Metagenomic Analysis of Bacterial Diversity on Reusable Tourniquets in Hospital Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
2.2. Sample Collection and Processing
2.3. DNA Extraction and Sequencing
2.4. Data Analysis
2.5. Ethical Consideration
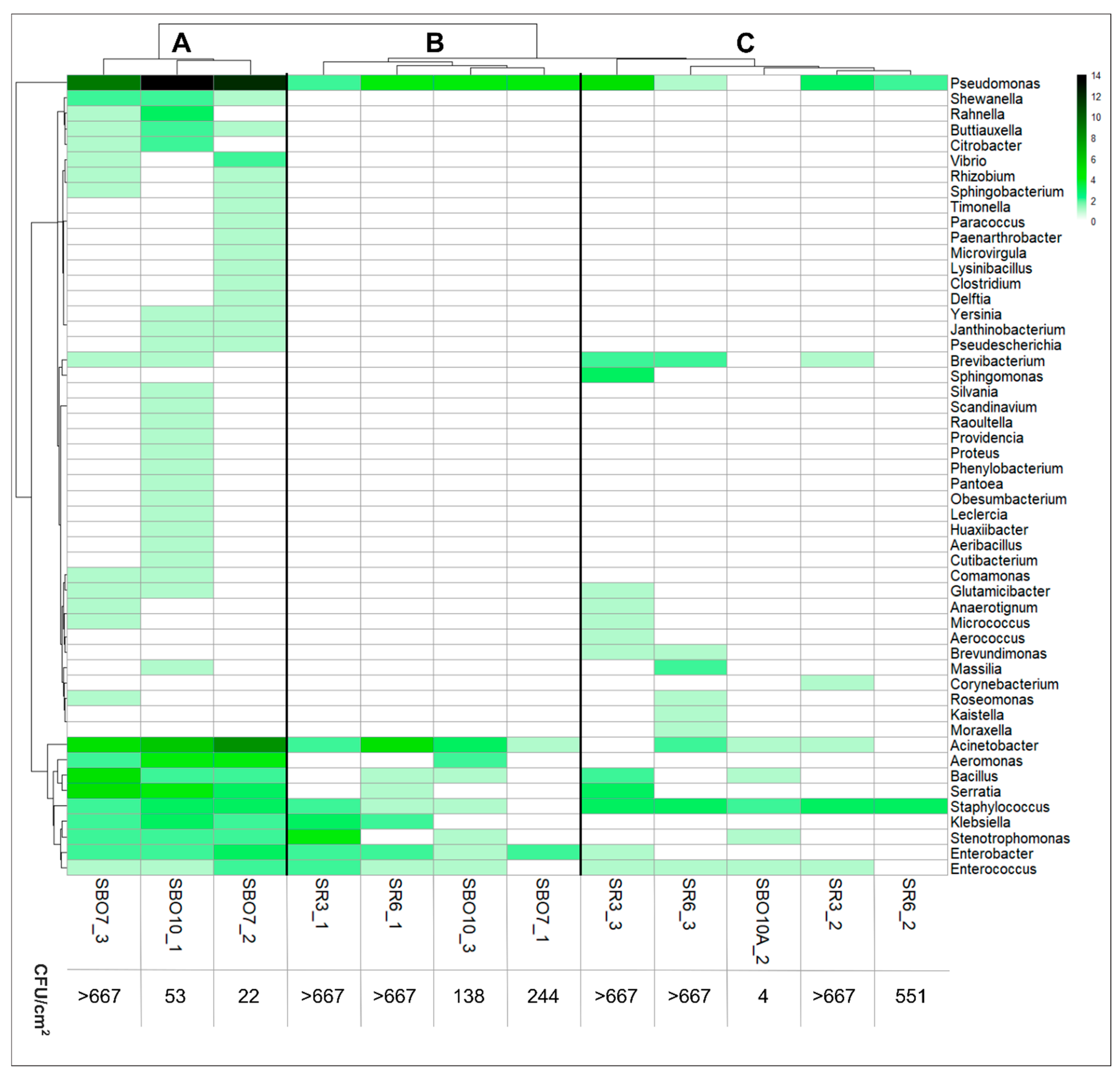
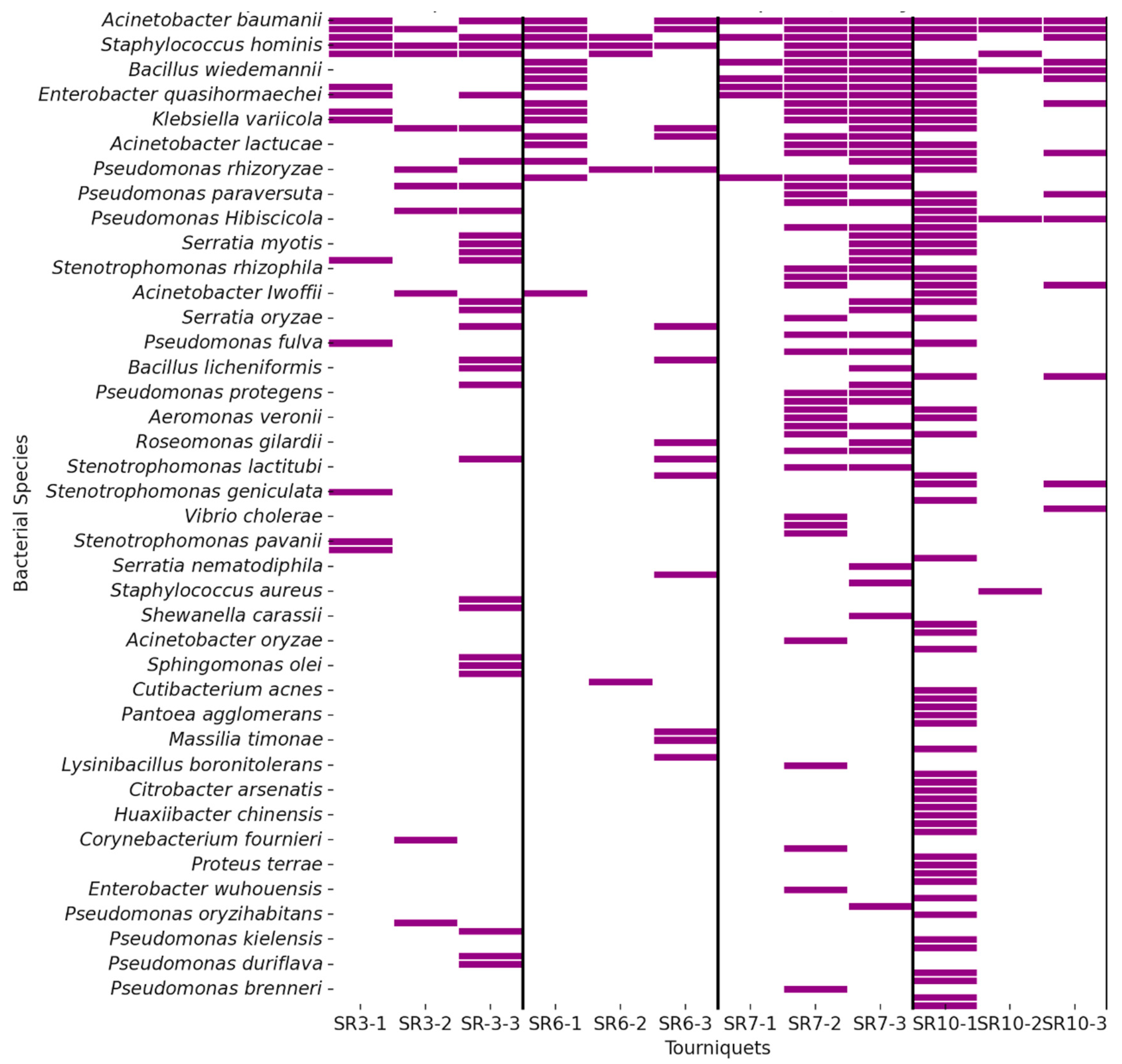
3. Results
3.1. Microbial Composition on the Surface of Reusable Tourniquets
3.2. Identification of Pathogenic Bacteria
3.2.1. Pathogenic Bacteria Cause HAI
3.2.2. Bacteria Pathogenic to Humans Found on Reusable Tourniquets
4. Discussion
Key Findings and Departmental Variability
5. Limitations
6. Conclusions
7. Implications for Practice
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Guidelines for the Prevention of Bloodstream Infections and Other Infections Associated with the Use of Intravascular Catheters. Part I: Peripheral Catheters; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Szymczyk, J.; Månsson, M.; Mędrzycka-Dąbrowska, W. Reusable Tourniquets for Blood Sampling as a Source of Multi-Resistant Organisms—A Systematic Review. Front. Public Health 2023, 11, 1258692. [Google Scholar] [CrossRef]
- Parreira, P.; Serambeque, B. Impact of an Innovative Securement Dressing and Tourniquet in Peripheral Intravenous Catheter-Related Complications and Contamination: An Interventional Study. Int. J. Environ. Res. Public Health 2019, 16, 3301. [Google Scholar] [CrossRef]
- Salgueiro-Oliveira, A.; Oliveira, V.; Costa, P.; Gama, F.; Graveto, J.; Parreira, P.; Osório, N. Tourniquets Used in Peripheral Venipuncture as a Potential Vehicle for Transmission of Microorganisms: Scoping Review. Infectio 2020, 24, 93. [Google Scholar] [CrossRef][Green Version]
- Culjak, M.; Gveric Grginic, A.; Simundic, A.M. Bacterial Contamination of Reusable Venipuncture Tourniquets in Tertiary-Care Hospital. Clin. Chem. Lab. Med. 2018, 56, e201. [Google Scholar] [CrossRef]
- Natarajan, A.; Das, S.; Chaudhary, N. Microbial Profile of Tourniquets Used in Phlebotomy at a Rural Tertiary Care Teaching Hospital. Cureus 2023, 15, e49328. [Google Scholar] [CrossRef]
