Formation Kinetics and Morphology Characteristics of Natural Gas Hydrates in Sandstone Fractures
Abstract
1. Introduction
2. Methodology
2.1. Experimental Apparatuses
2.2. Experimental Material
2.3. Experimental Procedures
3. Results and Discussion
3.1. Hydrate Formation
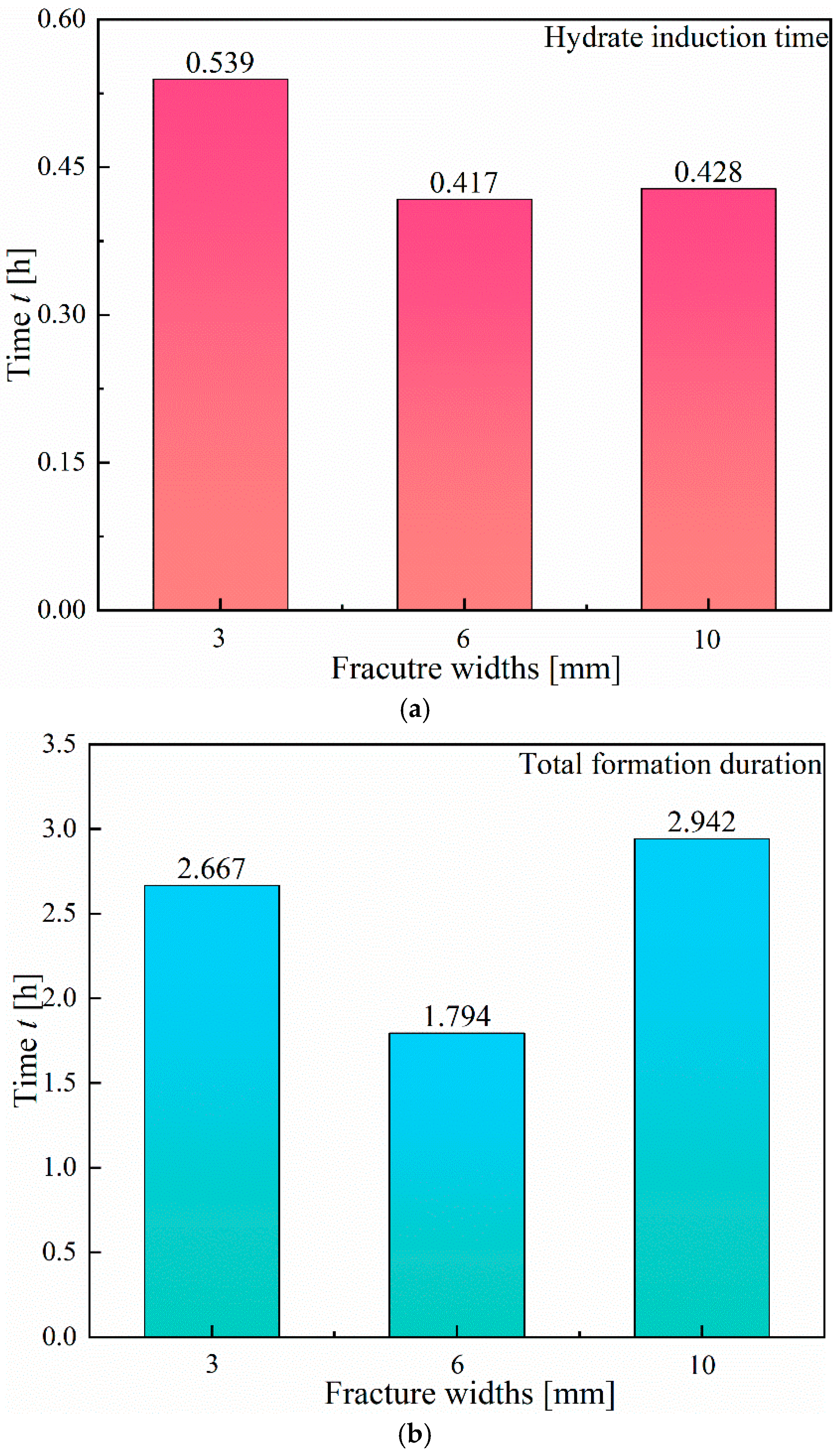
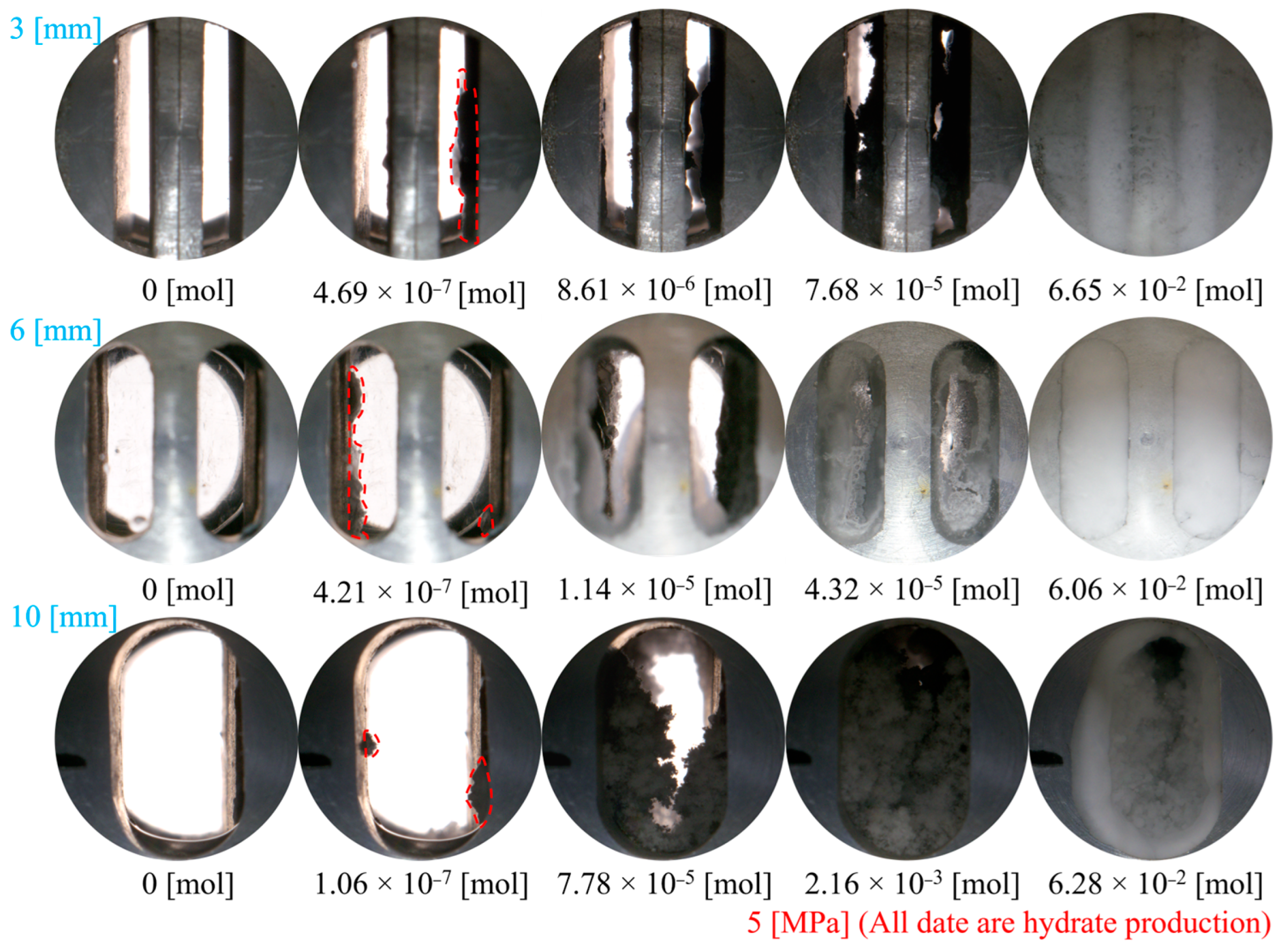
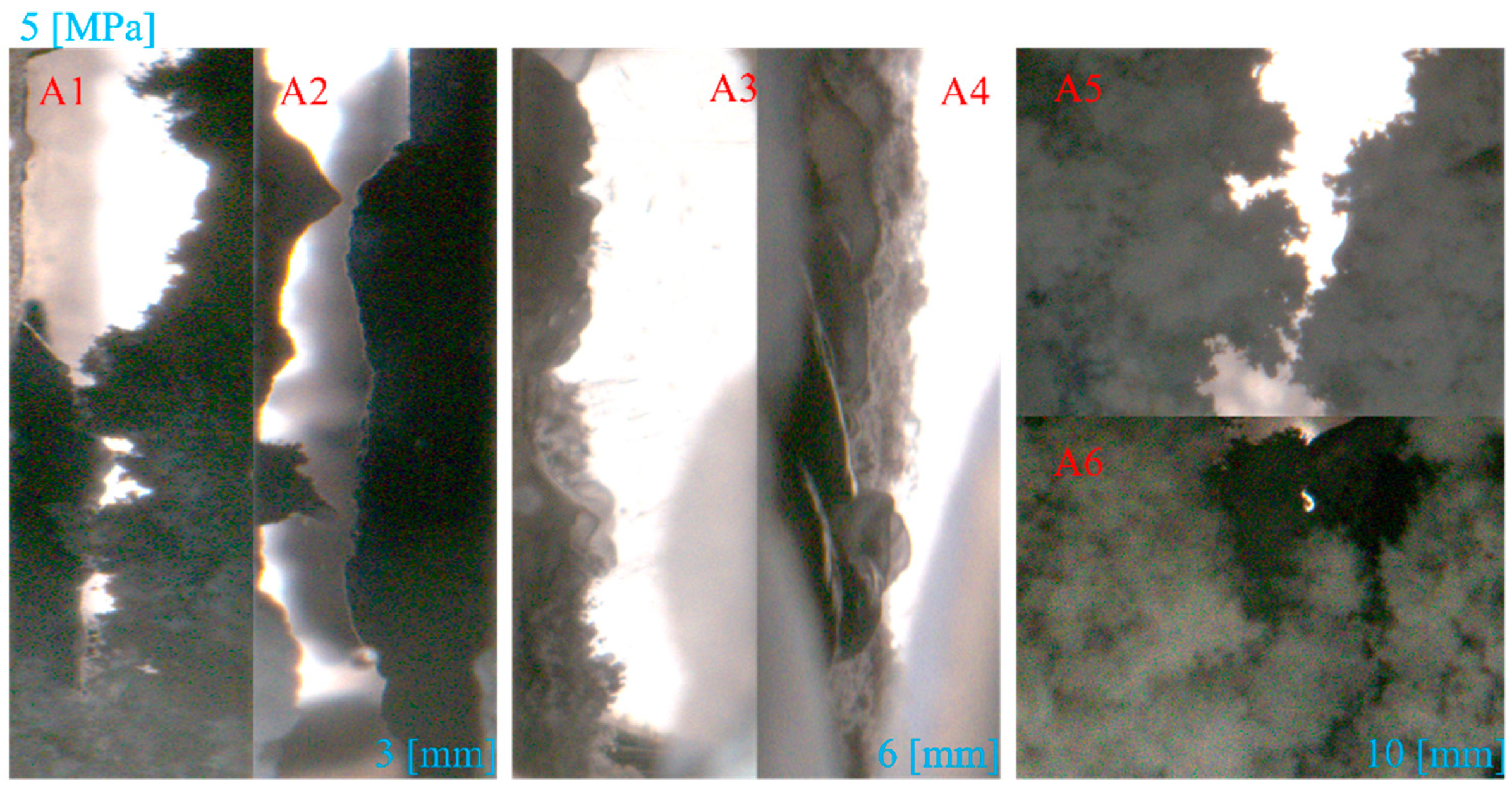
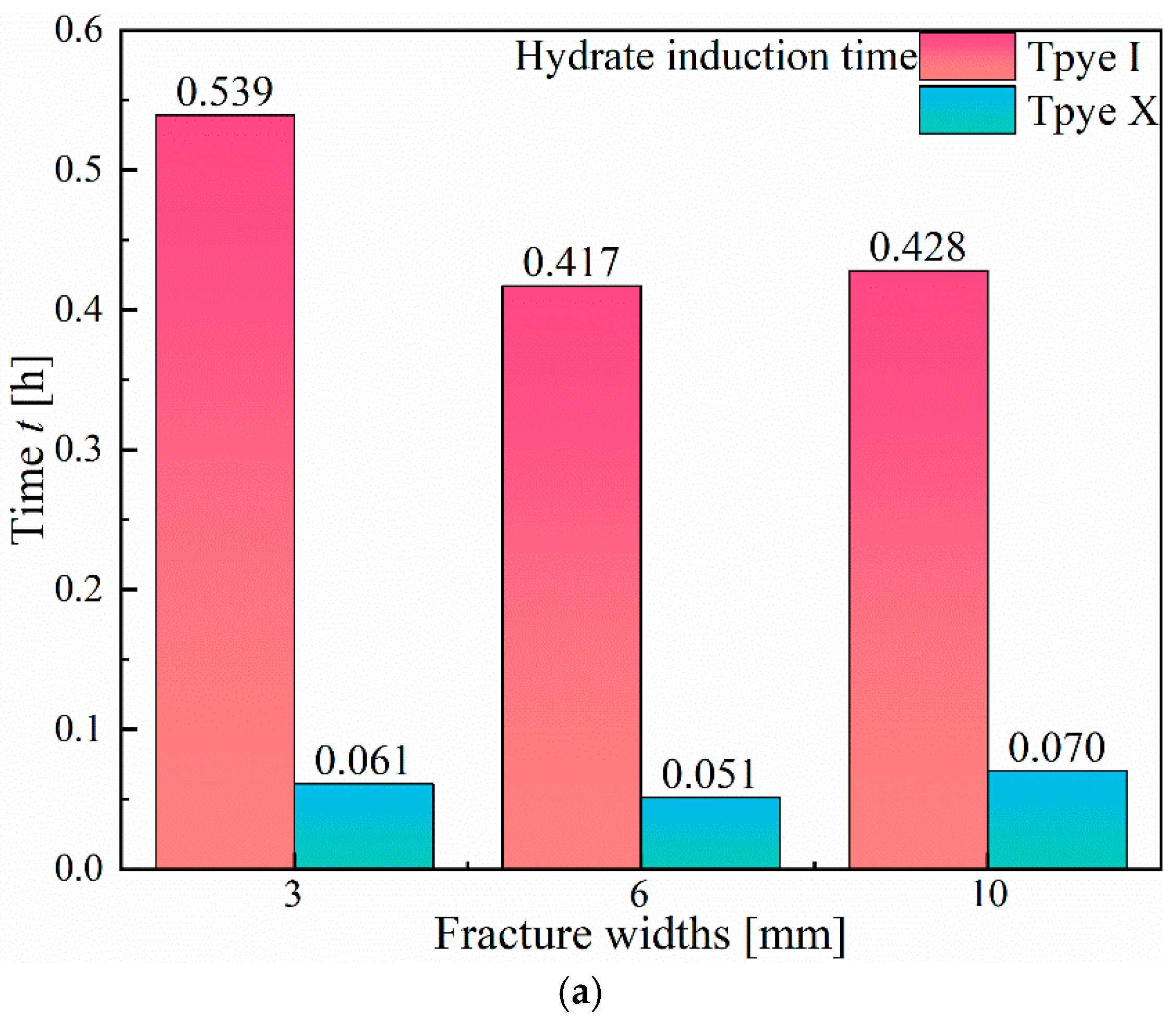
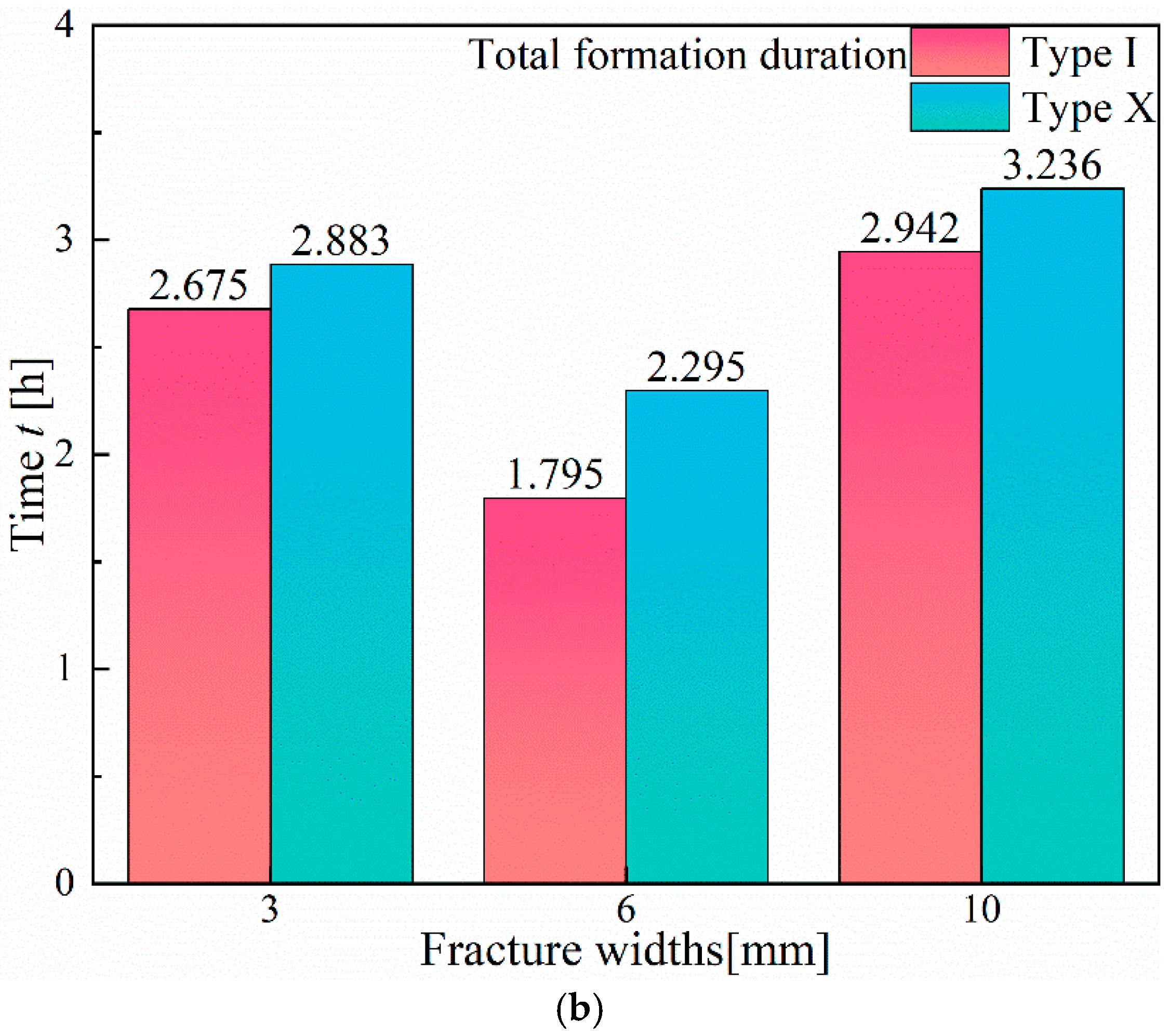
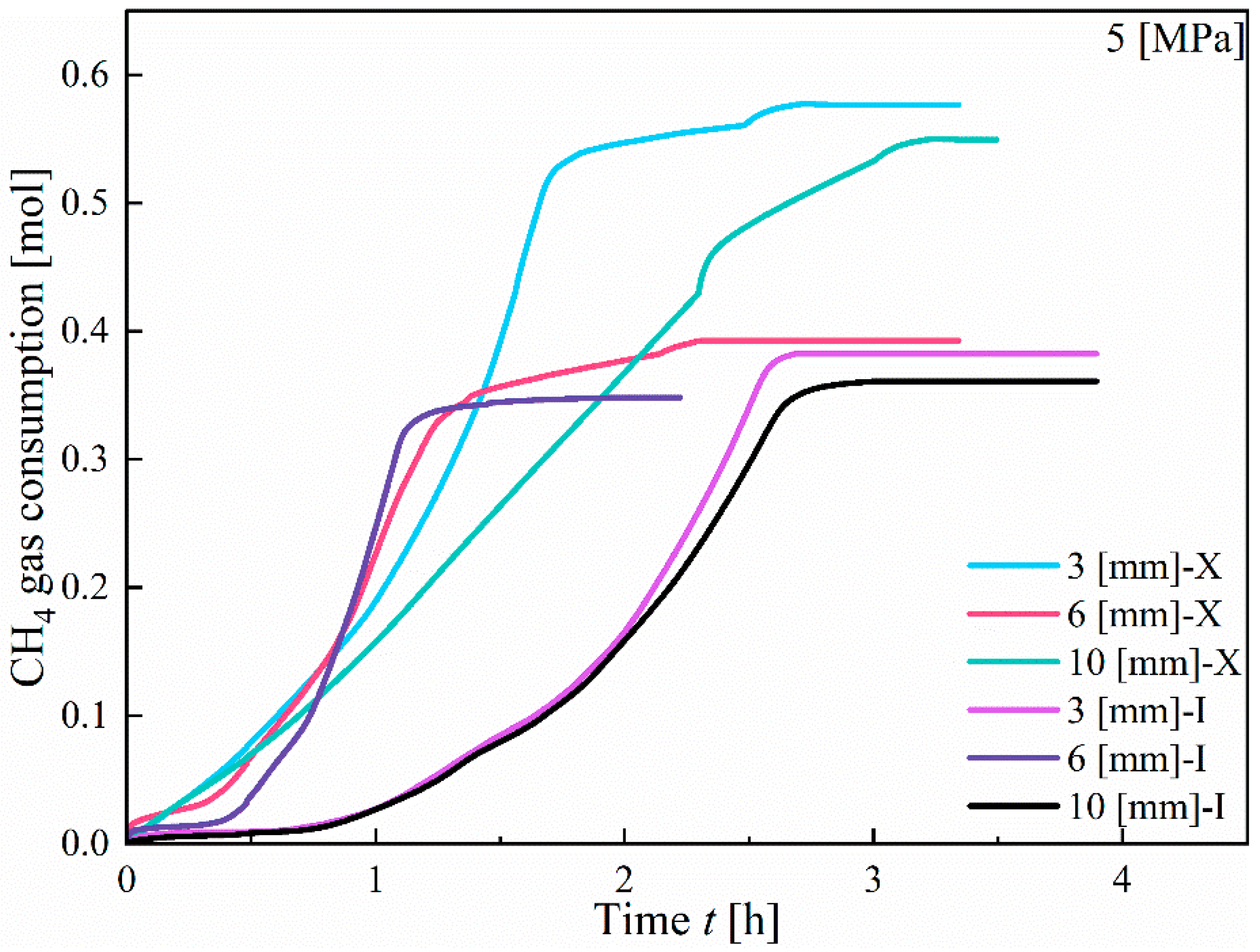
3.1.1. Effect of Fracture Width
