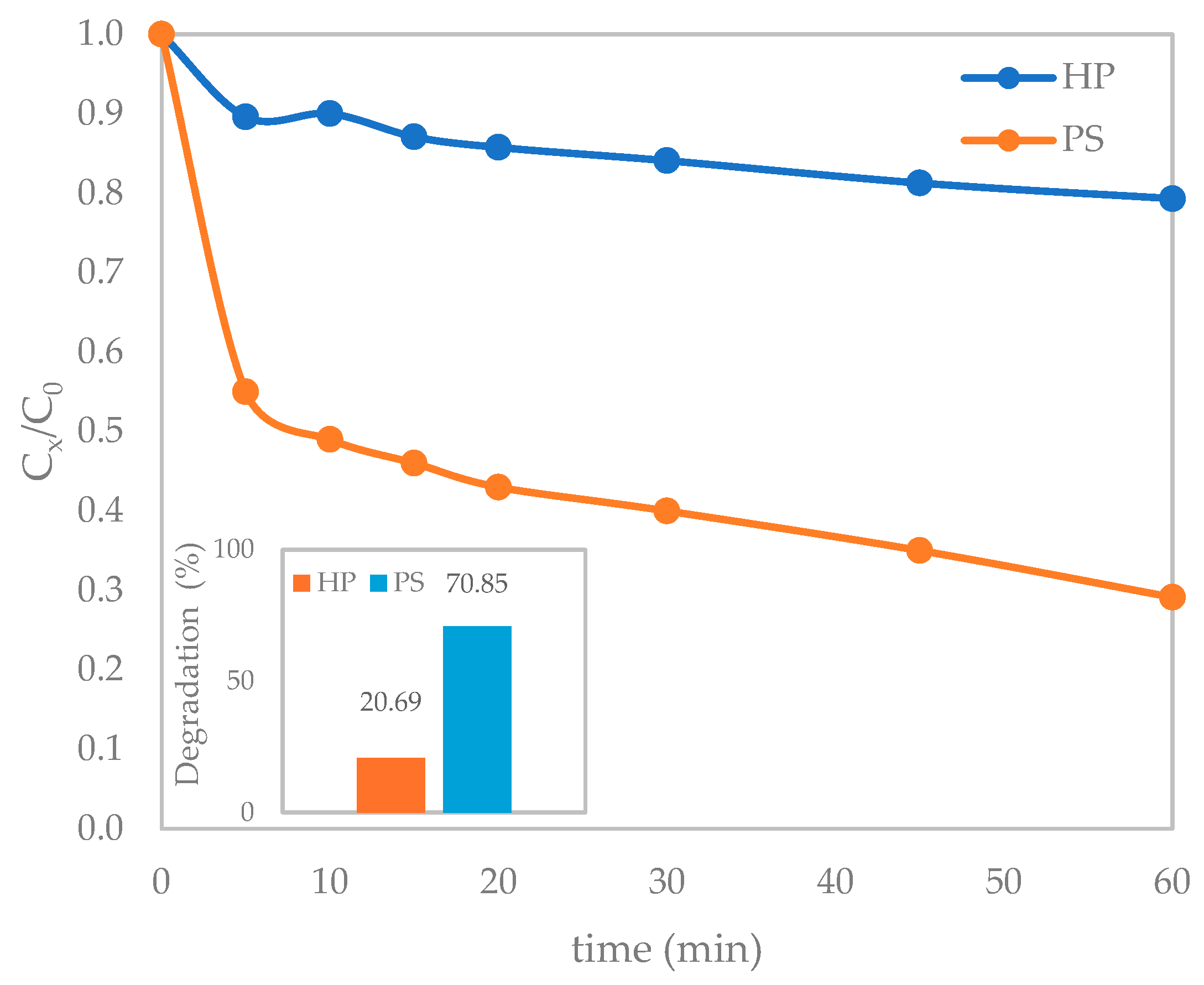
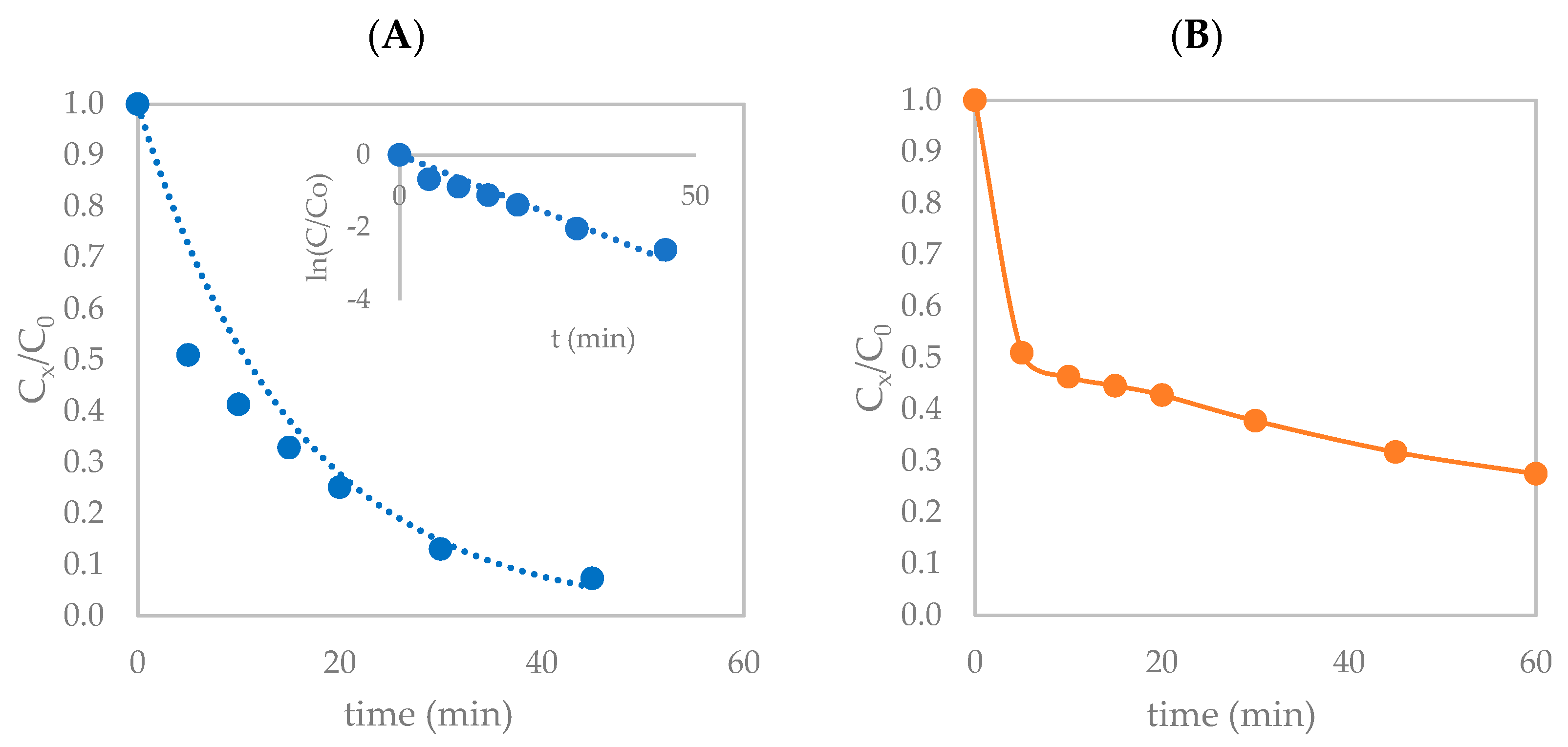
3.1.2. Photolysis with H2O2/S2O82−
3.2. Homogeneous Photocatalysis
3.2.1. Photo-Fenton and Photo-Fenton-like
Preliminary Study
