Comparative Assessment of Tooth Discoloration Following Premixed Calcium Silicate Cement Application with Various Surface Treatments: An In Vitro Study
Abstract
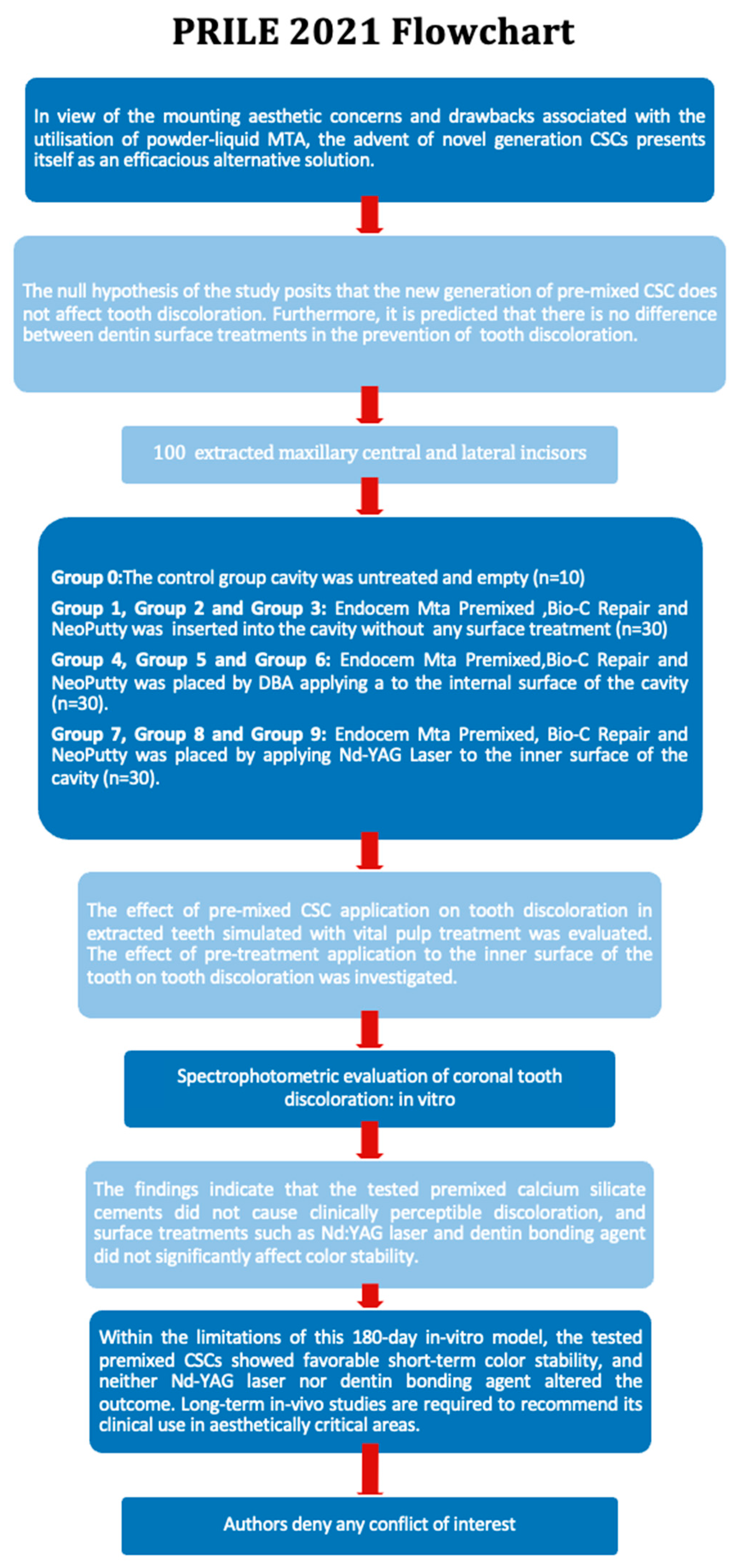
1. Introduction
2. Materials and Methods
2.1. Sample Selection
2.2. Tooth Preparation
2.3. Experimental Design
2.3.1. Nd-YAG Laser
2.3.2. Dentin Bonding Agent
2.4. Tooth Color Assessment
- ΔL′, ΔC′, and ΔH′ represent the differences in lightness, chroma, and hue, respectively;
- RT is the rotation function used to explain the interaction between chroma and hue in the blue region;
- SL, SC, and SH are the weighting functions for lightness, chroma, and hue;
- KL, KC, and KH are parametric factors (all set to 1 under standard laboratory conditions.
