Abstract
This study used Xinxin 2 (Juglans regia L. ‘Xinxin2’), a major cultivated walnut variety in Xinjiang, China, to clarify the response and adaptation mechanisms of the anatomical structures of walnut related to source–sink–flow under altered source–sink relationships. We anatomically observed the leaves, fruit stalks, and fruit of bearing branches by artificially adjusting the leaf-to-fruit ratio (LFR). The LFR substantially affected the leaf structure and thickness of the fruit-bearing branches obtained via girdled (p < 0.05). The results of the analysis of the leaf anatomy revealed that a low LFR impeded leaf growth and internal structural development while accelerating senescence, whereas a high LFR promoted leaf growth and delayed senescence. The same trend was observed for the phloem area (PA) of the fruit stalk with the increase in fruit load when the number of leaves on the fruit branch was the same. The maximum PA was reached when the number of fruits was high (except for 4L:3F). This indicates that the micro-anatomical structure of the fruit stalk is more developed under the treatment of a higher number of pinnate compound leaves and fruit level of LFRs. The cells of the 1L:3F and 2L:3F were considerably smaller in the green peel and kernel of the fruit on the branches obtained via girdled than those of 5L:1F plants (p < 0.05). No significant difference was found in the number of cells per unit area or the cross-sectional area of cells in the pericarp and kernel of the fruit under LFRs (p > 0.05); however, a large difference was noted in the microanatomical structure of the pericarp and kernel of fruit. Changes in the structural adaptation characteristics of walnut leaves (source), fruit stalk (flow), and fruit (sink) are related to source–sink regulation. A change in the LFR affects the carbohydrate synthesis in the leaves (source), transport in fruit stalks (flow), and the carbohydrate reception in fruits (sink).
1. Introduction
Walnut (Juglans regia L.) is a perennial tree of the Juglans genus in the Juglandaceae family. Walnut is an important tree species for producing woody grain and oil in China [1,2]. The walnut industry in Xinjiang province in China has rapidly developed. Producing high-quality walnuts is a challenge facing the development of the walnut industry in Xinjiang, China. As an important technical means in the production and quality improvement of economic forests and the management of fine cultivation, the regulation of the source–sink relationship is affected by the structure and function of the source, sink, and flow, affecting the distribution of carbohydrates in forest trees [3]. The structural traits of a tree form the basis of the functions of tree. However, information is lacking on whether and how the structural traits of leaves (sources), fruits (sinks), and conducting tissues (flow) that directly perform carbohydrate assimilation and distribution change with changes in the source–sink relationship. Therefore, in-depth explorations are required on the response and adaptation mechanisms of the anatomical structures of source–sink–flow in walnuts with changes in the carbohydrate source–sink relationship, which will increase our understanding of the source–sink relationships in economic forests and guide the development of precision cultivation management practices in the walnut industry.
The distribution of carbohydrates in most plants from the leaf (source) to the fruit (sink) is limited by the ability to transport carbohydrates between the source and sink [4,5] and the competitiveness of the sink [6]. Therefore, scholars at home and abroad have focused on the metabolic processes of carbohydrates have been studied in economic forests. These studies have focused on the photosynthetic physiology [7], sugar metabolism physiology [8], as well as the yield and quality formation physiology [9], in the process of the synthesis, transport, distribution, and use of carbohydrates in economic forest trees under source–sink regulation. The carbohydrate assimilation and distribution patterns under source–sink regulation have been determined. The leaf-to-fruit ratio (LFR) can be used to quantify these carbohydrate source–sink relationships. The morphological features of leaves have been used to characterize carbohydrate assimilation and transport capacity of plants. For example, the chloroplast numbers and chloroplast volumes increase in the leaves of Korla fragrant pear (Pyrus sinkiangensis Y.) under low-LFR conditions compared with normal conditions [10]. The leaves of Hosui Pear (Pyrus pyrifolia Nakai) have higher numbers of osmiophilic plastoglobuli within the chloroplasts, disorganized thylakoid membranes, and incipient chloroplast degradation under high fruit load conditions compared with control conditions [11]. The characteristics of the sink organ, such as the fruit, play a key role in fruit size, which is not only related to the ability of the sink organ to unload and absorb carbon assimilates but also to the size and number of cells in the sink organ [12]. The fruit volume as well as the number and volume of mesocarp cells differ within and between Prunus sibirica (Prunus armeniaca L.) trees. The within-tree difference in fruit volume is mainly due to the difference in the number of cells, whereas the between-tree differences in cell volume more strongly affect fruit volume [13]. The volume and number of cells affect the size of grapes (Vitis vinifera L.). The structure and properties of the conducting tissue, which is a channel for transporting water, nutrients, metabolites, and other substances, are key in coordinating plant growth and development [14]. The anatomical characteristics, yield, and components of the panicle stems of the seedlings of two rice varieties (Oryza sativa L.) strongly affected the floret load of large and small vascular bundles [15]. Studies in cotton (Gossypium hirsutum L.) have shown that defoliated branches can improve the photosynthetic and transpiration capacity of fruit branches and leaves, which is conducive to the formation and development of conducting tissues, thereby promoting assimilation and water transport [16].
The research on walnuts has mainly focused on how the source–sink relationship regulates carbohydrate assimilation and distribution. However, after the change of source–sink relationship, whether the structural traits of leaves (source), fruits (sink) and conducting tissues (flow), which directly carry the assimilation and distribution functions of carbohydrates, change, and how to change are poorly understood. In addition, studies have solely focused on one part of the source–sink–flow for one species, with no unified research on how regulating the source–sink relationship affects the structural traits of the carbohydrate sources, sinks, and flow. Therefore, the responses of the anatomical structures of walnut sources, sinks, and to flow to changes in the source–sink relationship and the mechanisms through which these adaptions occur must be thoroughly studied to deepen our understanding of the sources and sinks in economic forests.
2. Materials and Methods
2.1. Experimental Materials
The walnut production park in Daireyaboyi Village, Awati County, Aksu Prefecture (41°11′06.31″–41°12′47.74″ N, 79°12′12.76″–79°13′57.87″ E; 1394 m a.s.l.) was chosen as the study site. The orchard had a uniform stand structure, with the main cultivar being ‘Xinxin 2’ (J. regia ‘Xinxin 2’), which was 12 years old. The healthy walnut tree with the same growth potential was selected as the test sample tree, before the experiment.
