Abstract
Chemical endodontic irritants can lead to the demineralization of the inorganic tooth structure, its loss of integrity, microhardness changes, erosion, and an increased risk of fractures. We investigated the action of iron oxide nanomagnet particles (IONPs) as an irrigant solution for improving hardness and identifying the concentration of element ions in the root canal. There were six groups in total: a control group (no treatment) and experimental groups (UN: ultrasound agitation normal saline, UI: ultrasound agitation IONPs, MSI: magnetic field and endodontic needle with syringe agitation IONPs, MUI: magnetic field and ultrasound agitation IONPs, and EDTA: ethylenediaminetetraacetic acid). We hypothesized that IONPs with magnetic agitation would preserve microhardness better than EDTA. Vickers hardness testing was used to evaluate microhardness, which was then analyzed using energy-dispersive X-ray spectroscopy (EDS) to investigate the calcium/phosphorus ratio and the presence of iron. The IONP groups exhibit a higher VHN value than the EDTA group (p < 0.05). These results support our hypothesis, indicating that utilizing an IONP irrigant solution with an external magnetic field does not change microhardness but enhances it compared to the EDTA group, suggesting that employing an external magnetic field to deliver nanoparticles to the root canal wall does not affect the properties of the tooth structure compared to conventional instrumentation techniques, which lead to unnecessary loss of root structure.
1. Introduction
The smear layer is a 1–2 μm-thick thin film arranged on the root canal wall system that acts as a barrier blocking the opening of dentinal tubules [1]. This layer must be removed because it limits the diffusion purpose of the disinfecting agent and compromises the sealing ability of the root canal system [2]. Consequently, eradicating this layer during endodontic treatment is necessary for successful treatment and to prevent the recolonization of microorganisms within the prepared canal. Unfortunately, chemomechanical treatments, utilizing a chemical irrigant with excessive mechanical root canal preparation, cannot approach all anatomical depths of the canal to eradicate the microorganisms from the dentinal tubules and debridement debris from the root canal system [3]. Lateral canals, isthmi, fins, accessory canals, apical ramifications, and recesses are present in the root canal system, representing a harbor for bacterial biofilm and preventing the objective of preparation for treatment by impeding cleaning, disinfection, and the shaping of the root canal system [4,5]. In addition to the above, chemical chelating factors have been proposed for removing the smear layer [6]. These may also lead to the demineralization of the inorganic structure and the loss of the root canal’s structural integrity [7].
A sodium hypochlorite agent (NaOCl) can dissolve tissue and has antimicrobial properties. However, it can negatively reduce the physical characteristics of the dentine matrix and harm the periapical area due to accidental extrusion beyond the foramen [8].
Ethylenediaminetetraacetic acid (EDTA) is most commonly applied as a chelating factor. It can remove calcium ions from the root structure, resulting in changes to dentin microhardness [7] and eroding the root canal wall [9]. The chemical result of the irrigant used in endodontic treatment adversely affects the composition of the dentin structure, depending on the concentration and time of exposure [10]. Consequently, there is an increase in the susceptibility to root fractures [11].
Nanotechnology enhances the fabrication of new materials, creating novel materials, structures, and devices with special properties and functions [12]. It has been applied to various scientific disciplines, including medicine and dentistry. Magnetic nanoparticles (MNPs) are one type of material utilized in various technologies, such as in biomedicine for drug delivery [13]. For endodontic treatment, they are used as a root canal irrigant due to their association with peroxidase, which aids in improving antibacterial activity on the root canal wall [14] to mitigate the formation of dental plaque biofilms [15]. MNPs also enhance adhesion to dentin [16] and, under the control of a magnetic field, deeply penetrate root canal sealers to improve periapical healing [17].
Under a magnetic field, the nanoparticles are directed to a target area; this is the major application of IONPs in nanomedicine, which are coated with organic or inorganic materials for drug delivery [18,19]. Magnetite, with its chemical formula of Fe3O4, forms a spinel structure and is prevalent [20]. Hence, it is clear that successful cleaning and shaping in endodontic treatment require the use of irrigant solutions that neither affect the root canal surface nor cause alterations in the mechanical and chemical characteristics of the tooth structure [21].
Iron oxide nanoparticles (IONPs) have special properties, including excellent physicochemical properties, stability in aqueous solutions, minimal cytotoxicity, biocompatibility, and persistent magnetic characteristics. These attributes make them interesting for technological applications in biological and medicinal fields [22], including for imaging and diagnostics, where they are used in magnetic resonance imaging (MRI) probes and positron emission tomography (PET) [23,24]; for improving combination therapy (alongside chemotherapeutic drugs); as hyperthermia factors [25]; and for magnetic separation, drug delivery [18,26,27], iron detection, and cancer treatment [28]. The low cytotoxicity of IONPs, as well as their ability to produce a magnetic response under an external magnetic field, can improve their application in the biomedical field [29]. In 2018, Nosrati et al. suggested that uncoated magnetite nanoparticles or those coated with amino acids up to a concentration of 373 μg/mL showed no cytotoxicity [30]. Another study showed that IONPs functionalized with tri-sodium citrate solution have no cytotoxicity for fibroblasts at concentrations lower than 0.08 mg/mL [31]. This is consistent with the present study’s unpublished results, which recorded no toxic effect up to 312.5 μg/mL at 24, 48, and 72 h. It has been suggested that IONPs, at lower concentrations, do not harm cellular structures [32].
Metal oxide nanoparticles, including magnetite, have different mechanisms of antibacterial action. The first mechanism involves their small size, being smaller than bacterial pores. This improves their ability to access the cell membrane, resulting in continuous destructive activity. By penetrating the cytoplasmic membrane, they can interfere with nucleic acid or protein synthesis and disrupt the functional activity of the cell [33,34,35], thereby enhancing their antibacterial effects. The second mechanism that accentuates their antibacterial activity is the formation of reactive oxygen species (ROS) from ferrous ions, which includes superoxide radicals, hydroxyl radicals, hydrogen peroxide, and singlet oxygen free radicals. This occurs through the Fenton reaction, which can damage bacterial DNA, compromise lipids and proteins through oxidative stress, and restrict or limit further growth of the organism [36,37]. The third mechanism involves static electricity. In an experimental study, positively charged IONPs may be attracted to the negative charge on the surface of E. faecalis bacteria biofilm. When guided through an external magnetic field, the IONPs can eradicate biofilm from the treated root canal wall, preventing acidic formation and subsequently causing bacterial cell death through physical damage [38].