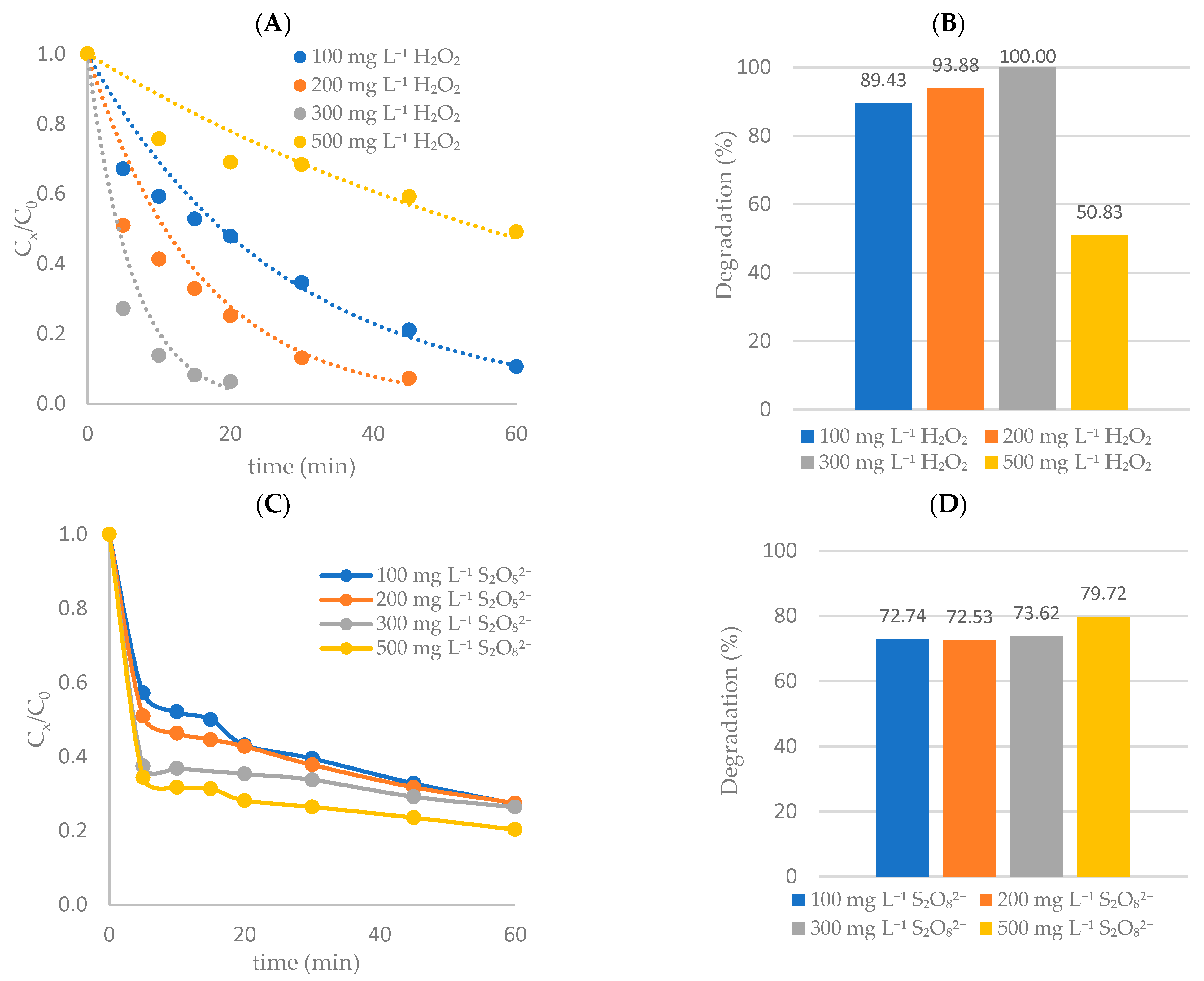
Effect of Oxidant Concentration
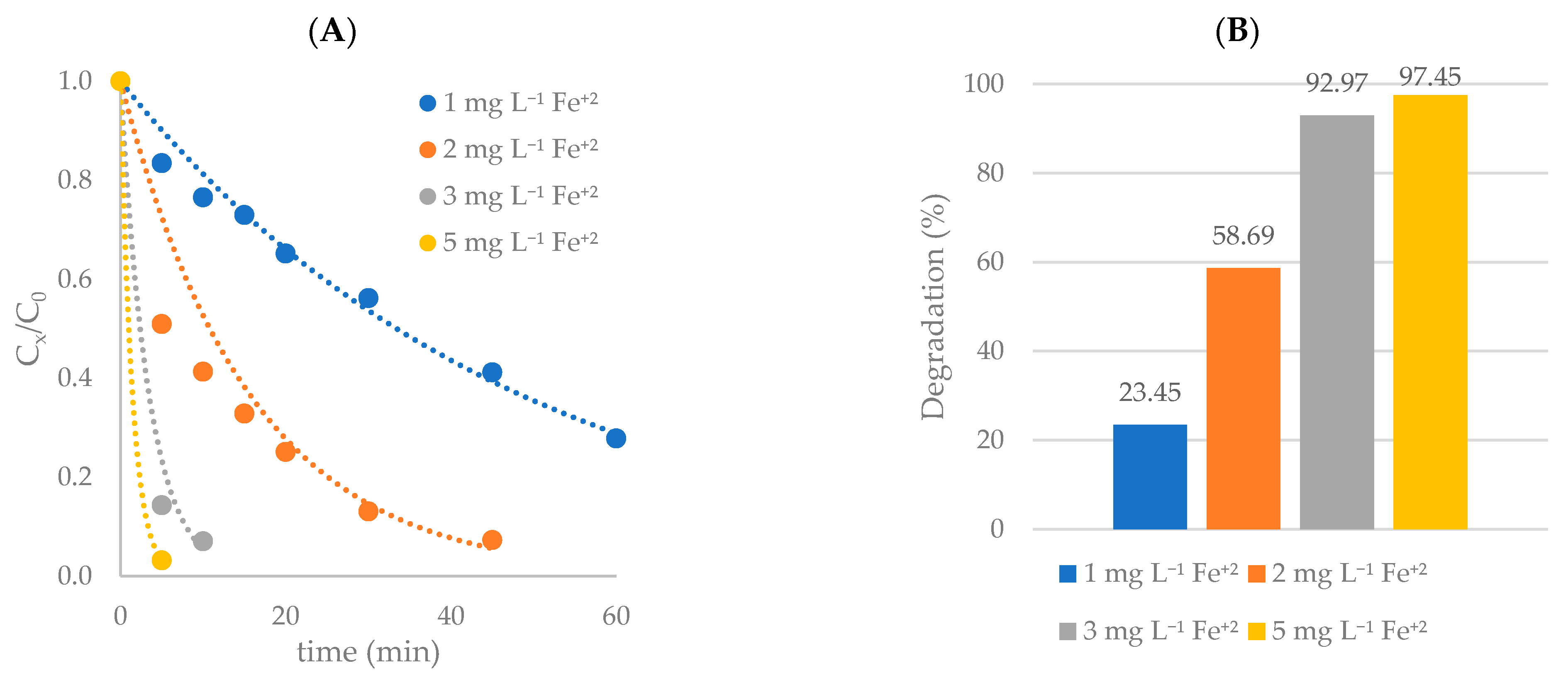
Photo-Fenton: Effect of Fe+2 Concentration
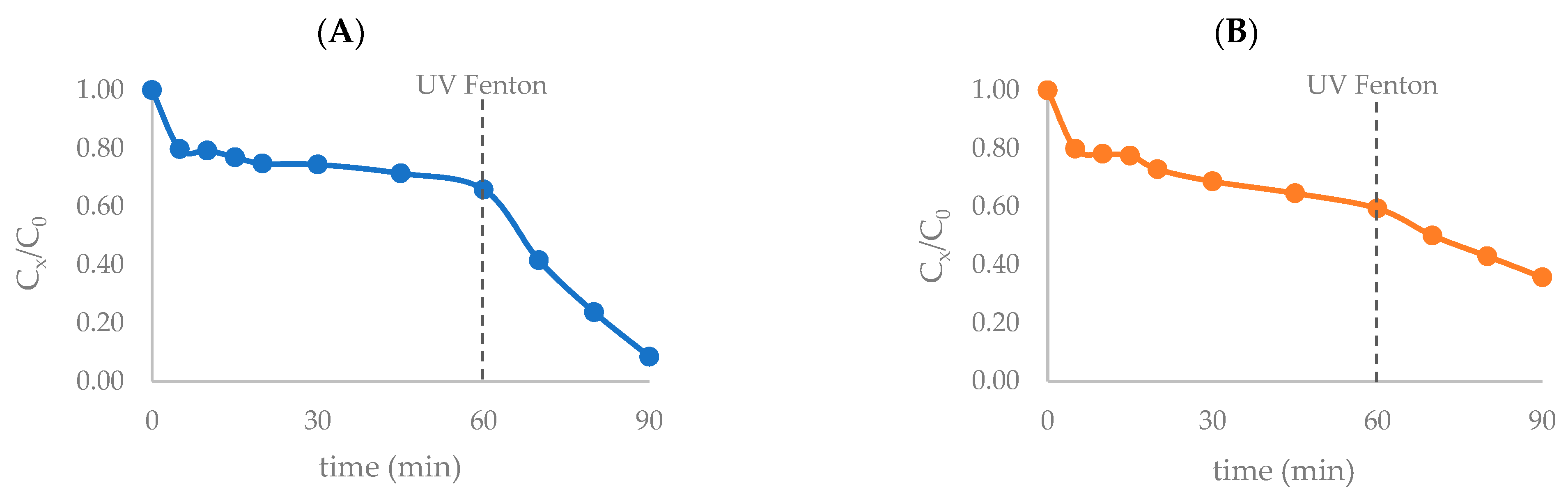