3.1.2. Effect of Fracture Shape
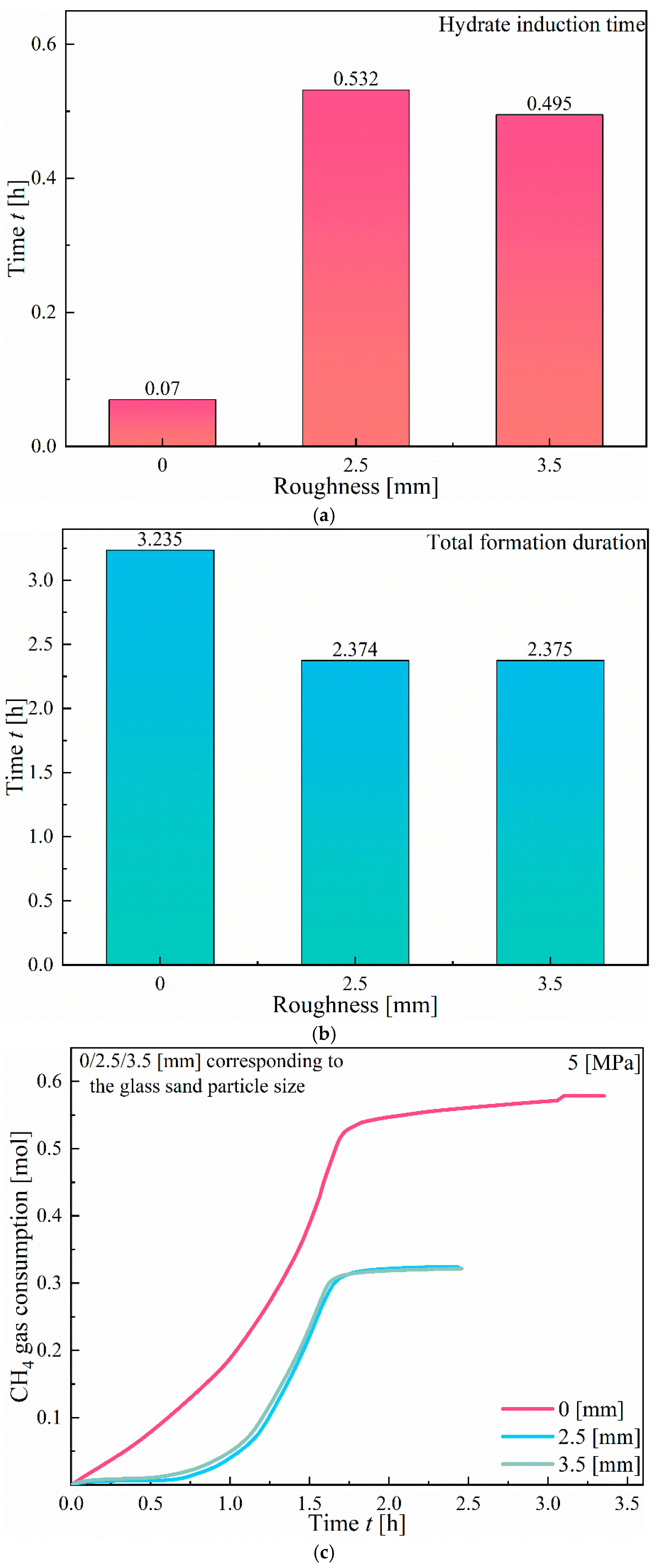
3.1.3. Effect of Roughness
3.2. Hydrate Dissociation
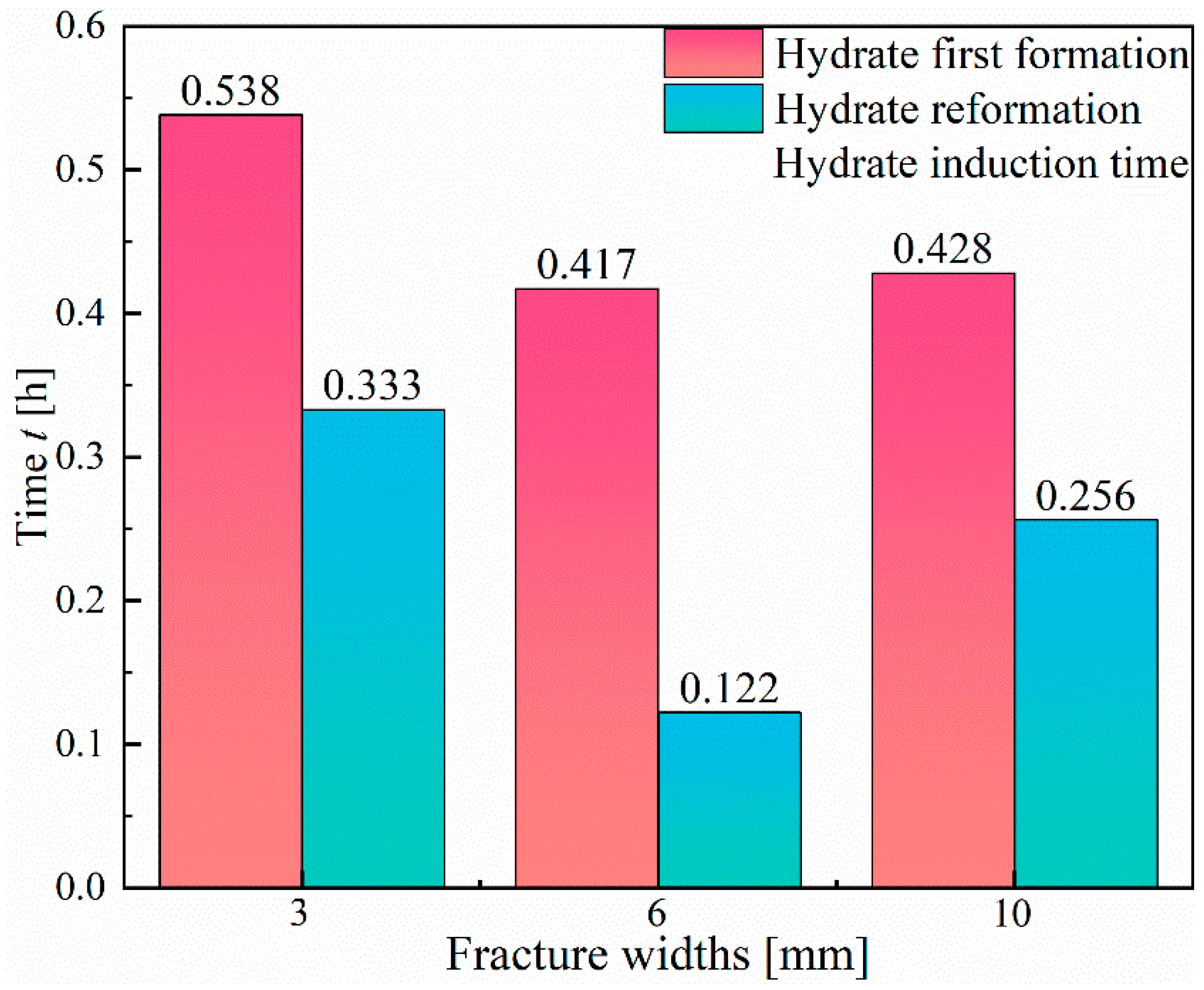
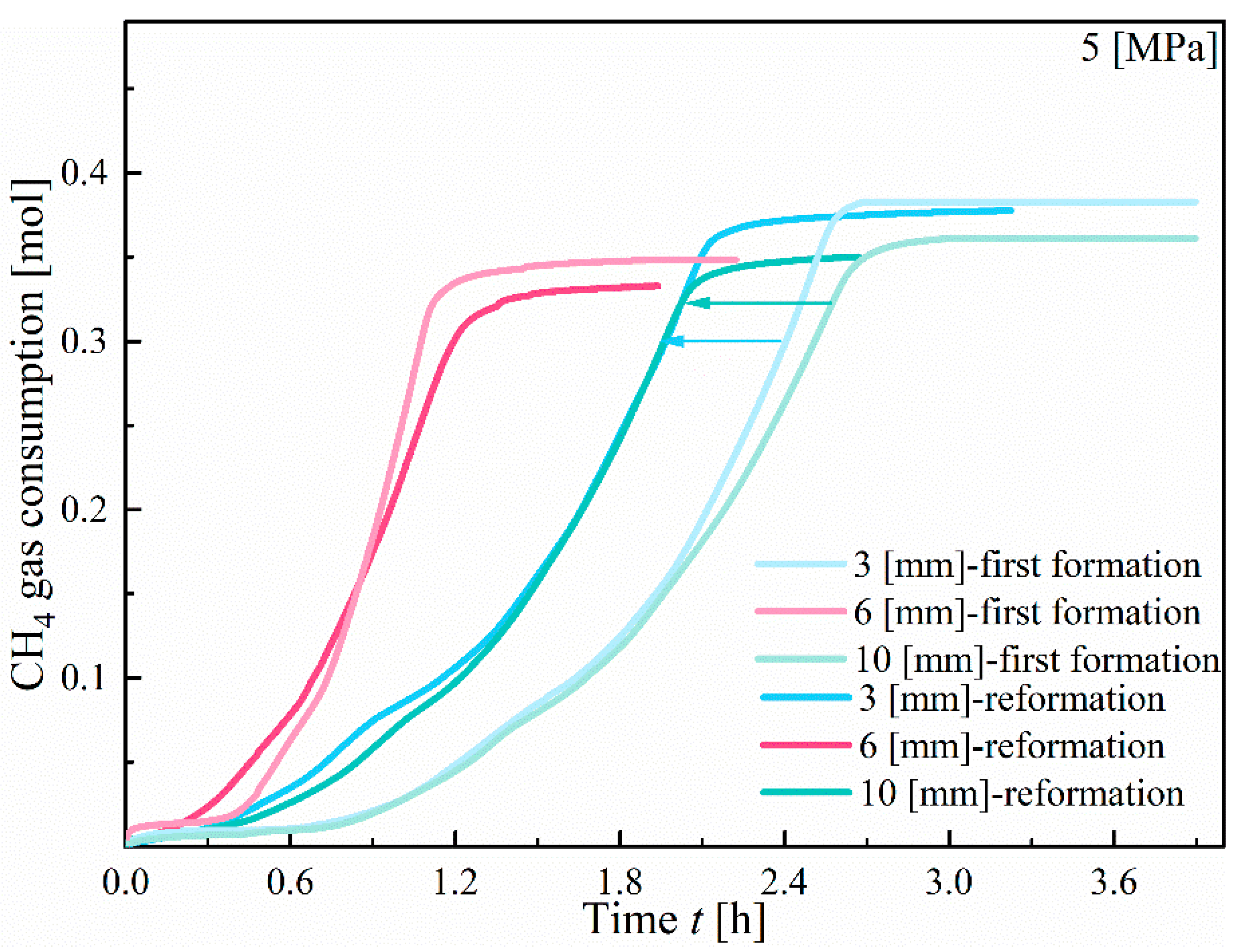
3.3. Hydrate Reformation
4. Conclusions
- (1)
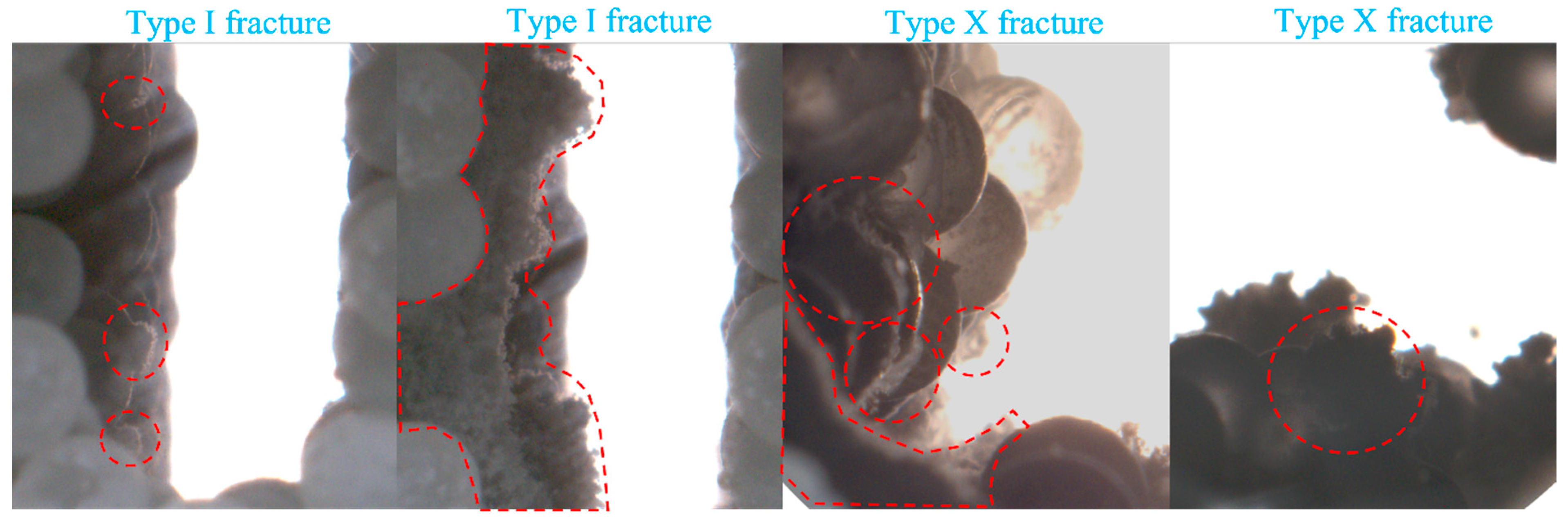
- In Type I fractures, under 3 mm fracture width conditions, the hydrate induction time is longer than those under the other two fracture width conditions, with a larger gas consumption of 3.82 × 10−1 mol. However, the differences in gas consumption among the three types of fractures are not significant. Hydrates preferentially form on the fracture wall and gradually expand to both the left and right side walls of the fracture.
- (2)
- Compared to Type I fractures, in Type X fractures, the hydrate induction time is shorter, and the final formation quantity is larger. Under the condition of a 3 mm fracture width, the induction time decreases by 88.68%. Gas diffuses into the fracture from multiple directions, leading to a multidirectional centripetal expansion of hydrates in the Type X fractures.