2.2. Artificial Regulation of Leaf-to-Fruit Ratio
According to Wang’s method [8], a total of 15 leaf-to-fruit ratio treatments (as shown in Figure 1) were set up, with three replicates per treatment. Ten bearing branches with the same growth potential after self-fruiting were selected as the sample from the south side of the crown of each sample plant in each treatment at a distance of 1.3 m–1.8 m from the ground. We ensured that all bearing branches in each treatment had the same LFR by removing fruit or and leaves and marked. In total, 45 trees and 450 bearing branches were selected.
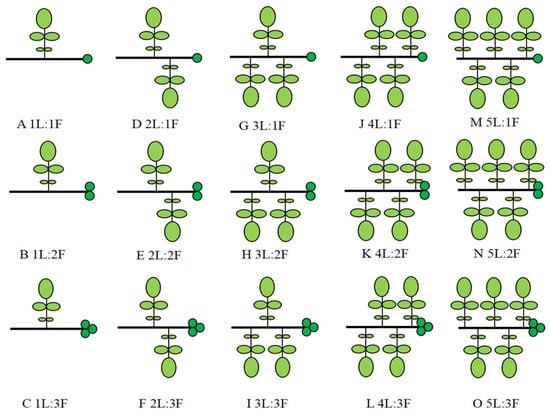
Figure 1.
Different leaf-to-fruit ratios (LFRs) manipulations on the girdled branches of walnut, in which light green represents leaves and dark green represents fruits, where light and dark green represent leaves and fruits, respectively.
2.3. Sample Collection
A 5 mm-wide complete phloem ring was cut 10 mm proximal to the base of defruited and defoliated bearing branches using a grafting knife. The bearing branches were collected at 10, 30, 50, 70, and 90 days after creating the different LFRs. Three bearing branches were collected from the three sample trees in each treatment. The leaves (source), fruit stalks (flow), and fruits (sink) were collected from the girdled bearing branches. The collection was performed as follows: The two leaflets closest to the parietal leaf on the compound leaf closest to the fruit were collected at 30 and 90 days after the LFR treatment. The fruit stalks were collected 10 and 90 days after LFR treatment. Fruit stalks and kernel samples were collected 10 and 90 days after LFR treatment, respectively. The middle area of the main vein on the third or fourth compound leaf was selected to observe the anatomical structure. The collected leaves, fruit stalks, and fruit samples were washed and wiped dry with deionized water, cut, and placed into a formalin–acetic acid–alcohol (FAA) fixative solution for paraffin sectioning.
2.4. Preparing and Observing of Paraffin Sections
Following the protocol established by Wang et al. [17], the materials in 2.3 FAA fixative solution were dehydrated, cleared, paraffin-embedded, sectioned, sliced, and baked. Finally, the sections were double-stained with safranin solid green. The neutral gel was sealed and dried in a 40 °C electric constant-temperature blast-drying oven. The microstructure of the slices was observed using a fluorescence microscopy system (Leica DM6000B; Leica Company, Weisra, Germany). The leaf thickness (LT), palisade tissue thickness (PTT), spongy tissue thickness (STT), upper epidermis thickness (UET), and lower epidermis thickness (LET) were measured. The main vein phloem (MVP), fruit stalk phloem (FSP), green husk cell (GHC), and kernel cells (KC) were photographed to determine their area. AutoCAD 2014 software was used to process the images and calculate the phloem area (PA). Each LFR treatment was performed in triplicate, and 10 visual fields for each replicate were observed. The leaf structure data on the bearing branches with the same LFR were reported as the average of the two measurement results at 30 and 90 days after the LFR treatment.
2.5. Data Analysis
The data were statistically analyzed using SPSS version 22.0. We used analysis of variance (ANOVA) to study the effect of the LFR on the anatomical structures of the leaves, fruit stalks, and fruits. The least significant difference multiple comparison method was used if the effect of LFR was significant (p < 0.05) to determine whether the response variables significantly differed between the LFR treatments.
3. Results
3.1. Effects of Different LFRs on Anatomical Structure of Branch Leaves
Walnut leaves anatomically consist of the upper epidermis, palisade tissue, spongy tissue, and lower epidermis. The LFR significantly affected the LT, PTT, STT, UET, and MVPA (p < 0.05), indicating that the LFR strongly impacted the structural parameters of the girdled bearing branches (Table 1). However, the LFR did not significantly affect the LET (p > 0.05).

Table 1.
ANOVA results of the effect of the LFR on anatomical leaf structures.
An increase in the LFR increased the LT, PTT, STT, and MVPA when the same number of leaves or fruits was retained on the girdled bearing branches. The results of multiple comparisons (Figure 2) showed that the LT did not significantly differ between 4L:1F and 5L:1F (p > 0.05, Figure 2A). The LT was significantly higher for 4L:1F and 5L:1F compared with the other LFRs (except for 4L:2F and 5L:2F, p < 0.05). The LT did not significantly differ between 1L:2F and 1L:3F (p > 0.05); however, the LT was significantly lower in 1L:2F and 1L:3F compared with the other LFRs (except 1L:1F, 2L:2F, and 2L:3F, p < 0.05). The maximum PTT was 104.64 μm at 5L:1F, which was significantly higher than that of the other LFRs (except for 4L:1F, p < 0.05, Figure 2B). The PTT did not significantly differ between the 1L:2F and2 L:3F treatments (p > 0.05). However, the PTT significantly lower in the 1L:2F and2 L:3F treatments than for the other LFRs (except for 1L:3F, 2L:2F, 3L:3F, and 5L:3F), where the PTT was 32.54% and 31.42% lower than that in the 5L:1F treatment, respectively. The STT did not significantly differ between the 4L:1F and 5L:1F treatments (p > 0.05, Figure 2C) and was significantly higher at LFRs of 4L:1F and 5L:1F than at the other LFRs (p < 0.05). The STT did not significantly differ between the LFRs of 2:3 and 1:2 (p > 0.05); however, the STT at 2L:3F and 1L:2F were significantly lower than that at high LFRs (p < 0.05). The UET did not significantly differ between 5L:1F and 5L:2F (p > 0.05, Figure 2D) or between 5L:1F and 4L:(1–3)F (p > 0.05); however, the UET was significantly higher for these LFRs for the other LFRs (p < 0.05). The maximum MVPA was observed for 4L:1F, 5L:1F, and 5L:2F (Figure 2E), averaging between 81,890 μm2 and 84,720 μm2; the MVPA was significantly higher for these LFRs than for the other LFRs (except for 4L:2F, p < 0.05). The MVPA was smallest at LFRs of 1L:2F, 1L:3F and 2L:3F, averaging between 53,817 μm2 and 58 040 μm2.