Effect of Ultraviolet Radiation
3.3. Heterogeneous Photocatalysis
3.3.1. Basolite F300 (MOF)
Preliminary Study
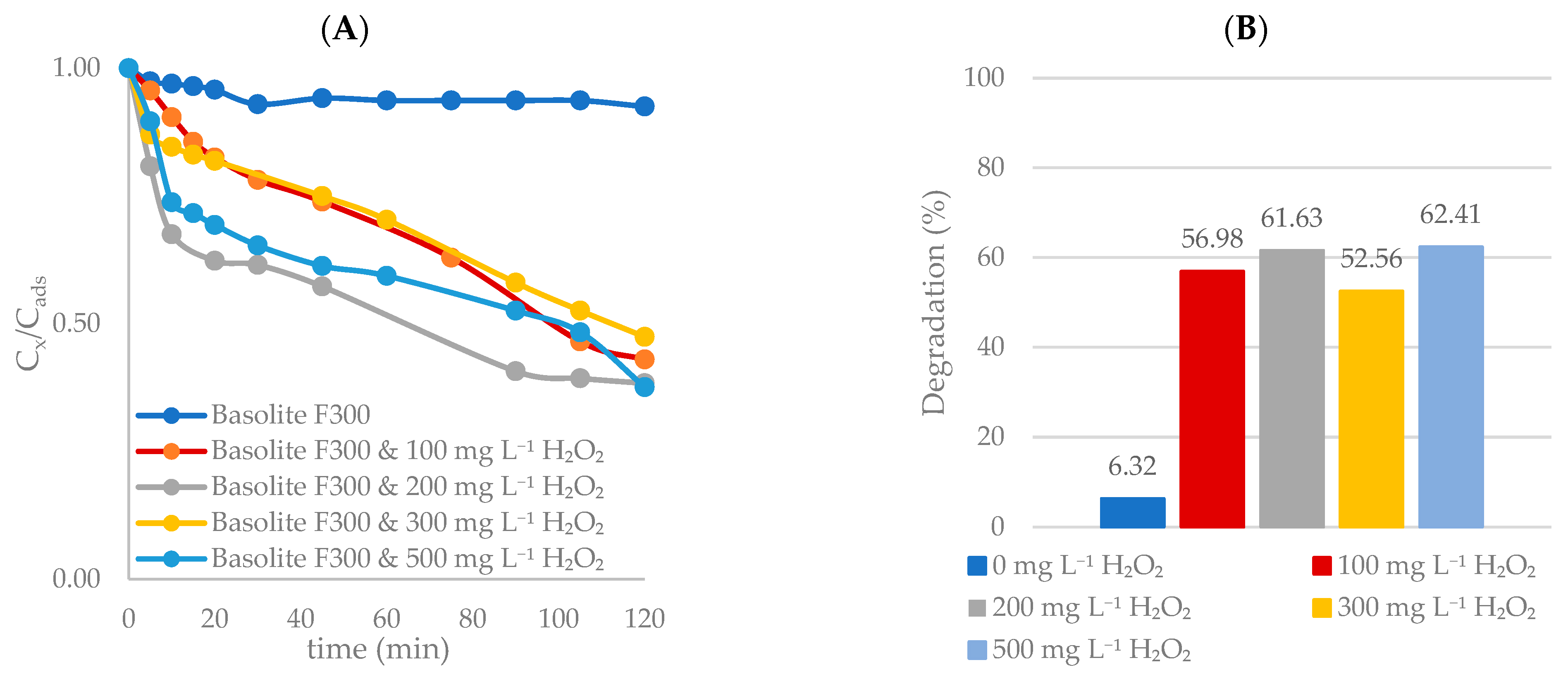
Effect of H2O2 Concentration
- To limit the hole–electron recombination.
