Efficacy of Oxygen Fluid (blue®m) on Human Gingival Fibroblast Viability, Proliferation and Inflammatory Cytokine Expression: An In Vitro Study
Abstract
1. Introduction
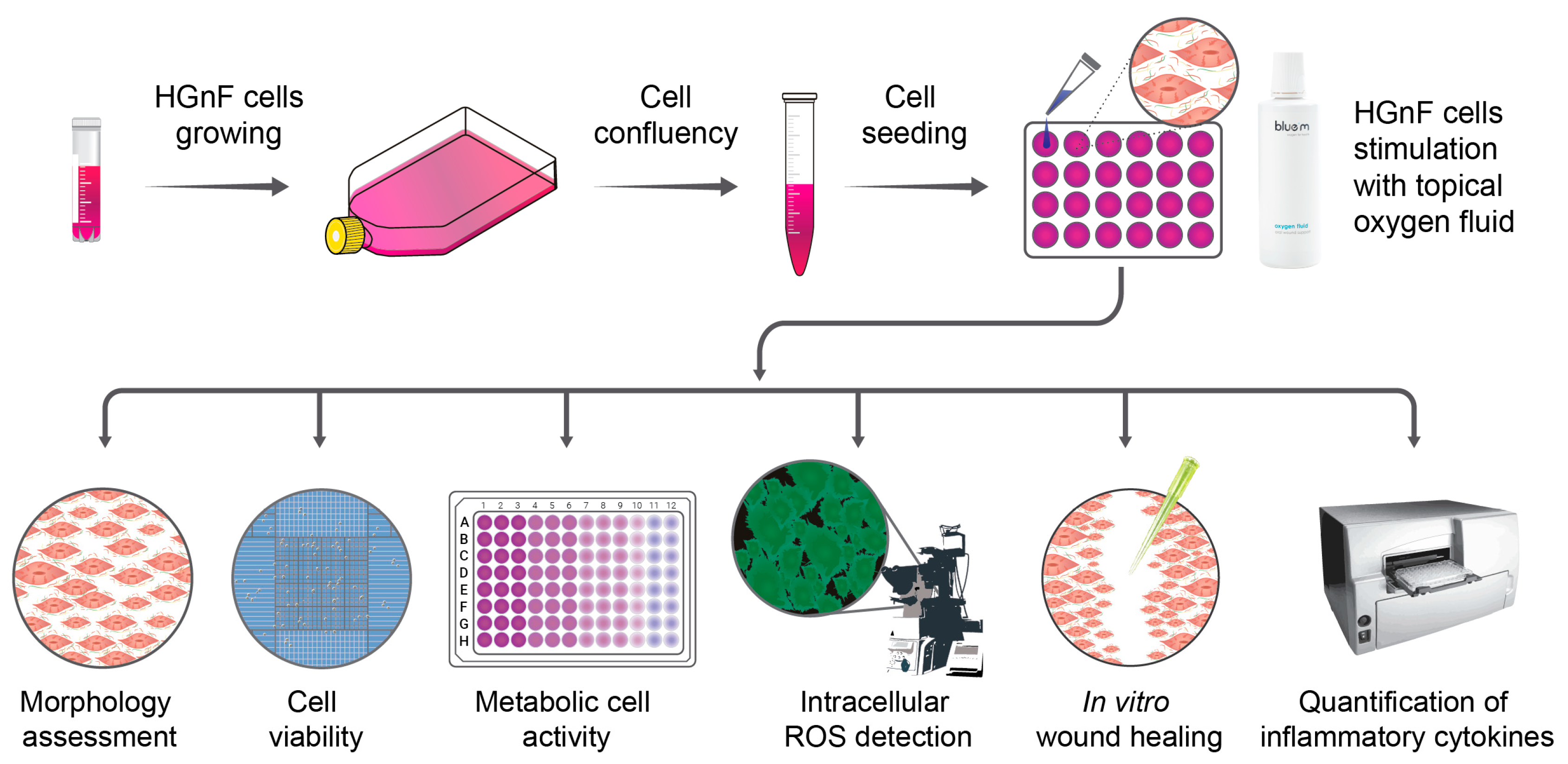
2. Materials and Methods
2.1. Cell Culture
2.2. Stimulation of Gingival Fibroblasts with Topical blue®m Oxygen Fluid Therapy
2.3. Cell Morphology
2.4. Immunofluorescence Staining
2.5. Cell Viability (AlamarBlue Assay)
2.6. Cell Proliferation (Trypan Blue Assay)
2.7. Wound-Healing Assay
2.8. Reactive Oxygen Species Measurement
2.9. Detection of Pro-Inflammatory Cytokine Expression Using ELISA (Enzyme-Linked Immunosorbent Assay)
2.10. Statistical Analysis
3. Results
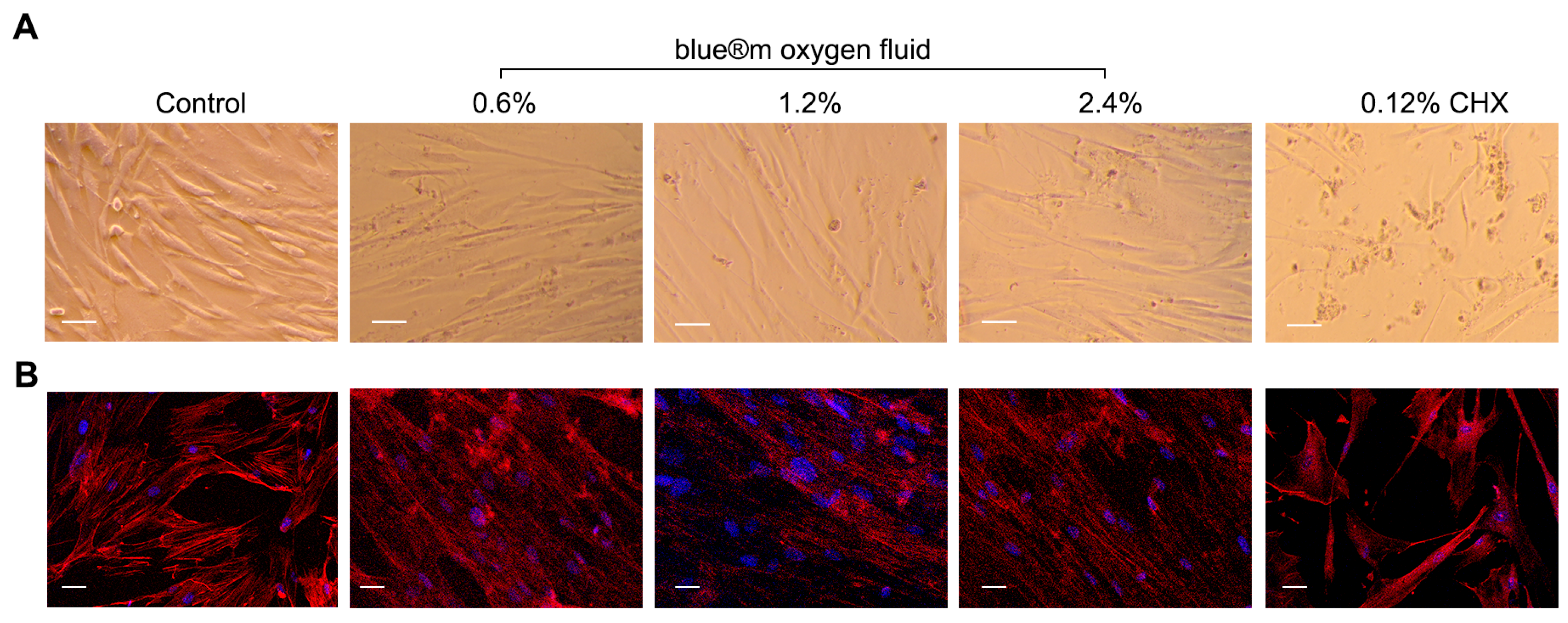
3.1. Cell Morphology and Immunofluorescence Staining
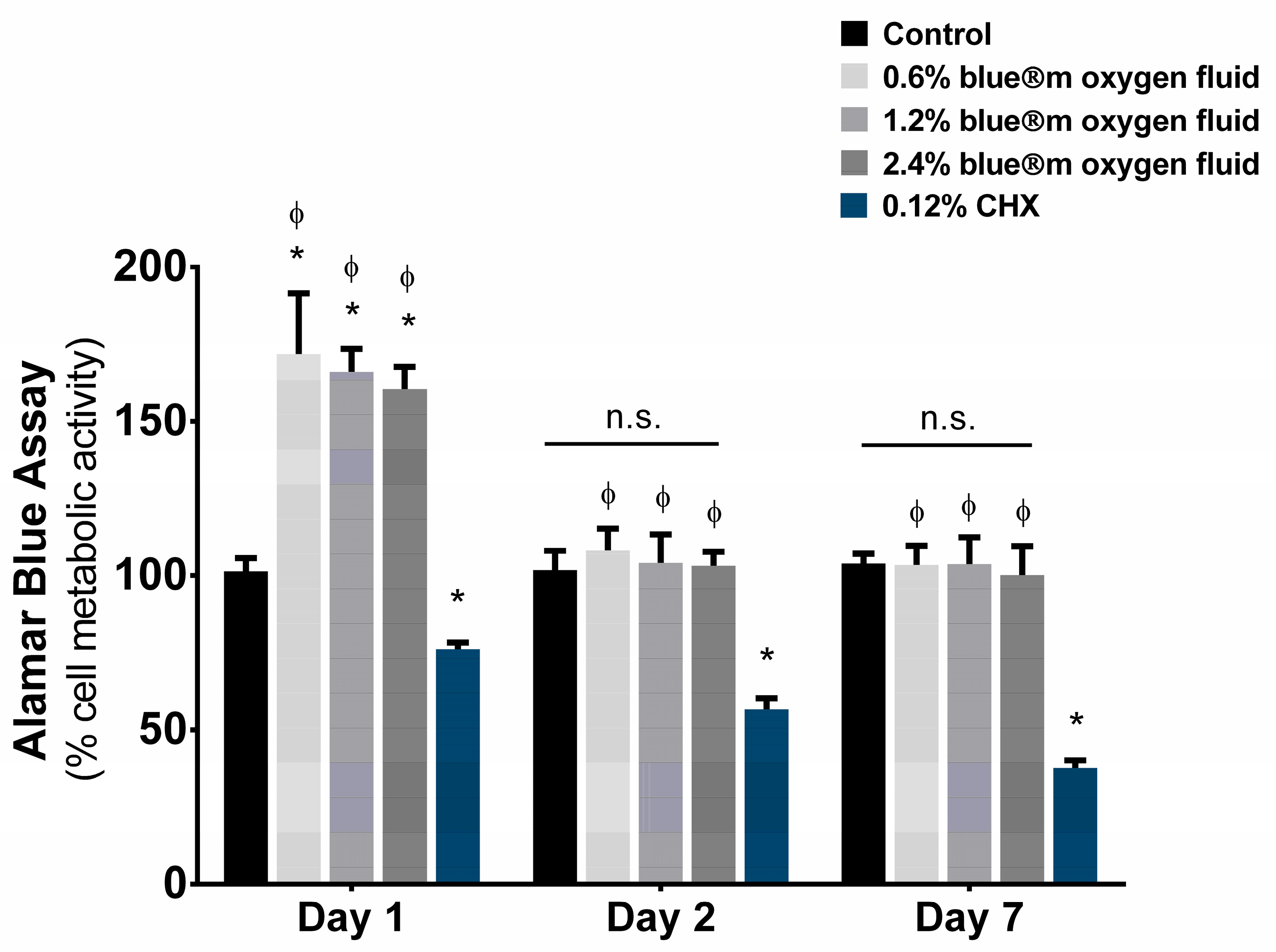
3.2. Cell Viability
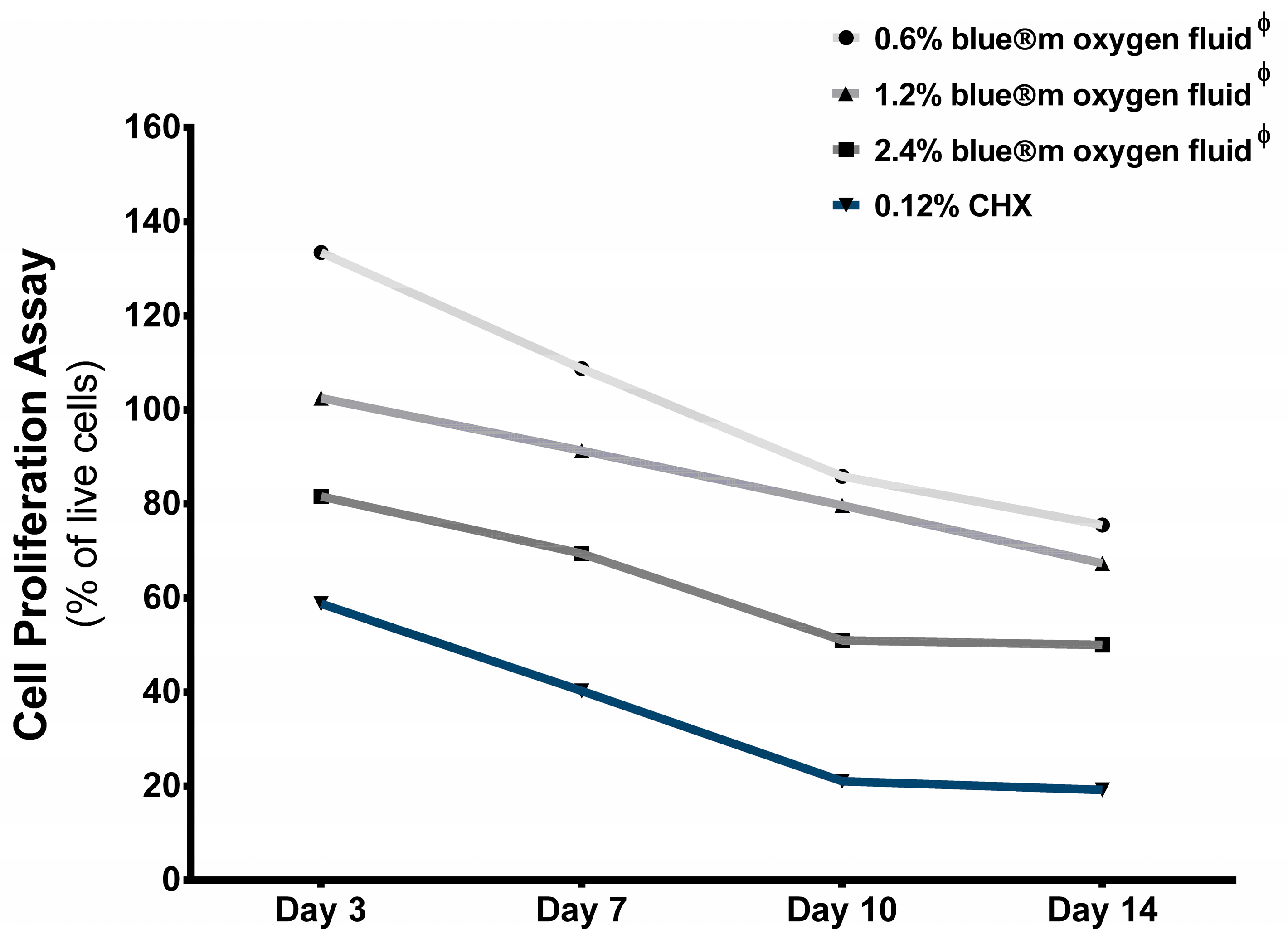
3.3. Cell Proliferation
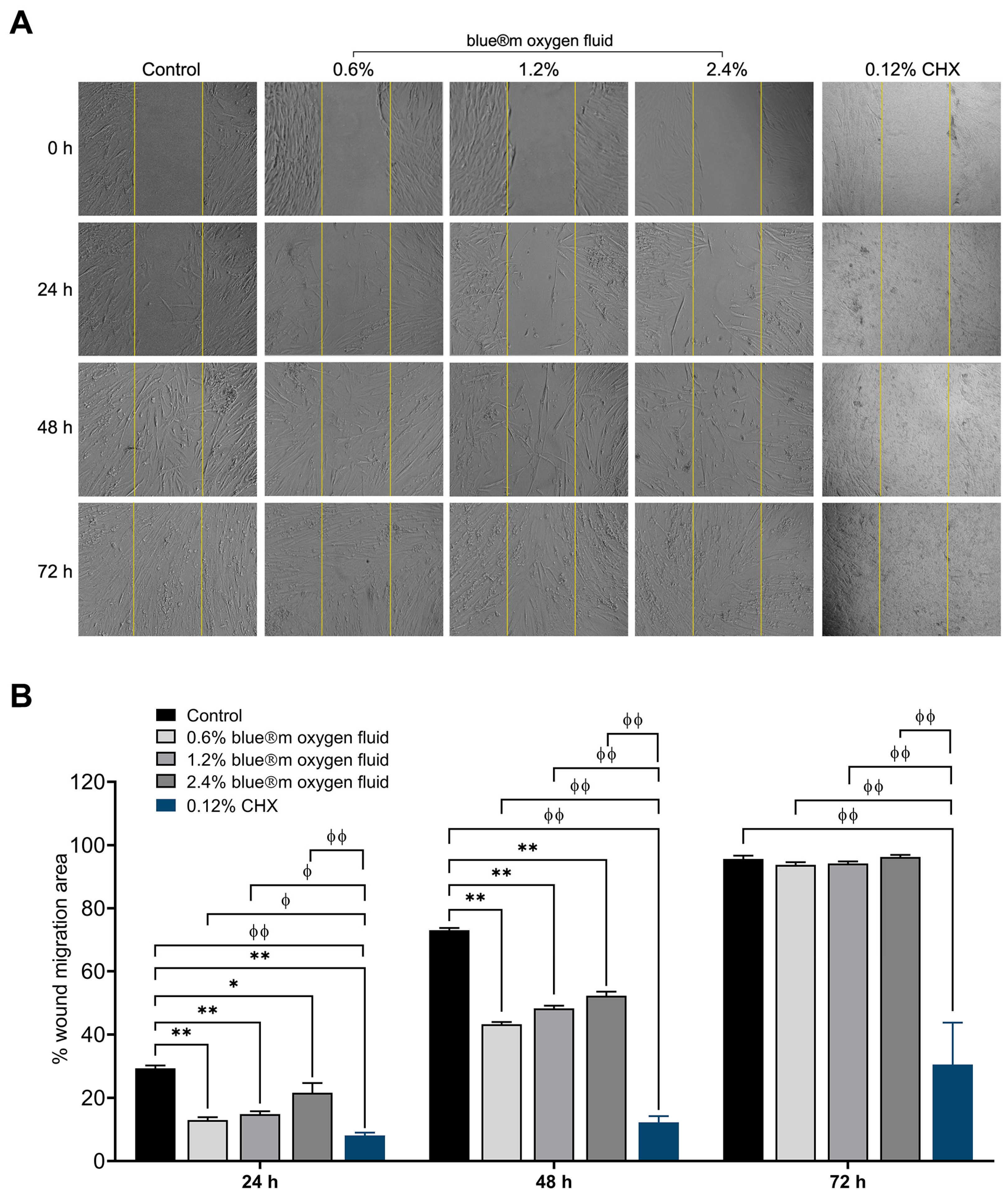
3.4. Wound Healing
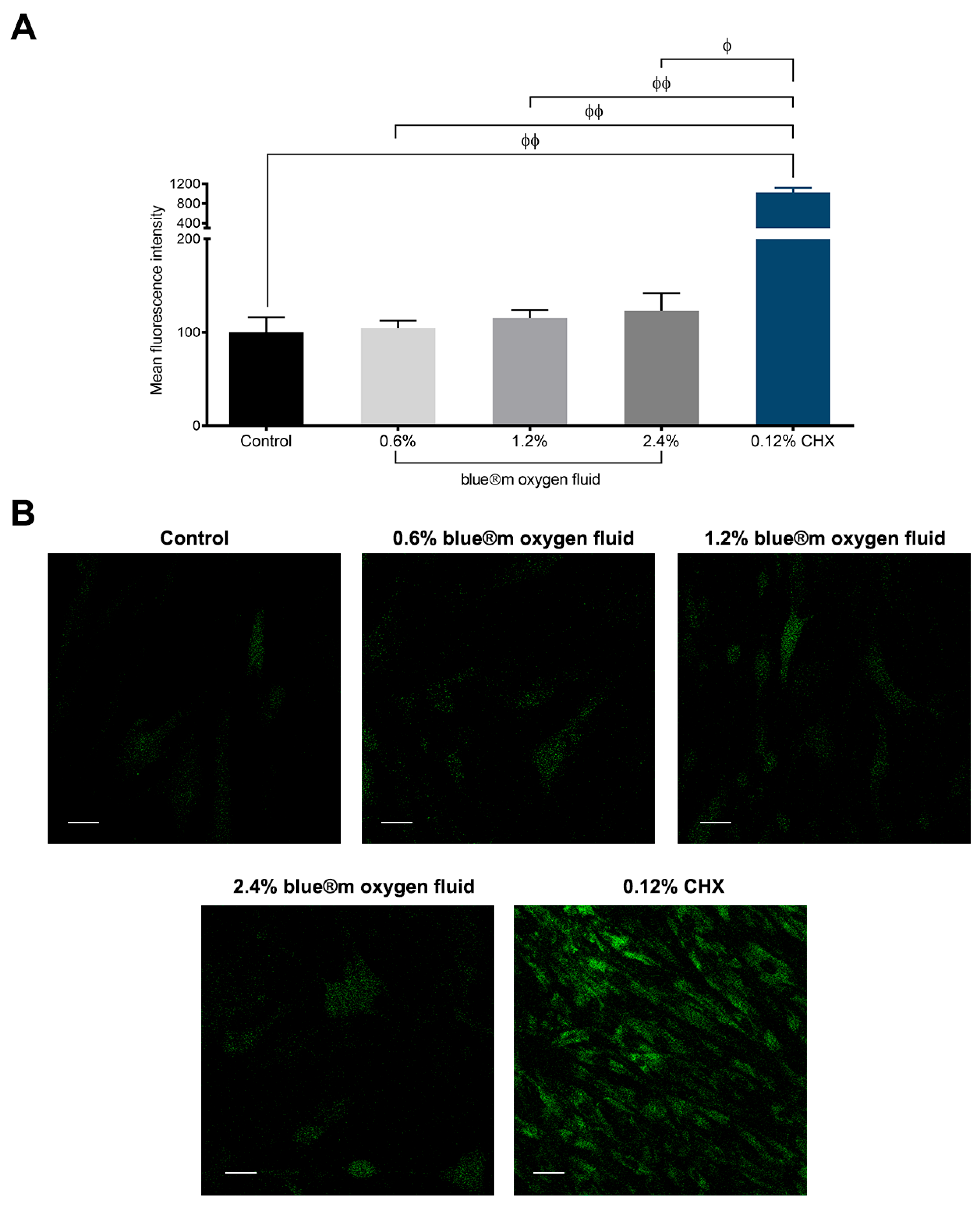
3.5. Results of Reactive Oxygen Species
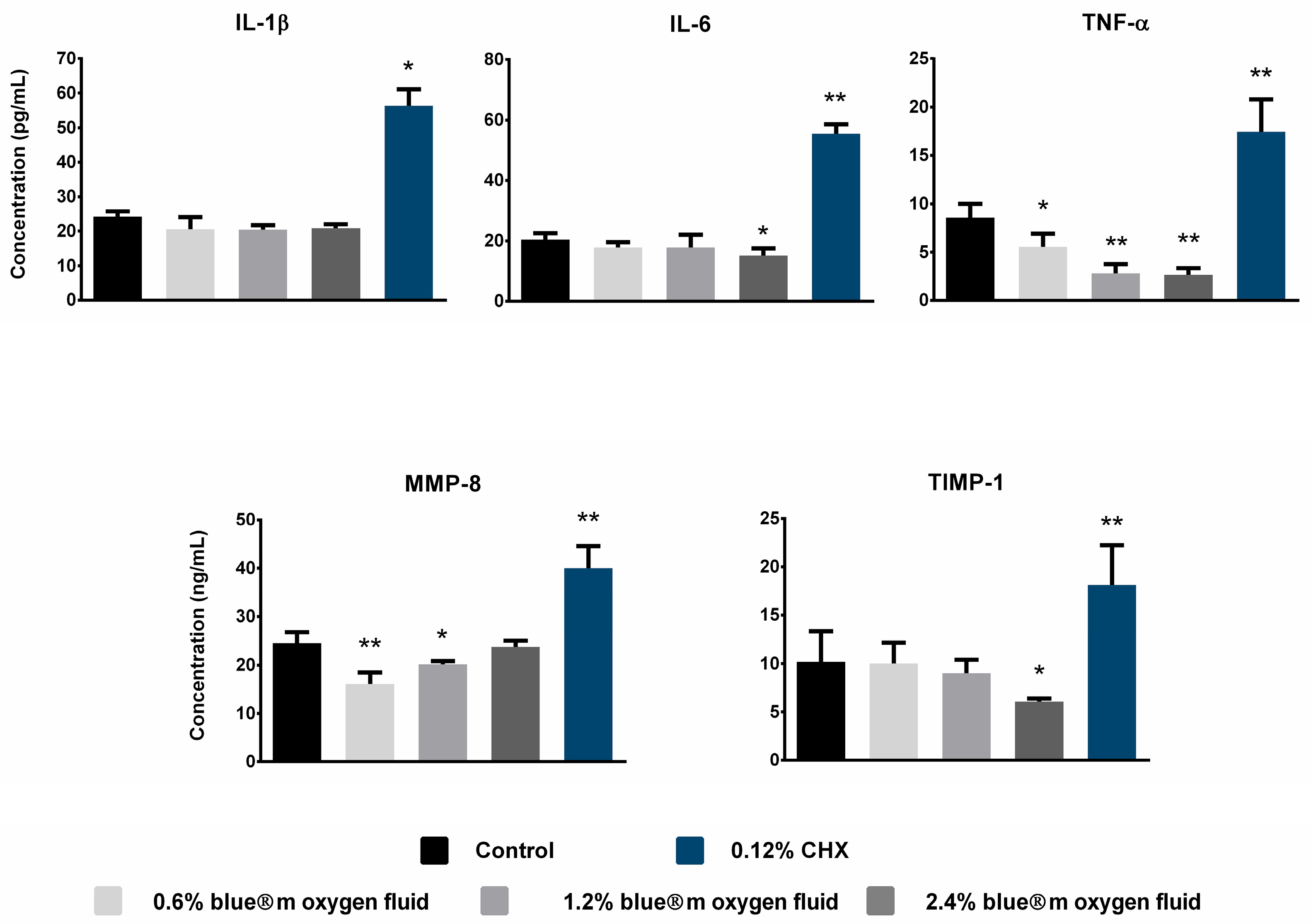
3.6. Detection of Pro-Inflammatory Cytokine Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HGnF | Human gingival fibroblast |
| ROS | Reactive oxygen species |
| IL | Interleukin |
| TNF | Tumor necrosis factor |
| MMP | Matrix metalloproteinases |
| TIMP | Tissue inhibitors of metalloproteinases |
