Antifungal Activity of Quaternary Pyridinium Salts Against Fusarium culmorum in Wheat Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Quaternary Pyridinium Salts
2.2. Isolation and Identification of Fusarium culmorum
DNA Extraction, Amplification, and Sequencing
2.3. Disease Evaluation
2.4. In Vivo Test on Wheat
- Sterile grains treated with a fungicide and sown in contaminated sand (F/CS);
- Sterile grains treated with a synthesized compound and sown in contaminated sand (C/CS);
- Sterile grains sown in contaminated sand (SG/CS).
2.5. Statistical Analysis
3. Results
3.1. Disease Index
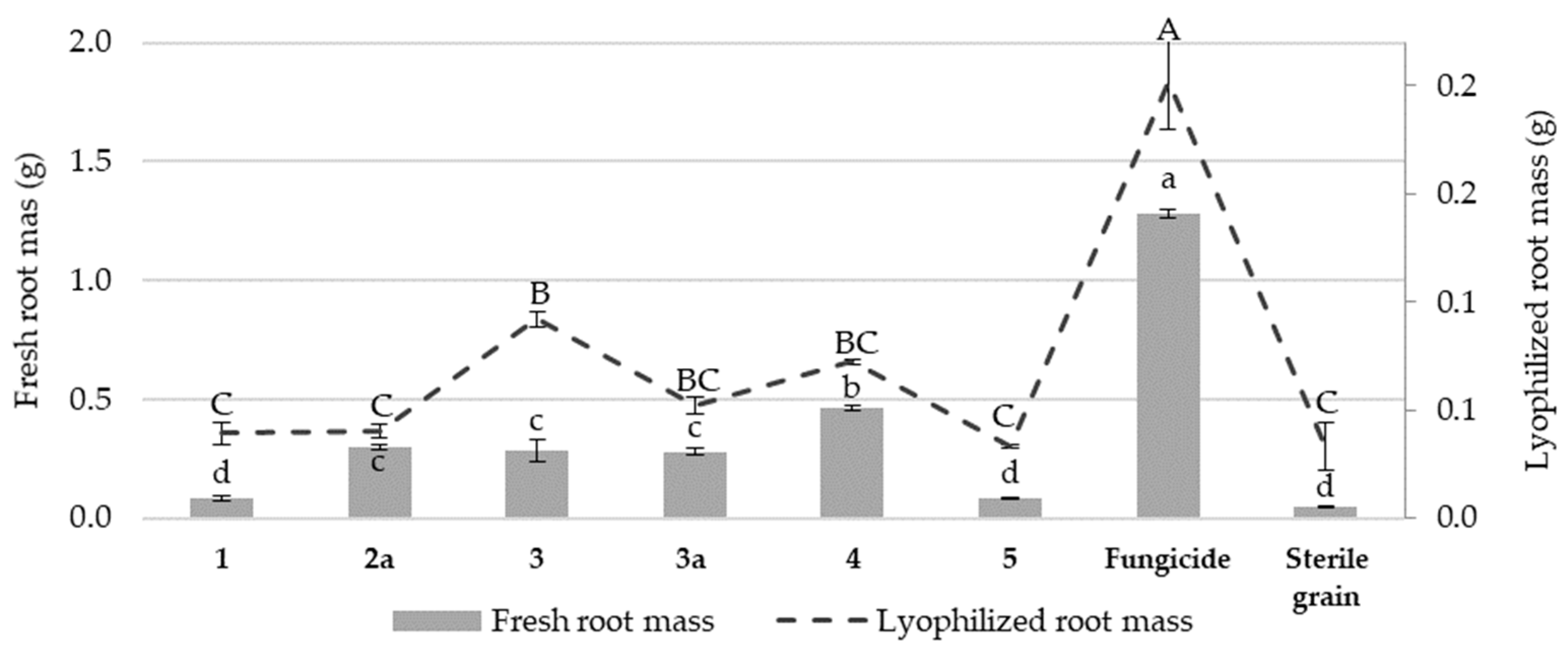
3.2. The Effect of Nicotinamide Derivatives on the Fresh and Lyophilized Root Mass of Wheat Seedlings
