Abstract
(1) Background: The present study was carried out to provide a state-of-the-art review of the prosthodontic factors related to customized subperiosteal implants (CSIs), and to offer clinical guidelines in this regard. (2) Methods: An expert consensus meeting was held in July 2024 in Santpedor (Manresa, Spain) to establish the most relevant clinical guidelines. (3) Results and (4) Conclusions: An interdisciplinary approach including surgeons, prosthodontists, bio-medical engineers and dental technicians, integrating both biological and mechanical considerations when designing CSI rehabilitations, is very important. While the reported survival rate of CSIs appears promising, their long-term performance beyond 5 years remains insufficiently documented. Thus, CSIs are a viable treatment option for patients with insufficient bone to place conventional implants, but there is a clear need to identify and analyze delayed-onset complications associated with these devices. The findings and their broader implications should be thoroughly examined, and potential future research directions should be highlighted.
1. Introduction
Customized subperiosteal implants (CSIs) were introduced in the 1940s as a treatment option for the rehabilitation of edentulous jaws and mandibles. Subsequently, they fell into disuse due to the development of important complications [1]. Indeed, the great majority of patients treated with first-generation CSIs had poor outcomes and low short- to medium-term survival rates. Nowadays, technological advances, such as computed tomography (CT), digital planning software and additive manufacturing by direct metal laser sintering, have allowed a new generation of CSIs to be manufactured with greater precision and better clinical outcomes. Indeed, the current devices are more biocompatible, have an improved fitting and are more stable, thus reducing the likelihood of developing complications [1,2,3,4].
These devices are specifically adapted to the anatomy of the patient and are indicated in cases where conventional endosseous dental implants are not feasible due to severe bone resorption. Other options such as zygomatic implants or major bone augmentation techniques are also available, but these treatments are usually technically demanding and time-consuming and have been associated with complications.
CSIs are indicated in patients with insufficient bone to place conventional dental implants, as in type V and VI extreme bone atrophies [1,5]. Furthermore, they are indicated for patients who refuse complex reconstructive techniques, or are unwilling to have removable prostheses. Thus, CSIs can be considered as an alternative to zygomatic implants [6] or endosseous implants requiring advanced bone grafting [2], or to correct severe defects after surgery in oncological patients [7]. CSIs are not recommended in patients with severe systemic pathologies or who are under therapies that contraindicate surgical procedures [6].
The reduced postoperative morbidity is among the main advantages of CSIs, since the surgical procedures are usually faster and simpler in comparison with more invasive options like bone reconstruction surgeries [1,2]. At present, CSIs are usually applied in patients with edentulous resorbed maxillas, and can be loaded immediately. This is an important feature, since the patients can recover their aesthetics and function immediately after the surgical procedure, and this in turn improves their quality of life [2].
Following completion of the restorative treatment, patients should undergo supportive therapy appointments every six months. These appointments are essential for the assessment of oral health status, the identification and management of risk factors and indicators, and the professional removal of plaque and biofilm from around the abutments and the implant-supported prosthesis [6].
Despite their benefits, CSIs also pose significant challenges. Complications related to implant stability, integration with surrounding tissues, soft tissue dehiscences and associated infections can occur. Other uncommon adverse events, such as CSI structural fractures associated with an inaccurate diagnosis or bone resorption due to infections, have also been reported [8]. The available literature contains limited long-term data regarding the success and complication rates for the new generation of CSIs and CAD-CAM prosthetic rehabilitations. Two studies report clinical data until 6 years of follow-up [2,8]. A previous consensus paper about CSIs published by the current authors [6] addressed several clinically relevant topics but did not provide an in in-depth analysis of the main prosthodontic considerations or the prevention and management of complications associated with the prosthesis, which remain under-reported in the current literature. Due to the lack of standardized protocols for prosthetic rehabilitation, the aim of this paper was to provide a structured state-of-the-art review of the prosthodontic factors related with CSIs and to provide clinical guidelines to support decision-making and improve treatment outcomes.
2. Materials and Methods
An on-site consensus meeting was held in July 2024 in Santpedor (Manresa, Spain) with 10 active experts in several fields like oral and maxillofacial surgery, prosthodontics, dentistry and biomedical engineering. The expert panel included clinicians from multiple regions of Spain, with more than 10 years of clinical experience in both private practices and large public hospitals. The following areas of interest were discussed to establish the most relevant clinical guidelines:
- Prosthodontic planning
- Provisionalization protocols
- Prosthetic design
- Surgical considerations
- Supportive therapy protocols
- General recommendations and future perspectives
Prior to the meeting, all participants were provided with the most relevant papers on the topic for review. Then, several cases were presented by the clinicians involved in the on-site meeting, focusing on the aforementioned key areas of interest. All participants were encouraged to share their opinions and clinical experience. One of the authors (RF) acted as moderator to ensure that all participants could openly share their opinions and present their results in alignment with the above-mentioned areas of interest. Afterwards, the authors established the most relevant clinical guidelines and, in the event of differing opinions, a consensus was reached. Following the meeting, three researchers (OC-F, AS-T and RF) drafted a document summarizing the main recommendations, and all the authors reviewed and edited the final manuscript.
