Application of Difenoconazole and Trichoderma Broth Combination for Synergistic Control of Corn Leaf Blight and Stalk Rot in Straw-Returned Fields in Liaoning Province, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Study Sites
2.2. Maize Cultivation and Experimental Design
2.2.1. Experimental Design for Investigating the Effects of Straw Returning on Northern Corn Leaf Blight and Stalk Rot Incidence
2.2.2. Field Plot Layout Design for Studying Fungicide Application on Crop Residues
2.3. Fungicides and Dosages
2.4. Investigation Methods
2.4.1. Investigation on Farmers’ Preference for Fungicide Types in Corn Planting in Fuxin City
2.4.2. Investigation on Disease Incidence, Disease Index, and Control Efficacy
plants) × 100;
Corresponding severity value)/(Total number of investigated leaves ×
Maximum severity value)] × 100;
of treatment group)/Disease index of control group] × 100.
2.5. Data Analysis
3. Results
3.1. Straw Return Aggravates Northern Corn Leaf Blight in Fuxin Region with Minimal Effects on Fusarium Stalk Rot
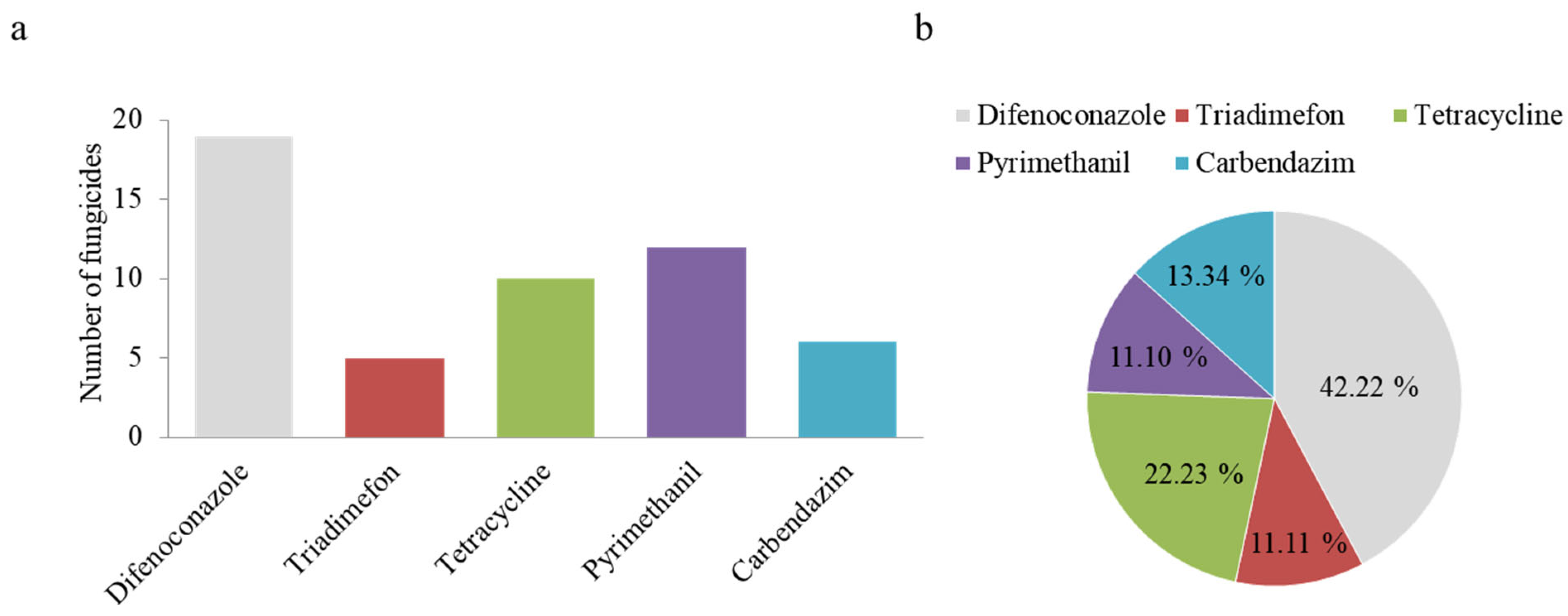
3.2. Survey of Commonly Used Fungicides in the Corn Fields of Fuxin Region
3.3. Synergistic Effect of Difenoconazole and Trichoderma Fermentate in Controlling Northern Corn Leaf Blight and Stem Rot
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. World Food and Agriculture—Statistical Yearbook 2022; FAO: Rome, Italy, 2022. [Google Scholar]
- National Bureau of Statistics of China. China Statistical Yearbook 2021; China Statistics Press: Beijing, China, 2021. [Google Scholar]
- Carson, M.L. Virulence in Exserohilum turcicum populations on maize in the United States. Plant Health Prog. 2016, 17, 170–173. [Google Scholar]
- Wegulo, S.N.; Bissonnette, K.M.; Arias, M.M.A.; Malvick, D.K. Epidemiology and management of northern corn leaf blight. Plant Dis. 2018, 102, 12–24. [Google Scholar]
- Munkvold, G.P.; Desjardins, A.E. Fumonisins in maize: Can we reduce their occurrence? Plant Dis. 1997, 81, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, C.; Chakraborty, S.; Manners, J.M.; Kazan, K. Population structure and genetic diversity of Fusarium graminearum in major maize-producing areas of China. Phytopathology 2019, 109, 702–715. [Google Scholar]
- Zhao, J.; Hu, Y.; Gao, W.; Chen, H.; Yang, M.; Quan, Z.; Fang, Y.; Chen, X.; Xie, H.; He, H.; et al. Effects of long-term conservation tillage on N2 and N2O emission rates and N2O emission microbial pathways in Mollisols. Sci. Total Environ. 2024, 908, 168440. [Google Scholar] [CrossRef]
- Wen, L.; Peng, Y.; Zhou, Y.; Cai, G.; Lin, Y.; Li, B. Effects of conservation tillage on soil enzyme activities of global cultivated land: A meta-analysis. J. Environ. Manag. 2023, 345, 118904. [Google Scholar] [CrossRef]