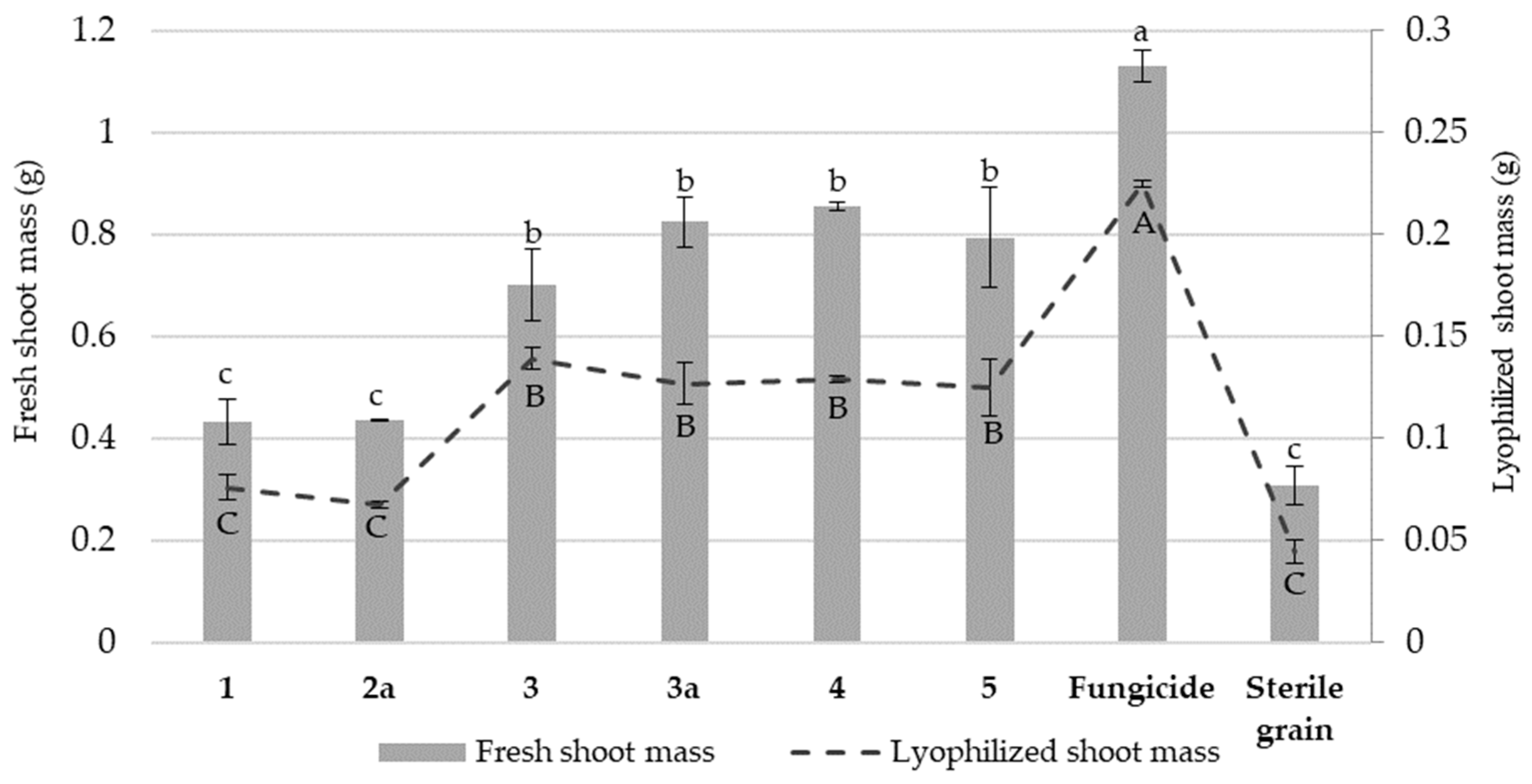
3.3. The Effect of Nicotinamide Derivatives on the Fresh and Lyophilized Shoot Mass of Wheat Seedlings
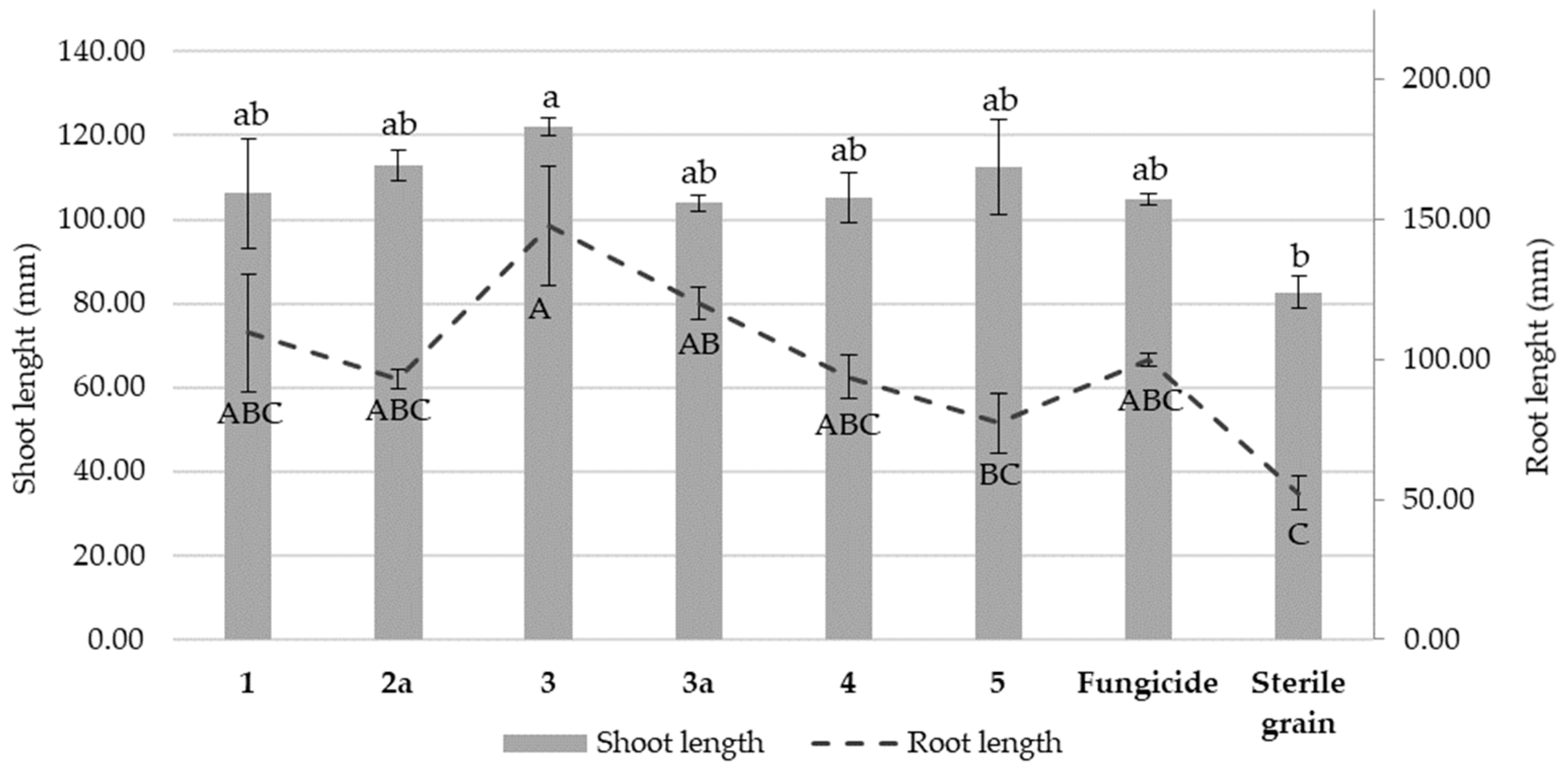
3.4. The Effect of Nicotinamide Derivatives on the Shoot and Root Length
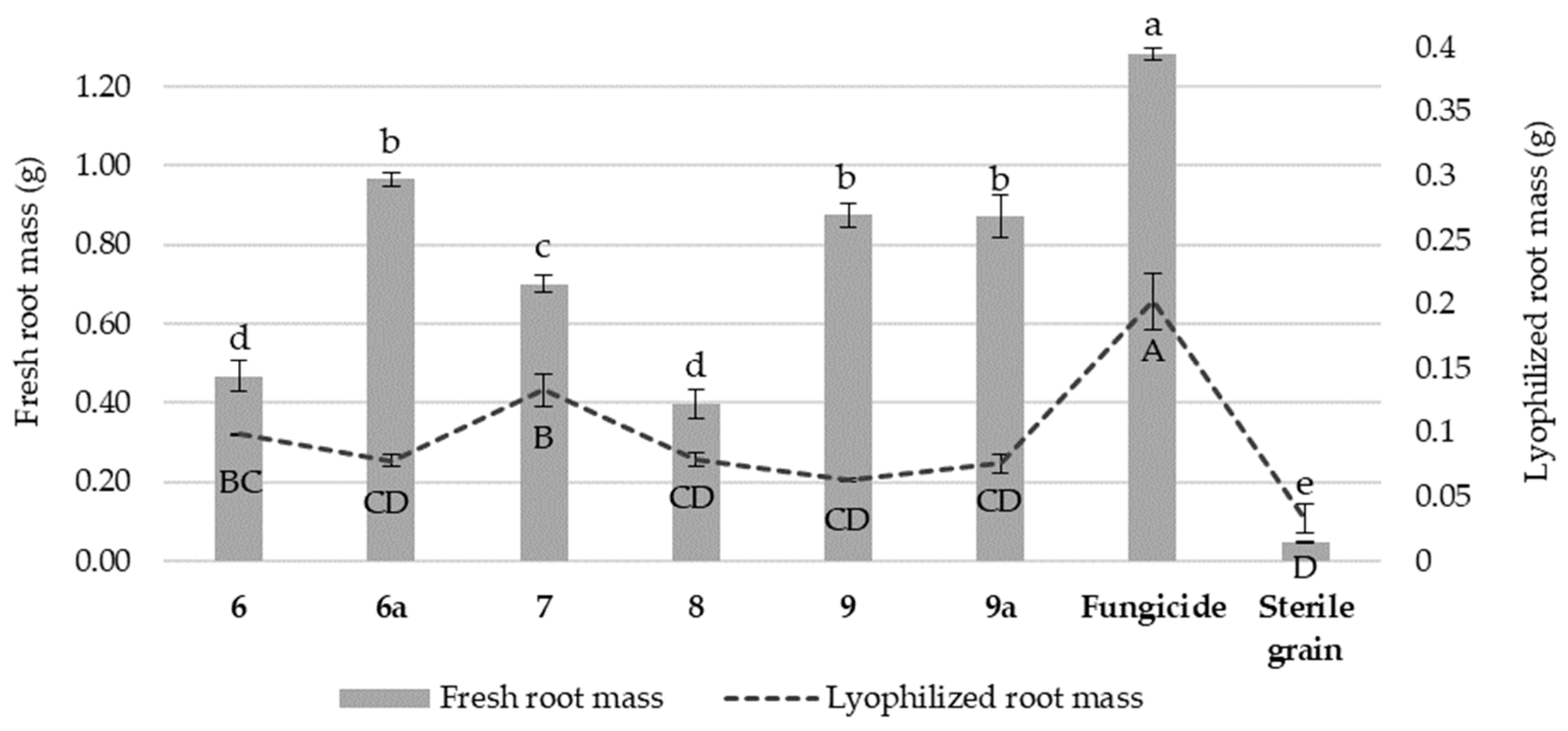
3.5. The Effect of Isonicotinamide Derivatives on the Fresh and Lyophilized Root Mass of Wheat Seedlings
3.6. The Effect of Isonicotinamide Derivatives on the Fresh and Lyophilized Shoot Mass of Wheat Seedlings
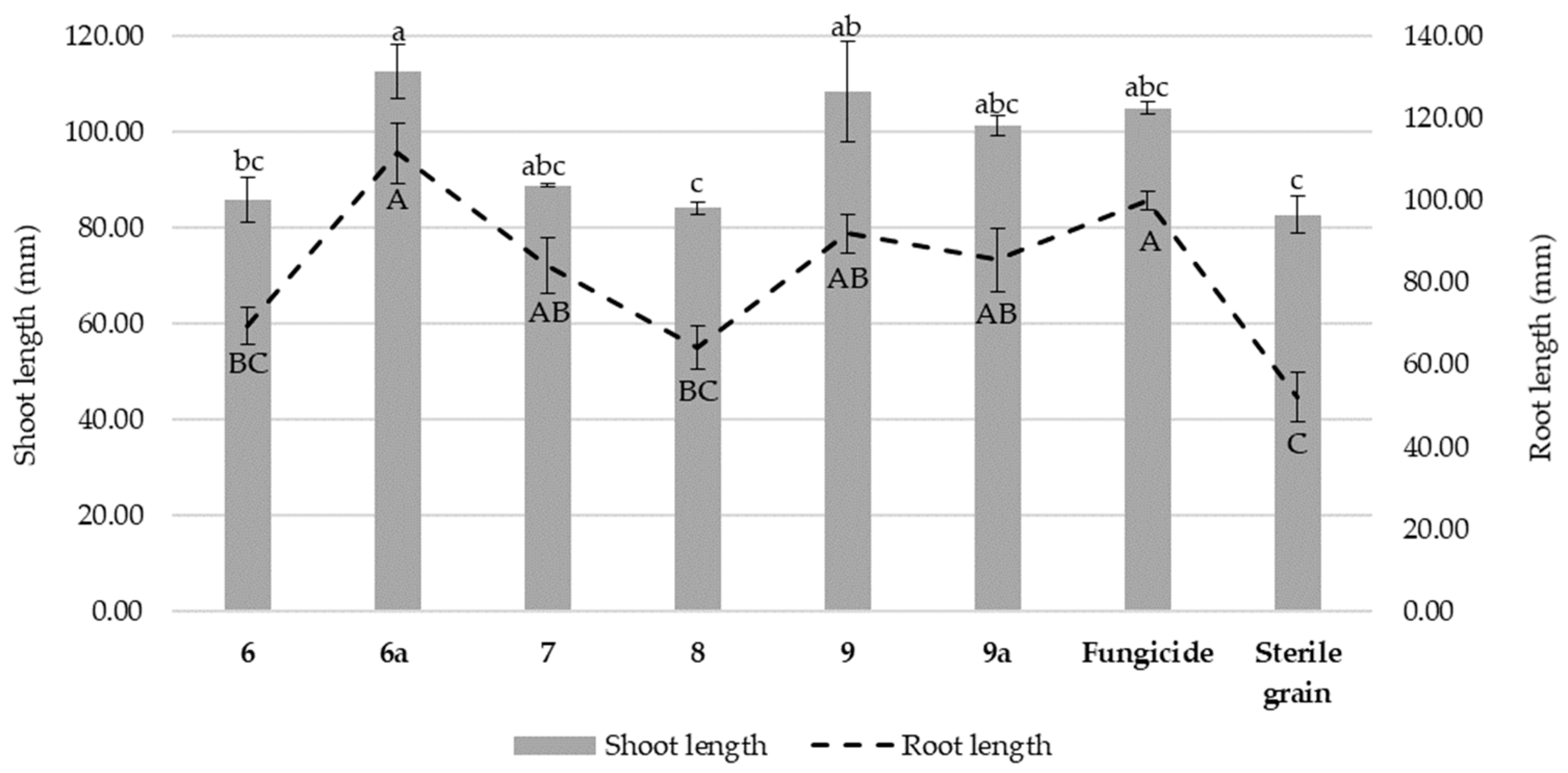
3.7. The Effect of Isonicotinamide Derivatives on the Shoot and Root Length
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okungbowa, F.I.; Shittu, H.O. Fusarium wilts: An overview. Environ. Res. J. 2012, 6, 83–102. [Google Scholar]