3. Results
3.1. Prosthodontic Planning
- Adequate preoperative prosthodontic planning is crucial. For this purpose, it is essential to have a high-resolution helical CT scan, an accurate impression of the soft tissues with the aid of an intraoral scan (Standard Tessellation Language (STL) file), information regarding the intermaxillary relationship and vertical dimension, intraoral and extraoral photographs and a digital wax-up of the final prosthesis [2]. Cone-beam computer tomography (CBCT) is not suitable to plan CSIs due to its suboptimal image quality [6]. All these steps provide valuable information that will allow us to correctly design the CSI and its prosthetic connections.
- All professionals involved in the treatment (prosthodontists, surgeons, CSI manufacturers and laboratory technicians) must have access to the patient data and should discuss the treatment plan before manufacturing the CSI (EO).
- The patient should be involved, together with the healthcare professionals, in the decision-making process regarding the rehabilitation options. Establishing an adequate dentist–patient relationship must be based on trust and patient-centered care, taking into account the patient’s personal motivations in order to choose the most appropriate treatment solution [9,10]. Active listening and empathetic communication concerning personal needs are encouraged to establish trust and improve patients’ adherence to clinical recommendations and future follow-ups [11].
3.2. Provisionalization Protocols
- CSIs can be loaded immediately, on the same day of the surgical procedure [7].
- It is essential to avoid pressure over the soft tissues with the provisional prosthesis. If possible, clinicians should consider leaving a visible space between the mucosa and the prosthesis to avoid pressure during healing and to facilitate oral hygiene (EO) (Figure 1).
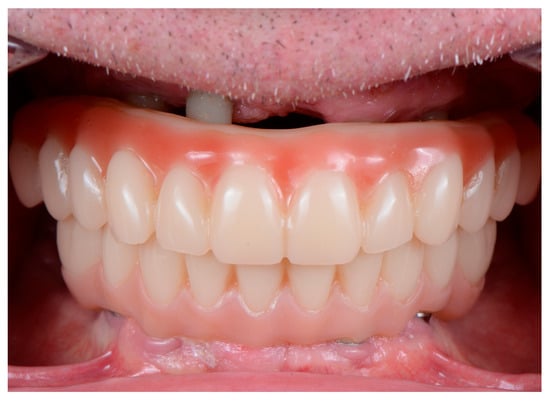 Figure 1. Provisional prostheses made of polymethylmethacrylate (PMMA) with a specific design to avoid pressure on the soft tissues and to allow for adequate hygiene during the healing process.
Figure 1. Provisional prostheses made of polymethylmethacrylate (PMMA) with a specific design to avoid pressure on the soft tissues and to allow for adequate hygiene during the healing process. - The provisional prosthesis must allow adequate access for oral hygiene (EO).
- The use of intermediate transepithelial abutments is recommended, and the emergence profile of the prosthesis should be straight (EO).
- CSI provisional prostheses can be made with the same materials used in the fabrication process for provisionals placed over conventional endosseous dental implants. The working group recommends the use of any CAD-CAM milled titanium-based material such as milled PMMA or acetal [7]. Additionally, 3D-printed resin prosthetic restorations can be considered but clinicians should be aware that the available evidence to support their use as a provisional prosthesis to be placed over a CSI is very scarce (EO).
- Although immediate placement of the provisional prosthesis using an intraoperative check bar is feasible, most authors recommend a delayed approach based on an impression made after CSI placement. This method ensures optimal accuracy, reduces discrepancies and favors the stability of the implant (EO).
- The final position of the CSI should not be jeopardized by the need to adapt the provisional prosthesis (EO).
3.3. Prosthetic Design
- At least four prosthetic connections are required. However, the number of connections should be adapted according to the patient’s characteristics, taking into account the risk factors associated with mechanical complications or CSI exposure. A greater number of connections seems to increase the incidence of exposures [2].
- Prosthetic connections should be placed with adequate spacing, leaving at least one tooth (pontic) between the connections, to ensure the correct distribution of functional loads and to minimize the risk of soft tissue dehiscences (EO).
- The working group recommends the use of removable intermediate transepithelial abutments (EO) (Figure 2).