| DMEM | Dulbecco’s modified eagle’s medium |
| PBS | Phosphate-buffered saline |
| DAPI | 4′,6-diamidino-2-phenylindole dihydrochloride |
| CLSM | Confocal laser scanning microscope |
| EDTA | Ethylenediaminetetraacetic acid |
| DCFH-DA | 2′,7′-Dichlorodihydrofluorescein diacetate |
| MFI | Mean fluorescence intensity |
| ELISA | Enzyme-linked immunosorbent assay |
| ANOVA | Analysis of variance |
| CHX | Chlorhexidine |
References
- Ngeow, W.C.; Tan, C.C.; Goh, Y.C.; Deliberador, T.M.; Cheah, C.W. A Narrative Review on Means to Promote Oxygenation and Angiogenesis in Oral Wound Healing. Bioengineering 2022, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Pippi, R. Post-Surgical Clinical Monitoring of Soft Tissue Wound Healing in Periodontal and Implant Surgery. Int. J. Med. Sci. 2017, 14, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Rizkalla, A.S.; Hamilton, D.W. FGF and TGF-beta growth factor isoform modulation of human gingival and periodontal ligament fibroblast wound healing phenotype. Matrix Biol. 2025, 136, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Ahangar, P.; Mills, S.J.; Smith, L.E.; Gronthos, S.; Cowin, A.J. Human gingival fibroblast secretome accelerates wound healing through anti-inflammatory and pro-angiogenic mechanisms. NPJ Regen. Med. 2020, 5, 24. [Google Scholar] [CrossRef]