- Ozdemir, F.; Koc, N.K.; Paulitz, T.; Nicol, J.M.; Schroeder, K.L.; Poole, G. Determination of fusarium crown rot resistance in wheat to Fusarium culmorum and Fusarium pseudogramineaum using real time PCR. Crop Prot. 2020, 135, 105204. [Google Scholar] [CrossRef]
- Wang, H.; Hwang, S.F.; Eudes, F.; Chang, K.F.; Howard, R.J.; Turnbull, G.D. Trichothecenes and aggressiveness of Fusarium graminearum causing seedling blight and root rot in cereals. Plant Pathol. 2006, 55, 224–230. [Google Scholar] [CrossRef]
- Miedaner, T.; Cumagun, C.J.R.; Chakraborty, S. Population genetics of three important head blight pathogens Fusarium graminearum, F. pseudograminearum and F. culmorum. J. Phytopathol. 2008, 156, 129–139. [Google Scholar] [CrossRef]
- Hogg, A.C.; Johnston, R.H.; Johnston, J.A.; Klouser, L.; Kephart, K.D.; Dyer, A.T. Monitoring Fusarium crown rot populations in spring wheat residues using quantitative real-time polymerase chain reaction. Phytopathology 2010, 100, 49–57. [Google Scholar] [CrossRef]
- Aoki, T.; O’Donnell, K.; Geiser, D.M. Systematics of key phytopathogenic Fusarium species: Current status and future challenges. J. Gen. Plant Pathol. 2014, 80, 189–201. [Google Scholar] [CrossRef]
- FAO—Food and Agriculture Organization of United Nations. Crops and Livestock Products. Available online: https://www.fao.org/faostat (accessed on 25 November 2024).