- To produce additional active radicals from self-activation of the oxidant by radiation.
- To achieve, apart from the heterogeneous photocatalytic process, a photo-Fenton reaction, as the catalyst is an iron-based MOF.
Effect of S2O82− Concentration
3.3.2. Goethite
Preliminary Study
Effect of H2O2 Concentration
Effect of S2O82− Concentration
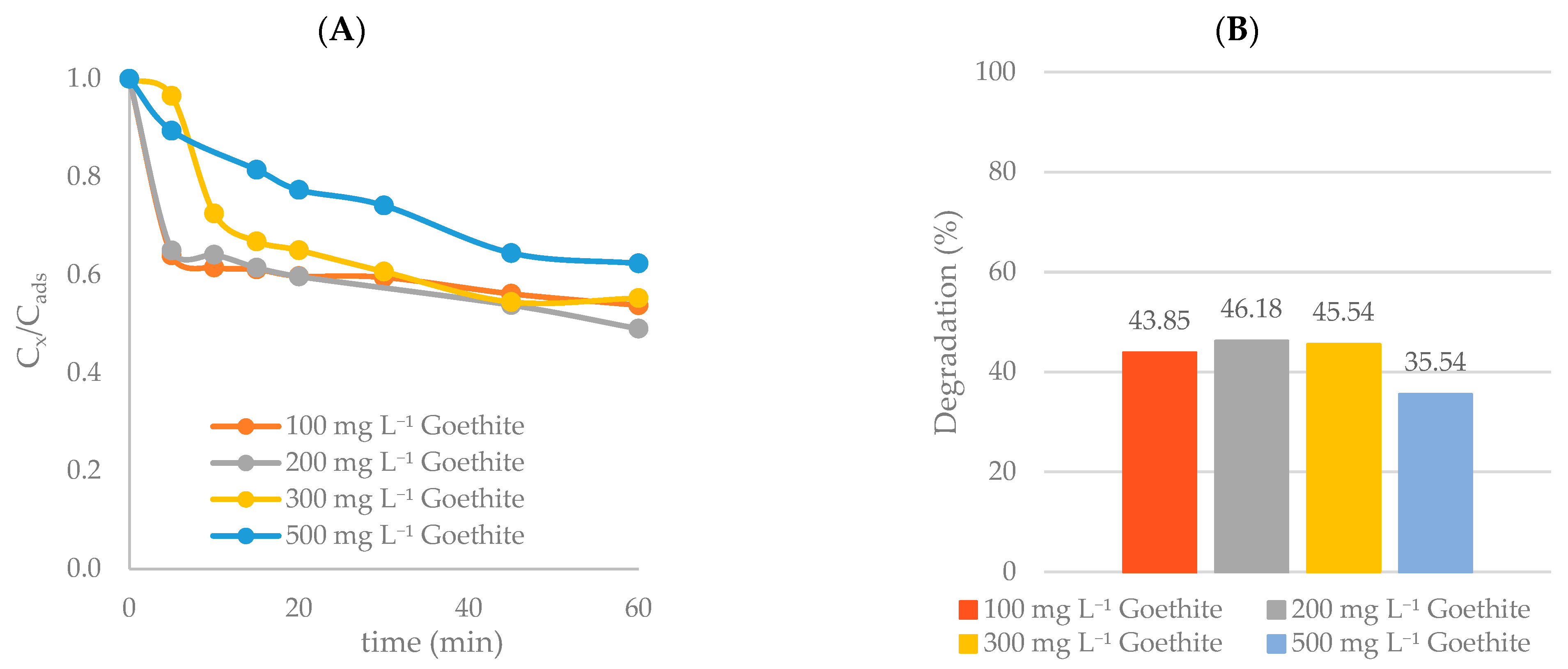
Effect of Goethite Concentration
Effect of pH
3.4. Comparison of Homogeneous and Heterogeneous Techniques
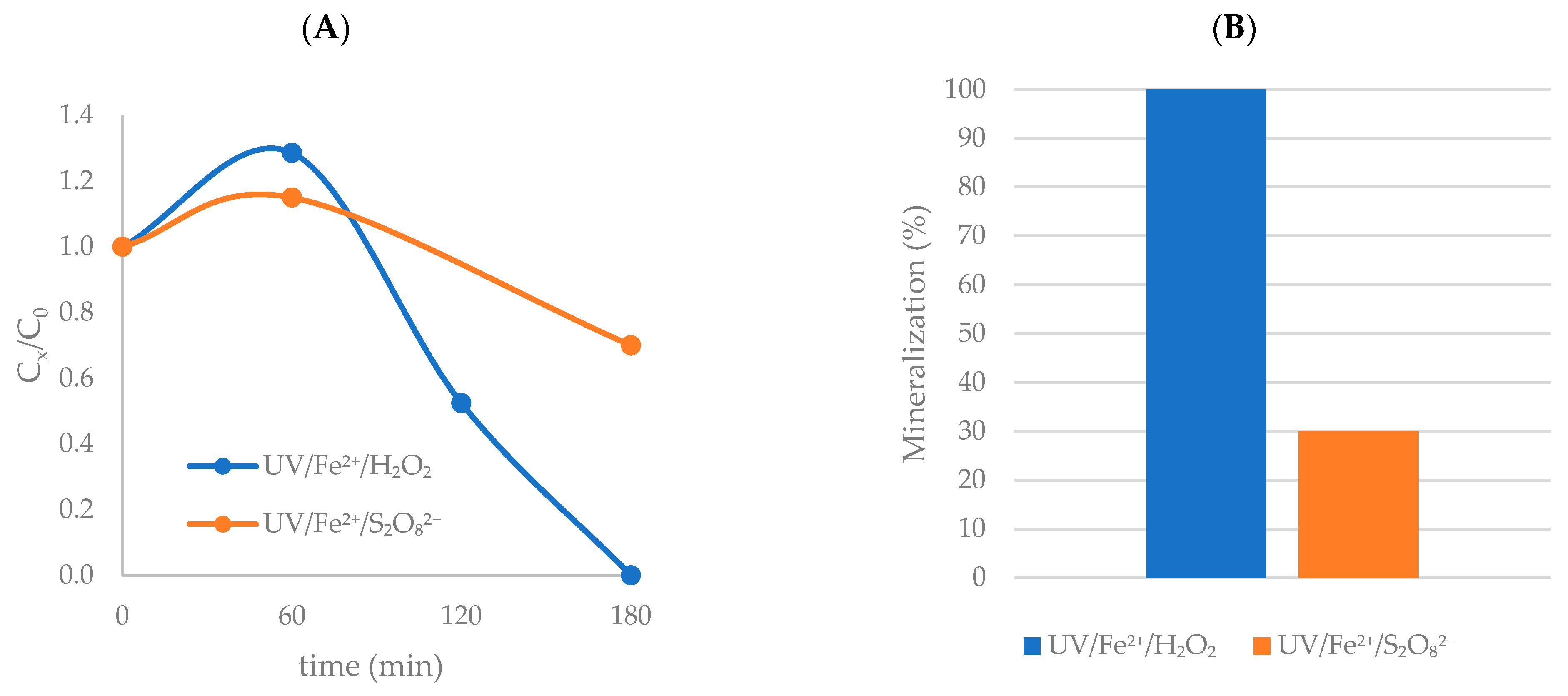
3.5. Mineralization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Islam, T.; Repon, M.R.; Islam, T.; Sarwar, Z.; Rahman, M.M. Impact of textile dyes on health and ecosystem: A review of structure, causes, and potential solutions. Environ. Sci. Pollut. Res. 2023, 30, 9207–9242. [Google Scholar] [CrossRef] [PubMed]
- Kathiresan, G.; Vijayakumar, K.; Sundarrajan, A.P.; Kim, H.-S.; Adaikalam, K. Photocatalytic degradation efficiency of ZnO, GO and PVA nanoadsorbents for crystal violet, methylene blue and trypan blue dyes. Optik 2021, 238, 166671. [Google Scholar] [CrossRef]
- Affat, S. Classifications, Advantages, Disadvantages, Toxicity Effects of Natural and Synthetic Dyes: A review. Univ. Thi-Qar J. Sci. 2021, 8, 130–135. [Google Scholar]
- Mohammed, A.F.M.; Sherif, M.M.; Hasan, A.I.; Makrahy, B.E.; Hasan, N.E. Toxic effects of chronic exposure to dyes among workers of synthetic textile industries. Egypt. J. Hosp. Med. 2019, 74, 744–751. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El Nemr, A. Health and Environmental Impacts of Dyes: Mini Review. Am. J. Environ. Sci. Eng. 2017, 1, 64–67. [Google Scholar] [CrossRef]
- Miller, J.A.; Baumann, C.A. The Carcinogenicity of Certain Azo Dyes Related to p-Dimethylaminoazobenzene. Cancer Res. 1945, 5, 227–234. [Google Scholar]
- Chung, K.-T. Azo dyes and human health: A review. J. Environ. Sci. Health Part C 2016, 34, 233–261. [Google Scholar] [CrossRef] [PubMed]
- Kupradinun, P.; Rienkijakarn, M.; Tanyakaset, M.; Tepsuwan, A.; Kusamran, W.R. Carcinogenicity Testing of the Cosmetic Dye: D&C Red No. 36. Asian Pac. J. Cancer Prev. 2002, 3, 55–60. [Google Scholar] [PubMed]
- Attri, P.; Garg, S.; Ratan, J.K.; Giri, A.S. Comparative study using advanced oxidation processes for the degradation of model dyes mixture: Reaction kinetics and biodegradability assay. Mater. Today Proc. 2022, 57, 1533–1538. [Google Scholar] [CrossRef]
- Khan, S.; Malik, A. Environmental and Health Effects of Textile Industry Wastewater. In Environmental Deterioration and Human Health: Natural and Anthropogenic Determinants; Springer: Dordrecht, The Netherlands, 2013; pp. 55–71. [Google Scholar] [CrossRef]