- Liu, J.X.; Werner, J.; Kirsch, T.; Zuckerman, J.D.; Virk, M.S. Cytotoxicity evaluation of chlorhexidine gluconate on human fibroblasts, myoblasts, and osteoblasts. J. Bone Jt. Infect. 2018, 3, 165–172. [Google Scholar] [CrossRef]
- Fiorillo, L.; D’Amico, C.; Mehta, V.; Cicciù, M.; Cervino, G. Chlorhexidine cytotoxicity on oral Behaviors: Last 20 Years systematic review. Oral Oncol. Rep. 2024, 9, 100245. [Google Scholar] [CrossRef]
- Sindhusha, V.B.; Rajasekar, A. Efficacy of Oxygen-Enriched Mouthwash as a Pre-procedural Mouth Rinse Against Oral Microbes Produced During Ultrasonic Scaling. Cureus 2023, 15, e49164. [Google Scholar] [CrossRef]
- Basudan, A.M.; Abas, I.; Shaheen, M.Y.; Alghamdi, H.S. Effectiveness of Topical Oxygen Therapy in Gingivitis and Periodontitis: Clinical Case Reports and Review of the Literature. J. Clin. Med. 2024, 13, 1451. [Google Scholar] [CrossRef]
- Shaheen, M.Y.; Abas, I.; Basudan, A.M.; Alghamdi, H.S. Local Oxygen-Based Therapy (blue®m) for Treatment of Peri-Implant Disease: Clinical Case Presentation and Review of Literature about Conventional Local Adjunct Therapies. Medicina 2024, 60, 447. [Google Scholar] [CrossRef]
- Leano, S.M.; De Souza, W.; De Vecchi, R.; Lopes, A.; Deliberador, T.; Granjeiro, J.M. A multimodal in vitro approach to assess the safety of oral care products using 2D and 3D cellular models. Front. Toxicol. 2024, 6, 1474583. [Google Scholar] [CrossRef]