- (3)
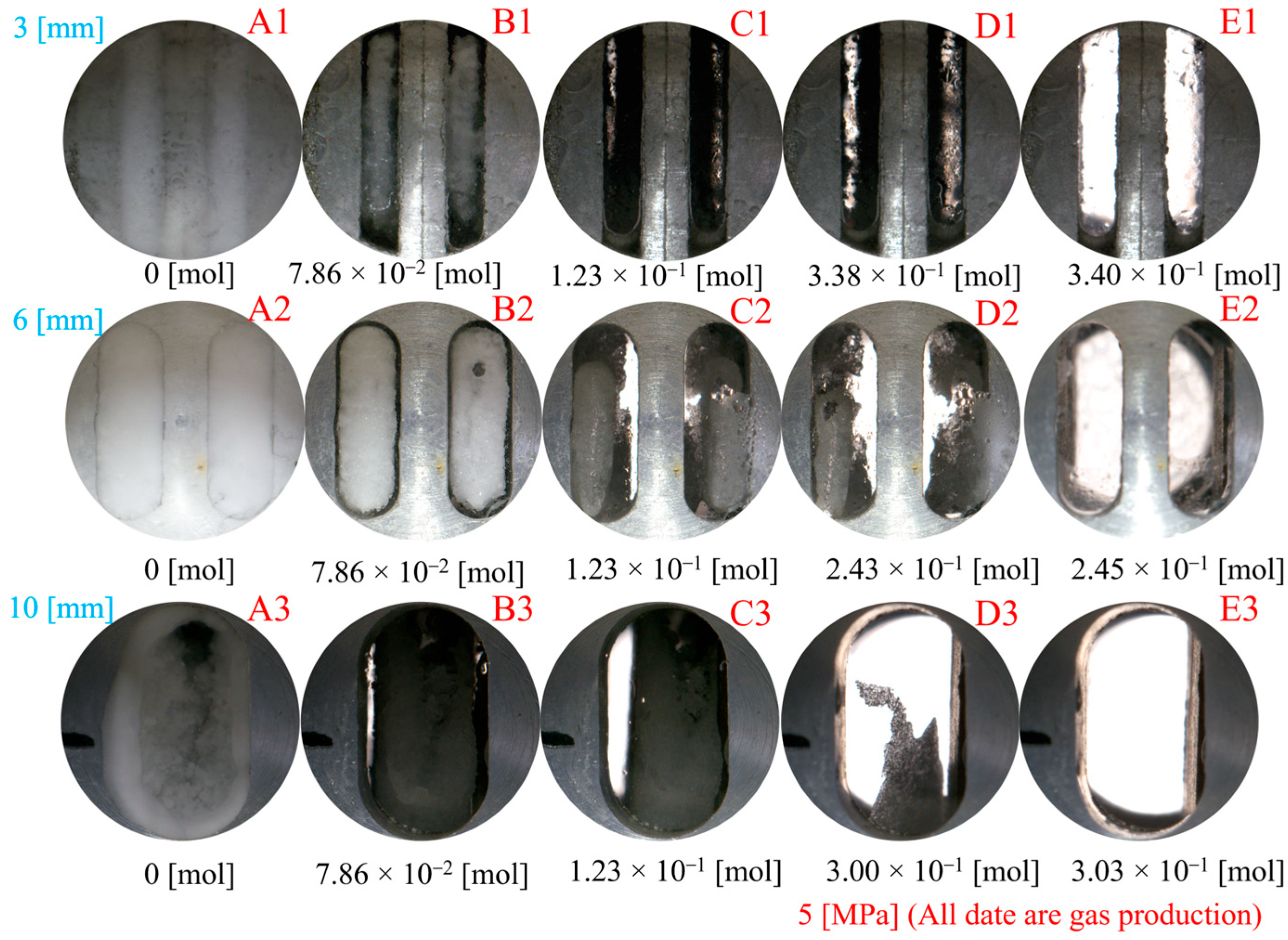
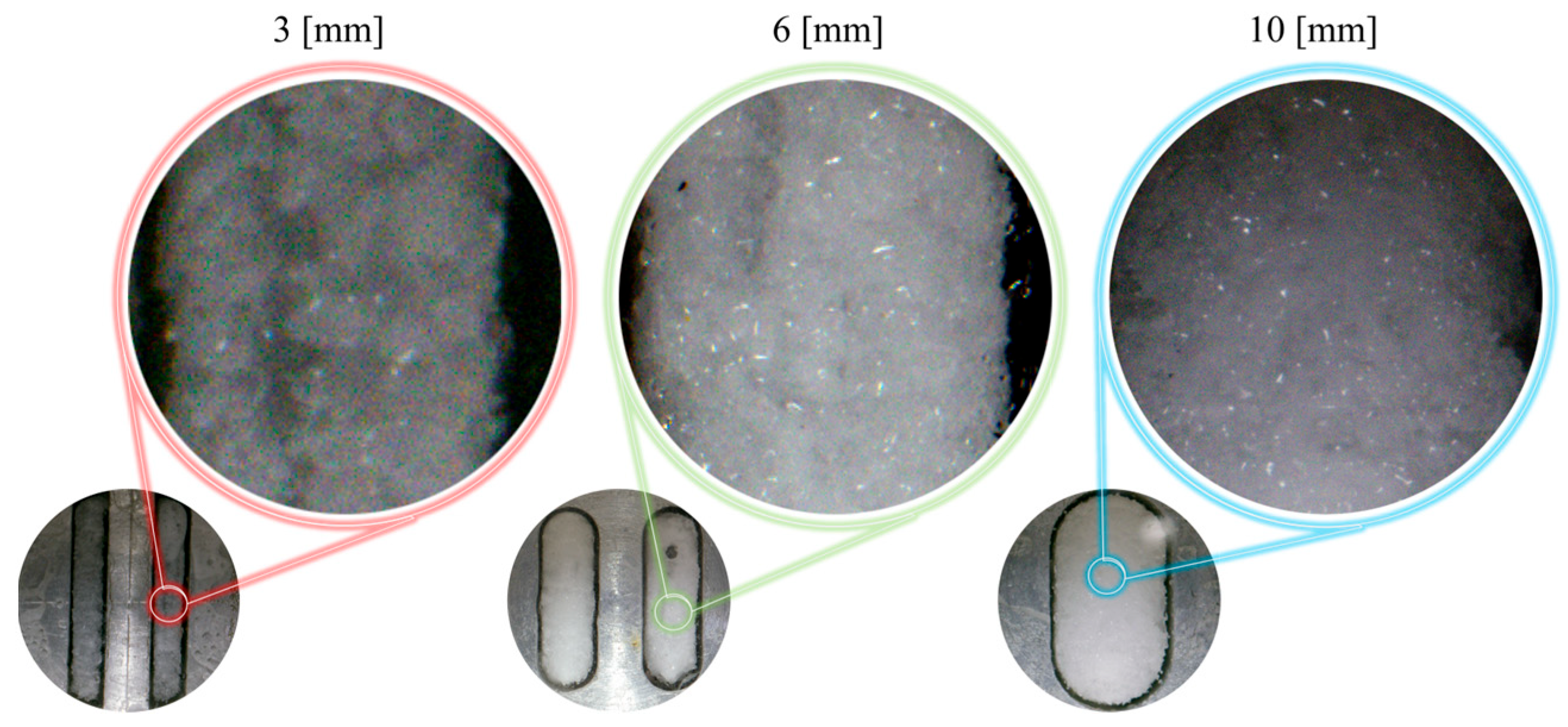
- An increase in fracture roughness promotes hydrate nucleation at surface protrusions but inhibits the final quantity of hydrates. In Type I fractures, rough walls significantly shorten the induction time, whereas in Type X fractures, rough walls extend the induction period.