- Jose, J.; Ueno, Y.; Burgess, K. Water-Soluble Nile Blue Derivatives: Syntheses and Photophysical Properties. Chem.—Eur. J. 2009, 15, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Tajalli, H.; Ghanadzadeh Gilani, A.; Zakerhamidi, M.S.; Tajalli, P. The photophysical properties of Nile red and Nile blue in ordered anisotropic media. Dye. Pigment. 2008, 78, 15–24. [Google Scholar] [CrossRef]
- Mehra, S.; Singh, M.; Chadha, P. Adverse Impact of Textile Dyes on the Aquatic Environment as well as on Human Beings. Toxicol. Int. 2021, 28, 165–176. [Google Scholar] [CrossRef]
- Selvaraj, V.; Swarna Karthika, T.; Mansiya, C.; Alagar, M. An over review on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications. J. Mol. Struct. 2021, 1224, 129195. [Google Scholar] [CrossRef]
- Mahbub, P.; Duke, M. Scalability of advanced oxidation processes (AOPs) in industrial applications: A review. J. Environ. Manag. 2023, 345, 118861. [Google Scholar] [CrossRef] [PubMed]
- M’Arimi, M.M.; Mecha, C.A.; Kiprop, A.K.; Ramkat, R. Recent trends in applications of advanced oxidation processes (AOPs) in bioenergy production: Review. Renew. Sustain. Energy Rev. 2020, 121, 109669. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef] [PubMed]
- Oturan, M.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Du, C.; Zhang, Y.; Zhang, Z.; Zhou, L.; Yu, G.; Wen, X.; Chi, T.; Wang, G.; Su, Y.; Deng, F.; et al. Fe-based metal organic frameworks (Fe-MOFs) for organic pollutants removal via photo-Fenton: A review. Chem. Eng. J. 2022, 431, 133932. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Kitagawa, S. Metal–Organic Frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.; Banerjee, S.; Mani, R.; Chattopadhyaya, M.C. Synthesis, characterization and application of goethite mineral as an adsorbent. J. Environ. Chem. Eng. 2013, 1, 281–289. [Google Scholar] [CrossRef]
- Ortiz de la Plata, G.B.; Alfano, O.M.; Cassano, A.E. Optical properties of goethite catalyst for heterogeneous photo-Fenton reactions. Comparison with a titanium dioxide catalyst. Chem. Eng. J. 2008, 137, 396–410. [Google Scholar] [CrossRef]
- Ortiz de la Plata, G.B.; Alfano, O.M.; Cassano, A.E. The heterogeneous photo-Fenton reaction using goethite as catalyst. Water Sci. Technol. 2010, 61, 3109–3116. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.; Henary, M. Nile Red and Nile Blue: Applications and Syntheses of Structural Analogues. Chem.—Eur. J. 2016, 22, 13764–13782. [Google Scholar] [CrossRef] [PubMed]