- Garima; Agarwal, T.; Costantini, M.; Pal, S.; Kumar, A. Oxygenation therapies for improved wound healing: Current trends and technologies. J. Mater. Chem. B 2022, 10, 7905–7923. [Google Scholar] [CrossRef] [PubMed]
- Njokweni, M. Adjunctive topical oxygen therapy in the management of complex diabetes-related wounds: A South African case study series. Foot 2023, 57, 101961. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G. Topical Wound Oxygen Therapy in the Treatment of Chronic Diabetic Foot Ulcers. Medicina 2021, 57, 917. [Google Scholar] [CrossRef] [PubMed]
- Roi, C.; Gaje, P.N.; Ceausu, R.A.; Roi, A.; Rusu, L.C.; Boia, E.R.; Boia, S.; Luca, R.E.; Rivis, M. Heterogeneity of Blood Vessels and Assessment of Microvessel Density-MVD in Gingivitis. J. Clin. Med. 2022, 11, 2758. [Google Scholar] [CrossRef]
- Cunha, G.; D’Angieri Saugo, G.; Gabrielli, M.A.C.; Barbeiro, C.O.; de Almeida, L.Y.; Bufalino, A.; Pereira-Filho, V.A. Cytotoxicity evaluation of Chlorhexidine and Blue(R)M applied to a human gingival fibroblast (HGF-1) and keratinocytes (NOK-SI): In vitro study. J. Stomatol. Oral Maxillofac. Surg. 2024, 125, 101923. [Google Scholar] [CrossRef]
- Joorabloo, A.; Liu, T. Recent advances in reactive oxygen species scavenging nanomaterials for wound healing. Exploration 2024, 4, 20230066. [Google Scholar] [CrossRef]