In this study, a dental irrigant incorporating IONPs under the control of an external magnetic field was developed to improve the microhardness of root canal systems without requiring extensive canal shaping. A simulated irrigant solution was tested to evaluate the influence of a magnetic field in mimicking clinical conditions. This approach demonstrated a potential protocol for avoiding conventional instrumentation techniques and the loss of unnecessary root structure. A magnet’s external field was applied to root canal dentinal walls, and an IONPs solution was agitated inside the root canal space. This study aimed to investigate the optimum effect of IONPs in enhancing hardness and identify the concentration of ions in the root canal walls. The null hypothesis states that there are no differences among all the irrigant protocols and the no-treatment group, and that the combination of magnetic activation with ultrasonics to agitate the IONP irrigant in the root canal space does not preserve or enhance dentin microhardness compared to other groups.
2. Materials and Methods
2.1. Sample Collection
The sample size was calculated by adopting an alpha (α) level of 0.05 (5%), a beta (β) level of 0.05 (5%), power = 95%, and an effect size (f) of (0.9501871), based on the results of Sahebi et al. [39]. The predicted sample size was 53 samples. Sample size calculation was performed using G*Power, version 3.1.9.7 (Windows software, Düsseldorf, Germany). The sample size was subsequently increased to 60 samples, n = 10 specimens per group. Sixty vital maxillary first molars were extracted from well-known dental centers within one month and were kept in thymol solution [40]. Informed consent was obtained from all subjects whose teeth were extracted due to extensive periodontitis. We explained that the extracted teeth would be used for the study after extraction. The Scientific Committee of the College of Dentistry-University of Baghdad (No. 1024 on 30 January 2025) approved this study.
2.2. Sample Selection
The teeth were inspected with a magnifying lens to exclude any teeth with carious lesions, cracks, or open root apices [41]. Radiographs—X-rays from the buccal and palatal areas—were used to identify the presence of a straight canal; the root specimens were calibrated at 12 mm and placed perpendicularly in a silicon rubber base (Zermak, Badia Polesine, Italy) held in clear acrylic molds to facilitate assessment of the instrumentation process.
2.3. Sample Preparation
A single operator performed all the experiments to minimize the variables during specimen preparation. The pulpal tissue was removed using a barbed broach. A stainless-steel K-file size 10 (Dentsply Maillefer, Ballaigues, Switzerland) was used until the file tip exited from the root specimen, observed under a magnifying lens. The root was instrumented with K-file sizes from size 15 to size 20, except for teeth in the control group, which were rotated in a clockwise direction with slight pressure, ensuring to remove any debris when pulling the file. An endodontic needle with a double-sided access gauge 30 was inserted 3 mm short of working length to irrigate each canal with 2 mL of normal saline between instruments. Any root that needed enlargement of more than file size 20 was excluded and replaced with another one. The two longitudinal grooves on both buccal and lingual sides were created using a double-sided diamond disc of a straight handpiece under water cooling (avoiding deeply exposing the canal space). This procedure aimed to separate the root into two halves with a chisel, following the groove’s path.
2.4. Generation of the Magnetic Field to Agitate IONPs
A combination of two types of magnets was used to generate the magnetic field, as depicted in Figure 1.
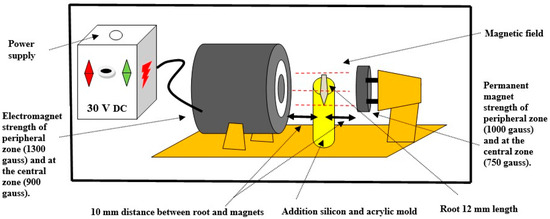
Figure 1.
Schematic diagram of the magnetic field device.
Type 1 (permanent magnet): consisted of an 18-mm-round super neodymium magnet (K&J Magnetics Inc., Pipersville, PA, USA: Neodymium 35) with constant power (750 gauss in the central area and 1000 gauss in the peripheral area) located 10 mm from the root side.
Type 2 (electromagnet): constructed from a coil of wire and an iron core (self-made). This self-made device could control the magnetic field strength by adjusting the direct current (DC) up to 30 volts (V). The polarity (on–off cycles) was defined, and the frequency was 10 times per second. Its magnetic strength (900 gauss in the central area and 1300 gauss in the peripheral area) was stronger than that of the Type 1 magnet to enhance the agitation of IONPs in the canal space. This magnet was located 10 mm from the other root side. The electromagnet was constructed from a soft iron cylindrical core measuring 6 cm in length and 2.5 cm in diameter. The coil consisted of AWG 24 enameled copper wire, conforming to approximately 1200 turns, mounted on a plastic spool. The DC power supply was 24 V DC. The operating current was typically maintained between 0.5 and 1 ampere. The magnetic strength between them was identified using a handheld digital gaussmeter device (Tunkia, Co., Ltd., Changsha, Hunan, China) provided by the Scientific Research Authority, Ministry of Higher Education and Scientific Research, Baghdad, Iraq. The magnetic strength at the root position between them was 450 gauss, measured using a gaussmeter device by calibrating the device probe at an intermediate distance between the center of each magnet, representing the root specimen.
2.5. Preparation of IONP Irrigation Solution
Ten grams of dried IONPs (Fe3O4) powder (25–50 nm; US Research Nanomaterials Inc., Houston, TX, USA) was mixed with one liter of deionized water. The solution concentration was 1%, selected based on the previous pilot study and cytotoxicity and bacteriological tests. The mixture was agitated under an ultrasonic device for 20 min to achieve a clear colloidal dispersion [42]. The physicochemical characterization of the IONPs was carried out using transmission electron microscopy (TEM) (Thermo Fisher Scientific, Eindhoven, Nederland). They appeared to be spherical with a dry core under vacuum, with diameters ranging from 6 to 12 nm [43]. Dynamic light scattering (DLS) showed a larger core size and hydrodynamic diameter in solution (50–100 nm), which included the coating thickness, depending on surface coatings or aggregation in aqueous suspension, often observed after sonication or dilution for uniform dispersion at room temperature [44]. The zeta potential represents the electrokinetic effects of uncoated iron oxides (−16 to −20 mV) and surface charge in a liquid and electric field environment, with positive pH behavior at low pH values [45].