2.5. Statistical Evaluation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Duncan, H.F.; Galler, K.M.; Tomson, P.L.; Simon, S.; El-Karim, I.; Kundzina, R.; Krastl, G.; Dammaschke, T.; Fransson, H.; Markvart, M.; et al. European Society of Endodontology Position Statement: Management of Deep Caries and the Exposed Pulp. Int. Endod. J. 2019, 52, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Pradittapong, P.; Chompu-inwai, P.; Chaipattanawan, N.; Nirunsittirat, A.; Phinyo, P.; Manmontri, C. Postoperative Pain Following Vital Pulp Therapy in Carious Permanent Teeth of Children and Adolescents: A Prospective Cohort Study. Int. J. Paediatr. Dent. 2025, 35, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Nagas, E.; Ertan, A.; Eymirli, A.; Uyanik, O.; Cehreli, Z.C. Tooth Discoloration Induced by Different Calcium Silicate-Based Cements: A Two-Year Spectrophotometric and Photographic Evaluation in Vitro. J. Clin. Pediatr. Dent. 2021, 45, 112–116. [Google Scholar] [CrossRef]
- Taha, N.A.; Aboyounes, F.B.; Tamimi, Z.Z. Root-End Microsurgery Using a Premixed Tricalcium Silicate Putty as Root-End Filling Material: A Prospective Study. Clin. Oral Investig. 2021, 25, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Lee, S.M.; Bang, J.Y.; Kim, R.H.; Kwak, S.W.; Kim, H.C. Chemomechanical Properties and Biocompatibility of Various Premixed Putty-Type Bioactive Ceramic Cements. J. Endod. 2023, 49, 1713–1721. [Google Scholar] [CrossRef]
- İpek, İ.; Ünal, M.; Güner, A.; Candan, M. Push-out Bond Strength of Biodentine, MTA Repair HP, and a New Pre-Mixed NeoPutty Bioactive Cement: Scanning Electron Microscopy Energy Dispersive X-Ray Spectroscopy Analysis. J. Aust. Ceram. Soc. 2022, 58, 171–179. [Google Scholar] [CrossRef]
- de Toubes, K.S.; Tonelli, S.Q.; Girelli, C.F.M.; Azevedo, C.G.d.S.; Thompson, A.C.T.; Nunes, E.; Silveira, F.F. Bio-C Repair-A New Bioceramic Material for Root Perforation Management: Two Case Reports. Braz. Dent. J. 2021, 32, 104–110. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, Y.; Lee, J.; Kim, J.; Lee, J.; Han, M.R.; Kim, J.; Shin, J. Evaluation of Setting Time, Solubility, and Compressive Strength of Four Calcium Silicate-Based Cements. J. Korean Acad. Pediatr. Dent. 2023, 50, 217–228. [Google Scholar] [CrossRef]
- Cruz Hondares, T.; Hao, X.; Zhao, Y.; Lin, Y.; Napierala, D.; Jackson, J.G.; Zhang, P. Antibacterial, Biocompatible, and Mineralization-Inducing Properties of Calcium Silicate-Based Cements. Int. J. Paediatr. Dent. 2024, 34, 843–852. [Google Scholar] [CrossRef]
- Maximiano, V.; Machado, A.C.; Lopes, R.M.; Rabelo, F.E.M.; Garófalo, S.A.; Zezell, D.M.; Aranha, A.C.C.; Scaramucci, T. Association of Nd:YAG Laser and Calcium-Phosphate Desensitizing Pastes on Dentin Permeability and Tubule Occlusion. J. Appl. Oral Sci. 2021, 29, e20200736. [Google Scholar] [CrossRef]
- Liu, H.C.; Lin, C.P.; Lan, W.H. Sealing Depth of Nd:YAG Laser on Human Dentinal Tubules. J. Endod. 1997, 23, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.L.; Jang, Y.E.; Kim, B.S.; Kim, J.W.; Kim, Y. Pre-Application of Dentin Bonding Agent Prevents Discoloration Caused by Mineral Trioxide Aggregate. BMC Oral Health 2020, 20, 163. [Google Scholar] [CrossRef]