- Grootveld, M.; Lynch, E.; Page, G.; Chan, W.; Percival, B.; Anagnostaki, E.; Mylona, V.; Bordin-Aykroyd, S.; Grootveld, K.L. Potential Advantages of Peroxoborates and Their Ester Adducts Over Hydrogen Peroxide as Therapeutic Agents in Oral Healthcare Products: Chemical/Biochemical Reactivity Considerations In Vitro, Ex Vivo and In Vivo. Dent. J. 2020, 8, 89. [Google Scholar] [CrossRef]
- Settem, R.P.; Sharma, A. Oral bacterium contributes to periodontal inflammation by forming advanced glycation end products. Infect. Immun. 2025, 93, e0056024. [Google Scholar] [CrossRef]
- Naruishi, K. Biological Roles of Fibroblasts in Periodontal Diseases. Cells 2022, 11, 3345. [Google Scholar] [CrossRef]
- Sadek, K.M.; El Moshy, S.; Radwan, I.A.; Rady, D.; Abbass, M.M.S.; El-Rashidy, A.A.; Dorfer, C.E.; Fawzy El-Sayed, K.M. Molecular Basis beyond Interrelated Bone Resorption/Regeneration in Periodontal Diseases: A Concise Review. Int. J. Mol. Sci. 2023, 24, 4599. [Google Scholar] [CrossRef]
- Radzki, D.; Negri, A.; Kusiak, A.; Obuchowski, M. Matrix Metalloproteinases in the Periodontium-Vital in Tissue Turnover and Unfortunate in Periodontitis. Int. J. Mol. Sci. 2024, 25, 2763. [Google Scholar] [CrossRef] [PubMed]
- Fadl, A.; Leask, A. Hiding in Plain Sight: Human Gingival Fibroblasts as an Essential, Yet Overlooked, Tool in Regenerative Medicine. Cells 2023, 12, 2021. [Google Scholar] [CrossRef] [PubMed]