- Stringer, B.; Infante, T.D.; Fainstein, V. Bacterial Contamination of Tourniquets Used in Routine Blood Collection. Am. J. Infect. Control 2011, 39, 744. [Google Scholar] [CrossRef]
- Szymczyk, J.; Kurpas, M.; Krasiński, B.; Zorena, K.; Mędrzycka-Dąbrowska, W. Reusable Tourniquets as Potential Transmitters of Infection: A Microbiological Analysis. Microorganisms 2025, 13, 152. [Google Scholar] [CrossRef]
- Field, N.; Cohen, T. Strengthening the Reporting of Molecular Epidemiology for Infectious Diseases (STROME-ID): An Extension of the STROBE Statement. Lancet Infect. Dis. 2014, 14, 341. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals; ECDC: Stockholm, Sweden, 2024. [Google Scholar]
- Mędrzycka-Dąbrowska, W.; Lange, S.; Zorena, K.; Dąbrowski, S.; Ozga, D.; Tomaszek, L. Carbapenem-Resistant Klebsiella pneumoniae Infections in ICU COVID-19 Patients—A Scoping Review. J. Clin. Med. 2021, 10, 2067. [Google Scholar] [CrossRef]
- Duszynska, W.; Litwin, A. Analysis of Acinetobacter baumannii Hospital Infections in Patients Treated at the Intensive Care Unit of the University Hospital, Wroclaw, Poland: A 6-Year, Single-Center, Retrospective Study. Infect. Drug Resist. 2018, 11, 629. [Google Scholar] [CrossRef]
- Rafa, E.; Wałaszek, M.Z. The Incidence of Healthcare-Associated Infections, Their Clinical Forms, and Microbiological Agents in Intensive Care Units in Southern Poland in a Multicentre Study from 2016 to 2019. Int. J. Environ. Res. Public Health 2021, 18, 2238. [Google Scholar] [CrossRef]
- Rafa, E.; Kołpa, M. Healthcare-Acquired Infection Surveillance in Neurosurgery Patients, Incidence and Microbiology, Five Years of Experience in Two Polish Units. Int. J. Environ. Res. Public Health 2022, 19, 7544. [Google Scholar] [CrossRef]
- Wałaszek, M.Z.; Słowik, R. Five-Year Analysis of Surgical Site Infections in Three Orthopaedics and Trauma Wards under HAI-Net from the South of Poland in 2014-2018 Considering the Standardized Infection Ratio. Medicina 2022, 58, 682. [Google Scholar] [CrossRef]