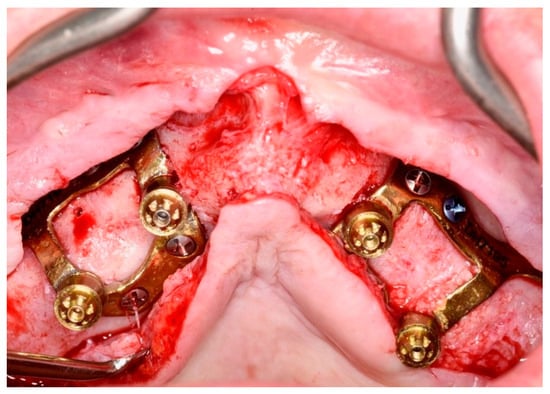 Figure 2. Maxillary CSI with transepithelial abutments. Laser sintering titanium Grade 23 (Ti6AI4V ELI alloy) with thickness of 0.8 mm using an EOS M290 printer. (Avinent, Santpedor, Spain).
Figure 2. Maxillary CSI with transepithelial abutments. Laser sintering titanium Grade 23 (Ti6AI4V ELI alloy) with thickness of 0.8 mm using an EOS M290 printer. (Avinent, Santpedor, Spain). - The materials used to fabricate definite prostheses over conventional endosseous dental implants can also be used in CSI prostheses [3,7,12]. New CAD-CAM materials (titanium or PEEK) with PMMA or composite resin coatings can be considered in order to reduce the weight of the prosthesis and/or to minimize overload on the CSI (EO) (Figure 3).
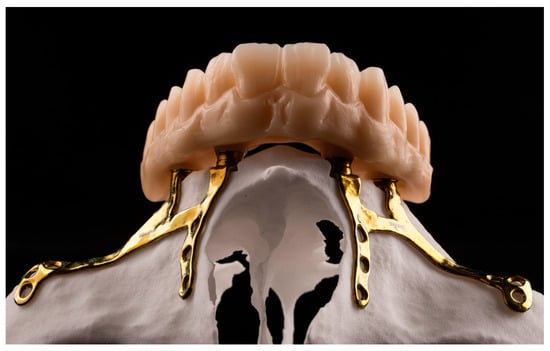 Figure 3. Maxillary definite prosthesis made of polymethylmethacrylate (PMMA).
Figure 3. Maxillary definite prosthesis made of polymethylmethacrylate (PMMA). - The prosthetic screws should be placed with the recommended torques to avoid mechanical complications such as screw loosening (EO).
3.4. Surgical Considerations
- Buccal fat pad flaps may be an interesting approach to treat complications and exposures of CSIs. However, their routine use to prevent soft tissue dehiscences is not recommended, since these complications are associated with the CSI design and the amount of keratinized mucosa, rather than with the thickness of the buccal flap [4].
- An adequate thickness and width of keratinized mucosa is essential to avoid complications. Occasionally, soft tissue augmentation may be necessary prior to CSI placement. Proper soft tissue management is essential to avoid dehiscences [12,13].
- Dental extractions should be done prior to CSI placement to allow proper tissue healing. If this is not feasible, basic periodontal therapy should be applied to the remaining teeth to remove biofilm and reduce inflammation (EO).
- The flap design should maximize the keratinized mucosa (EO). Distal vertical releasing incisions improve surgical access but should be placed far away from the posterior connections of the CSI [14].
- The prosthetic connection areas should have adequate keratinized mucosa to prevent possible exposures of the CSI (EO).
- The fixation screw protocol should be adapted according to the bone type involved. The fixation screw direction and primary stability are critical surgical factors (EO).
- Fixation screws should be placed perpendicular to the bone and in the center of the CSI insertion hole, maintaining the planned direction (EO) (Figure 4).
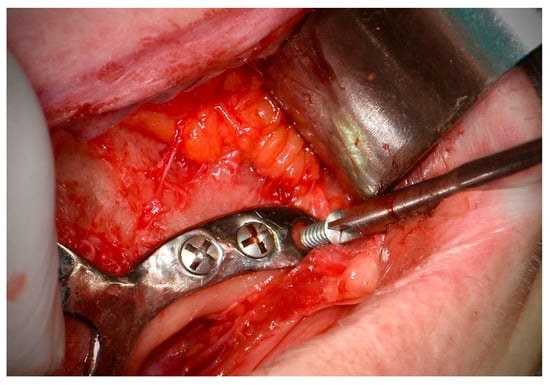 Figure 4. Fixation screw placement (Avinent, Santpedor, Spain).
Figure 4. Fixation screw placement (Avinent, Santpedor, Spain). - The working group recommends the use of self-drilling screws to avoid the use of drills, which may compromise screw stability in areas of alveolar bone in cases of grade V atrophy. In cortical bone areas, such as the nasal buttress and zygomatic bone, drilling prior to screw placement is recommended in order to avoid excessive insertion torque, screw head deformation and/or screw fracture. Depending on the surgeon’s experience, the use of drills may be omitted in certain areas [2] (Figure 5).