- Evgenidou, E.; Konstantinou, I.; Fytianos, K.; Poulios, I.; Albanis, T. Photocatalytic oxidation of methyl parathion over TiO2 and ZnO suspensions. Catal. Today 2007, 124, 156–162. [Google Scholar] [CrossRef]
- Sarwan, B.; Pare, B.; Acharya, A.D.; Jonnalagadda, S.B. Mineralization and toxicity reduction of textile dye neutral red in aqueous phase using BiOCl photocatalysis. J. Photochem. Photobiol. B Biol. 2012, 116, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Paul Guin, J.; Bhardwaj, Y.; Varshney, L. Mineralization and biodegradability enhancement of Methyl Orange dye by an effective advanced oxidation process. Appl. Radiat. Isot. 2017, 122, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Tsamos, P.; Kolias, P.; Lambropoulou, D.; Noli, F. Distribution and temporal variability of uranium and toxic metal(loid)s in snow and rainwater from an oil industry and urban area in Thessaloniki-Greece. Sci. Total Environ. 2022, 838, 155604. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yu, Y.; Liang, L.; Duan, X.; Li, R.; Lu, X.; Yan, B.; Li, N.; Wang, S. Remediation of antibiotic wastewater by coupled photocatalytic and persulfate oxidation system: A critical review. J. Hazard. Mater. 2021, 408, 124461. [Google Scholar] [CrossRef] [PubMed]
- Scaria, J.; Nidheesh, P.V. Comparison of hydroxyl-radical-based advanced oxidation processes with sulfate radical-based advanced oxidation processes. Curr. Opin. Chem. Eng. 2022, 36, 100830. [Google Scholar] [CrossRef]
- Evgenidou, E.; Konstantinou, I.; Fytianos, K.; Poulios, I. Oxidation of two organophosphorous insecticides by the photo-assisted Fenton reaction. Water Res. 2007, 41, 2015–2027. [Google Scholar] [CrossRef] [PubMed]
- Parthenidis, P.; Faka, A.; Aristidou, N.; Evgenidou, E.; Lambropoulou, D.A. Removal of antifungal agents fluconazole and voriconazole in aquatic media by homogeneous and heterogeneous photocatalytic applications: Efficiency, optimization and transformation products. J. Water Process Eng. 2025, 70, 107030. [Google Scholar] [CrossRef]
- Kontogiannis, A.; Evgenidou, E.; Nannou, C.; Bikiaris, D.; Lambropoulou, D. MOF-based photocatalytic degradation of the antibiotic lincomycin enhanced by hydrogen peroxide and persulfate: Kinetics, elucidation of transformation products and toxicity assessment. J. Environ. Chem. Eng. 2022, 10, 108112. [Google Scholar] [CrossRef]