- Ćosić, J.; Jurković, D. Fusarium vrste s različitih domaćina i njihova patogenost za klijance pšenice. Poljoprivreda 2001, 7, 5–9. [Google Scholar]
- Scherm, B.; Balmas, V.; Spanu, F.; Pani, G.; Delogu, G.; Pasquali, M.; Migheli, Q. Fusarium culmorum: Causal agent of foot and root rot and head blight on wheat. Mol. Plant Pathol. 2013, 14, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Balmas, V.; Scherm, B.; Marcello, A.; Beyer, M.; Hoffmann, L.; Migheli, Q.; Pasquali, M. Fusarium species and chemotypes associated with fusarium head blight and fusarium root rot on wheat in Sardinia. Plant Pathol. 2015, 64, 972–979. [Google Scholar] [CrossRef]
- Summerell, B.A. Resolving Fusarium: Current status of the genus. Annu. Rev. Phytopathol. 2019, 57, 323–339. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on agriculture, food, and human health and their management strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef]
- Desjardins, A.E. Fusarium mycotoxins: Chemistry, Genetics, and Biology; APS Press: St. Paul, MN, USA, 2006. [Google Scholar]
- Lemmens, M.; Scholz, U.; Berthiller, F.; Dall’Asta, C.; Koutnik, A.; Schuhmacher, R.; Ruckenbauer, P. The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for Fusarium head blight resistance in wheat. Mol. Plant Microbe Interact. 2005, 18, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Ansari, K.I.; Walter, S.; Brennan, J.M.; Lemmens, M.; Kessans, S.; McGahern, A.; Doohan, F.M. Retrotransposon and gene activation in wheat in response to mycotoxigenic and non-mycotoxigenic-associated Fusarium stress. Theor. Appl. Genet. 2007, 114, 927–937. [Google Scholar] [CrossRef]
- Diamond, M.; Reape, T.J.; Rocha, O.; Doyle, S.M.; Kacprzyk, J.; Doohan, F.M.; McCabe, P.F. The Fusarium mycotoxin deoxynivalenol can inhibit plant apoptosis-like programmed cell death. PLoS ONE 2013, 8, e69542. [Google Scholar] [CrossRef]
- Ansari, K.I.; Doyle, S.M.; Kacprzyk, J.; Khan, M.R.; Walter, S.; Brennan, J.M.; Doohan, F.M. Light influences how the fungal toxin deoxynivalenol affects plant cell death and defense responses. Toxins 2014, 6, 679–692. [Google Scholar] [CrossRef]
- Perincherry, L.; Lalak-Kańczugowska, J.; Stępień, Ł. Fusarium-produced mycotoxins in plant-pathogen interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef]
- Dweba, C.C.; Figlan, S.; Shimelis, H.A.; Motaung, T.E.; Sydenham, S.; Mwadzingeni, L.; Tsilo, T.J. Fusarium head blight of wheat: Pathogenesis and control strategies. Crop Prot. 2017, 91, 114–122. [Google Scholar] [CrossRef]
- Rojas, E.C.; Jørgensen, H.J.; Jensen, B.; Collinge, D.B. Fusarium diseases: Biology and management Perspectives. In Integrated Disease Management of Wheat and Barley; Burleigh Dodds Science Publishing: Cambridge, UK, 2018. [Google Scholar] [CrossRef]
- Islam, T.; Danishuddin; Tamanna, N.T.; Matin, M.N.; Barai, H.R.; Haque, M.A. Resistance mechanisms of plant pathogenic fungi to fungicide, environmental impacts of fungicides, and sustainable solutions. Plants 2024, 13, 2737. [Google Scholar] [CrossRef]
- Gikas, G.D.; Parlakidis, P.; Mavropoulos, T.; Vryzas, Z. Particularities of fungicides and factors affecting their fate and removal efficacy: A review. Sustainability 2022, 14, 4056. [Google Scholar] [CrossRef]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.; Knäbel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An overlooked pesticide class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef]
- Bušić, V.; Pavlović, H.; Roca, S.; Vikić-Topić, D.; Gašo-Sokač, D. Microwave-assisted quaternization of various pyridine derivatives and their antibacterial activity. Croat. Chem. Acta 2017, 90, 425–433. [Google Scholar] [CrossRef]
- Patel, N.B.; Shaikh, F.M. New 4-thiazolidinones of nicotinic acid with 2-amino-6-methylbenzothiazole and their biological activity. Sci. Pharm. 2010, 78, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Surendra Kumar, R.K.; Idhayadhulla, A.; Nasser, J.A. Synthesis of some new series of Mannich base derivatives and their antimicrobial activity. Orbital Electron. J. Chem. 2011, 3, 32–38. [Google Scholar]