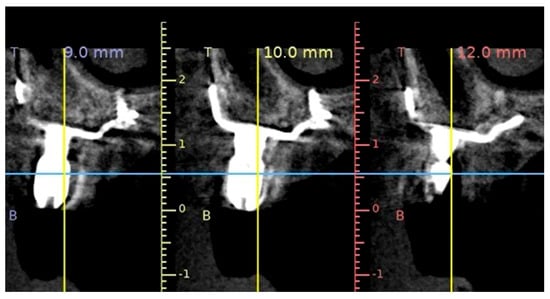 Figure 5. A sagittal cone-beam computed tomography slice showing the radiological setting of the CSI (Avinent, Santpedor, Spain).
Figure 5. A sagittal cone-beam computed tomography slice showing the radiological setting of the CSI (Avinent, Santpedor, Spain). - The number of fixation screws should ensure adequate mechanical stability of the CSI. A minimum of two screws per fixation area (palatal, zygomatic or nasal) is recommended [14]. Since the stability of the CSI depends on the number and length of the screws, their placement in the fixation areas should be maximized. Fixation screws in areas close to the alveolar ridge may increase the risk of CSI exposure, since they affect the design of the implant [2] (Figure 6).
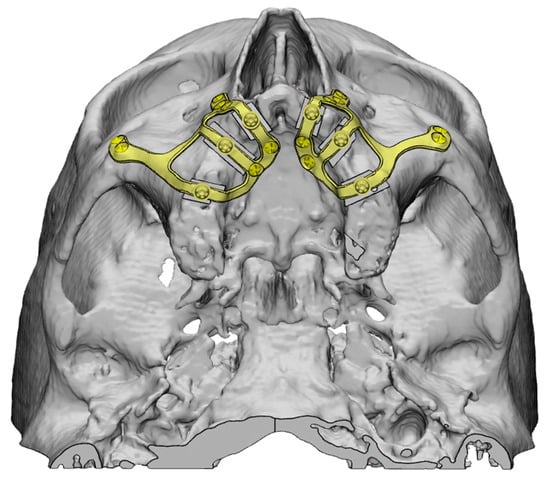 Figure 6. Planning and fixation screw distribution of a maxillary CSI (Avinent, Santpedor, Spain).
Figure 6. Planning and fixation screw distribution of a maxillary CSI (Avinent, Santpedor, Spain). - The use of a surgical cutting guide is essential in patients with grade V atrophy [5]. Placement of the CSI over the alveolar ridge should be avoided in order to minimize the presence of abrupt transitions, which are often associated with implant exposures. Titanium surgical cutting guides are rigid, thus providing greater precision and reducing the risk of particle release during the procedure. Other materials, such as a semirigid polyamide can be used, but further research is required to conduct a correct cost-effectiveness analysis (EO).
3.5. Supportive Therapy Protocols
- Clinicians should consider indicating an occlusal splint in all patients (EO).
- After placement of the CSI provisional prosthesis, patients should be followed-up on regularly (1, 3 and 6 months). At these appointments, clinicians should reassess both occlusion and proper access to oral hygiene (EO).
- All patients should undergo lifelong regular supportive therapy appointments at least every 6 months. However, patients considered to be at high risk of developing complications and peri-implant diseases (i.e., history of periodontal disease or poor oral hygiene) should have more frequent appointments (every 3–4 months). Follow-up controls should be primarily clinical. In the event of any complications (such as implant exposures or mobility), a cone-beam CT would be indicated (EO).
- Removal of the prosthesis is advisable in patients with inadequate biofilm control or when inflammation around the CSI connection areas is observed (EO).
- The main objective of regular supportive therapy appointments is to remove biofilm and calculus without changing the surface of the transepithelial abutment. For this purpose, mechanical debridement should be performed and may be complemented with the use of an antiseptic agent [12].
3.6. General Recommendations and Future Perspectives
3.6.1. General Recommendations
The professionals involved, including engineers, surgeons, prosthodontists and dental technicians, should receive specific training in the use of CSIs (EO).
3.6.2. Future Perspectives
- The literature clearly provides limited long-term information on the occurrence of mechanical and/or biological complications. There are also few data regarding the use of CSIs in partial edentulous patients.
- Additional biomechanical approaches such as finite element studies or other experimental methods like strain-gauge analysis are required to compare the mechanical behavior of different CSIs and prosthesis designs.
- Bruxism could be a risk factor for complications in patients with CSIs. Further clinical data are needed to assess the risks, as its effects remain uncertain [15].