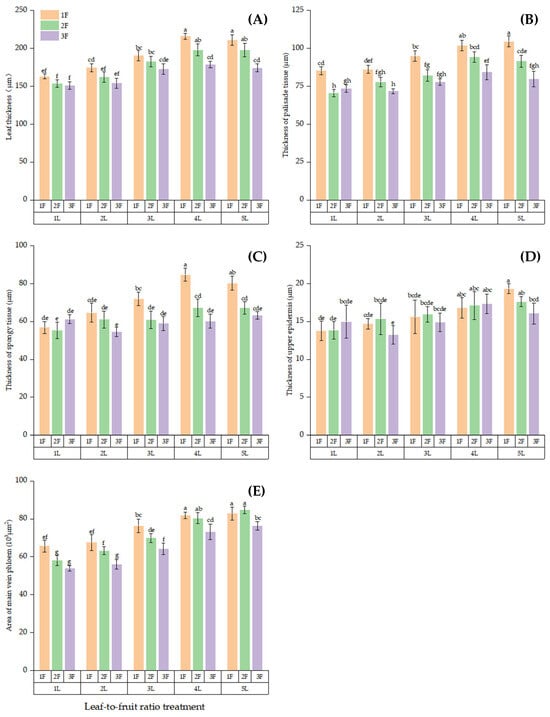
Figure 2.
Comparison of anatomical structure in leaf structure on girdled bearing branches at different LFRs. (A–E) The differences in leaf thickness (LT), palisade tissue thickness (PTT), sponge tissue thickness (STT), upper epidermis thickness (UET), and phloem area of main vein (MVPA) between treatments. Note: Different lowercase letters denote significant differences (p < 0.05) between treatments for the same index.
3.2. Morphological Structure of Leaf from Girdled Bearing Branches at Different LFRs
The cell morphology and structure of the walnut leaves differed among the LFRs. The walnut fruits entered the rapid growth stage and the walnut leaves reached maturity after 30 days of LFR treatment. The mesophyll PT cells were long, columnar, rich in chloroplasts, and neatly arranged. The gaps between the mesophyll PT cells were small, and the cells were arranged in two layers in the leaf. In contrast, the chloroplast content of the sponge tissue was lower, the cell morphology was irregular, the cell arrangement was loose, and the gaps between cells were was large. The morphological structures of the leaf cells differed among the LFRs. The LT in the (1–2)L:(1–3)F treatments was smaller than in the other treatments, and the PT in these treatments was mostly double-layered, accounting for less than 1/2 of the LT. The PT cells were only arranged in a single layer in some areas, with fewer chloroplasts, and the leaves were lighter at LFRs of (1–2):(1–3) compared with those for the other LFRs (Figure 3A,B,F,G,K,L). The LT in the (3–5)L:(1–3)F treatments was larger, and most of the palisade structure in the leaf was double-layered, accounting for more than 1/2 of the LT, at LFRs of 1–2:1–3 than for the other LFRs. The (4–5)L:(1–2)F treatments resulted in PT cells in some parts of the leaves; the cells were tightly arranged, the number of chloroplasts was high, and the leaf color was darker than for the other LFRs (Figure 3C–E, H–J, M–O). A lower leaf-to-fruit ratio or a higher fruit load hindered leaf growth and development.
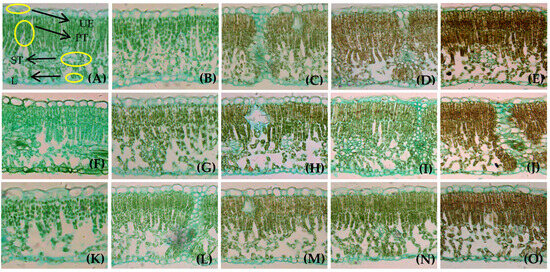
Figure 3.
Anatomical structure of leaves from girdled branches at different LFRs during fast-growth period: (A–O) 1L:1F (A), 2L:1F (B), 3L:1F (C), 4L:1F (D), 5L:1F (E), 1L:2F (F), 2L:2F (G), 3L:2F (H), 4L:2F (I), 5L:2F (J), 1L:3F (K), 2L:3F (L), 3L:3F (M), 4L:3F (N) and 5L:3F (O), respectively. (A) Location of each tissue type is indicated. Images were captured with a 20× objective lens. UE, upper epidermis; PT, palisade tissue; ST, spongy tissue; LE, lower epidermis.
The walnut fruit was ripening, and the leaves entered senescence after 90 days of LFR treatment. The leaf color markedly lightened, and the morphological cell structure was considerably different. The morphological structure of the leaf cells substantially differed among the LFR treatments. The leaves in the (1–3)L:(1–3)F treatments more quickly senesced, some of the UE cells were distorted and deformed, some mesophyll cells disintegrated, cavities appeared in the ST area, more free and damaged cells were observed in the mesophyll cells, and the lower epidermal cells disintegrated, bulged and fractured (Figure 4A–C,F–H,K–M). However, leaf senescence was not observed in the 5L:1F and 4L:1F treatments (Figure 4D,E); the ST area contained no cavities, and the epidermal cells showed no protrusion fractures. Leaf senescence was noted on the (4–5)L:(2–3)F branches. The upper epidermal cells were partially distorted and deformed, and the individual mesophyll cells began to disintegrate. A small number of cavities were observed in the ST areas of the leaves. Free damaged mesophyll cells were noted, and the LE cells were distorted and deformed; however, no convex fractures were observed (Figure 4I,J,N,O). A lower LFR or a higher fruit load accelerated leaf senescence.