2.6. Sample Grouping
The root specimens were randomly assigned to control and experimental groups using a Microsoft Excel random number generator (RAND function) (Microsoft Corporation, Redmond, Washington, USA; Version 16.0). Each root was assigned a unique identifier, and random numbers were formatted and sorted.
2.6.1. Control Group
- No instrumentation or irrigation treatment.
2.6.2. Five Experimental Groups
- UN group: an ultrasound device (Guilin Woodpecker U 600 Medical Instrument Co., Ltd., Guilin, China) used continuous irrigation to agitate normal saline irrigant solution.
- UI group: an ultrasound device was used to agitate the IONPs irrigant solution.
- MSI group: a magnetic field and an endodontic needle with a syringe were used to agitate the IONPs irrigant solution.
- MUI group: a magnetic field and an ultrasound device were used to agitate the IONPs irrigant solution.
The needle had a double-sided access gauge 30 (Stardent Equipment Co., Ltd., Foshan, Guangdong, China) under the control of an electrically programmable pump tool to control the flow rate of irrigant at 10 mL/min. The ultrasound device tip file was E 62, the length was 16 mm, and the dimension was 0.3 mm with zero taper. The file was inserted at 10 mm into the center of the canal and was moved 2–3 mm in the up-and-down direction. The device was set at half power as recommended by the manufacturer. All agitated irrigant solution was applied at 50 mL for five minutes.
- EDTA group: Irrigation with ethylenediaminetetraacetic acid solution (Meta BioMed’s, Cheongju-si, Chungcheong Buk-do, Korea) using a double-sided needle access gauge 30 at a concentration of 17% for one minute.
Finally, all experimental groups were irrigated with 50 mL of normal saline solution using continuous ultrasound devices for five minutes.
2.7. Vickers Hardness Testing Machine
The two longitudinal grooves depicted in Figure 2a were separated using a chisel and mallet; one half was used for the Vickers microhardness test [46,47] and was mounted in the plastic mold with acrylic resin. The surface of the root halves was ground under water flow utilizing a sequence of carbide abrasive papers. Finally, it was polished with a 0.1 mm alumina suspension on the felt disc of a rotary grinder polisher device (MoPao, E160, Jinan Kason Testing Equipment Co., Ltd., China) for five seconds at 60 revolutions per minute (rpm) to remove roughness and achieve a finely smoothed, glossy, mirror-like surface prior to microscopic examination. The microhardness of the root dentine surface was analyzed using a digital Vickers hardness tester (Laryee, Beijing, China) with a load of 0.98 newtons and a dwell time of 15 s [48]. The measurements were performed at the coronal, middle, and apical sections at four indentations. To reduce measurement bias, all recordings were performed by an examiner blinded to the group assignments throughout the analysis. This case is depicted in Figure 2b,c; the first and second indentations were made in subsurface, 50 µm and 100 µm from the lumen (intermediary distance of each section) of each third section [49,50]. Third and fourth indentations were made in the lumen surface along lines parallel to the border of the canal lumen (1 mm above and another 1 mm below the midpoint of each third section).
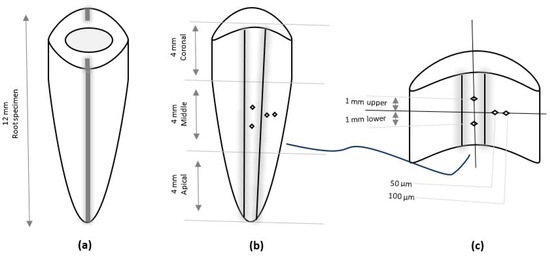
Figure 2.
A schematic diagram of root canal preparation: (a) single root specimen with two longitudinal grooves, (b) one-half of the splitting of the root with canal lumen, and (c) the middle section with four identical Vickers microhardness points.
2.8. EDS (Energy-Dispersive X-Ray Spectroscopy) Analysis
Each group section was examined using EDS (Thermo Fisher Scientific, Eindhoven, Nederland). This assessment is depicted in Figure 3. Half the root specimen was mounted on the device’s aluminum stubs and sputter-coated with gold. Then, each specimen was split at the center point of the canal lumen into three sections: coronal, middle, and apical. The analysis determined the weight percentages of iron (Fe), calcium (Ca), and phosphorus (P) concentrations [51,52].
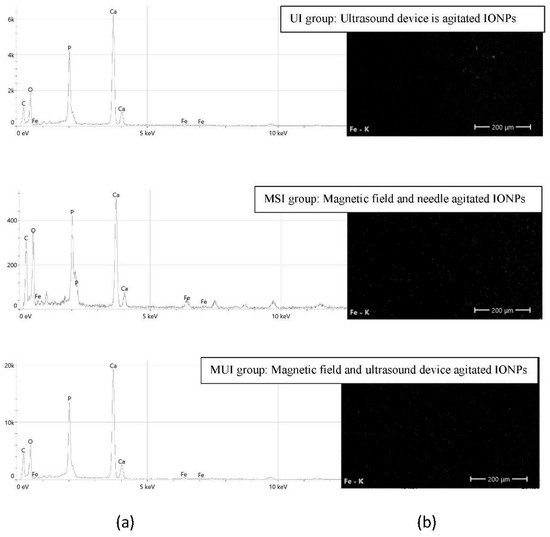
Figure 3.
The experimental groups of agitated IONPs. (a) EDS micrograph spectrum and (b) the distribution of Fe in elemental mapping.
2.9. Statistical Analysis
Data were analyzed using SPSS, IBM Statistics software (Version 26, Chicago: SPSS Inc. USA), and the normality of the data was assessed using the Shapiro–Wilk test. A one-way ANOVA and Tukey post hoc test were utilized to compare continuous data, and statistical significance was set at p < 0.05.
3. Results
3.1. Analysis of the First Point at 50 µm Subsurface from the Border of the Root Canal Lumen
A significant difference (p < 0.05) between groups was observed only in the coronal region. Group MSI (magnetic field and endodontic needle, agitated IONPs irrigant) showed the lowest mean dentin microhardness, followed by the EDTA group. In contrast, the highest mean values were observed in the UN group (ultrasound device, agitated normal saline irrigant), UI group (ultrasound device, an agitated IONPS irrigant), and MUI group (magnetic field and ultrasound device, agitated IONPs irrigant). All groups had lower microhardness values than the control at 50 µm and higher values than the EDTA 17% group, as shown in Table 1.