- Li, Y.; Li, T.; Zhao, D.; Wang, Z.; Liao, Y. Different tillage practices change assembly, composition, and co-occurrence patterns of wheat rhizosphere diazotrophs. Sci. Total Environ. 2021, 767, 144252. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Chang, S.X.; Cui, S.; Jagadamma, S.; Zhang, Q.; Cai, Y. Residue retention promotes soil carbon accumulation in minimum tillage systems: Implications for conservation agriculture. Sci. Total Environ. 2020, 740, 140147. [Google Scholar] [CrossRef]
- Nandan, R.; Singh, V.; Singh, S.S.; Kumar, V.; Hazra, K.K.; Nath, C.P.; Poonia, S.; Malik, R.K.; Bhattacharyya, R.; McDonald, A. Impact of conservation tillage in rice-based cropping systems on soil aggregation, carbon pools and nutrients. Geoderma 2019, 340, 104–114. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Tu, C.; Hoyt, G.D.; DeForest, J.L.; Hu, S. Long-term no-tillage and organic input management enhanced the diversity and stability of soil microbial community. Sci. Total Environ. 2017, 609, 341–347. [Google Scholar] [CrossRef]
- Liu, X.; Song, X.; Li, S.; Liang, G.; Wu, X. Understanding how conservation tillage promotes soil carbon accumulation: Insights into extracellular enzyme activities and carbon flows between aggregate fractions. Sci. Total Environ. 2023, 897, 165408. [Google Scholar] [CrossRef] [PubMed]
- Devkota, M.; Devkota, K.P.; Kumar, S. Conservation Agriculture Improves Agronomic, Economic, and Soil Fertility Indicators for a Clay Soil in a Rainfed Mediterranean Climate in Morocco. Agric. Syst. 2022, 201, 103470. [Google Scholar] [CrossRef]
- Xiao, L.; Kuhn, N.; Zhao, R.; Cao, L. Net effects of conservation agriculture principles on sustainable land use: A synthesis. Glob. Change Biol. 2021, 27, 6321–6330. [Google Scholar] [CrossRef]
- Nazu, S.B.; Saha, S.M.; Hossain, M.E.; Haque, S.; Khan, M.A. Willingness to pay for adopting conservation tillage technologies in wheat cultivation: Policy options for small-scale farmers. Environ. Sci. Pollut. Res. 2022, 29, 63458–63471. [Google Scholar] [CrossRef]
- Shen, Y.; Kong, W.; Shi, R.; Du, R.; Zhao, M. Farmers’ adoption behavior of conservation tillage technology: A multidimensional heterogeneity perspective. Environ. Sci. Pollut. Res. 2023, 30, 37744–37761. [Google Scholar] [CrossRef]
- Ren, Z.; Han, X.; Feng, H.; Wang, L.; Ma, G.; Li, J.; Lv, J.; Tian, W.; He, X.; Zhao, Y. Long-term conservation tillage improves soil stoichiometry balance and crop productivity based on a 17-year experiment in a semi-arid area of northern China. Sci. Total Environ. 2023, 908, 168283. [Google Scholar] [CrossRef]
- Meng, X.; Meng, F.; Chen, P.; Hou, D.; Zheng, E.; Xu, T. A meta-analysis of conservation tillage management effects on soil organic carbon sequestration and soil greenhouse gas flux. Sci. Total Environ. 2024, 954, 176315. [Google Scholar] [CrossRef]