- The use of CSIs in systemically compromised patients and patients under antiresorptive or antiangiogenic treatment is controversial. Further research on this topic is required.
4. Discussion
Customized subperiosteal implants (CSIs) are an interesting solution for the prosthetic rehabilitation of patients with severe bone atrophy, where traditional approaches such as endosseous dental implants are not feasible [13]. CSIs sit directly on the bone surface and are screwed into anatomical buttress areas, thus avoiding structures such as the maxillary sinus, which might reduce the complications rate in comparison with zygomatic implants [8].
These devices have been successfully used in cases of bone augmentation failure [12,16] or after oncological surgery [16,17]. A recent systematic review [18] including data from 227 patients recorded a high short-term survival rate of 97.8%, with a median follow-up time of approximately two years.
However, several limitations and complications associated with CSIs have been described in the literature. Some patients might present with soft-tissue inflammation that can be reverted by improving the oral hygiene or by changing the prosthetic design. Several reports have stated that soft tissue dehiscences leading to CSI exposure are common findings. On the other hand, CSI mobility is considered a major complication since it usually leads to implant failure, especially in patients with insufficient bone support. Finally, mechanical complications like the fracture of the prosthetic components or of the CSI structure can also appear [2,8].
The use of CSIs allows patients to restore functions such as chewing, phonation and swallowing, and also improves aesthetics, even in challenging cases with major defects [2,13]. Oral health-related quality of life (QoL) is a relevant outcome parameter in implant dentistry, since it takes into account the impact of treatments on different dimensions of patient life. A multicenter study [19] evaluated the quality of life of 40 patients rehabilitated with CSIs, showing very positive outcomes. Indeed, the results of the Oral Health Impact Profile (OHIP-14) questionnaire and of the visual analogue scales (VASs), measuring the patient’s self-reported perception on aesthetics, comfort, masticatory function, phonation, hygiene and general satisfaction, were positive. Interestingly, the authors suggested that the improved prosthesis retention might play a crucial role, since most of the included patients had previously explored other treatment options or required multiple visits for adjustments and prosthetic relining. On the other hand, the impact of CSIs on the quality of life of the patients might also be related to the fact that these devices allow for an immediate loading protocol, thus limiting impairment of their social and working activities [16].
The customized design of these implants allows for a precise and passive fit on the remaining bone, optimizing both the initial stability and functional load distribution through robust structures [2,16]. To this end, the accuracy and quality of the preoperative CT scan is key to fabricating accurate and stable CSIs. For this reason, some authors recommend removing all teeth and/or implants prior to the CT scan, in order to avoid the presence of radiographic artifacts [14]. Moreover, the materials used in CSIs are non-ferromagnetic and are generally considered MRI-safe, although their presence may cause artifacts that could reduce the image quality.
An osteotomy to remove remaining residual alveolar bone using surgical guides is an important step that should be carefully planned preoperatively. Vatteroni et al. [7] recommend osteotomies that are 3 mm in depth and 4 mm in width, starting apical to the mucogingival line. Proper placement and sizing of the osteotomies will allow for the correct seating of the CSI, close to the basal bone, which may reduce postsurgical bone remodeling [14]. This surgical maneuver reduces the risk of dehiscences around the CSI abutments, facilitates tissue management and may decrease CSI fatigue [14,15]. A retrospective study reported a mean resorption rate of <0.5 mm in the crestal and in the supporting flap areas after one year of loading [20]. Indeed, the maxillary regions where the CSIs rest, which are mainly the piriform aperture and the zygomatic process, experience less bone resorption when subjected to occlusal forces [21], and should have a minimum thickness of 4 mm [7].
Follow-up appointments are highly recommended to evaluate the degree of resorption and to assess other risk factors such as bruxism, which could increase CSI fatigue. Some authors even advise against the placement of these implants in patients with parafunctions, and further advise the close monitoring of patients who have implant-supported antagonist rehabilitations, as they lack proprioception [15].
Bone atrophy is usually associated with soft tissue deficiencies, and both are crucial for ensuring long-lasting results when CSIs are involved [2]. In this regard, soft tissue dehiscences or mucosal recessions are very common (in up to 25.6% of cases [18]), especially on the buccal aspect. The main risk factors include a thin phenotype or the absence of keratinized mucosa. This can lead to inflammation of the surrounding tissues and can favor the development of infections [12]. Smoking also has a detrimental effect on healing and tissue quality, and has even been found to increase the likelihood of mucosal recession up to 7-fold [12]. Again, patient selection is paramount when suggesting this type of treatment, especially in the case of smokers and diabetics [21]. Other planning and surgical factors may also affect the outcome. Firstly, a tension-free suture of the surgical flap must be obtained. On the other hand, the design of the CSI plays a major role in the development of biological complications. The presence of abrupt buccal areas or of other irregularities may cause mucosal compression leading to tissue dehiscence. The treatment of this complication usually requires additional surgeries consisting of advancement flaps, connective tissue grafts, the use of a Bichat’s fad pad flap or rotated palatal flaps [13,16]. Thus, it is important to avoid irregular designs to favor an adequate distribution of mechanical loads [8].