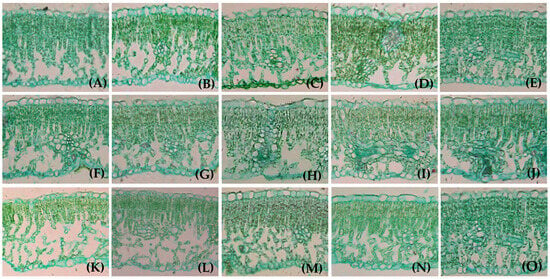
Figure 4.
Anatomical structure of leaves on girdled branches with different LFRs in mature period: (A–O) 1L:1F (A), 2L:1F (B), 3L:1F (C), 4L:1F (D), 5L:1F (E), 1L:2F (F), 2L:2F (G), 3L:2F (H), 4L:2F (I), 5L:2F (J), 1L:3F (K), 2L:3F (L), 3L:3F (M), 4L:3F (N) and 5L:3F (O), respectively. Location of each tissue type is indicated in Figure 2A. All images were captured with a 20× objective lens.
The main vein in mature leaves developed into the xylem, and the cells were large and radially arranged according to the microscopy observations. The phloem was composed of multiple layers of smaller cells (Figure 5). The larger the LFR, the more developed the main vein of the leaves. The lengths of the main vein and main vein vascular bundle were significantly higher in the high-LFR than in the low-LFR groups (p < 0.05). Figure 6 shows that the vein was longest at an LFR of 5:1 and significantly longer than that at the other LFRs (p < 0.05, Figure 6A). The vein was the shortest at 1L:3F, being significantly shorter than that at the other LFRs (p < 0.05). The leaf vein was widest at 3L:2F and most narrow at 1L:2F (Figure 6B). The leaf vein vascular bundle was significantly longer at 5L:1F than of the other LFRs (p < 0.05, Figure 6C). The leaf vein vascular bundle was significantly shorter at 1L:3F compared with the other LFRs (p < 0.05).
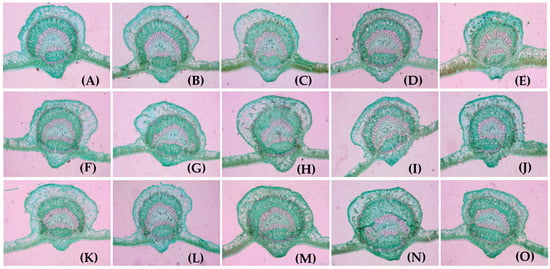
Figure 5.
Microstructure of main leaf veins on bearing branches with different LFRs: (A–O) 1L:1F (A), 2L:1F (B), 3L:1F (C), 4L:1F (D), 5L:1F (E), 1L:2F (F), 2L:2F (G), 3L:2F (H), 4L:2F (I), 5L:2F (J), 1L:3F (K), 2L:3F (L), 3L:3F (M), 4L:3F (N)and 5L:3F (O), respectively. All images were captured with a 5× objective lens.
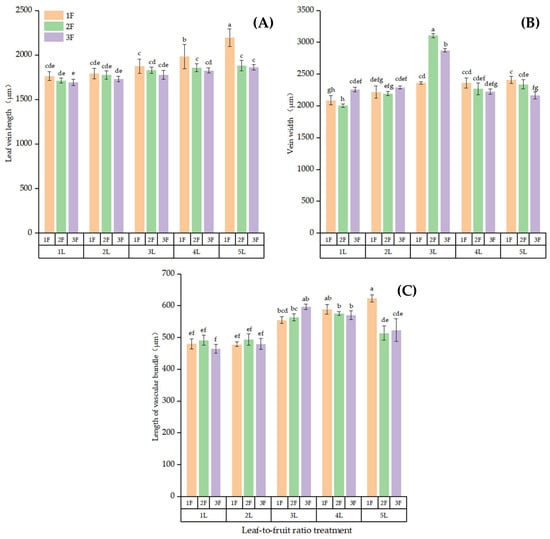
Figure 6.
Anatomical structure of the main leaf vein on bearing branches with different LFRs. Differences in (A) vein length, (B) vein width, and (C) vascular bundle length. Note: Different lowercase letters denote significant differences (p < 0.05) between treatments for the same index.
3.3. Anatomical Structure of Fruit Stalk on Girdled Branches at Different LFRs
Fruit stalk as the channel for the transporting carbohydrates and nutrients. The ANOVA results (Table 2) showed that the LFR significantly affected the FSPA (p < 0.05). The results of multiple comparison results (Table 3) showed that for branches with the same number of leaves, the FSPA increased with the increase in fruit load. The FSPA was generally the highest when the number of fruits was high, (1–5)L:3F > (1–5)L:2F > (1–5)L:1F, except for 4L:3F. However, when the number of fruits was the same on the girdled fruit branches, the FSPA of the stalk on the fruit-bearing branch did not significantly differ among the LFRs (p > 0.05). The FSPA was the largest at 5L:3F, 4L:2F, and 3L:3F, averaging 126,000 μm2–131,000 μm2. The FSPA values were smallest in the 1L:1F, 2L:1F, 3L:1F, 4L:1F, and 5L:1F treatments, averaging 104,000 μm2–108,000 μm2.

Table 2.
ANOVA results of the effect of LFR on fruit stalk trait.

Table 3.
Results of multiple comparisons of phloem area of fruit stalk of girdled branches for different leaf-to-fruit ratio (LFR) (mean ± SD, n = 10). FSPA is area of fruit stalk phloem.
3.4. Cell Structure of Fruit Stalks on Girdled Bearing Branches at Different LFRs
The changes in the anatomical structures of the fruit stalk cells were similar for each LFR over time according to the microscopy observations. However, the anatomical structures of the fruit stalks substantially differed among the LFR treatments. The fruit stalk was anatomically more developed at the higher LFRs. The pith of the fruit stalk gradually degenerates and disappears as walnuts grow and develop; therefore, only a few tissues are shown in Figure 7.