- Gašo-Sokač, D.; Katalinić, M.; Kovarik, Z.; Bušić, V.; Kovač, S. Synthesis and evaluation of novel analogues of vitamin B6 as reactivators of tabun and paraoxon inhibited acetylcholinesterase. Chem.-Biol. Interact. 2010, 187, 234–237. [Google Scholar] [CrossRef]
- Pidlypnyi, N.; Kaul, S.; Wolf, S.; Drafz, M.H.; Schmidt, A. Syntheses and characterization of N-(indolyl) pyridinium salts and of their ylides. Z. Naturforsch. B. 2014, 69, 605–614. [Google Scholar] [CrossRef]
- Zorbaz, T.; Braïki, A.; Maraković, N.; Renou, J.; de La Mora, E.; Maček Hrvat, N.; Renard, P.Y. Potent 3-hydroxy-2-pyridine aldoxime reactivators of organophosphate-inhibited cholinesterases with predicted blood–brain barrier penetration. Chem. Eur. J. 2018, 24, 9675–9691. [Google Scholar] [CrossRef]
- Lee, H.M.; Andrys, R.; Jonczyk, J.; Kim, K.; Vishakantegowda, A.G.; Malinak, D.; Musilek, K. Pyridinium-2-carbaldoximes with quinolinium carboxamide moiety are simultaneous reactivators of acetylcholinesterase and butyrylcholinesterase inhibited by nerve agent surrogates. J. Enzyme Inhib. Med. Chem. 2021, 36, 437–449. [Google Scholar] [CrossRef]
- Singh, B.S.; Lobo, H.R.; Pinjari, D.V.; Jarag, K.J.; Pandit, A.B.; Shankarling, G.S. Ultrasound and deep eutectic solvent (DES): A novel blend of techniques for rapid and energy efficient synthesis of oxazoles. Ultrason. Sonochemistry 2013, 20, 287–293. [Google Scholar] [CrossRef]
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Guillena, G.; Pastor, I.M.; Ramón, D.J. Deep eutectic solvents: The organic reaction medium of the century. Eur. J. Org. Chem. 2016, 2016, 612–632. [Google Scholar] [CrossRef]
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations. J. Mol. Liq. 2016, 215, 345–386. [Google Scholar] [CrossRef]
- Prabhune, A.; Dey, R. Green and sustainable solvents of the future: Deep eutectic solvents. J. Mol. Liq. 2023, 379, 121676. [Google Scholar] [CrossRef]
- Bénit, P.; Goncalves, J.; El Khoury, R.; Rak, M.; Favier, J.; Gimenez-Roqueplo, A.P.; Rustin, P. Succinate dehydrogenase, succinate, and superoxides: A genetic, epigenetic, metabolic, environmental explosive crossroad. Biomedicines 2022, 10, 1788. [Google Scholar] [CrossRef]
- Wu, J.; Kang, S.; Luo, L.; Shi, Q.; Ma, J.; Yin, J.; Yang, S. Synthesis and antifungal activities of novel nicotinamide derivatives containing 1, 3, 4-oxadiazole. Chem. Cent. J. 2013, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Bouillaud, F. Inhibition of succinate dehydrogenase by pesticides (SDHIs) and energy metabolism. Int. J. Mol. Sci. 2023, 24, 4045. [Google Scholar] [CrossRef] [PubMed]
- Bušić, V.; Vrandečić, K.; Siber, T.; Roca, S.; Vikić Topić, D.; Gašo Sokač, D. A rapid microwave induced synthesis of isonicotinamide derivatives and their antifungal activity. Croat. Chem. Acta 2019, 92, 125–135. [Google Scholar] [CrossRef]
- Bušić, V.; Roca, S.; Vikić-Topić, D.; Vrandečić, K.; Ćosić, J.; Molnar, M.; Gašo-Sokač, D. Eco-friendly quaternization of nicotinamide and 2-bromoacetophenones in deep eutectic solvents. Antifungal activity of the products. Environ. Chem. Lett. 2020, 18, 889–894. [Google Scholar] [CrossRef]
- Messali, M.; Ahmed, S.A. A green microwave-assisted synthesis of new pyridazinium-based ionic liquids as an environmentally friendly alternative. Green Sustain. Chem. 2011, 1, 70–75. [Google Scholar] [CrossRef]
- Tăbăcaru, A.; Dediu Botezatu, A.V.; Horincar, G.; Furdui, B.; Dinică, R.M. Green accelerated synthesis, antimicrobial activity and seed germination test of quaternary ammonium salts of 1, 2-bis (4-pyridyl) ethane. Molecules 2019, 24, 2424. [Google Scholar] [CrossRef]
- Fernando, P.A.I.; Kosgei, G.K.; Schutt, T.C.; Jernberg, J.; Koval, A.M.; Thornell, T.L.; Kimble, A.N. Efficient microwave-assisted synthesis of 1-hexylpyridin-1-ium bromide: Anion exchange to acetate and fundamentals tools to probe its interaction with RuCl3. J. Mol. Liq. 2024, 397, 124080. [Google Scholar] [CrossRef]