- Antunes, L.C.; Visca, P.; Towner, K.J. Acinetobacter baumannii: Evolution of a Global Pathogen. Pathog. Dis. 2014, 71, 292. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic Resistance in Pseudomonas aeruginosa: Mechanisms and Alternative Therapeutic Strategies. Biotechnol. Adv. 2019, 37, 177. [Google Scholar] [CrossRef]
- Wyres, K.L.; Holt, K.E. Klebsiella pneumoniae as a Key Trafficker of Drug Resistance Genes from Environmental to Clinically Important Bacteria. Curr. Opin. Microbiol. 2018, 45, 131. [Google Scholar] [CrossRef]
- Fiore, E.; Van Tyne, D.; Gilmore, M.S. Pathogenicity of Enterococci. Microbiol. Spectr. 2019, 7, GPP3-0053-2018. [Google Scholar] [CrossRef]
- Looney, W.J.; Narita, M.; Mühlemann, K. Stenotrophomonas maltophilia: An emerging opportunist human pathogen. Lancet Infect. Dis. 2009, 9, 312–323. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603. [Google Scholar] [CrossRef]
- Elhassan, H.A.; Dixon, T. MRSA Contaminated Venepuncture Tourniquets in Clinical Practice. Postgrad. Med. J. 2012, 88, 194. [Google Scholar] [CrossRef]
- Golder, M.; Chan, C.L.; O’Shea, S.; Corbett, K.; Chrystie, I.L.; French, G. Potential Risk of Cross-Infection during Peripheral-Venous Access by Contamination of Tourniquets. Lancet 2000, 355, 44. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, S.; Marsh, N.; Ray-Barruel, G.; Flynn, J.; Larsen, E.; Rickard, C.M. Infection Risks Associated with Peripheral Vascular Catheters. J. Infect. Prev. 2016, 17, 207. [Google Scholar] [CrossRef]
- Choudhury, M.A.; Marsh, N.; Banu, S.; Paterson, D.L.; Rickard, C.M. Molecular Comparison of Bacterial Communities on Peripheral Intravenous Catheters and Matched Skin Swabs. PLoS ONE 2016, 11, e0146354. [Google Scholar] [CrossRef]
- Mermel, L.A. Short-Term Peripheral Venous Catheter-Related Bloodstream Infections: A Systematic Review. Clin. Infect. Dis. 2017, 65, 1757. [Google Scholar] [CrossRef]
- Weber, D.J.; Anderson, D.; Rutala, W.A. The role of the surface environment in healthcare-associated infections. Curr. Opin. Infect. Dis. 2013, 26, 338–344. [Google Scholar] [CrossRef]

| Sample Name | Observation Time | Area |
|---|---|---|