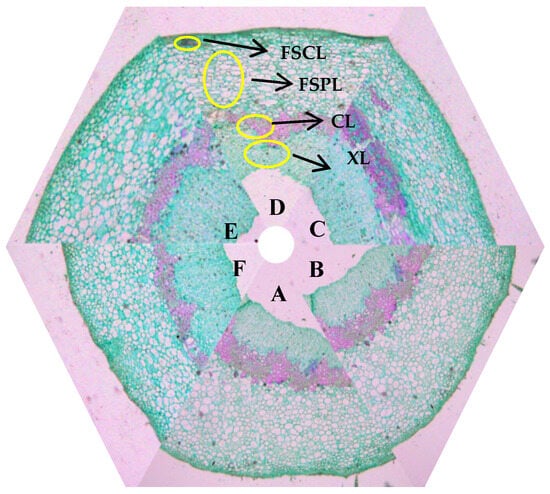
Figure 7.
Microstructure of transverse section of fruit stalk at walnut fruit maturity: (A–F) 1L:1F, 1L:2F, 1L:3F, 5L:1F, 5L:2F and 5L:3F, respectively. The image was captured with a 5× objective lens. FSCL, fruit stalk cortex length; FSPL, fruit stalk phloem length; CL, cambium length; XL, xylem length.
The length of Xylem (XL), Phloem (FSPL), Cambium (CL), Cortex (FSCL), long with Pith (PL) and the width of pith (PW) in the cross-section of fruit stalk showed the same trend with the change of LFRs, most reaching a maximum value when the fruit quantity was high: (1–5)L:3F > (1–5)L:2F > (1–5)L:1F (Figure 8). The PL was significantly longer at 3L:3F than at the other LFRs (p < 0.05; Figure 8A). The PW was significantly wider at 5L:3F than at the other LFRs (p < 0.05, Figure 8B). The XL did not significantly differ between the 5L:3F and 1L:2F treatments but was significantly higher in these two treatments than in the other LFR treatments (p < 0.05, Figure 8C). The CL was the significantly longer at 5L:3F compared with that for the other LFRs (p < 0.05, Figure 8D). The FSPL was the shortest at 1L:1F, but the difference in the phloem length among the LFRs was small (Figure 8E). The cortex was significantly shorter at 1L:1F than at the other LFRs (p < 0.05, Figure 8F).
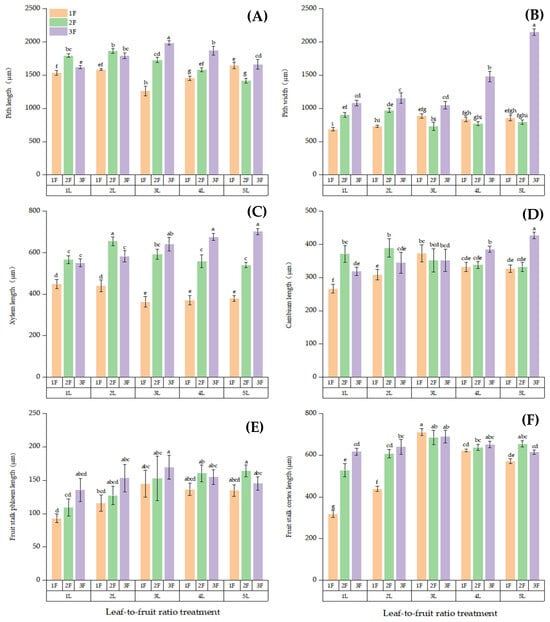
Figure 8.
Differences in the anatomical structures of the main leaf vein on the bearing branches among the different LFR treatments: (A) pith length, (B) pith width, (C) xylem length, (D) cambium length, (E) fruit stalk phloem length, and (F) fruit stalk cortex length. Note: Different lowercase letters denote significant differences (p < 0.05) between treatments for the same index.
3.5. Anatomical Structure of Fruits on Branches with Different LFRs After Girdled
The ANOVA results for mature fruit cells from the girdled fruit branches under different LFRs are shown in Table 4. The cell number in the green husks (GHCN), cell area in the green husks (GHCA), number of cells in the kernel (KCN), and cell area in the kernels (KCA) significantly differed among the LFR treatments (p < 0.05).

Table 4.
Effect of LFR on characteristics of walnut fruit.
The fruit cells of the girdled branches significantly differed among the LFRs (p < 0.05, Figure 9). The number of cells per unit area in the green fruit husks at 5L:1F was significantly lower than that at 1L:3F and 2L:3F (p < 0.05), averaging 51.04 cells·10−5 μm−2, 79.12 cells·10−5 μm−2, and 80.65 cells·10−5 μm−2, respectively. The cross-sectional area of the green fruit skin cells at 5L:1F was significantly larger than at 1L:3F and 2L:3F (p < 0.05), averaging 1830 μm2, 1027 μm2, and 1033 μm2, respectively. The number of cells per unit area in the fruit kernels was significantly higher at 1L:3F than at the other LFRs (p < 0.05), averaging 93.29 cells·10−5 μm−2 among all LFRs. The cross-sectional area of fruit kernel cells in the 5L:1F treatment was significantly larger than in the 1L:3F and 2L:3F treatments (p < 0.05), averaging 1830 μm2, 1030 μm2, and 1040 μm2, respectively.
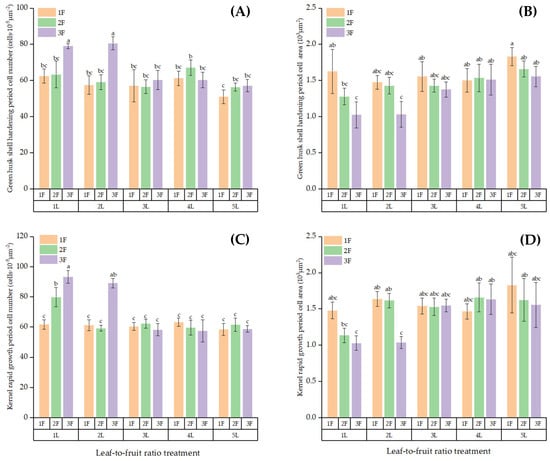
Figure 9.