- Siber, T.; Bušić, V.; Zobundžija, D.; Roca, S.; Vikić-Topić, D.; Vrandečić, K.; Gašo-Sokač, D. An improved method for the quaternization of nicotinamide and antifungal activities of its derivatives. Molecules 2019, 24, 1001. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.E.; Toussoun, T.A.; Marasas, W.F.O. Fusarium Species: An Illustrated Manual for Identification; Pennsylvania State University Press: University Park, PA, USA, 1983. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Lević, J. Vrste Roda Fusarium u Oblasti Poljoprivrede, Veterinarske i Humane Medicine; Institut za kukuruz “Zemun Polje”: Beograd, Srbija, 2008. [Google Scholar]
- Karlsson, I.; Edel-Hermann, V.; Gautheron, N.; Durling, M.B.; Kolseth, A.K.; Steinberg, C.; Friberg, H. Genus-specific primers for study of Fusarium communities in field samples. Appl. Environ. Microbiol. 2016, 82, 491–501. [Google Scholar] [CrossRef] [PubMed]
- McKinney, H.H. Investigations of the rosette disease of wheat and its control. J. Agric. Res. 1923, 23, 771. [Google Scholar]
- Juber, K.S.; Al-Juboory, H.H.; Al-Juboory, S.B. Fusarium wilt disease of strawberry caused by Fusarium oxysporum f. sp. Fragariae in Iraq and its control. J. Exp. Biol. Agric. Sci. 2014, 2, 419–427. [Google Scholar]
- Kumar, V.; Chandel, S. Effect of Epidemiological Factors on Percent Disease Index of Rose Powdery Mildew Caused by Podosphaera pannosa (Wallr.) de Bary. 2018. Unpublished Manuscript. Available online: https://www.researchgate.net/publication/327866159 (accessed on 12 April 2025).
- Mesterházy, A. Comparative analysis of artificial inoculation methods with Fusarium spp. on winter wheat varieties. J. Phytopathol. 1978, 93, 12–25. [Google Scholar] [CrossRef]
- Papadopulos, F.; Spinelli, M.; Valente, S.; Foroni, L.; Orrico, C.; Alviano, F.; Pasquinelli, G. Common tasks in microscopic and ultrastructural image analysis using ImageJ. Ultrastruct. Pathol. 2007, 31, 401–407. [Google Scholar] [CrossRef]
- Goulart, A.C.P. Effectiveness of fungicide seed treatment in the control of soybean seedling damping off caused by Rhizoctonia solani under greenhouse conditions. Summa Phytopathol. 2022, 48, 121–125. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.J.; Lin, G.T.; Wu, J.P.; Xu, G.; Xu, D. Novel N-(1H-pyrazol-5-yl) nicotinamide derivatives: Design, synthesis and antifungal activity. Chem. Biodivers. 2022, 19, e202101032. [Google Scholar] [CrossRef]
- Peng, Y.; Chang, J.; Xiao, Z.; Huang, J.; Xu, T.; Chen, S.; Luo, H. Synthesis and antifungal activity of novel tetrahydrogeranyl quaternary ammonium salts. Nat. Prod. Commun. 2022, 17, 1–10. [Google Scholar] [CrossRef]
- Sidiq, Y.; Nakano, M.; Mori, Y.; Yaeno, T.; Kimura, M.; Nishiuchi, T. Nicotinamide effectively suppresses fusarium head blight in wheat plants. Int. J. Mol. Sci. 2021, 22, 2968. [Google Scholar] [CrossRef]
- Mohammed, H.F.; Imara, D.A. Evaluation of the efficacy of salicylic acid and nicotinic acid to control collar rot and root rot caused by Sclerotium rolfsii and enhance the productivity of fenugreek (Trigonella foenum-groecum L.) in Egypt. Egypt. J. Bot. 2023, 63, 931–950. [Google Scholar] [CrossRef]
- Ueda, K.; Nakajima, Y.; Inoue, H.; Kobayashi, K.; Nishiuchi, T.; Kimura, M.; Yaeno, T. Nicotinamide mononucleotide potentiates resistance to biotrophic invasion of fungal pathogens in barley. Int. J. Mol. Sci. 2021, 22, 2696. [Google Scholar] [CrossRef] [PubMed]
- Smolik, B.; Sędzik-Wójcikowska, M. Examining nicotinamide application methods in alleviating lead-induced stress in Spring Barley. Agronomy 2024, 14, 1314. [Google Scholar] [CrossRef]
- Louw, A.E.; Dubery, I.A. Plant defence responses in isonicotinamide-treated tobacco cells. Evidence supporting a role for nicotinamide related metabolites as stress mediators in plant defense metabolism. J. Plant Physiol. 2000, 156, 26–32. [Google Scholar] [CrossRef]
- Yan, S.; Weng, B.; Jing, L.; Bi, W. Effects of drought stress on water content and biomass distribution in summer maize (Zea mays L.). Front. Plant Sci. 2023, 14, 1118131. [Google Scholar] [CrossRef]
- Sadak, M.S.; Rady, M.M.; Badr, N.M.; Gaballah, M.S. Increasing sunflower salt tolerance using nicotinamide and α--tocopherol. Int. J. Acad. Res. 2010, 2, 263–270. [Google Scholar]