- Dong, H.Y.; Dong, Z.; Liu, K.J.; Wang, L.J.; Liu, P.B.; Hou, Z.Y. Effects of Different Maize Straw Returning Modeson Occurrence of Main Diseases of Maize. Crops J. 2020, 6, 104–108. (In Chinese) [Google Scholar]
- Li, Z.; Chen, J.; Liu, C.; He, S.; Wang, M.; Wang, L.; Bhadauria, V.; Wang, S.; Cheng, W.; Liu, H.; et al. Natural variations of maize ZmLecRK1 determine its interaction with ZmBAK1 and resistance patterns to multiple pathogens. Mol. Plant 2024, 17, 1606–1623. [Google Scholar] [CrossRef]
- Sucher, J.; Boni, R.; Yang, P.; Rogowsky, P.; Büchner, H.; Kastner, C.; Kumlehn, J.; Krattinger, S.G.; Keller, B. The durable wheat disease resistance gene Lr34 confers common rust and northern corn leaf blight resistance in maize. Plant Biotechnol. J. 2016, 15, 489–496. [Google Scholar] [CrossRef]
- Parihar, C.M.; Parihar, M.D.; Sapkota, T.B.; Nanwal, R.K.; Singh, A.K.; Jat, S.L.; Nayak, H.S.; Mahala, D.M.; Singh, L.K.; Kakraliya, S.K.; et al. Long-term impact of conservation agriculture and diversified maize rotations on carbon pools and stocks, mineral nitrogen fractions and nitrous oxide fluxes in Inceptisol of India. Sci. Total Environ. 2018, 640–641, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Pramanick, B.; Kumar, M.; Naik, B.M.; Singh, S.K.; Kumar, M.; Singh, S.V. Soil Carbon-Nutrient Cycling, Energetics, and Carbon Footprint in Calcareous Soils with Adoption of Long-Term Conservation Tillage Practices and Cropping Systems Diversification. Sci. Total Environ. 2024, 912, 169421. [Google Scholar] [CrossRef] [PubMed]
- Langemeier, C.B.; Robertson, A.E.; Wang, D.; Jackson-Ziems, T.A.; Kruger, G.R. Factors Affecting the Development and Severity of Goss’s Bacterial Wilt and Leaf Blight of Corn, Caused by Clavibacter michiganensis subsp. nebraskensis. Plant Dis. 2017, 101, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Camera, J.N.; Forcellini, C.A.; Koefender, J.; Golle, D.P.; Schoffel, A.; Deuner, C.C. Reaction of Maize Hybrids to Northern Corn Leaf Blight and Common Rust, and Chemical Control of Northern Corn Leaf Blight. Plant. Pathol. 2019, 86, e0082018. [Google Scholar] [CrossRef]
- Betancur González, J.A.; Barbosa Felipini, R.; Zeist, A.R.; Ogliari, J.B. Resistance and Tolerance to Exserohilum turcicum in Landrace Sweet Corn Varieties from a Diversity Microcenter in Southern Brazil. Trop. Plant Pathol. 2025, 50, 46. [Google Scholar] [CrossRef]
- Kumari, N.; Kaur, S.; Sharma, V. Dissecting the role of salicylic acid in mediating stress response in mungbean cultivars concurrently exposed to Macrophomina phaseolina infection and drought stress. Plant. Physiol. Biochem. 2024, 210, 108660. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, H.; Wang, J. Synthesis and application of a compound microbial inoculant for effective soil remediation. Environ. Sci. Pollut. Res. 2023, 30, 120915–120929. [Google Scholar] [CrossRef]