Table 1.
Descriptive data of groups’ dentine microhardness in each section at the depth of 50 µm subsurface from the root canal lumen after using different techniques with different materials.
3.2. Analysis of the Second Point at 100 µm Subsurface from the Border of the Root Canal Lumen
A significant difference (p < 0.05) between groups was observed only in the coronal region. The lowest mean value of the dentin microhardness test was observed in the EDTA group, followed by the MSI group (magnetic field and endodontic needle, agitated with IONPs irrigant). In contrast, the highest mean values were observed in the UI group (ultrasound device, agitated IONPS irrigant), UN group (ultrasound device, agitated normal saline irrigant), and MUI group (magnetic field and ultrasound device, agitated IONPs irrigant). All groups had lower microhardness values than the control at 100 µm and higher than the EDTA 17% group, as shown in Table 2.

Table 2.
Descriptive data of groups’ dentine microhardness in each section at a depth of 100 µm subsurface from the root canal lumen after using different techniques with different materials.
The mean value was higher at a depth of 100 µm than at 50 µm. However, all groups, at both the first 50 µm point and the second 100 µm point subsurface from the border of the root canal lumen showed lower mean values compared to the control group, but higher values than the EDTA group. The data are shown in Table 1 and Table 2.
3.3. Analysis of the Third Point at 1 mm Above the Midpoint of Each Third of the Root Section at the Lumen Surface
A significant difference (p < 0.05) between groups was only observed in the apical section. The lowest mean dentin microhardness value was observed in the EDTA group. In contrast, the MUI group (magnetic field and ultrasound device, agitated IONPs irrigant) showed a higher mean value than the MSI group (magnetic field and endodontic needle, agitated IONPs irrigant), followed by the UN group (ultrasound device, agitated normal saline irrigant). No significant effect was observed in the UI group (ultrasound device, an agitated IONPS irrigant). However, all groups except for the EDTA group had higher mean values than the control group. The data are shown in Table 3. Typically, the MUI group (magnetic field and ultrasound device, agitated IONPs irrigant) showed a higher mean level of dentin microhardness value in the apical section, followed by the middle section, and a lower level in the coronal section than the control group (p < 0.05).

Table 3.
Descriptive data of groups’ dentine microhardness in each section at 1 mm above the midpoint of each third at the lumen surface of the root canal after using different techniques with different materials.
3.4. Analysis of the Fourth Point at 1 mm Below Midpoint of Each Third of the Root Section at the Lumen Surface
A significant difference (p < 0.05) was only observed in the middle section, where the mean value of dentin microhardness was lowest in the EDTA group, followed by the MSI group (magnetic field and endodontic needle, agitated IONPs irrigant), the UI group (ultrasound device, agitated IONPs irrigant), and the UN group (ultrasound device, agitated normal saline irrigant). The MUI group (magnetic field and ultrasound device, agitated IONPs irrigant) showed higher mean values than the control group. However, all other groups had lower mean values than the control group, except for the MUI group (magnetic field and ultrasound device, agitated IONPs irrigant). The data are shown in Table 4. Compared with the control group, the MUI group showed a higher mean value of dentin microhardness in the middle section, followed by the apical section, with a lower level in the coronal section.

Table 4.
Descriptive data of groups’ dentine microhardness in each section at 1 mm below the midpoint of each third at the lumen surface of the root canal after using different techniques with different materials.
3.5. Assessment of the Concentrations of Calcium (Ca) and Phosphorus (P) Ratio and Concentration of Iron Ion by Energy-Dispersive X-Ray Spectroscopy (EDS)
The root specimen was coated with a thin gold film, and a semi-quantitative analysis of elements was performed at an accelerating voltage of 15 kV. The specimens were bombarded with an electron beam, and then the detector of the device captured the emitted X-rays, and subsequently analyzed the height peak area of each element. The measured intensities were coincident with background and normalized to provide relative weight percentages of the elements [53].
When comparing the Ca/P ratios of the irrigant groups after using different protocols with no treatment of the control group, the Ca/P ratio of the IONPs irrigant group was higher than that of the EDTA group. The UN group (ultrasound device, agitated normal saline irrigant) had the lowest concentration of iron ions, followed by the MUI group (magnetic field and ultrasound device, agitated IONPs irrigant). There was a high concentration in the MSI group (magnetic field and endodontic needle). The data are shown in Table 5.

Table 5.
Descriptive data of the elements’ weight concentrations and Ca/P ratio in the root structure after using different techniques with different materials.
4. Discussion
One of the primary factors influencing the clinical success of root canal treatment is the irrigation technique, as no perfect irrigation protocol can meet all the treatment criteria [54]. A previous study demonstrated that certain irrigants may remove the smear layer with bacteria and penetrate at a distance of up to 130 μm from the canal wall [55]. However, another study revealed that bacteria and their byproducts could persist at depths of more than 100 μm [56]. Therefore, the present study focused on evaluating dentin hardness at 50 μm and 100 μm from the canal [50].
This study used different irrigants, including IONPs, EDTA, and normal saline. To enhance the microhardness of the root structure while conserving the tooth structure, roots were prepared using various protocols involving ultrasonic activation [57] and an external magnetic field [19], either alone or with ultrasonic oscillation.
No clinical data have been investigated to determine the effect of a magnetic field and IONPs on root dentin hardness or tooth structure, nor has the Ca/P ratio in root canals been examined. All prior studies of root canal cleaning, shaping, and irrigation techniques have focused on the microhardness efficiency of each system as the primary goal, but have overlooked the conservative structure of the root canal system. Previous research used the Vickers indenter technique to assess the change in dentin hardness after treatment with different chemical agents [7,50,58]. Microhardness measurement can serve as a direct indicator of mineral loss or acquisition in dental hard tissue [59]. A reduction in dentin hardness leads to a decrease in the fracture resistance of the tooth [60]. A study found that five-minute periods of ultrasound irrigation were more effective in eradicating the smear layer than one-minute periods [61]. This aligns with our study of using irrigation for five-minute periods.