- Haekkinen, L.; Uitto, V.-J.; Larjava, H. Cell biology of gingival wound healing. Periodontol. 2000 2000, 24, 127–152. [Google Scholar] [CrossRef]
- AlMobarak, S.; AlMadi, E.; Almohaimede, A.; Badran, M.; Lambarte, R.A. The Effect of Commiphora molmol Nanoparticles as an Endodontic Irrigant on the Morphology, Viability, Migration, and Proliferation of Human Bone Marrow Mesenchymal Stem Cells: An In Vitro Study. Int. J. Mol. Sci. 2025, 26, 1412. [Google Scholar] [CrossRef]
- Staehlke, S.; Brief, J.; Senz, V.; Eickner, T.; Nebe, J.B. Optimized Gingiva Cell Behavior on Dental Zirconia as a Result of Atmospheric Argon Plasma Activation. Materials 2023, 16, 4203. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Bhavikatti, S.K.; Karobari, M.I.; Zainuddin, S.L.A.; Marya, A.; Nadaf, S.J.; Sawant, V.J.; Patil, S.B.; Venugopal, A.; Messina, P.; Scardina, G.A. Investigating the Antioxidant and Cytocompatibility of Mimusops elengi Linn Extract over Human Gingival Fibroblast Cells. Int. J. Environ. Res. Public Health 2021, 18, 7162. [Google Scholar] [CrossRef]
- Aldoss, A.; Lambarte, R.; Alsalleeh, F. High-Glucose Media Reduced the Viability and Induced Differential Pro-Inflammatory Cytokines in Human Periodontal Ligament Fibroblasts. Biomolecules 2023, 13, 690. [Google Scholar] [CrossRef]
- Rasband, W.S. US National Institutes of Health: Bethesda, MD, USA. 2011. Available online: http://imagej.nih.gov/ij/ (accessed on 23 March 2025).