Differences in the anatomical structures of fruit cells on the fruiting ring-cut branches among the LFR treatments: (A) number and (B) area of green husk cells during the shell hardening period; (C) number and (D) area of grain cells during the rapid kernel growth period. Note: Different lowercase letters denote significant differences (p < 0.05) between treatments for the same index.
3.6. Cell Structure of Fruits on Girdled Branches with Different LFRs
The volume of walnut fruit increases through increases in the vertical division of epidermal cells. However, pericycle division also occurs in some locations, which gradually increases the number of cell layers to thicken the skin. The microscopy observations (Figure 10) showed that the anatomical structure of the green peel substantially differed among the LFRs. The thin-walled tissue cells were more densely arranged and the cell volume was larger on the branches with more feather compound leaves and more fruits.
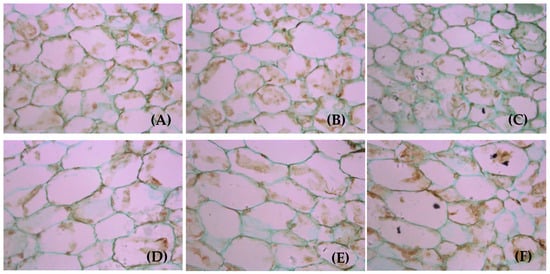
Figure 10.
Anatomical structure of green peel of mature walnut fruit: (A–F)1L:1F, 1L:2F, 1L:3F, 5L:1F, 5L:2F, and 5L:3F, respectively. The images were captured with a 40× objective lens.
The anatomical structure of the kernels at the microscopy level differed among the LFRs (Figure 11). The numbers of pinnate compound leaves and fruit were higher, nothing was observed in the intercellular space, and the growth in the kernel cell morphology was relatively balanced in all directions at higher LFRs compared with those at lower LFRs. The cross-sectional area of the kernel cells in the 5L:(1–3)F fruit was larger than that in the 1L:(1–3)F fruit, and the kernel cells in the 5L:(1–3)F fruit tended to be regular polygons or close to circles. The cell arrangement was more compact and orderly, with the organelles in the cells showing more replacement and decomposition in the 5L:(1–3)F fruit than in the other LFR treatments.
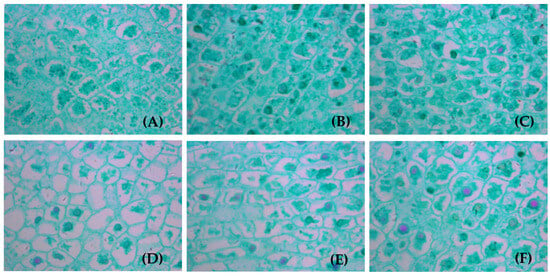
Figure 11.
Anatomy and structure of mature walnut kernel: (A–F) 1L:1F, 1L:2F, 1L:3F, 5L:1F, 5L:2F, and 5L:3F, respectively. The images were captured with a 40× objective lens.
4. Discussion
4.1. The Relationship Between Walnut Leaf Traits and LFR on the Girdled Bearing Branches
Leaf structure and function are strongly related in plants, and the traits of a leaf are closely related to photosynthesis [18]. Various indicators reflect the traits of leaves, one of which is the CO2 assimilation rate (photosynthetic capacity). The CO2 assimilation rate of palisade tissue is higher than that of spongy tissue; therefore, palisade tissue thickness is an indicator of photosynthetic capacity [19]. The carbohydrate assimilation capacity of a leaf increases with increases in the thicknesses of the spongy and palisade mesophyll tissues in the leaf as well as with increases in the size of the stomatal aperture and the net photosynthetic rate [20,21]. We found that the leaf and palisade tissue thicknesses gradually increased as the LFR increased. This finding indicates that when the fruit load is small and the sink is weak, smaller amounts of the carbohydrates assimilated by the leaves are distributed to the sink organs; the carbohydrates accumulate in the source leaves or are used for leaf growth and development. This hypothesis is supported by the microscopy observations of the leaf cell morphology during the rapid fruit growth period (Figure 3). The LT and PTT gradually decreased with an increase in the number of fruits when 1–2 leaves were retained on the ring-cut bearing branches, indicating that low LFRs affected the leaf growth and internal structure formation.. In addition, ring cutting affected the leaf growth and development on the bearing branches through blocking carbohydrate transport to other parts of the tree as well as the input of carbohydrates from other parts of the tree through the phloem to the leaves on the bearing branches so that the leaves were not self-sufficient, which hindered leaf development [22]. Walnut leaves entered senescence 90 days after the LFR treatment. A higher fruit load accelerated leaf senescence, which was reflected in the morphological changes of the cell structure (Figure 4). The upper epidermis cells on the leaves were partially distorted and deformed, individual mesophyll cells began to disintegrate, many cavities appeared in the leaf spongy tissue area, the number of free and damaged cells increased in the mesophyll cells, and the lower epidermal cells began to disintegrate when 1–2 leaves were retained on the ring-cut bearing branches compared with the other treatments. But the morphological structure of the leaves on the branches of 5L:1F and 4L:1F circumcision did not show the above obvious changes. This result is similar to that reported for Korla fragrant pear [10]. This finding indicates that increasing the number of leaves on bearing branches on walnut trees can optimize the leaf microstructure characteristics and thus increase the carbohydrate assimilation capacity of walnut leaves.
4.2. Relationship Between Fruit Stalk Traits and LFR on Girdled Bearing Branches
The fruit stalks is the organ that connects fruits and bearing branches, acting as channels for nutrient and water transport. The traits of the fruit stalk are closely related to the quality of fruit development [23,24]. The results of phloem structure and function models showed that the phloem juice volume is affected by various factors. Increasing the phloem area increases cambium activity, which increases the transport of carbohydrates from the leaves to the fruits [25]. We found that when the same number of leaves was retained on the ring-cut fruit branches, the area of the stalk phloem increased with the increase in the number of fruit (except 4L:3F)., The microscopic observation results of the morphological structure of fruit stalk cells showed that the change trend of the microanatomy of the fruit stalk with the LFRs was about the same, and the microanatomy of the fruit stalk was significantly different at different levels of LFRs Ananas comosus [26] and citrus [27]. Therefore, the carbohydrate availability in the high loading or low LFR treatment was likely lower, and fewer carbohydrates were allocated to the leaves and fruit stalks, which restricted the growth of the leaves and fruit stalks. The carbohydrate availability was likely higher in the low loading or high LFR treatment, and surplus carbohydrates were distributed to the leaves and stalks, which increased the leaf and stalk growth, and most of the carbohydrates were distributed to the fruits.