However, EDTA 17% was used as an irrigant for one minute to remove the smear layers in dentin [62]. New approaches are essential, as obtaining optimal root canal microhardness is associated with a conservative root structure. To achieve this, we investigated the combination of IONPs with magnetic and continuous ultrasound activation as an innovative approach to removing debris without adverse effects on the mechanical and chemical properties of dentin structure, comparing it to chemical methods, such as EDTA, to ensure the success of clinical treatment [21] and improve the effectiveness of the depth of irrigant penetration. The application of ultrasonics in conjunction with an external magnetic field is more effective with deeper irrigant penetration. This idea aligns with Paragliola et al.’s study, as they suggest that hydrodynamic irrigation progresses to deepen the penetration of irrigant solution into the canal wall dentine [63]. This finding consistently showed that all IONPs irrigant activation methods were more effective than normal saline or EDTA.
Furthermore, the EDS test revealed that when comparing the Ca/P ratio of the irrigant groups to that of the control group, the IONPs irrigant had a higher ratio than the EDTA 17% irrigant. The EDTA group effectively removed the smear layer but caused a significantly greater decrease in dentin microhardness than the other groups. These findings are consistent with results reported in other studies [64,65]. A notable direct relationship was observed between root dentin calcium reduction and its microhardness. Panighi and G’Sell reported a straightforward linear correlation between dentin hardness and calcium ion concentration [66].
However, all groups had lower microhardness values at 50 µm and 100 µm compared to the control group, but values were higher in the EDTA group. This corroborates another study, which concluded that utilizing EDTA 17% can reduce dentin microhardness. This may explain why all experimental groups exhibited significantly higher microhardness than the chelating agents compared to the control group [49]. When comparing the microhardness at 50 µm and 100 µm, it showed a lower mean value at 50 µm than at 100 µm because there is an inverse relationship between dentin hardness and depth of radicular dentin at the distance between the pulp lumen and cementodentinal junction within the same tooth. The area near the pulp lumen at 50 µm had low microhardness because of the increased number and width of dentinal tubules, which was associated with a lower amount of peritubular dentin than the area close to the cementodentinal junction at 100 µm [67]. In the present study, the coronal section had lower dentin microhardness than the middle and apical sections in all groups because of the increased tubular density of coronal dentin compared to middle and apical dentin, reflecting a histological pattern of radicular root canal dentin [68]. Changes in dentin microhardness from the coronal to apical sections are related to changes in mineral amount during different treatment protocols [69]. In the present study, the analysis of the points at 1 mm above and below the midpoint of the root section at the lumen surface showed an increase in the microhardness of root canal dentin after irrigation with IONPs, especially for the MUI group compared with the control group and the EDTA group. This occurred due to the change in calcium/phosphorus ratio in the root lumen due to the effect of iron ions. In contrast, phosphate ions may attract calcium ions to form a satisfactory layer for the nucleation of crystals, resulting in the construction of a calcium–phosphate surface [70]. This study has several limitations. First, the analysis of the antibacterial effect, cytotoxicity, and fracture resistance is based on preliminary data from unpublished work and has not yet undergone peer review. Second, using an external magnetic field to guide the irrigant may present practical challenges in routine clinical settings. Third, this study did not assess long-term effects on dentin structure and canal healing and only assessed the immediate effects (e.g., microhardness or smear layer removal). Consequently, the technique has not been clinically validated, given that it has not yet been tested in actual patients. Future clinical trials are necessary to confirm these findings and evaluate the method’s effectiveness and safety in real-world settings.
5. Conclusions
The utilization of an external magnetic field to agitate IONP irrigants did not change dentin microhardness or dentinal structure in terms of the Ca/P ratio, but enhanced microhardness compared to EDTA irrigation. This new approach is a promising tool for endodontic treatment with a conservative tooth structure. We suggest that future work optimize magnetic field parameters with practical clinical implications and assess the need for in vivo studies to evaluate long-term dentin strength.
Author Contributions
Authorship Contribution: Conceptualization, E.S.A.-M.; Data curation, E.S.A.-M.; Methodology, E.S.A.-M.; Investigation, E.S.A.-M.; Project administration, E.S.A.-M. and H.F.A.-H.; Resources, H.F.A.-H.; Funding Acquisition, E.S.A.-M. and H.F.A.-H.; Software, E.S.A.-M.; Writing—Original Draft, E.S.A.-M.; Writing—Review & Editing, E.S.A.-M. and H.F.A.-H.; Supervision, Validation, H.F.A.-H.; Visualization, H.F.A.-H.; Formal analysis, E.S.A.-M. and H.F.A.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Scientific Committee of the College of Dentistry-University of Baghdad (No. 1024 on 30 January 2025) has approved.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ANOVA | analysis of variance |
| AWG | american wire gauge |
| Ca | calcium |