- Saramet, V.; Stan, M.S.; Ripszky Totan, A.; Tancu, A.M.C.; Voicu-Balasea, B.; Enasescu, D.S.; Rus-Hrincu, F.; Imre, M. Analysis of Gingival Fibroblasts Behaviour in the Presence of 3D-Printed versus Milled Methacrylate-Based Dental Resins-Do We Have a Winner? J. Funct. Biomater. 2024, 15, 147. [Google Scholar] [CrossRef]
- AlJunaydil, N.A.; Lambarte, R.N.A.; Sumague, T.S.; Alghamdi, O.G.; Niazy, A.A. Lovastatin and Resveratrol Synergistically Improve Wound Healing and Inhibit Bacterial Growth. Int. J. Mol. Sci. 2025, 26, 851. [Google Scholar] [CrossRef]
- Basso, F.G.; Pansani, T.N.; Turrioni, A.P.; Soares, D.G.; de Souza Costa, C.A.; Hebling, J. Tumor Necrosis Factor-alpha and Interleukin (IL)-1beta, IL-6, and IL-8 Impair In Vitro Migration and Induce Apoptosis of Gingival Fibroblasts and Epithelial Cells, Delaying Wound Healing. J. Periodontol. 2016, 87, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Yu, X.Z.; Zhang, S.Q.; Zhang, Y.X.; Chen, X.L.; Long, Z.J.; Hu, H.Z.; Xie, D.H.; Zhang, W.H.; Chen, J.X.; et al. Hydrogel with ROS scavenging effect encapsulates BR@Zn-BTB nanoparticles for accelerating diabetic mice wound healing via multimodal therapy. iScience 2023, 26, 106775. [Google Scholar] [CrossRef] [PubMed]
- Mattei, B.M.; Imanishi, S.A.W.; de Oliveira Ramos, G.; de Campos, P.S.; Weiss, S.G.; Deliberador, T.M. Mouthwash with Active Oxygen (blue®m) Reduces Postoperative Inflammation and Pain. Case. Rep. Dent. 2021, 2021, 5535807. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Meher, B.; Mishra, L.; Panda, S.; Sokolowski, K.; Lapinska, B. Evaluation of Blue® m Mouthwash vs. Chlorhexidine Mouthwash in Patients with Gingivitis—A Pilot Study. Appl. Sci. 2024, 14, 11862. [Google Scholar] [CrossRef]
- Furukawa, M.; JR, K.K.; Yamada, M.; Senda, A.; Manabe, A.; Miyazaki, A. Cytotoxic Effects of Hydrogen Peroxide on Human Gingival Fibroblasts In Vitro. Oper. Dent. 2015, 40, 430–439. [Google Scholar] [CrossRef]
- Berner, T.; Nakahara, K.; Kobayashi, E.; Tanaka, A.; Taniguchi, Y.; Iizuka, T.; Sawada, K. Investigating the effect of antiseptic solution on the release of interleukin-6 and transforming growth factor beta 1 from human gingival fibroblasts using wound healing assays. J. Oral Sci. 2020, 62, 293–297. [Google Scholar] [CrossRef]
- Pilloni, A.; Ceccarelli, S.; Bosco, D.; Gerini, G.; Marchese, C.; Marini, L.; Rojas, M.A. Effect of Chlorhexidine Digluconate in Early Wound Healing of Human Gingival Tissues. A Histological, Immunohistochemical and Biomolecular Analysis. Antibiotics 2021, 10, 1192. [Google Scholar] [CrossRef]
- Sterczala, B.; Grzech-Lesniak, K.; Michel, O.; Trzeciakowski, W.; Dominiak, M.; Jurczyszyn, K. Assessment of Human Gingival Fibroblast Proliferation after Laser Stimulation In Vitro Using Different Laser Types and Wavelengths (1064, 980, 635, 450, and 405 nm)-Preliminary Report. J. Pers. Med. 2021, 11, 98. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Zheng, M.; Luan, Q.X. Mitochondrial reactive oxygen species mediate the lipopolysaccharide-induced pro-inflammatory response in human gingival fibroblasts. Exp. Cell Res. 2016, 347, 212–221. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Z. ROS-scavenging materials for skin wound healing: Advancements and applications. Front. Bioeng. Biotechnol. 2023, 11, 1304835. [Google Scholar] [CrossRef]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Stavroullakis, A.T.; Carrilho, M.R.; Levesque, C.M.; Prakki, A. Profiling cytokine levels in chlorhexidine and EGCG-treated odontoblast-like cells. Dent. Mater. 2018, 34, e107–e114. [Google Scholar] [CrossRef] [PubMed]