- Hilton, T.J. Keys to Clinical Success with Pulp Capping: A Review of the Literature. Oper. Dent. 2009, 34, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Fagogeni, I.; Metlerska, J.; Lipski, M.; Falgowski, T.; Maciej, G.; Nowicka, A. Materials Used in Regenerative Endodontic Procedures and Their Impact on Tooth Discoloration. J. Oral Sci. 2019, 61, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Chaves, E.T.; Morel, L.L.; Pappen, F.G.; Demarco, F.F.; Santos, L.G.P. Can a Dentin Bonding Agent Prevent Color Change in Regenerative Endodontic Procedures? An in Vitro Evaluation. Braz. Dent. J. 2024, 35, e24-5550. [Google Scholar] [CrossRef]
- Shokouhinejad, N.; Khoshkhounejad, M.; Alikhasi, M.; Bagheri, P.; Camilleri, J. Prevention of Coronal Discoloration Induced by Regenerative Endodontic Treatment in an Ex Vivo Model. Clin. Oral Investig. 2018, 22, 1725–1731. [Google Scholar] [CrossRef]
- Sesen Uslu, Y.; Arıcan Alpay, B.; Sesen, P.; Özyürek, T. Preventive Effects of Laser Irradiation and Dentin Bonding Agent Application on Tooth Discoloration Induced by Mineral Trioxide Aggregate. Appl. Sci. 2024, 14, 1048. [Google Scholar] [CrossRef]
- Nagendrababu, V.; Murray, P.E.; Ordinola-Zapata, R.; Peters, O.A.; Rôças, I.N.; Siqueira, J.F.; Priya, E.; Jayaraman, J.; Pulikkotil, S.J.; Camilleri, J.; et al. PRILE 2021 Guidelines for Reporting Laboratory Studies in Endodontology: A Consensus-Based Development. Int. Endod. J. 2021, 54, 1482–1490. [Google Scholar] [CrossRef]
- Kınay Taran, P.; Kara, Ö. Prevention Efficacy of Dentin Tubule Sealing with Nd:YAG Laser against Tooth Discoloration Induced by Vital Pulp Treatment. Int. J. Paediatr. Dent. 2024, 34, 153–159. [Google Scholar] [CrossRef]
- de Jesus, L.S.; Volpato, C.A.M.; Bortoluzzi, E.A.; da Silveira Teixeira, C.; Rossetto, H.L.; de Carvalho Panzeri Pires-de-Souza, F.; da Fonseca Roberti Garcia, L. Tooth Discoloration Induced by the Different Phases of a Calcium Aluminate Cement: One-Year Assessment. J. Esthet. Restor. Dent. 2021, 33, 999–1009. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, R.; Zhu, M. Comparative Evaluation of Bonding Performance between Universal and Self-Etch Adhesives: In Vitro Study. Heliyon 2024, 10, e35226. [Google Scholar] [CrossRef] [PubMed]
- Paravina, R.D.; Ghinea, R.; Herrera, L.J.; Bona, A.D.; Igiel, C.; Linninger, M.; Sakai, M.; Takahashi, H.; Tashkandi, E.; Del Mar Perez, M. Color Difference Thresholds in Dentistry. J. Esthet. Restor. Dent. 2015, 27 (Suppl. 1), S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Aljanahi, M.; Alhussin, A.; Elbishari, H. Challenges Faced When Masking a Single Discoloured Tooth—Part 1: Aetiology and Non-Invasive Management. Br. Dent. J. 2025, 238, 919–924. [Google Scholar] [CrossRef]