| Ca/P ratio | calcium phosphorus ratio |
| DC | direct current |
| EDS | energy-dispersive X-ray spectroscopy |
| EDTA | ethylenediaminetetraacetic acid |
| Fe | iron |
| FE-SEM | field emission scan electron microscopy |
| Fe3O4 | iron oxide |
| Gauss | unit of magnetic field density |
| IONP | iron oxide nanomagnet particles |
| mL/min | milliliters per minute |
| µm | micrometer |
| mm | millimeter |
| MNPs | magnetic nanoparticles |
| MSI | magnetic field, endodontic needle, and IONP |
| MUI | magnetic field, ultrasound device, and IONP |
| NaOCl | sodium hypochlorite |
| nm | nanometer |
| P | phosphorus |
| rpm | revolutions Per Minute. |
| SPIONs | superparamagnetic iron oxide nanoparticles solution |
| UN | ultrasound device and normal saline |
| UI | ultrasound device and IONPs |
| V | voltage |
| VHN | vicker hardness number |
References
- Virdee, S.; Seymour, D.W.; Farnell, D.; Bhamra, G.; Bhakta, S. Efficacy of irrigant activation techniques in removing intracanal smear layer and debris from mature permanent teeth: A systematic review and meta-analysis. Int. Endod. J. 2018, 51, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-E.; Bae, K.-S. Scanning electron microscopy study of the adhesion of Prevotella nigrescens to the dentin of prepared root canals. J. Endod. 2002, 28, 433–437. [Google Scholar] [CrossRef] [PubMed]
- de Paz, L.C. Redefining the persistent infection in root canals: Possible role of biofilm communities. J. Endod. 2007, 33, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Versiani, M.; Martins, J.; Ordinola-Zapata, R. Anatomical complexities affecting root canal preparation: A narrative review. Aust. Dent. J. 2023, 68, S5–S23. [Google Scholar] [CrossRef]
- Alves, F.R.F.; Squeira, F.S., Jr.; Carmo, F.L.; Dos Santos, A.L.; Peixoto, R.S.; Rôças, I.N.; Rosado, A.S. Bacterial community profiling of cryogenically ground samples from the apical and coronal root segments of teeth with apical periodontitis. J. Endod. 2009, 35, 486–492. [Google Scholar] [CrossRef]
- Prado, M.; Gusman, H.; Gomes, B.P.; Simão, R.A. Scanning electron microscopic investigation of the effectiveness of phosphoric acid in smear layer removal when compared with EDTA and citric acid. J. Endod. 2011, 37, 255–258. [Google Scholar] [CrossRef]
- Taneja, S.; Kumari, M.; Anand, S. Effect of QMix, peracetic acid and ethylenediaminetetraacetic acid on calcium loss and microhardness of root dentine. J. Conserv. Dent. Endod. 2014, 17, 155–158. [Google Scholar] [CrossRef]
- Hülsmann, M.; Hahn, W. Complications during root canal irrigation—Literature review and case reports. Int. Endod. J. 2000, 33, 186–193. [Google Scholar] [CrossRef]
- Qian, W.; Shen, Y.; Haapasalo, M. Quantitative analysis of the effect of irrigant solution sequences on dentin erosion. J. Endod. 2011, 37, 1437–1441. [Google Scholar] [CrossRef]
- Ozdemir, H.O.; Buzoglu, H.D.; Çalt, S.; Çehreli, Z.C.; Varol, E.; Temel, A. Chemical and ultramorphologic effects of ethylenediaminetetraacetic acid and sodium hypochlorite in young and old root canal dentin. J. Endod. 2012, 38, 204–208. [Google Scholar] [CrossRef]
- Saha, S.G.; Sharma, V.; Bharadwaj, A.; Shrivastava, P.; Saha, M.K.; Dubey, S.; Kala, S.; Gupta, S. Effectiveness of various endodontic irrigants on the micro-hardness of the root canal dentin: An in vitro study. J. Clin. Diagn. Res. JCDR 2017, 11, ZC01–ZC04. [Google Scholar] [CrossRef] [PubMed]
- Brüngel, R.; Rückert, J.; Müller, P.; Babick, F.; Friedrich, C.M.; Ghanem, A.; Hodoroaba, V.-D.; Mech, A.; Weigel, S.; Wohlleben, W.; et al. Nanodefiner framework and e-tool revisited according to the European commission’s nanomaterial definition 2022/C 229/01. Nanomaterials 2023, 13, 990. [Google Scholar] [CrossRef] [PubMed]
- El-Boubbou, K. Magnetic iron oxide nanoparticles as drug carriers: Clinical relevance. Nanomedicine 2018, 13, 953–971. [Google Scholar] [CrossRef]
- Bukhari, S.; Kim, D.; Liu, Y.; Karabucak, B.; Koo, H. Novel endodontic disinfection approach using catalytic nanoparticles. J. Endod. 2018, 44, 806–812. [Google Scholar] [CrossRef]
- Al-Bazaz, F.A.; Radhi, N.J.; Hubeatir, K.A. Sensitivity of Streptococcus mutans to selected nanoparticles (in vitro study). J. Baghdad Coll. Dent. 2018, 30, 69–75. [Google Scholar] [CrossRef]
- Garcia, I.M.; Balhaddad, A.A.; Lan, Y.; Simionato, A.; Ibrahim, M.S.; Weir, M.D.; Masri, R.; Xu, H.H.K.; Collares, F.M.; Melo, M.A.S. Magnetic motion of superparamagnetic iron oxide nanoparticles-loaded dental adhesives: Physicochemical/biological properties, and dentin bonding performance studied through the tooth pulpal pressure model. Acta Biomater. 2021, 134, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Sun, Y.; Wang, Z.; Ren, B.; Xu, H.H.K.; Peng, X.; Li, M.; Wang, S.; Wang, H.; Wu, Y.; et al. The preventive effect of a magnetic nanoparticle-modified root canal sealer on persistent apical periodontitis. Int. J. Mol. Sci. 2022, 23, 13137. [Google Scholar] [CrossRef]
- El-Boubbou, K. Magnetic iron oxide nanoparticles as drug carriers: Preparation, conjugation and delivery. Nanomedicine 2018, 13, 929–952. [Google Scholar] [CrossRef]
- Al-Badr, R.J.; Al-Huwaizi, H.F. Antimicrobial Evaluation for Novel Solution of Iron Oxide Nanoparticles Functionalized with Glycine and Coated by Chitosan as Root Canal Final Irrigation. Syst. Rev. Pharm. 2020, 11, 633–642. [Google Scholar]
- Teja, A.S.; Koh, P.-Y. Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Ari, H.; Erdemir, A.; Belli, S. Evaluation of the effect of endodontic irrigation solutions on the microhardness and the roughness of root canal dentin. J. Endod. 2004, 30, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N.; Lee, J.-S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflores, O.B.; Ger, T.-R.; Hsiao, C.-D. Potential toxicity of iron oxide magnetic nanoparticles: A review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef]