- Chatzigiannidou, I.; Teughels, W.; Van de Wiele, T.; Boon, N. Oral biofilms exposure to chlorhexidine results in altered microbial composition and metabolic profile. NPJ Biofilms Microbiomes 2020, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.M.; Pansani, T.N.; de Souza Costa, C.A.; Basso, F.G. Regulation of interleukin-6 and matrix metalloproteinases syntheses by bioflavonoids and photobiomodulation in human gingival fibroblasts. Lasers Med. Sci. 2022, 37, 2973–2987. [Google Scholar] [CrossRef]
- Smith, P.C.; Martinez, C.; Martinez, J.; McCulloch, C.A. Role of Fibroblast Populations in Periodontal Wound Healing and Tissue Remodeling. Front. Physiol. 2019, 10, 270. [Google Scholar] [CrossRef]
| In Vitro Assays | Experimental Groups | ||||
|---|---|---|---|---|---|
| Control | blue®m Oxygen Fluid | 0.12% CHX | |||
| 0.6% | 1.2% | 2.4% | |||
| Cell viability | |||||
| Day 1 | 100.00 ± 4.27 | 171.82 ± 19.71 | 166.08 ± 7.41 | 160.54 ± 7.15 | 76.14 ± 2.11 |
| Day 2 | 100.00 ± 6.19 | 108.07 ± 7.23 | 104.16 ± 9.27 | 102.18 ± 4.59 | 56.66 ± 3.58 |
| Day 7 | 100.00 ± 3.21 | 102.48 ± 6.23 | 103.72 ± 8.71 | 100.14 ± 9.44 | 37.63 ± 2.52 |
| Cell proliferation | |||||
| Day 3 | - | 133.47 ± 10.49 | 102.54 ± 15.90 | 80.86 ± 12.71 | 58.62 ± 3.63 |
| Day 7 | - | 108.75 ± 17.88 | 92.03 ± 2.84 | 75.43 ± 14.31 | 40.24 ± 2.11 |
| Day 10 | - | 86.74 ± 4.05 | 80.70 ± 5.46 | 57.01 ± 17.80 | 20.31 ± 5.84 |
| Day 14 | - | 75.53 ± 13.35 | 72.38 ± 14.87 | 50.03 ± 12.14 | 19.54 ± 2.25 |
| Wound healing | |||||
| Day 1 | 29.28 ± 2.28 | 12.97 ± 2.25 | 14.82 ± 2.22 | 21.58 ± 7.62 | 8.08 ± 0.92 |
| Day 2 | 73.02 ± 1.76 | 43.23 ± 1.78 | 48.26 ± 2.11 | 52.27 ± 3.23 | 12.24 ± 1.95 |
| Day 3 | 95.64 ± 2.58 | 93.74 ± 2.14 | 93.74 ± 2.14 | 96.24 ± 1.70 | 30.51 ± 13.26 |
| Intracellular ROS detection | 98.75 ± 13.86 | 104.83 ± 7.63 | 115.09 ± 8.79 | 122.87 ± 19.04 | 1027.01 ± 93.36 |
| Quantification of inflammatory cytokines | |||||
| IL-1β | 24.25 ± 1.52 | 19.87 ± 2.48 | 20.42 ± 1.33 | 20.87 ± 1.14 | 56.37 ± 4.75 |
| IL-6 | 20.42 ± 2.15 | 17.98 ± 2.52 | 17.77 ± 2.83 | 15.17 ± 2.35 | 55.48 ± 3.15 |
| TNF-α | 8.56 ± 1.46 | 5.55 ± 1.36 | 2.81 ± 0.82 | 2.67 ± 0.69 | 17.45 ± 3.34 |
| MMP-8 | 24.52 ± 2.26 | 16.01 ± 2.49 | 20.19 ± 0.68 | 23.78 ± 1.26 | 40.03 ± 4.54 |
| TIMP-1 | 10.18 ± 3.08 | 10.02 ± 1.41 | 9.03 ± 1.37 | 6.06 ± 0.33 | 18.13 ± 4.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambarte, R.N.A.; Basudan, A.M.; Shaheen, M.Y.; Sumague, T.S.; AlAhmari, F.M.; BinShwish, N.M.; Alzawawi, A.S.; Niazy, A.A.; Alfhili, M.A.; Alghamdi, H.S. Efficacy of Oxygen Fluid (blue®m) on Human Gingival Fibroblast Viability, Proliferation and Inflammatory Cytokine Expression: An In Vitro Study. Appl. Sci. 2025, 15, 7459. https://doi.org/10.3390/app15137459
Lambarte RNA, Basudan AM, Shaheen MY, Sumague TS, AlAhmari FM, BinShwish NM, Alzawawi AS, Niazy AA, Alfhili MA, Alghamdi HS. Efficacy of Oxygen Fluid (blue®m) on Human Gingival Fibroblast Viability, Proliferation and Inflammatory Cytokine Expression: An In Vitro Study. Applied Sciences. 2025; 15(13):7459. https://doi.org/10.3390/app15137459
Chicago/Turabian StyleLambarte, Rhodanne Nicole A., Amani M. Basudan, Marwa Y. Shaheen, Terrence S. Sumague, Fatemah M. AlAhmari, Najla M. BinShwish, Abeer S. Alzawawi, Abdurahman A. Niazy, Mohammad A. Alfhili, and Hamdan S. Alghamdi. 2025. "Efficacy of Oxygen Fluid (blue®m) on Human Gingival Fibroblast Viability, Proliferation and Inflammatory Cytokine Expression: An In Vitro Study" Applied Sciences 15, no. 13: 7459. https://doi.org/10.3390/app15137459
APA StyleLambarte, R. N. A., Basudan, A. M., Shaheen, M. Y., Sumague, T. S., AlAhmari, F. M., BinShwish, N. M., Alzawawi, A. S., Niazy, A. A., Alfhili, M. A., & Alghamdi, H. S. (2025). Efficacy of Oxygen Fluid (blue®m) on Human Gingival Fibroblast Viability, Proliferation and Inflammatory Cytokine Expression: An In Vitro Study. Applied Sciences, 15(13), 7459. https://doi.org/10.3390/app15137459