- Evgenidou, E.; Vasilopoulou, K.; Koronaiou, L.A.; Kyzas, G.; Bikiaris, D.; Lambropoulou, D. AOP-Based Transformation of Abacavir in Different Environments: Evolution Profile of Descyclopropyl-Abacavir and In Silico Toxicity Assessment of the Main Transformation Products. Molecules 2023, 28, 1866. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, P.; Yang, X.; Shan, L.; Zhang, W.; Shao, X.; Niu, R. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide. J. Hazard. Mater. 2010, 179, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.; Kumar, S.; Raju, K.; Rajashekhar, K.E. Photo Fenton and Photo Fenton like processes for the degradation of Methyl Orange an azo dye in aqueous medium: Influence of oxidation states of Iron. Chem. Pap. 2010, 64, 378–385. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Lin, Z.; Lin, W.; Chen, R.; Chen, P.; Lv, W.; Liu, G. Harnessing synergistic effects of UV/MgO2/PMS system for enhanced antibiotic degradation. Sep. Purif. Technol. 2025, 353, 128407. [Google Scholar] [CrossRef]
- Alvear-Daza, J.J.; Sanabria, J.; Castaño-Rodriguez, V.A.; Correa-Betancourt, A.; Binet, S.; Sánchez, F.H.; Muñoz-Medina, G.A.; Gutiérrez-Zapata, H.M.; Pizzio, L.R.; Rengifo-Herrera, J.A. Goethite (α-FeOOH) photocatalytic activity at natural concentrations by the addition of H2O2 at neutral pH and the simultaneous presence of fluoride and bicarbonate. Appl. Res. 2024, 3, e202300015. [Google Scholar] [CrossRef]
- Leland, J.K.; Bard, A.J. Photochemistry of colloidal semiconducting iron oxide polymorphs. J. Phys. Chem. 1987, 91, 5076–5083. [Google Scholar] [CrossRef]
- Wu, H.; Dou, X.; Deng, D.; Guan, Y.; Zhang, L.; He, G. Decolourization of the azo dye Orange G in aqueous solution via a heterogeneous Fenton-like reaction catalysed by goethite. Environ. Technol. 2011, 33, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Li, Y.; Mao, X.; Zhang, H. Electro-enhanced goethite activation of peroxydisulfate for the decolorization of Orange II at neutral pH: Efficiency, stability and mechanism. J. Taiwan Inst. Chem. Eng. 2016, 65, 390–398. [Google Scholar] [CrossRef]
- Schwertmann, U.; Fechter, H. The point of zero charge of natural and synthetic ferrihydrites and its relation to adsorbed silicate. Clay Miner. 1982, 17, 471–476. [Google Scholar] [CrossRef]
- Cristiano, E.; Hu, Y.-J.; Sigfried, M.; Kaplan, D.; Nitsche, H. A Comparison of Point of Zero Charge Measurement Methodology. Clays Clay Miner. 2011, 59, 107–115. [Google Scholar] [CrossRef]
- Antonopoulou, M. Homogeneous and Heterogeneous Photocatalysis for the Treatment of Pharmaceutical Industry Wastewaters: A Review. Toxics 2022, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Nasalevich, M.A.; van der Veen, M.; Kapteijn, F.; Gascon, J. Metal–organic frameworks as heterogeneous photocatalysts: Advantages and challenges. CrystEngComm 2014, 16, 4919–4926. [Google Scholar] [CrossRef]