- Xie, J.; Chen, K.; Huang, J.; Lee, S.; Wang, J.; Gao, J.; Li, X.; Chen, X. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials 2010, 31, 3016–3022. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Zhang, H.; Yu, J.; Tong, S.; Tian, N.; Wang, Z.; Wang, X.; Su, X.; Chu, X.; Lin, J.; et al. Monodisperse Au–Fe2C Janus nanoparticles: An attractive multifunctional material for triple-modal imaging-guided tumor photothermal therapy. ACS Nano 2017, 11, 9239–9248. [Google Scholar] [CrossRef]
- Ren, Y.; Ren, Y.; Zhang, H.; Cheng, J.; Cai, X.; Liu, R.; Xia, G.; Wu, W.; Wang, S.; Ding, J.; et al. Multifunctional magnetic Fe3O4 nanoparticles combined with chemotherapy and hyperthermia to overcome multidrug resistance. Int. J. Nanomed. 2012, 7, 2261–2269. [Google Scholar]
- Vasir, J.K.; Labhasetwar, V. Targeted drug delivery in cancer therapy. Technol. Cancer Res. Treat. 2005, 4, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, I.; Aghazadeh, M.; Doroudi, T.; Ganjali, M.R.; Kolivand, P.H. Superparamagnetic iron oxide (Fe3O4) nanoparticles coated with PEG/PEI for biomedical applications: A facile and scalable preparation route based on the cathodic electrochemical deposition method. Adv. Phys. Chem. 2017, 2017, 9437487. [Google Scholar] [CrossRef]
- Liu, G.; Men, P.; Harris, P.L.; Rolston, R.K.; Perry, G.; Smith, M.A. Nanoparticle iron chelators: A new therapeutic approach in Alzheimer disease and other neurologic disorders associated with trace metal imbalance. Neurosci. Lett. 2006, 406, 189–193. [Google Scholar] [CrossRef]
- Balhaddad, A.A.; Xia, Y.; Lan, Y.; Mokeem, L.; Ibrahim, M.S.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Magnetic-responsive photosensitizer nanoplatform for optimized inactivation of dental caries-related biofilms: Technology development and proof of principle. ACS Nano 2021, 15, 19888–19904. [Google Scholar] [CrossRef]
- Nosrati, H.; Salehiabar, M.; Attari, E.; Davaran, S.; Danafar, H.; Manjili, H.K. Green and one-pot surface coating of iron oxide magnetic nanoparticles with natural amino acids and biocompatibility investigation. Appl. Organomet. Chem. 2018, 32, e4069. [Google Scholar] [CrossRef]
- Ferraz, F.S.; López, J.L.; Lacerda, S.M.; Procópio, M.S.; Figueiredo, A.F.; Martins, E.M.; Guimarães, P.P.; Ladeira, L.O.; Kitten, G.T.; Dias, F.F.; et al. Biotechnological approach to induce human fibroblast apoptosis using superparamagnetic iron oxide nanoparticles. J. Inorg. Biochem. 2020, 206, 111017. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Perlstein, B.; Houbara, O.; Felner, I.; Banin, E.; Margel, S. Synthesis and characterization of zinc/iron oxide composite nanoparticles and their antibacterial properties. Colloids Surf. A Physicochem. Eng. Asp. 2011, 374, 1–8. [Google Scholar] [CrossRef]
- Das, S.; Diyali, S.; Vinothini, G.; Perumalsamy, B.; Balakrishnan, G.; Ramasamy, T.; Dharumadurai, D.; Biswas, B. Synthesis, morphological analysis, antibacterial activity of iron oxide nanoparticles and the cytotoxic effect on lung cancer cell line. Heliyon 2020, 6, e04953. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, H.; Gielis, J.; Acke, M.; Cools, F.; Cos, P.; Coenye, T. The role of reactive oxygen species in antibiotic-induced cell death in Burkholderia cepacia complex bacteria. PLoS ONE 2016, 11, e0159837. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.Y.; Lee, W.I.; Nelson, K.L.; Yoon, J.; Sedlak, D.L. Bactericidal effect of zero-valent iron nanoparticles on Escherichia coli. Environ. Sci. Technol. 2008, 42, 4927–4933. [Google Scholar] [CrossRef]
- Rodrigues, G.R.; López-Abarrategui, C.; Gómez, I.d.l.S.; Dias, S.C.; Otero-González, A.J.; Franco, O.L. Antimicrobial magnetic nanoparticles based-therapies for controlling infectious diseases. Int. J. Pharm. 2019, 555, 356–367. [Google Scholar] [CrossRef]
- Sahebi, S.; Mofidi, H.; Abbaszadegan, A.; Gholami, A.; Eskandari, F. The effect of nanobased irrigants on the root canal dentin microhardness: An ex-vivo study. BMC Oral Health 2023, 23, 581. [Google Scholar] [CrossRef]
- Unnikrishnan, M.; Mathai, V.; Sadasiva, K.; Santakumari, R.S.M.; Girish, S.; Shailajakumari, A.K. The Evaluation of Dentin Microhardness After Use of 17% EDTA, 17% EGTA, 10% Citric Acid, MTAD Used as Chelating Agents Combined With 2.5% Sodium Hypochlorite After Rotary Instrumentation: An: In Vitro: SEM Study. J. Pharm. Bioallied Sci. 2019, 11 (Suppl. 2), S156–S163. [Google Scholar] [CrossRef]
- Aksel, H.; Arslan, E.; Puralı, N.; Uyanık, Ö.; Nagaş, E. Effect of ultrasonic activation on dentinal tubule penetration of calcium silicate-based cements. Microsc. Res. Tech. 2019, 82, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Al Bazaz, F.; Radhi, N.J.; Hubeatir, K.A.; Alghazali, M.W. Effect of CO2 laser and selected nanoparticles on the microhardness of human dental enamel in vitro study. J. Med. Chem. Sci. 2023, 6, 1487–1497. [Google Scholar]
- Wu, K.; Liu, J.; Saha, R.; Peng, C.; Su, D.; Wang, Y.A.; Wang, J.-P. Investigation of commercial iron oxide nanoparticles: Structural and magnetic property characterization. ACS Omega 2021, 6, 6274–6283. [Google Scholar] [CrossRef]
- Aksu Demirezen, D.; Yılmaz, Ş.; Yılmaz, D.D.; Yıldız, Y.Ş. Green synthesis of iron oxide nanoparticles using Ceratonia siliqua L. aqueous extract: Improvement of colloidal stability by optimizing synthesis parameters, and evaluation of antibacterial activity against Gram-positive and Gram-negative bacteria. Int. J. Mater. Res. 2022, 113, 849–861. [Google Scholar] [CrossRef]