| SR3_1 | Indefinite | Adult observation |
| SR3_2 | 14 days | |
| SR3_3 | 28 days | |
| SR6_1 | Indefinite | Resuscitation area |
| SR6_2 | 14 days | |
| SR6_3 | 28 days | |
| SBO7_1 | Indefinite | Pediatric surgery |
| SBO7_2 | 14 days | |
| SBO7_3 | 28 days | |
| SBO10_1 | Indefinite | Adult general surgery |
| SBO10_2 | 14 days | |
| SBO10_3 | 28 days |
| Microorganism | Percent of HAI Cases in Europe 2022–2023 [cyt] | Number of Tourniquets All | Number of Tourniquets Emergency Department | Number of Tourniquets Operating Theater |
|---|---|---|---|---|
| E. coli | 12.7% | 0/12 | 0/6 | 0/6 |
| Klebsiella spp. | 11.7% | 5/12 | 2/6 | 3/6 |
| Enterococcus spp. | 10% | 9/12 | 4/6 | 5/6 |
| Staphylococcus aureus | 9% | 1/12 | 0/6 | 1/6 |
| Clostridium difficile | 8% | 0/12 | 0/6 | 0/6 |
| Pseudomonas aeruginosa | 7.9% | 9/12 | 4/6 | 5/6 |
| Coagulase-negative staphylococci | 5.8% | 11/12 | 6/6 | 5/6 |
| Proteus spp. | 3.2% | 0/12 | 0/6 | 0/6 |
| Acinetobacter spp. | 3.2% | 10/12 | 4/6 | 6/6 |
| Enterobacter spp. | 3% | 0/12 | 0/6 | 0/6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymczyk, J.; Jaskulak, M.; Kurpas, M.; Zorena, K.; Mędrzycka-Dąbrowska, W. Metagenomic Analysis of Bacterial Diversity on Reusable Tourniquets in Hospital Environments. Appl. Sci. 2025, 15, 7545. https://doi.org/10.3390/app15137545
Szymczyk J, Jaskulak M, Kurpas M, Zorena K, Mędrzycka-Dąbrowska W. Metagenomic Analysis of Bacterial Diversity on Reusable Tourniquets in Hospital Environments. Applied Sciences. 2025; 15(13):7545. https://doi.org/10.3390/app15137545
Chicago/Turabian StyleSzymczyk, Julia, Marta Jaskulak, Monika Kurpas, Katarzyna Zorena, and Wioletta Mędrzycka-Dąbrowska. 2025. "Metagenomic Analysis of Bacterial Diversity on Reusable Tourniquets in Hospital Environments" Applied Sciences 15, no. 13: 7545. https://doi.org/10.3390/app15137545
APA StyleSzymczyk, J., Jaskulak, M., Kurpas, M., Zorena, K., & Mędrzycka-Dąbrowska, W. (2025). Metagenomic Analysis of Bacterial Diversity on Reusable Tourniquets in Hospital Environments. Applied Sciences, 15(13), 7545. https://doi.org/10.3390/app15137545