Some mechanical complications associated with CSIs have also been described in the literature. Fracture of the prosthetic abutments, especially in anterior areas [13], and of the fixation screws [14], are among the most common complications. These may be associated with inadequate structural design or with an unbalanced distribution of functional loads. Thus, Chamorro-Pons et al. [3] has recommended a minimum thickness of the CSI structure of 0.8 mm in order to prevent plastic deformation and fractures. Hence, the CSI design should guarantee adequate stability and promote long-term function [21].
The design and placement of CSIs requires specific training, and the surgeon’s experience may be an important variable to consider. Indeed, several technical difficulties that are usually not found in conventional dental implants may occur [6]. In this regard, Onică et al. [2] stress the role of human error in determining the treatment success rates, since current technology allows for the manufacturing of very precise structures. Therefore, in order to reduce possible discrepancies between the radiological data and the patient’s anatomy due to bone resorption, it is advisable to perform the preoperative CT scan less than three months before surgery and with a high resolution (CT slices < 1 mm) [13].
These implants allow the placement of immediately-loaded polymethyl methacrylate (PMMA) or polyetheretherketone (PEEK) prostheses [7], shortening the overall treatment time and improving patient quality of life. Advances in 3D printing seem to offer promising solutions for implant-supported provisional restorations although the available data on this context is very limited. Some authors mention that fitting the provisional prosthesis can be easier if it is done 2–3 weeks after surgery. This approach ensures the absence of dehiscences and reduces the need for readjustments of the provisional prosthesis [8].
The use of intermediate abutments in CSIs allows us to place screw-retained prostheses. This is an important advantage and makes the prosthetic rehabilitation protocol very similar to that employed for conventional dental implants. The healing time required until the final prosthesis is placed differs from study to study. While some authors place the final restoration after 3 months [13], others wait from 6 to 12 months [2,8]. The materials commonly used in conventional dental implant-supported prostheses (both metal–resin and metal–ceramic) can also be used in CSIs [7]. It is of the utmost importance to ensure an adequate fit of the prosthetic structure in order to obtain an optimal result and to avoid the development of mechanical complications such as screw loosening or ceramic chipping [13].
The prosthetic rehabilitation of patients with severe atrophies presents additional challenges, such as the presence of muscular insertions near the alveolar ridge, which may hinder the design of the CSI and compromise the prosthetic design. In addition, these implants may limit access to oral hygiene which, in turn, may increase the risk of biological complications. Therefore, it is essential to consider this limitation when designing the prosthesis, and it is imperative that patients receive specific training on how to remove the oral biofilm, especially in the abutment areas.
Finally, this report has some limitations that need to be considered. On one hand, the involved clinicians had a limited number of cases with a long follow-up period, which did not allow to draw conclusions about the long-term success of CSIs. On the other hand, some sources of bias could be present since the authors did not employ a structured communication technique (for example, the Delphi method [22]). Nevertheless, the moderator involved had previous experience in consensus reports.
5. Conclusions
All the abovementioned factors underscore the need for an interdisciplinary approach (surgeons, prosthodontics, biomedical engineers, dental technicians, etc.), considering both biological and mechanical aspects, when designing CSI rehabilitations. The reported survival rate of CSIs seems to be promising, although long-term data (>5 years) is still scarce. Thus, future research through longitudinal studies with larger patient cohorts, quality-of-life assessments or biomechanical performance over time should be performed, and should clearly provide information about the delayed-onset complications associated with these implants. Authors should discuss the results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted.
Author Contributions
Conceptualization, Á.T.-P., J.H.-L., M.d.C.-P., R.S.-G., C.R.-A., P.G.-M., J.R.-M., O.C.-F., A.S.-T. and R.F.; methodology, A.S.-T., O.C.-F. and R.F.; formal analysis, A.S.-T., O.C.-F. and R.F.; investigation, Á.T.-P., J.H.-L., M.d.C.-P., R.S.-G., C.R.-A., P.G.-M., J.R.-M., O.C.-F., A.S.-T. and R.F.; resources, A.S.-T., O.C.-F. and R.F.; data curation, Á.T.-P., J.H.-L., M.d.C.-P., R.S.-G., C.R.-A., P.G.-M., J.R.-M., O.C.-F., A.S.-T. and R.F.; writing—original draft preparation, A.S.-T., O.C.-F. and R.F.; writing—review and editing, A.S.-T., O.C.-F. and R.F.; visualization, R.F.; supervision, R.F.; project administration, R.F.; funding acquisition, R.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Càtedra UB-AVINENT of Digital Dentistry.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created.