- Pourgholi, M.; Jahandizi, R.M.; Miranzadeh, M.; Beigi, O.H.; Dehghan, S. Removal of Dye and COD from Textile Wastewater Using AOP (UV/O3, UV/H2O2, O3/H2O2 and UV/H2O2/O3). J. Environ. Health Sustain. Dev. 2018, 3, 630–636. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y. A comprehensive review on persulfate activation treatment of wastewater. Sci. Total Environ. 2022, 831, 154906. [Google Scholar] [CrossRef] [PubMed]
- Shokoohi, R.; Salari, M.; Shabanloo, A.; Shabanloo, N.; Marofi, S.; Faraji, H.; Vanaei Tabar, M.; Moradnia, M. Catalytic activation of persulphate with Mn3O4 nanoparticles for degradation of acid blue 113: Process optimisation and degradation pathway. Int. J. Environ. Anal. Chem. 2022, 102, 3786–3805. [Google Scholar] [CrossRef]
- Asgari, G.; Seid-mohammadi, A.; Rahmani, A.; Samadi, M.T.; Salari, M.; Alizadeh, S.; Nematollahi, D. Diuron degradation using three-dimensional electro-peroxone (3D/E-peroxone) process in the presence of TiO2/GAC: Application for real wastewater and optimization using RSM-CCD and ANN-GA approaches. Chemosphere 2021, 266, 129179. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Wang, C.-W. Assessing acute toxicity potential of persulfate ISCO treated water. Chemosphere 2013, 93, 2711–2716. [Google Scholar] [CrossRef] [PubMed]
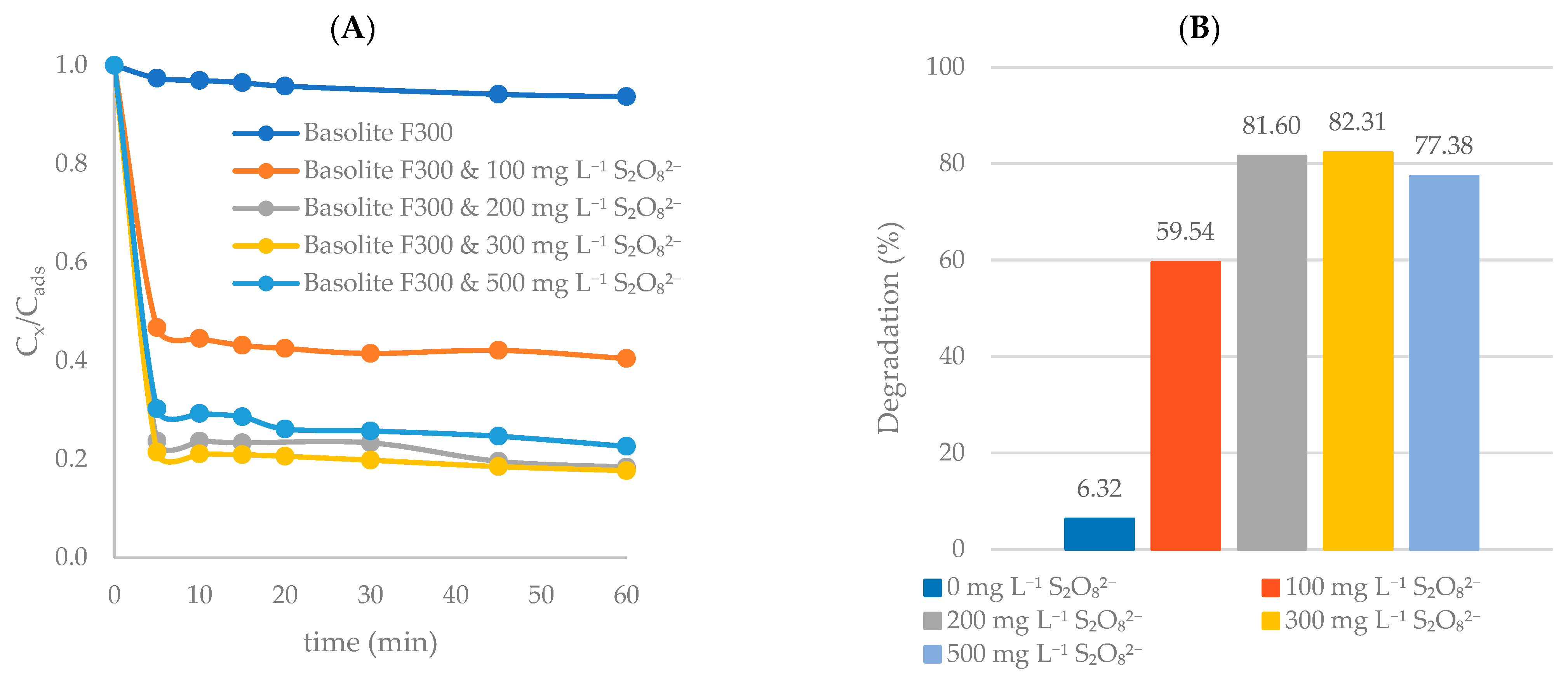
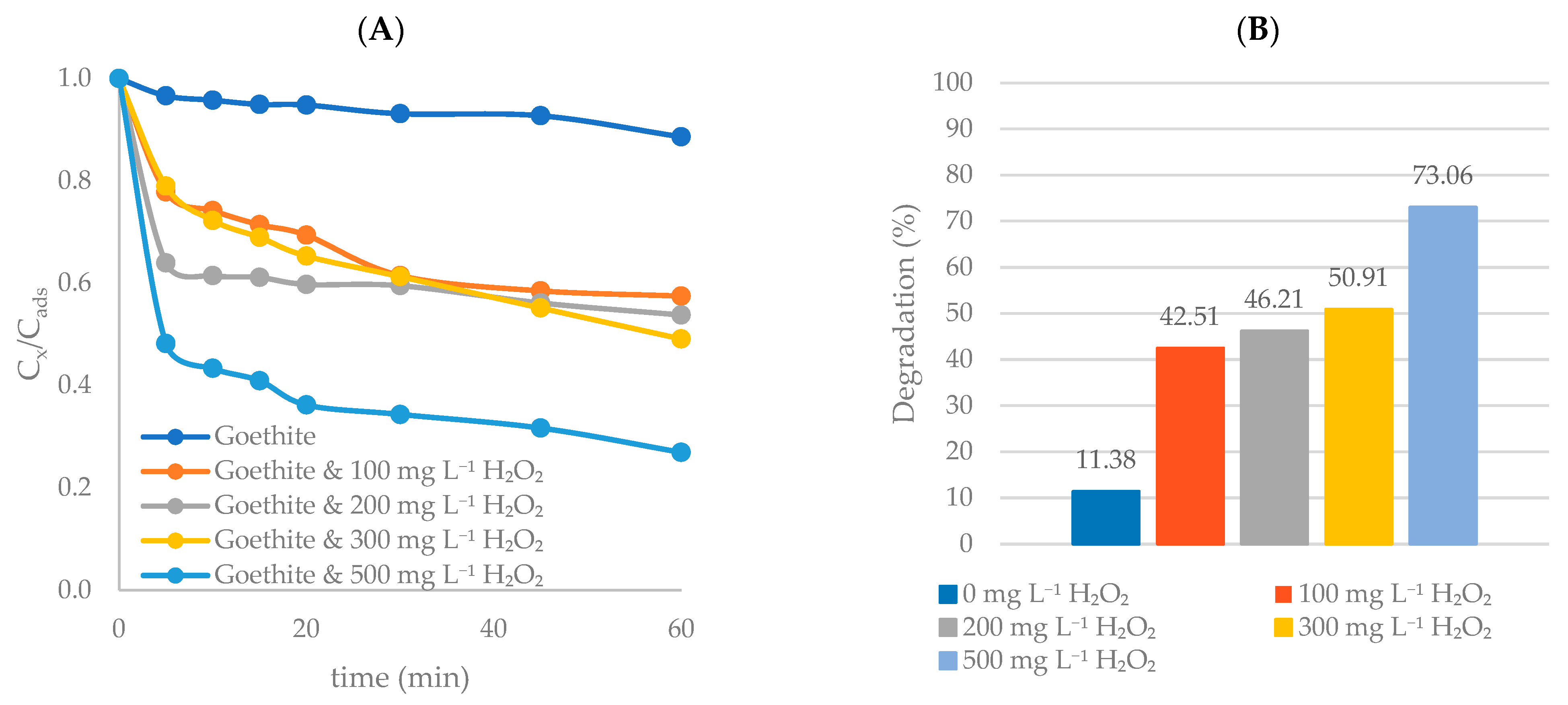


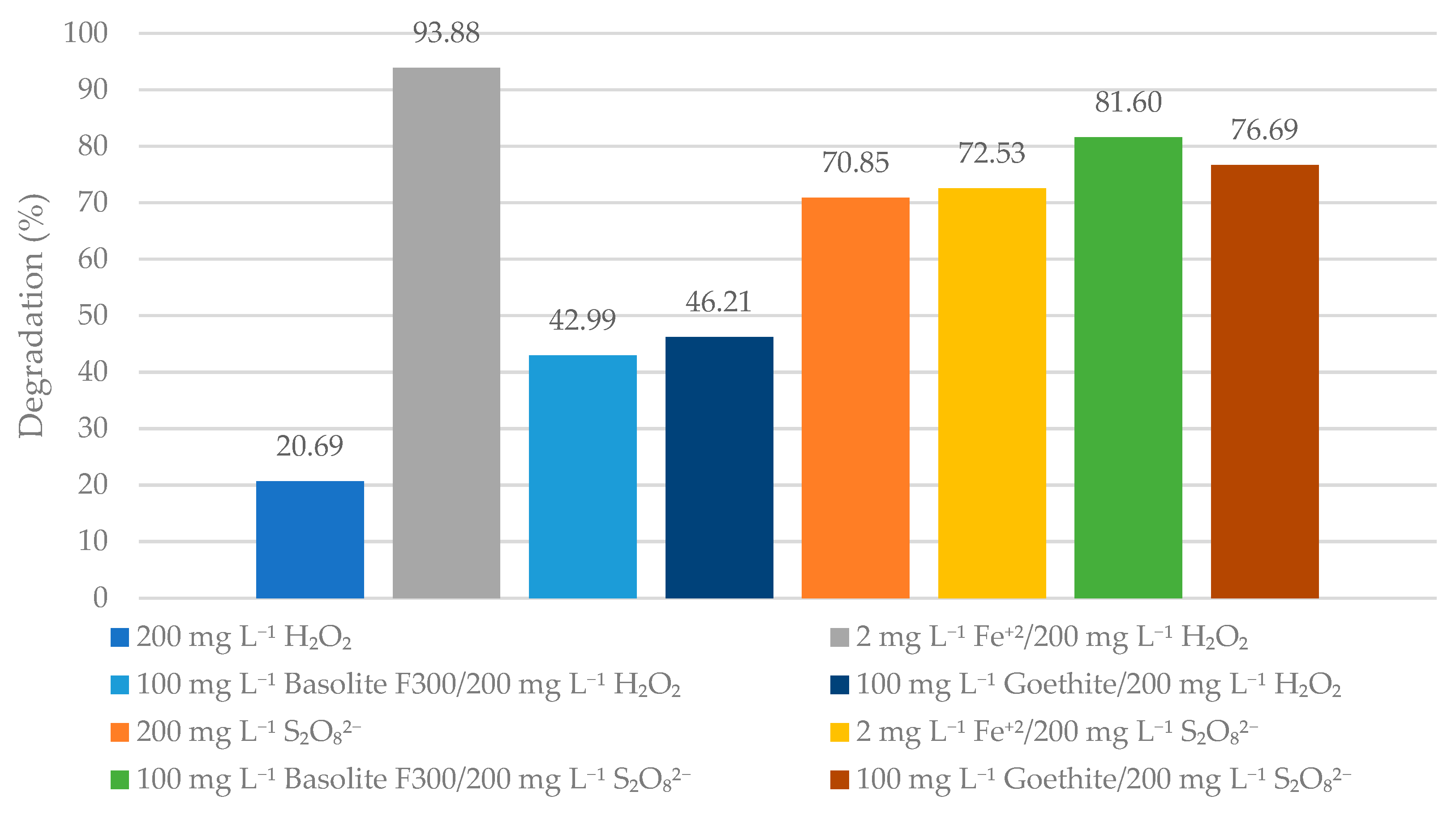
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulou, G.; Evgenidou, E.; Lambropoulou, D. Homogeneous and Heterogeneous Photo-Fenton-Based Photocatalytic Techniques for the Degradation of Nile Blue Dye. Appl. Sci. 2025, 15, 7917. https://doi.org/10.3390/app15147917
Papadopoulou G, Evgenidou E, Lambropoulou D. Homogeneous and Heterogeneous Photo-Fenton-Based Photocatalytic Techniques for the Degradation of Nile Blue Dye. Applied Sciences. 2025; 15(14):7917. https://doi.org/10.3390/app15147917
Chicago/Turabian StylePapadopoulou, Georgia, Eleni Evgenidou, and Dimitra Lambropoulou. 2025. "Homogeneous and Heterogeneous Photo-Fenton-Based Photocatalytic Techniques for the Degradation of Nile Blue Dye" Applied Sciences 15, no. 14: 7917. https://doi.org/10.3390/app15147917
APA StylePapadopoulou, G., Evgenidou, E., & Lambropoulou, D. (2025). Homogeneous and Heterogeneous Photo-Fenton-Based Photocatalytic Techniques for the Degradation of Nile Blue Dye. Applied Sciences, 15(14), 7917. https://doi.org/10.3390/app15147917