- Carvalho, J.A.; Franco, C.; Proença, L.; Neves, J.A.; Polido, M.; Mendes, J.J.; Azul, A.M. Spectrophotometric Analysis of Coronal Discoloration In Vitro Induced by Bioceramic Cements. Dent. J. 2023, 11, 180. [Google Scholar] [CrossRef] [PubMed]
- Al-Hiyasat, A.S.; Ahmad, D.M.; Khader, Y.S. The Effect of Different Calcium Silicate-Based Pulp Capping Materials on Tooth Discoloration: An in Vitro Study. BMC Oral Health 2021, 21, 330. [Google Scholar] [CrossRef]
- Yoldaş, S.E.; Bani, M.; Atabek, D.; Bodur, H. Comparison of the Potential Discoloration Effect of Bioaggregate, Biodentine, and White Mineral Trioxide Aggregate on Bovine Teeth: In Vitro Research. J. Endod. 2016, 42, 1815–1818. [Google Scholar] [CrossRef]
- Fundaoğlu Küçükekenci, F.; Küçükekenci, A.S.; Çakici, F. Evaluation of the Preventive Efficacy of Three Dentin Tubule Occlusion Methods against Discoloration Caused by Triple-Antibiotic Paste. Odontology 2019, 107, 186–189. [Google Scholar] [CrossRef]
- Marconyak, L.J.; Kirkpatrick, T.C.; Roberts, H.W.; Roberts, M.D.; Aparicio, A.; Himel, V.T.; Sabey, K.A. A Comparison of Coronal Tooth Discoloration Elicited by Various Endodontic Reparative Materials. J. Endod. 2016, 42, 470–473. [Google Scholar] [CrossRef]
- Sirintawat, N.; Leelaratrungruang, T.; Poovarodom, P.; Kiattavorncharoen, S.; Amornsettachai, P. The Accuracy and Reliability of Tooth Shade Selection Using Different Instrumental Techniques: An in Vitro Study. Sensors 2021, 21, 7490. [Google Scholar] [CrossRef]
- Szalewski, L.; Wójcik, D.; Tokarczuk, O.; Ozdas, T.; Durlej, G. The Role of Lighting Type in Dental Photography for Tooth Shade Assessment. medRxiv 2025. [Google Scholar] [CrossRef]
- Cui, C.; Zhou, X.N.; Chen, W.M. Self-Etching Adhesives: Possible New Pulp Capping Agents to Vital Pulp Therapy. Front. Med. China 2011, 5, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Koruyucu, M.; Akay, C.; Solakoglu, S.; Gencay, K. Investigation of the Cytotoxic Effect of Current Dentine Bonding Agents on Human Dental Pulp Cells. BMC Oral Health 2024, 24, 1207. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.; Lee, T.; Jeong, S.J.; Lee, J.H.; Song, J.S.; Kang, C.M. A Randomized Controlled Clinical Trial of Premixed Calcium Silicate-Based Cements for Pulpotomy in Primary Molars. J. Dent. 2023, 137, 104684. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kim, G.; Lee, J. An in Vitro Study of the Effects of Different Dentin Bonding Agents on the Prevention of Tooth Discoloration and the Sealing Ability of Calcium Silicate-Based Cement in Regenerative Endodontic Procedures. J. Korean Acad. Pediatr. Dent. 2023, 50, 277–291. [Google Scholar] [CrossRef]
- Taha, N.A.; Hamdan, A.M.; Al-Hiyasat, A.S. Coronal Discoloration Induced by Calcium Silicate-Based Cements Used in Full Pulpotomy in Mature Permanent Molars: A Randomized Clinical Trial. Clin. Oral Investig. 2023, 27, 1723–1730. [Google Scholar] [CrossRef]
- Sarshari, M.G.; Shakeri, K.; Parhizkar, A. Effect of Vital Pulp Therapy Biomaterials on Tooth Discolouration: A Review of the Literature. Int. J. Biomater. 2025, 2025, 3080084. [Google Scholar] [CrossRef]