- Carlson, J.; Kawatra, S. Factors affecting zeta potential of iron oxides. Miner. Process. Extr. Metall. Rev. 2013, 34, 269–303. [Google Scholar] [CrossRef]
- Gutiérrez-Salazar, M.d.P.; Reyes-Gasga, J. Microhardness and chemical composition of human tooth. Mater. Res. 2003, 6, 367–373. [Google Scholar] [CrossRef]
- Salem-Milani, A.; Zand, V.; Asghari-Jafarabadi, M.; Zakeri-Milani, P.; Banifatemeh, A. The effect of protocol for disinfection of extracted teeth recommended by center for disease control (CDC) on microhardness of enamel and dentin. J. Clin. Exp. Dent. 2015, 7, e552. [Google Scholar][Green Version]
- Zhang, Y.-R.; Du, W.; Zhou, X.-D.; Yu, H.-Y. Review of research on the mechanical properties of the human tooth. Int. J. Oral Sci. 2014, 6, 61–69. [Google Scholar] [CrossRef]
- Baldasso, F.E.R.; Roleto, L.; da Silva, V.D.; Morgental, R.D.; Kopper, P.M.P. Effect of final irrigation protocols on microhardness reduction and erosion of root canal dentin. Braz. Oral Res. 2017, 31, e40. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Delvarani, A.; Mehrvarzfar, P.; Malganji, G.; Lotfi, M.; Dadresanfar, B.; Saghiri, A.M.; Dadvand, S. A study of the relation between erosion and microhardness of root canal dentin. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, e29–e34. [Google Scholar] [CrossRef]
- Zheng, J.; Nagashima, K.; Parmiter, D.; de la Cruz, J.; Patri, A.K. SEM X-ray microanalysis of nanoparticles present in tissue or cultured cell thin sections. Charact. Nanoparticles Intend. Drug Deliv. 2011, 697, 93–99. [Google Scholar]
- Ali, M.M.M. Testing Different Properties of A Light-Cured Denture Base Material After Addition of Silicon Oxide Nanofiller (An in Vitro Study). J. Baghdad Coll. Dent. 2017, 29, 47–54. [Google Scholar] [CrossRef]
- Bazrafshan, A.A.; Hajati, S.; Ghaedi, M.; Asfaram, A. Synthesis and characterization of antibacterial chromium iron oxide nanoparticle-loaded activated carbon for ultrasound-assisted wastewater treatment. Appl. Organomet. Chem. 2018, 32, e3981. [Google Scholar] [CrossRef]
- Haapasalo, M.; Shen, Y.; Wang, Z.; Gao, Y. Irrigation in endodontics. Dent. Clin. 2010, 54, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Berutti, E.; Marini, R.; Angeretti, A. Penetration ability of different irrigants into dentinal tubules. J. Endod. 1997, 23, 725–727. [Google Scholar] [CrossRef]
- Haapasalo, M.; Ørstavik, D. In vitro infection and of dentinal tubules. J. Dent. Res. 1987, 66, 1375–1379. [Google Scholar] [CrossRef]
- Van der Sluis, L.W.M.; Versluis, M.; Wu, M.K.; Wesselink, P.R. Passive ultrasonic irrigation of the root canal: A review of the literature. Int. Endod. J. 2007, 40, 415–426. [Google Scholar] [CrossRef]
- Ghisi, A.C.; Kopper, P.M.P.; Baldasso, F.E.R.; Stürmer, C.P.; Rossi-Fedele, G.; Steier, L.; de Figueiredo, J.A.P.; Morgental, R.D.; Vier-Pelisser, F.V. Effect of super-oxidized water, sodium hypochlorite and EDTA on dentin microhardness. Braz. Dent. J. 2014, 25, 420–424. [Google Scholar] [CrossRef]
- Arends, J.; Ten Bosch, J. Demineralization and remineralization evaluation techniques. J. Dent. Res. 1992, 71, 924–928. [Google Scholar] [CrossRef]
- Gupta, C.; Singh, G.; Singh, M.P.; Agarwal, M.; Singh, K.S.; Mishra, A. Effect of QMix 2 in 1, BioPure MTAD and 17% Ethylenediaminetetraacetic Acid on Microhardness of Root Canal Dentin: An in vitro Study. Int. J. Prosthodont. Restor. Dent. 2017, 7, 17–20. [Google Scholar]
- Cameron, J. The use of ultrasonics in the removal of the smear layer: A scanning electron microscope study. J. Endod. 1983, 9, 289–292. [Google Scholar] [CrossRef]
- Koga, E.; NaimKassis, E.; Filho, I.; Linhares de Castro, F.P. EDTA as final irrigating gold standard in endodontics. Int. J. Recent Sci. Res. 2015, 6, 7818–7821. [Google Scholar]
- Paragliola, R.; Franco, V.; Fabiani, C.; Mazzoni, A.; Nato, F.; Tay, F.R.; Breschi, L.; Grandini, S. Final rinse optimization: Influence of different agitation protocols. J. Endod. 2010, 36, 282–285. [Google Scholar] [CrossRef]
- Alyahya, A.A.; Rekab, M.S.; O Al-Ostwani, A.E.; Abdo, A.; Kayed, K. The Effect of a Novel Silver-Citrate Root Canal Irrigation Solution (BioAkt), Ethylenediamine Tetraacetic Acid (EDTA), and Citric Acid on the Microhardness of Root Canal Dentin: A Comparative In Vitro Study. Cureus 2022, 14, e31255. [Google Scholar] [CrossRef]
- El-Banna, A.; Elmesellawy, M.Y.; Elsayed, M.A. Flexural strength and microhardness of human radicular dentin sticks after conditioning with different endodontic chelating agents. J. Conserv. Dent. Endod. 2023, 26, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Panighi, M.; G’Sell, C. Effect of the tooth microstructure on the shear bond strength of a dental composite. J. Biomed. Mater. Res. 1993, 27, 975–981. [Google Scholar] [CrossRef]
- Pashley, D.H.; Okabe, A.; Parham, P.L. The relationship between dentin microhardness and tubule density. Endod. Dent. Traumatol. 1985, 1 5, 176–179. [Google Scholar] [CrossRef]
- Carrigan, P.; Morse, D.R.; Furst, M.L.; Sinai, I.H. A scanning electron microscopic evaluation of human dentinal tubules according to age and location. J. Endod. 1984, 10, 359–363. [Google Scholar] [CrossRef] [PubMed]
- De-Deus, G.; Reis, C.; Fidel, S.; Fidel, R.A.S.; Paciornik, S. Longitudinal and quantitative evaluation of dentin demineralization when subjected to EDTA, EDTAC, and citric acid: A co-site digital optical microscopy study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 105, 391–397. [Google Scholar] [CrossRef]
- Ratih, D.N.; Enggardipta, R.A.; Kartikaningtyas, A.T. The effect of chitosan nanoparticle as a final irrigation solution on the smear layer removal, micro-hardness and surface roughness of root canal dentin. Open Dent. J. 2020, 14, 19–26. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).