- Li, H.R.; Zhang, X.Y.; He, K.L.; Xu, N.; Chen, N.W.; Ullah, Y.; Zhang, T.T.; Chen, Y.; Dai, C.-C.; Zhang, W. Differential responses of root and leaf-associated microbiota to continuous monocultures. Environ. Microbiome 2025, 20, 13. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Y.; Huang, X.; Shi, J.; Xu, J.; He, Y. Does straw returning affect the root rot disease of crops in soil? A systematic review and meta-analysis. J. Environ. Manag. 2023, 336, 117673. [Google Scholar] [CrossRef]
- Wang, W.; Wang, B.; Sun, X.; Qi, X.; Zhao, C.; Chang, X.; Khaskheli, M.I.; Gong, G. Symptoms and pathogens diversity of Corn Fusarium sheath rot in Sichuan Province, China. Sci. Rep. 2021, 11, 2835. [Google Scholar] [CrossRef]
- Wang, B.; Li, M.; Zhang, H.; Zhu, J.; Chen, S.; Ren, D. Effect of straw-derived dissolved organic matter on the adsorption of sulfamethoxazole to purple paddy soils. Ecotox. Environ. Saf. 2020, 203, 110990. [Google Scholar] [CrossRef] [PubMed]
| City | County (Latitude and Longitude) | Agrotype |
|---|---|---|
| Shenyang | Kangping (42.75° N, 123.35° E) | Loam soil |
| Faku (42.50° N, 123.40° E) | Loam soil | |
| Fushun | Qingyuan (42.10° N, 124.92° E) | Loam soil |
| Chaoyang | Beipiao (41.80° N, 120.75° E) | Loam soil |
| Fuxin | Fumeng (42.05° N, 121.75° E) | Sandy loam soil |
| Dandong | Fengcheng (40.45° N, 124.07° E) | Loam soil |
| Treatment Number | Treatment Combinations | Formulation | Dosage per Hectare |
|---|---|---|---|
| 1 | 10% Difenoconazole | WP (Wettable powder) | 300 g |
| 2 | 10% Difenoconazole + Biocontrol agent | WP (Wettable powder) + AS (Aqueous solution) | 300 g + 1.5 L |
| 3 | 15% Triadimefon | WP (Wettable powder) | 1200 g |
| 4 | 0.3% Tetramycin | AS (Aqueous solution) | 1050 g |
| 5 | 250 g/L Azoxystrobin | SC (Suspension concentrate) | 300 mL |
| 6 | 50% Carbendazim | WP (Wettable powder) | 1500 g |
| 7 | Biocontrol agent | AS (Aqueous solution) | 1.5 L |
| 8 | CK | - | - |
| Disease Severity Level | Symptom Description |
|---|---|
| 1 | Few lesions on leaves, lesion area ≤5% of the total leaf area |
| 3 | Sparse lesions, lesion area accounting for 6–10% of the leaf area |
| 5 | Moderate lesions, lesion area accounting for 11–30% of the leaf area |
| 7 | Abundant lesions, lesions merging, lesion area accounting for 31–70% of the leaf area |
| 9 | Leaves nearly covered by lesions, leading to withering |
| City | County (Latitude and Longitude) | Northern Corn Leaf Blight (Disease Index) | Fusarium Stalk Rot (Disease Incidence, %) | ||
|---|---|---|---|---|---|
| Non-Straw Return | Straw Return | Non-Straw Return | Straw Return | ||
| Shenyang | Kangping (42.75° N, 123.35° E) | 11.11 | 12.25 | 0 | 0 |