- Silva, E.C.A.; Pradelli, J.A.; da Silva, G.F.; Cerri, P.S.; Tanomaru-Filho, M.; Guerreiro-Tanomaru, J.M. Biocompatibility and Bioactive Potential of NeoPUTTY Calcium Silicate-Based Cement: An in Vivo Study in Rats. Int. Endod. J. 2024, 57, 713–726. [Google Scholar] [CrossRef]
- Abrão, S.M.S.; Gregorio, D.; De Azevedo, M.K.C.; Mori, G.G.; Poli-Frederico, R.C.; Maia, L.P. Cytotoxicity and Genotoxicity of Bio-C Repair, Endosequence BC Root Repair, MTA Angelus and MTA Repair HP. Braz. Dent. J. 2023, 34, 14–20. [Google Scholar] [CrossRef]


| Materials | Manufacturer | Chemical Composition | Application Steps as Recommended by the Manufacturer |
|---|---|---|---|
| Nd-YAG Laser | Smart file, Deka Laser Technologies, Firenze, Italy | N/A | The laser parameters were set to 1 W/cm2 power, 10 Hz frequency, and 100 mJ/cm2 energy density. The dentin surface was exposed to a pulsed beam at 10 Hz and 1 W for a total of 60 s to simulate clinical manipulation. |
| Clearfil™ Tri-S Bond Universal | Kuraray Noritake Dental Inc., Niigata, Japan | Bis-GMA, HEMA, ethanol,10-MDP, hydrophilic aliphatic dimethacrylate, colloidal silica, camphoroquinone, silane coating agent, accelerator, initiators, and water. | Universal adhesive system (SE technique) 1. Apply adhesive for 20 s 2. Gentle air stream 5 s 3. Light-polymerize 10 s |
| Endocem MTA Premixed | Maruchi, Wonju, Republic of Korea Serial number: M1240518 exp date: May 2026 | Tricalcium silicate, calcium aluminate, calcium sulfate, dimethyl sulfoxide, thickening agents (lithium carbonate, hydroxypropyl methylcellulose, phyllosilicate mineral), and zirconium dioxide. | Setting time: Initial: 7 min The material should be applied with a thickness of at least 3 mm. After application, a sterile, moist cotton pellet should be placed on it for 3 min, and then the material should be gently pressed with moist cotton. |
| Bio-C Repair | Angelus, Londrina, PR, Brazil Serial number: 74055 exp date: May 2026 | Calcium silicate, calcium aluminate, calcium oxide, zirconium oxide, silicon oxide, polyethylene glycol, and iron oxide. | Setting time: ≤120 min Place a lightly moistened sterile cotton ball over BIO-C® REPAIR and wait 15 min for the material’s initial setting. |
| NeoPutty | NuSmile, Houston, TX, USA Lot: 2023 102607 Exp date: September 2026 | Tantalite, tricalcium silicate, calcium aluminate, dicalcium silicate, tricalcium aluminate, calcium sulfate, proprietary organic liquid, and stabilizers. | Applying a minimum thickness of 1.5 mm Setting time: ~4 h. |
| Groups (n = 10) | Materials | Surface Pretreatments |
|---|---|---|
| G0 | No | No |
| G1 | EndoCem MTA Premixed | No |
| G2 | Bio-C Repair | No |
| G3 | NeoPUTTY | No |
| G4 | EndoCem MTA Premixed | Dentin-Bonding Agents |
| G5 | Bio-C Repair | Dentin-Bonding Agents |
| G6 | NeoPUTTY | Dentin-Bonding Agents |
| G7 | EndoCem MTA Premixed | Nd-YAG Laser |
| G8 | Bio-C Repair | Nd-YAG Laser |
| G9 | NeoPUTTY | Nd-YAG Laser |
| T0–T7 | T0–T30 | T0–T90 | T0–T180 | p † | ||
|---|---|---|---|---|---|---|