Acknowledgments
The manuscript has been edited by an experienced native English medical translator, Joe (Ira) Perkins.
Conflicts of Interest
The funders had no role in the design of the study, in the collection, analyses or interpretation of data, in the writing of the manuscript or in the decision to publish the results. Octavi Camps-Font reports grants and non-financial support from Inibsa Dental (Lliçà de Vall, Spain) and Dentaid SL (Cerdanyola del Vallès, Spain), and non-financial support from Nobel Biocare and Avinent SLU outside of the submitted work. Alba Sánchez-Torres reports grants, personal fees and nonfinancial support from MozoGrau (Valladolid, Spain), Mundipharma (Cambridge, UK) and Unither Pharmaceuticals (Paris, France) outside the submitted work. Rui Figueiredo reports grants, personal fees and nonfinancial support from MozoGrau (Valladolid, Spain), Avinent (Santpedor, Spain), Inibsa Dental (Lliçà de Vall, Spain) and Dentaid SL (Cerdanyola del Vallés, Spain), nonfinancial support from Nobel Biocare (Zürich, Switzerland) and personal fees from Geistlich Pharma AG (Wolhusen, Switzerland), BioHorizons Iberica (Madrid, Spain), Araguaney Dental (Barcelona, Spain), Septodont (Saint-Maur-des-fossés, France), Dentaid SL (Cerdanyola del Vallés, Spain) and Laboratorios Silanes (Mexico city, Mexico) outside the submitted work. Figueiredo has also participated as a principal investigator in a randomized clinical trial sponsored by Mundipharma (Cambridge, UK) and in another clinical trial as a co-investigator for Menarini Richerche (Florence, Italy). Other authors report no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CSIs | Customized subperiosteal implants |
| CT | Computed tomography |
| CAD-CAM | Computer-aided design/Computed-aided manufacture |
| EO | Expert opinion |
| PMMA | Polymethyl methacrylate |
| PEEK | Polyether ether ketone |
| QoL | Quality of life |
| OHIP | Oral health impact profile |
| VAS | Visual analogue scale |
References
- Łoginoff, J.; Majos, A.; Elgalal, M. The evolution of custom subperiosteal implants for treatment of partial or complete edentulism in patients with severe alveolar ridge atrophy. J. Clin. Med. 2024, 13, 3582. [Google Scholar] [CrossRef] [PubMed]
- Onică, N.; Budală, D.G.; Baciu, E.R.; Onică, C.A.; Gelețu, G.L.; Murariu, A.; Balan, M.; Pertea, M.; Stelea, C. Long-term clinical outcomes of 3d-printed subperiosteal titanium implants: A 6-year follow-up. J. Pers. Med. 2024, 14, 541. [Google Scholar] [CrossRef]
- Chamorro-Pons, M.; Arias-Gallo, J.; Margarit-Pérez, L.; Demaría-Martínez, G.; Cidad-Vicario, A. Implantes subperiósticos personalizados para la rehabilitación completa del maxilar superior atrófico. Revisión de una serie clínica de 8 casos. Rev. Esp. Cir. Oral Maxilofac. 2021, 43, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Tofé-Povedano, Á.; Parras-Hernández, J.; Herce-López, J.; Matute-García, D.; Astolfi-González-Moguena, V.; Rollón-Mayordomo, Á. Design modifications in subperiosteal implants to avoid complications. Presentation of a case series study and literature review. Rev. Esp. Cir. Oral Maxilofac. 2023, 45, 57–63. [Google Scholar]
- Cawood, J.I.; Howell, R.A. A classification of the edentulous jaws. Int. J. Oral Maxillofac. Surg. 1988, 17, 232–236. [Google Scholar] [CrossRef]
- Herce-López, J.; Pingarrón, M.D.C.; Tofé-Povedano, Á.; García-Arana, L.; Espino-Segura-Illa, M.; Sieira-Gil, R.; Rodado-Alonso, C.; Sánchez-Torres, A.; Figueiredo, R. Customized subperiosteal implants for the rehabilitation of atrophic jaws: A consensus report and literature review. Biomimetics 2024, 9, 61. [Google Scholar] [CrossRef]
- Vatteroni, E.; Covani, U.; Menchini Fabris, G.B. The new generation of subperiosteal implants for patient-specific treatment of atrophic dental arches: Literature review and two case reports. Int. J. Periodontics Restor. Dent. 2023, 43, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Mommaerts, M.Y. Management of adverse effects following additively manufactured subperiosteal jaw implantation in the maxilla. J. Stomatol. Oral Maxillofac. Surg. 2024; 102206, in press. [Google Scholar] [CrossRef]