| Faku (42.50° N, 123.40° E) | 12.04 | 13.08 | 13.89 | 11.52 | |
| Fushun | Qingyuan (42.10° N, 124.92° E) | 23.82 | 26.54 | 19.00 | 18.75 |
| Chaoyang | Beipiao (41.80° N, 120.75° E) | 48.58 | 51.52 | 6.00 | 7.52 |
| Fuxin | Fumeng (42.05° N, 121.75° E) | 35.64 | 42.78 | 12.00 | 12.80 |
| Dandong | Fengcheng (40.45° N, 124.07° E) | 32.86 | 28.95 | 7.00 | 8.00 |
| Treatment Number | Treatment Combinations | Disease Index | Disease Inhibition Rate (%) |
|---|---|---|---|
| 1 | 10% Difenoconazole | 23.74 ± 1.47 *** | 56.21 ± 2.73 c |
| 2 | 10% Difenoconazole + Biocontrol agent | 14.96 ± 0.28 *** | 72.40 ±0.52 a |
| 3 | 15% Triadimefon | 24.70 ± 1.91 *** | 54.44 ± 3.53 c |
| 4 | 0.3% Tetramycin | 23.94 ± 2.51 ** | 55.84 ± 4.62 c |
| 5 | 250 g/L Azoxystrobin | 16.31 ± 1.21 ** | 69.91 ± 2.24 b |
| 6 | 50% Carbendazim | 29.22 ± 2.86 ** | 46.11 ± 5.27 d |
| 7 | Biocontrol agent | 17.83 ± 0.78 *** | 67.11 ± 1.44 b |
| 8 | CK | 54.22 ± 0.71 | - |
| Treatment Number | Treatment Combinations | Disease Incidence (%) | Disease Inhibition Rate (%) |
|---|---|---|---|
| 1 | 10% Difenoconazole | 2.15 ± 1.18 | 2.09 ± 2.13 |
| 2 | 10% Difenoconazole + Biocontrol agent | 0.61 ± 0.70 * | 71.03 ± 71.51 |
| 3 | 15% Triadimefon | 4.61 ± 6.01 | 0 |
| 4 | 0.3% Tetramycin | 2.70 ± 1.76 | 0 |
| 5 | 250 g/L Azoxystrobin | 2.34 ± 2.47 | 0 |
| 6 | 50% Carbendazim | 3.28 ± 2.51 | 0 |
| 7 | Biocontrol agent | 3.98 ± 0.64 | 0 |
| 8 | CK | 2.11 ± 2.47 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Wang, L.; Liu, K.; Liang, B.; Gong, H.; Chen, L.; Dong, H. Application of Difenoconazole and Trichoderma Broth Combination for Synergistic Control of Corn Leaf Blight and Stalk Rot in Straw-Returned Fields in Liaoning Province, China. Appl. Sci. 2025, 15, 7834. https://doi.org/10.3390/app15147834
Wang P, Wang L, Liu K, Liang B, Gong H, Chen L, Dong H. Application of Difenoconazole and Trichoderma Broth Combination for Synergistic Control of Corn Leaf Blight and Stalk Rot in Straw-Returned Fields in Liaoning Province, China. Applied Sciences. 2025; 15(14):7834. https://doi.org/10.3390/app15147834
Chicago/Turabian StyleWang, Ping, Lijuan Wang, Kejie Liu, Bingbing Liang, Hanxuan Gong, Le Chen, and Huaiyu Dong. 2025. "Application of Difenoconazole and Trichoderma Broth Combination for Synergistic Control of Corn Leaf Blight and Stalk Rot in Straw-Returned Fields in Liaoning Province, China" Applied Sciences 15, no. 14: 7834. https://doi.org/10.3390/app15147834
APA StyleWang, P., Wang, L., Liu, K., Liang, B., Gong, H., Chen, L., & Dong, H. (2025). Application of Difenoconazole and Trichoderma Broth Combination for Synergistic Control of Corn Leaf Blight and Stalk Rot in Straw-Returned Fields in Liaoning Province, China. Applied Sciences, 15(14), 7834. https://doi.org/10.3390/app15147834