4.3. Relationship Between Fruit Traits and LFR on Girdled Bearing Branches
Fruit growth is affected by carbohydrate assimilation, transportation, and use [28], which are related to the source–sink relationship in the transport of photosynthetic products from the leaves to fruits [29]. We found no significant difference in the number of cells per unit area or the cross-sectional area of cells in the pericarp or kernel of most of the leaves and fruits on the ring-cut fruit branches among the different LFRs (p > 0.05) (except for 5L:1F, 1L:3F, and 2L:3F). We inferred that the carbohydrate synthesis and transport ability of the plants in the low LFR treatments were low, which led to the slowdown or even early cessation of fruit cell mitosis, consistent with the previously reported early control of carbohydrate competition of cell division [30]. Fruit size also depends largely on the distribution of carbohydrates after cell division. After cell division, the cell volume began to become larger, and the low content of carbohydrate on the branch of lower LFR would affect the process of cell enlargement, and ultimately lead to smaller cell volume. In the process of fruit growth and development, the continuous and effective supply of carbohydrates and nutrients to the fruit is an important basis for ensuring the size of the fruit, kernel enrichment and the formation of kernel nutrients. Our results showed that the number of cells per unit area in the green peel and kernel of fruit on the girdled branch with lower LFR was significantly higher, but the unit area was smaller, which may be related to the lower LFR treatment, which affected the morphological construction and physiological homeostasis of leaf and fruit stalk organs, and then adversely affected the synthesis and transportation of carbohydrates, which may ultimately affect the size of fruit and the formation of nutrients.
5. Conclusions
The LFR on walnut tree branches was artificially adjusted to impact the carbohydrate source–flow–sink supply relationship of girdled bearing branches. The anatomical structure of the walnut leaves (upper epidermis, lower epidermis, palisade tissue, and sponge tissue), fruit stalks (xylem, phloem, and cambium), and fruit (cell size and number) changed with changes in the LFR of the branches. The amount of photosynthetic carbohydrates in the leaves at a high LFR was sufficient for distribution to the fruits, and the excess carbohydrates were used for leaves and fruit stalk growth as well as the formation of their internal structures. A high LFR thus increased the size of the fruit cells as well as strengthened the development of the microanatomical structures of the leaves and fruit stalks. A low LFR or a high fruit load led to most of the carbohydrates being distributed to the fruit, which restricted the growth of the leaves and fruit stalks as well as the formation of their internal structures. The mitosis of fruit cells slowed or even stopped, cell growth was restricted, and leaf senescence accelerated at the lowest LFRs. In this study, only the effects of the anatomical structures of walnut branches under different LFRs on the source–sink–flow relationship were examined. However, the LFR may also affect branch growth, flower bud differentiation, and physiological and biochemical indices. As such, the effects of the LFR on these aspects require further study to obtain a more comprehensive theoretical understanding of the mechanisms that regulate walnut growth and development. These studies will help increase the efficiency and sustainable development of the walnut industry.
Author Contributions
Conceptualization, C.Z. and S.W.; methodology, Z.Z.; software, L.L.; validation, C.Z., S.W. and L.L.; formal analysis, L.L.; investigation, Z.Y.; data curation, L.L.; writing—original draft preparation, L.L.; writing—review and editing, S.W.; visualization, C.Z.; supervision, S.W.; project administration, C.Z.; funding acquisition, S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Regional Fund of the National Natural Science Foundation of China (grant number 32060361).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available from the corresponding author upon request.
Acknowledgments
We sincerely thank everyone involved in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Özcan, M.M.; Lemiasheuski, V. The Effect of Harvest Times on Mineral Contents of Almond and Walnut Kernels. Erwerbs-Obstbau 2020, 62, 455–458. [Google Scholar] [CrossRef]
- Quan, X.; Guō, Z.; Wang, S.; Li, H.; Gao, Z.; Wang, Y.; Cha, J. Analysis of morphological characteristics and fatty acid composition of walnut fruits from different varieties. Hunan For. Sci. 2020, 47, 7–14. [Google Scholar]
- Zhang, S.B. Association Analysis of the Impact of Leaf-to-Fruit Ratio on Carbohydrate Assimilation, Translocation, and Fruit Growth in Walnuts. Ph.D. Thesis, Xinjiang Agricultural University, Urumqi, China, 2023. [Google Scholar]
- Wang, X.Q.; Huang, W.D. Effects of weak light on the ultrastructure of phloem tissue in leaves of three-year-old oil peach (Prunus persica). Acta Bot. Sin. 2003, 45, 688–697. [Google Scholar]
- Chen, J.W.; Zhang, S.L.; Zhang, L.C.; Zhao, Z.Z.; Xu, J.G. Fruit Photosynthesis and Assimilate Translocation and Partitioning: Their Characteristics and Role in Sugar Accumulation in Developing Citrus unshiu Fruit. J. Integr. Plant Biol. 2002, 44, 158–163. [Google Scholar]