- Yuan, S.; Freeman, R.; Hill, K.; Newton, T.; Humphris, G. Communication, trust and dental anxiety: A person-centred approach for dental attendance behaviours. Dent. J. 2020, 8, 118. [Google Scholar] [CrossRef]
- Yamalik, N. Dentist-patient relationship and quality care 1. Introduction. Int. Dent. J. 2005, 55, 110–112. [Google Scholar] [CrossRef]
- Sánchez-Torres, A.; Camps-Font, O.; Figueiredo, R.; Valmaseda-Castellón, E. Validity and reliability of the Spanish version of the consultation and relational empathy measure in dental students (Sp-Dent-CARE): A cross-sectional study. Eur. J. Dent. Educ. 2024, 28, 267–274. [Google Scholar] [CrossRef]
- Van den Borre, C.; De Neef, B.; Loomans, N.A.J.; Rinaldi, M.; Nout, E.; Bouvry, P.; Naert, I.; Van Stralen, K.J.; Mommaerts, M.Y. Soft tissue response and determination of underlying risk drivers for recession and mucositis after AMSJI implantation in the maxilla. Int. J. Oral Maxillofac. Implant. 2024, 39, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, M.; Ozturk Muhtar, M.; Kundakcioglu, A.; Kucukcakir, O.; Cansiz, E. Evaluation of clinical success of the 3D-printed custom-made subperiosteal implants. J. Craniofac. Surg. 2024, 35, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; Biglio, A.; Roy, M.; Salzano, G.; Troise, S.; Abbate, V.; Mayo-Yanez, M.; Lechien, J.R.; Piombino, P.; De Riu, G. Full-arch rehabilitation of severely atrophic maxilla with additively manufactured custom-made subperiosteal implants: A multicenter retrospective study. J. Cranio-Maxillofac. Surg. 2024, 52, 991–998. [Google Scholar] [CrossRef]
- De Moor, E.; Huys, S.E.F.; van Lenthe, G.H.; Mommaerts, M.Y.; Vander Sloten, J. Mechanical evaluation of a patient-specific additively manufactured subperiosteal jaw implant (AMSJI) using finite-element analysis. Int. J. Oral Maxillofac. Surg. 2022, 51, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.R.G.R.; Grillo, R. Maxillary rehabilitation after zygomatic implant sequelae using custom subperiosteal implants: A case study. J. Stomatol. Oral Maxillofac. Surg. 2024, 126, 102154. [Google Scholar] [CrossRef]
- Cebrián-Carretero, J.L.; Del Castillo-Pardo de Vera, J.L.; Montesdeoca-García, N.; Garrido-Martínez, P.; Pampín-Martínez, M.M.; Aragón-Niño, I.; Navarro-Cuéllar, I.; Navarro-Cuéllar, C. Virtual surgical planning and customized subperiosteal titanium maxillary implant (CSTMI) for three dimensional reconstruction and dental implants of maxillary defects after oncological resection: Case series. J. Clin. Med. 2022, 11, 4594. [Google Scholar] [CrossRef]
- Anitua, E.; Eguia, A.; Staudigl, C.; Alkhraisat, M.H. Clinical performance of additively manufactured subperiosteal implants: A systematic review. Int. J. Implant. Dent. 2024, 10, 4. [Google Scholar] [CrossRef]
- Van den Borre, C.; De Neef, B.; Loomans, N.A.J.; Rinaldi, M.; Nout, E.; Bouvry, P.; Naert, I.; Mommaerts, M.Y. Patient satisfaction and impact on oral health after maxillary rehabilitation using a personalized additively manufactured subperiosteal jaw implant (AMSJI). J. Pers. Med. 2023, 13, 297. [Google Scholar] [CrossRef]
- Van den Borre, C.; Rinaldi, M.; De Neef, B.; Loomans, N.A.J.; Nout, E.; Van Doorne, L.; Naert, I.; Politis, C.; Schouten, H.; Klomp, G.; et al. Radiographic evaluation of bone remodeling after additively manufactured subperiosteal jaw implantation (AMSJI) in the maxilla: A one-year follow-up study. J. Clin. Med. 2021, 10, 3542. [Google Scholar] [CrossRef]
- Kundakcioglu, A.; Ayhan, M. Evaluation of different subperiosteal implant thicknesses on mechanical strength and stress on bone by finite element analysis. Int. J. Med. Sci. 2024, 21, 1672–1680. [Google Scholar] [CrossRef]
- Dalkey, N.; Helmer, O. An experimental application of the Delphi method to the use of experts. Manag. Sci. 1963, 9, 458–467. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).