| Group 0 | Mean ± SD | 2.03 ± 1.2 | 1.6 ± 0.82 | 2.1 ± 1.18 | 1.83 ± 1.12 | 0.323 |
| Median (IQR) | 1.85 (1.04–3.11) | 1.63 (0.77–2.21) | 1.9 (1.12–3.26) | 1.86 (0.89–2.43) | ||
| Group 1 | Mean ± SD | 1.76 ± 0.99 | 2.14 ± 1.32 | 1.94 ± 1.13 | 1.94 ± 0.84 | 0.668 |
| Median (IQR) | 1.87 (0.67–2.66) | 2.61 (0.56–3.25) | 1.9 (0.82–2.85) | 1.72 (1.24–2.87) | ||
| Group 2 | Mean ± SD | 1.49 ± 1.28 | 1.88 ± 0.95 | 1.68 ± 1.01 | 1.57 ± 1.27 | 0.668 |
| Median (IQR) | 1.28 (0.39–2.34) | 1.43 (1.2–2.82) | 1.79 (0.58–2.62) | 0.84 (0.72–2.53) | ||
| Group 3 | Mean ± SD | 2.31 ± 0.95 | 2.54 ± 0.94 | 2.68 ± 1.18 | 2.62 ± 0.5 | 0.976 |
| Median (IQR) | 2.18 (1.62–3.2) | 2.62 (1.92–3.33) | 2.61 (1.88–3.35) | 2.48 (1.91–3.2) | ||
| Group 4 | Mean ± SD | 1.85 ± 0.7 | 2.38 ± 1.59 | 2.02 ± 0.81 | 1.85 ± 1.12 | 0.668 |
| Median (IQR) | 1.88 (1.35–2.43) | 2.21 (0.81–3.32) | 2.29 (1.38–2.52) | 1.73 (0.89–2.78) | ||
| Group 5 | Mean ± SD | 2.16 ± 1.13 | 2.59 ± 1.57 | 1.87 ± 1.09 | 1.88 ± 0.87 | 0.356 |
| Median (IQR) | 2.47 (0.83–3.03) | 2.19 (1.28–4.32) | 1.9 (0.84–2.72) | 1.86 (1.26–2.51) | ||
| Group 6 | Mean ± SD | 2.22 ± 1.35 | 1.78 ± 0.75 | 2.35 ± 1.28 | 2.85 ± 1.22 | 0.566 |
| Median (IQR) | 2.01 (0.94–3.27) | 1.67 (1.18–2.38) | 2.03 (1.75–2.85) | 2.52 (1.81–4.4) | ||
| Group 7 | Mean ± SD | 2.23 ± 0.92 | 1.77 ± 1.09 | 1.68 ± 1.08 | 1.53 ± 0.64 | 0.430 |
| Median (IQR) | 2.03 (1.64–2.86) | 1.48 (1.12–2.21) | 1.5 (0.68–2.78) | 1.34 (1.01–1.89) | ||
| Group 8 | Mean ± SD | 2.4 ± 1.12 | 1.74 ± 1.31 | 2.63 ± 1.26 | 1.72 ± 0.97 | 0.101 |
| Median (IQR) | 2.34 (1.47–3.04) | 1.59 (0.62–3.16) | 2.35 (1.72–3.61) | 1.87 (0.65–2.68) | ||
| Group 9 | Mean ± SD | 3.1 ± 1.59 | 2.63 ± 1.13 | 2.45 ± 1.58 | 1.62 ± 1.32 | 0.062 |
| Median (IQR) | 2.83 (1.72–4.58) | 2.56 (1.71–3.25) | 1.59 (1.13–4.38) | 1.09 (0.64–2.23) | ||
| p ‡ | 0.352 | 0.377 | 0.598 | 0.073 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kara Şimşek, N.; Ayrancı, L.B.; Şimşek, H. Comparative Assessment of Tooth Discoloration Following Premixed Calcium Silicate Cement Application with Various Surface Treatments: An In Vitro Study. Appl. Sci. 2025, 15, 7709. https://doi.org/10.3390/app15147709
Kara Şimşek N, Ayrancı LB, Şimşek H. Comparative Assessment of Tooth Discoloration Following Premixed Calcium Silicate Cement Application with Various Surface Treatments: An In Vitro Study. Applied Sciences. 2025; 15(14):7709. https://doi.org/10.3390/app15147709
Chicago/Turabian StyleKara Şimşek, Nagihan, Leyla Benan Ayrancı, and Hüseyin Şimşek. 2025. "Comparative Assessment of Tooth Discoloration Following Premixed Calcium Silicate Cement Application with Various Surface Treatments: An In Vitro Study" Applied Sciences 15, no. 14: 7709. https://doi.org/10.3390/app15147709
APA StyleKara Şimşek, N., Ayrancı, L. B., & Şimşek, H. (2025). Comparative Assessment of Tooth Discoloration Following Premixed Calcium Silicate Cement Application with Various Surface Treatments: An In Vitro Study. Applied Sciences, 15(14), 7709. https://doi.org/10.3390/app15147709