- Shen, C.H.; Chen, R.F.; Liu, Z.; Xi, R. Characteristics of Photosynthetic Product Transportation and Allocation During Fruit Expansion Period of Gaozhou Camellia oleifera. J. Cent. South Univ. For. Technol. 2024, 44, 58–66. [Google Scholar]
- Ma, Y.L.; Guo, S.J.; Liao, Y.N.; Wang, F. Effects of Different Canopy Layer Burr Loads on Photosynthetic Characteristics and Fruit Quality in Chestnut. Sci. Silvae Sin. 2022, 58, 90–97. [Google Scholar]
- Wang, S.; Pan, C.; Zhang, C.; Zhao, S. Key enzymes of sucrose metabolism play an important role in source-sink regulation of walnut fruit growth and development. N. Z. J. Crop Hortic. Sci. 2023, 51, 400–419. [Google Scholar] [CrossRef]
- Zhang, S.B.; Chen, H.; Pan, C.D. Effects of the Source-Sink Relationship on Walnut Nut Quality at the Scale of the Fruit-Bearing Branchs. Forest 2022, 13, 1034. [Google Scholar] [CrossRef]
- He, C.C.; Zhare, M.; Abudureyimu, A.; Yili, K. Effects of Different Load Levels on Photosynthetic Efficiency and Chloroplast Ultrastructure of Korla Fragrant Pear. J. Xinjiang Agric. Univ. 2012, 35, 368–372. [Google Scholar]
- Wu, T.; Tao, S.T.; Zhang, H.P.; Song, Y.; Yao, G.; Zhang, S. Effects of fruit thinning on sugar accumulastion in pear fruits and photosynthetic characteristics of leaves. Acta Hortic. Sin. 2011, 38, 2041–2048. [Google Scholar]
- Wu, H.B. Photosynthetic Physiological Characteristics and Photosynthate Allocation during the Development of Korean Pine Cones. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2022. [Google Scholar]
- Jackson, D.; Coombe, B. The growth of apricot fruit. I. Morphological changes during development and the effects of various tree factors. Aust. J. Agric. Res. 1966, 17, 465. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Peng, Y.B.; Pelleschi-Travier, S.; Fan, Y.; Lu, Y.F.; Lu, Y.M.; Gao, X.P.; Shen, Y.Y.; Delrot, S.; Zhang, D.P. Evidence for Apoplasmic Phloem Unloading in Developing Apple Fruit. Plant Physiol. 2004, 135, 574–586. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.; Peng, J.; Liu, Y.; Liang, K.; Li, C.; Mao, X.; Pan, J. Study on the “Source-Sink-Flow” Characteristics and Super High-Yield Potential of the Extra-Large Panicle Rice Germplasm DS23 in South China. Guangdong Agric. Sci. 2023, 50, 150–159. [Google Scholar]
- Zhang, Y.; Ren, F.X.; Jing, J.J.; Du, H.; Sun, H.C.; Liu, L.T.; Bai, Z.Y.; Li, C.D. Effects of leaf and branch removal on the anatomical structure of cotton leaves, petioles, boll stalks, and yield. Cotton Sci. 2016, 28, 268–275. [Google Scholar]
- Wang, Q.J.Y.; Li, J.; Li, T.; Xing, Y.; Qing, L.; Fang, K. Anatomical Structure and Cell Wall Components Changes in Chestnut ‘Yanshan Hongli’ Female Flowers at Developmental Stages. Bull. Bot. Res. 2024, 44, 670–680. [Google Scholar]
- Xu, J.W.; Tang, J.M.; Jiang, H.L.; Zou, R.; Wei, X. A comparative study on leaf anatomy and photosynthetic characteristics of different growth stages of Horsfieldia hainanensis. PeerJ 2024, 12, e18640. [Google Scholar] [CrossRef]
- Li, K.T.; Syvertsen, J.P. Morphological Responses Induced by Walnut Leaf Blight Disease. Nonwood For. Res. 2024, 42, 188–196. [Google Scholar]
- Zhang, S.B.; Zhang, R.; Xu, C.Z.; Guo, L.; Gao, S.; Guo, L.; Zhang, J. Effects of shading on physiological characteristics of Juglans regia ‘Wen 185’ leaves. North. Hortic. 2017, 18, 28–34. [Google Scholar]
- Zhang, S.; Chongzhi, X.U.; Zhang, R.; Zhou, W.; Gao, S.; Guo, L. Effects of shading on photosynthetic characteristics of Juglans regia ‘Wen 185’. China Fruits 2017, 3, 22–27. [Google Scholar]
- Fang, J.B.; Zheng, F.; Chen, J.Y.; Tian, L.; Zhang, W. The Effect of Source-Sink Relationship Changes on Photosynthetic Intensity in Kiwifruit Leaves. In Proceedings of the 9th Annual Academic Conference of the Chinese Society for Horticultural Science, Chongqing, China, 1 October 2001. [Google Scholar]
- Sun, H.Q.; Wang, N.N.; Liu, W.J.; Ma, C.H. Analysis of pear fruit stalk characteristics and their correlation with main economic traits of fruits. Acta Bot. Boreali-Occident. Sin. 2019, 39, 1416–1424. [Google Scholar]
- Cao, Y.J.; Maimaiti, A.; Tayifu, X.; Shi, Z.; Abulitipu, Y. Comparative Analysis of Different Types of Fruit Stalks in Korla Fragrant Pear. Xinjiang Agric. Sci. 2023, 60, 1442–1450. [Google Scholar]
- Lalonde, S.; Tegeder, M.; Throne-Holst, M.; Frommer, W.B.; Patrick, J.W. Phloem loading and unloading of sugars and amino acids. Plant Cell Environ. 2010, 26, 37–56. [Google Scholar] [CrossRef]
- Cai, X.L.; Pan, J.C.; Zhou, Y.M.; Liu, H.H.; Huang, G.X. Research on the Mathematical Model of Fruit Growth and Development of Taiwan Pineapple Sugar Apple. J. Anhui Agric. Sci. 2016, 44, 44–45. [Google Scholar]
- Garcia-Luis, A.; Oliveira, M.E.M.; Bordón, Y.; Siqueira, D.L.; Tominaga, S.; Guardiola, J.L. Dry Matter Accumulation in Citrus Fruit is Not Limited by Transport Capacity of the Pedicel. Ann. Bot. 2002, 90, 755–764. [Google Scholar] [CrossRef]
- Zhao, P.; Xia, Y.; He, J.Y.; Wu, J.; Ma, J.Q.; Huang, C.S. Effects of leaf-fruit ratio on 13C assimilate partitioning and fruit quality in mulberry during veraison. Acta Sericologica Sin. 2023, 49, 1–7. [Google Scholar]
- Cannell, M.G.R.; Dewar, R.C. Carbon Allocation in Trees: A Review of Concepts for Modelling. Trees 1994, 25, 59–104. [Google Scholar]
- Smith, A.M.; Stitt, M. Coordination of carbon supply and plant growth. Plant Cell Environ. 2007, 30, 1126–1149. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).