- Erofeeva, E.A. Hormesis in plants: Its common occurrence across stresses. Curr. Opin. Toxicol. 2022, 30, 100333. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Blain, R.B. Hormesis and plant biology. Environ. Pollut. 2009, 157, 42–48. [Google Scholar] [CrossRef]
- Jodynis-Liebert, J.; Kujawska, M. Biphasic dose-response induced by phytochemicals: Experimental evidence. J. Clin. Med. 2020, 9, 718. [Google Scholar] [CrossRef]
- Zandona, A.; Lihtar, G.; Maraković, N.; Miš, K.; Bušić, V.; Gašo-Sokač, D.; Katalinić, M. Vitamin B3-based biologically active compounds as inhibitors of human cholinesterases. Int. J. Mol. Sci. 2020, 21, 8088. [Google Scholar] [CrossRef]




| Chemical Name | Label |
|---|---|
| Nicotinamide derivatives | |
| 3-carbamoyl-1-methylpyridinium iodide | (1) |
| 3-carbamoyl-1-(2-(4-chlorophenyl)-2-oxoethyl)pyridinium bromide | (2) |
| 1-(2-phenyl-2-oxoethyl)-3-carbamoylpyridinium bromide | (3) |
| 3-carbamoyl-1-(2-(4-nitrophenyl)-2-oxoethyl)pyridinium bromide | (4) |
| 1-(3-bromopropyl)-3-carbamoylpyridinium bromide | (5) |
| Isonicotinamide derivatives | Label |
| 4-carbamoyl-1-(2-(4-methoxyphenyl)-2-oxoethyl)pyridinium bromide | (6) |
| 1-(2-([1,1′-biphenyl]-4-yl)-2-oxoethyl)-4-carbamoylpyridinium bromide | (7) |
| 4-carbamoyl-1-(2-(2-methoxyphenyl)-2-oxoethyl)pyridinium bromide | (8) |
| 4-carbamoyl-1-[2-(4-nitrophenyl)-2-oxoethyl]pyridinium bromide | (9) |
| Phase | Temperature (°C) | Duration |
|---|---|---|
| Initial denaturation | 95 °C | 5 min |
| 35 cycles: | ||
| Denaturation | 94 °C | 30 s |
| Annealing | 55 °C | 45 s |
| Elongation | 72 °C | 1 min 30 s |
| Final elongation | 72 °C | 10 min |
| Treatment | Concentration (µg/mL) | Disease Index (± SD) * | Notes |
|---|---|---|---|
| (1) | 100 | 6.7 ± 2.03 b | Moderate effect |
| (2) | 10 | 0.9 ± 0.78 c | Most effective with the lowest disease index among this group |
| (3) | 10 | 0.9 ± 0.95 c | Most effective with the lowest disease index among this group |
| (3) | 100 | 2.2 ± 1.91 bc | Very effective |
| (4) | 100 | 6.7 ± 2.39 b | Moderate effect |
| (5) | 100 | 11.6 ± 4.91 a | Less effective |
| SG/CS | - | 14.2 ± 3.73 a | Negative control, least effective |
| F/CS | Fludioxonil + Difenoconazole 25 + 25 g/L (16.7%) | 2.2 ± 1.54 bc | Positive control, highly effective |
| Treatment | Concentration (µg/mL) | Disease Index (± SD) * | Notes |
|---|---|---|---|
| (6) | 10 | 4.9 ± 1.64 bc | Moderate to strong effect |
| (6) | 100 | 12 ± 8.33 ab | Less effective |
| (7) | 100 | 5.3 ± 5.29 bc | Moderate to strong effect |
| (8) | 100 | 3.5 ± 3.11 c | Very effective, with the lowest disease index among this group |
| (9) | 10 | 8.9 ± 5.37 abc | Less effective |
| (9) | 100 | 5.8 ± 2.37 bc | Moderate to strong effect |
| SG/CS | - | 14.2 ± 3.73 a | Negative control, least effective |
| F/CS | Fludioxonil + Difenoconazole 25 + 25 g/L (16.7%) | 2.2 ± 1.54 c | Positive control, highly effective |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siber, T.; Petrović, E.; Ćosić, J.; Bušić, V.; Gašo-Sokač, D.; Vrandečić, K. Antifungal Activity of Quaternary Pyridinium Salts Against Fusarium culmorum in Wheat Seedlings. Appl. Sci. 2025, 15, 7889. https://doi.org/10.3390/app15147889
Siber T, Petrović E, Ćosić J, Bušić V, Gašo-Sokač D, Vrandečić K. Antifungal Activity of Quaternary Pyridinium Salts Against Fusarium culmorum in Wheat Seedlings. Applied Sciences. 2025; 15(14):7889. https://doi.org/10.3390/app15147889
Chicago/Turabian StyleSiber, Tamara, Elena Petrović, Jasenka Ćosić, Valentina Bušić, Dajana Gašo-Sokač, and Karolina Vrandečić. 2025. "Antifungal Activity of Quaternary Pyridinium Salts Against Fusarium culmorum in Wheat Seedlings" Applied Sciences 15, no. 14: 7889. https://doi.org/10.3390/app15147889
APA StyleSiber, T., Petrović, E., Ćosić, J., Bušić, V., Gašo-Sokač, D., & Vrandečić, K. (2025). Antifungal Activity of Quaternary Pyridinium Salts Against Fusarium culmorum in Wheat Seedlings. Applied Sciences, 15(14), 7889. https://doi.org/10.3390/app15147889