- (4)
- The dissociation of hydrates typically expands from the fracture wall inward, accompanied by a wavy surface topography. Gas production is affected by the fracture width, with the highest observed in the 3 mm fractures, reaching 0.34 mol.
- (5)
- Due to the memory effect, the induction time for hydrate reformation is significantly shorter than that for primary formation, and the formation rate is faster. However, the volume of hydrate reformation is lower than that of primary formation. Therefore, during hydrate extraction, it is crucial to carefully control the temperature and pressure conditions, as well as the dissociation rate, to avoid a rapid decrease in pressure, which may lead to hydrate reformation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| n: | Gas consumption | P0: | Initial system pressure |
| Pt: | System pressure at time t | Z0: | Gas compressibility factors at P0 |
| Zt: | Gas compressibility factors at Pt | Vg: | Gas volume with the reaction vessel |
| T: | Experimental temperature | R: | Molar gas constant |
| t: | Time | P: | Pressure |
References
- Fang, X.; He, Y.; Li, Z.; Wang, G.; Qiu, S. Design and Performance Analysis of a Composite Separator with Enhanced De-Bonding Efficiency of Gas Hydrate-Bearing Sediments. Appl. Sci. 2025, 15, 5323. [Google Scholar] [CrossRef]
- Mukhsaf, M.H.; Li, W.; Jani, G.H. Optimizing Methanol Injection Quantity for Gas Hydrate Inhibition Using Machine Learning Models. Appl. Sci. 2025, 15, 3229. [Google Scholar] [CrossRef]
- Wang, L.; Shen, S.; Wu, Z.; Wu, D.; Li, Y. Strength and creep characteristics of methane hydrate-bearing clayey silts of the South China Sea. Energy 2024, 294, 130789. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, S.; Ning, F.; Li, P.; Wei, H.; Wei, C. Strength estimation for hydrate-bearing sediments from direct shear tests of hydrate-bearing sand and silt. Geophys. Res. Lett. 2018, 45, 715–723. [Google Scholar] [CrossRef]
- Yan, R.; Lu, D.; Song, Y.; Yu, H.; Zhang, Q.; Zhou, J. Modeling the mechanical behavior of methane hydrate-bearing soil considering the influences of temperature and pore pressure. Energy Fuels 2022, 36, 12510–12523. [Google Scholar] [CrossRef]
- You, Z.; Hao, Y.; Hu, W.; Shen, S.; Wu, P.; Li, Y. Strength analysis of hydrate-bearing sandy sediments in excess gas and excess water based on drained triaxial compression tests. Eng. Geol. 2023, 325, 107292. [Google Scholar] [CrossRef]
- Chong, Z.R.; Yang, S.H.B.; Babu, P.; Linga, P.; Li, X. Review of natural gas hydrates as an energy resource: Prospects and challenges. Appl. Energy 2016, 162, 1633–1652. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, C.; Luo, T.; Wang, A.; Ma, C.; Song, Y.; Yang, W. Triaxial Shearing Characteristics of Hydrate-Bearing Silty-Clayey Sediments under Deviatoric Consolidation. Energy Fuels 2025, 39, 6865–6880. [Google Scholar] [CrossRef]
- He, Y.; Tang, Y.; Zhou, Y.; Xie, N.; Wang, G. Experimental Study on Sand Removal by a Downhole in Situ Spiral-Swirl Natural Gas Hydrate Coupling Separator. Appl. Sci. 2025, 15, 2833. [Google Scholar] [CrossRef]
- Luo, T.; Ma, C.; Chen, Y.; Song, J.; Wang, Z.; Yan, H.; Kang, H.; Li, Z.; Sun, X.; Song, Y.; et al. Numerical Study of Long-Term Natural Gas Production of Hydrate Composite Reservoirs in the South China Sea by Depressurization Using Horizontal Wells. Energy Fuels 2025, 39, 2568–2583. [Google Scholar] [CrossRef]
- Ma, H.; Liu, J.; Zhang, Y.; Li, J.; Kan, J.; Li, N. Prediction of phase equilibrium conditions and thermodynamic stability of CO2-CH4 gas hydrate. Appl. Sci. 2024, 14, 2320. [Google Scholar] [CrossRef]
- Yu, J.; Cheng, C.; Yang, J. Evolution Characteristics of the Strength and Dilation Parameters of Gas Hydrate-Bearing Sediments and a Modeling Study. Appl. Sci. 2024, 14, 2517. [Google Scholar] [CrossRef]
- Song, Y.; Yang, L.; Zhao, J.; Liu, W.; Yang, M.; Li, Y.; Liu, Y.; Li, Q. The status of natural gas hydrate research in China: A review. Renew. Sust. Energy Rev. 2014, 31, 778–791. [Google Scholar] [CrossRef]
- Zheng, J.; Bhatnagar, K.; Khurana, M.; Zhang, P.; Zhang, B.; Linga, P. Semiclathrate based CO2 capture from fuel gas mixture at ambient temperature: Effect of concentrations of tetra-n-butylammonium fluoride (TBAF) and kinetic additives. Appl. Energy 2018, 217, 377–389. [Google Scholar] [CrossRef]
- Ghaani, M.R.; Takeya, S.; English, N.J. Hydrogen storage in propane-hydrate: Theoretical and experimental study. Appl. Sci. 2020, 10, 8962. [Google Scholar] [CrossRef]
- Song, J.; Luo, T.; Chen, Y.; Wang, A.; Ma, C.; Ma, C.; Han, T.; Zhang, Y.; Wang, T.; Yang, W. Formation Kinetics and Morphology Characteristics of Fracture-Filling Natural Gas Hydrate under the Influence of Multiple Parameters. Energy Fuels 2024, 38, 17619–17633. [Google Scholar] [CrossRef]
- Teng, Y.; Li, Y.; Huang, T.; Chen, Y.; Wang, P.; Wang, B.; An, S.; Li, Y.; Han, S.; Zhu, J.; et al. Hydrogen purification via hydrate-based methods: Insights into H2-CO2-CO hydrate structures, thermodynamics, and kinetics. Gas Sci. Eng. 2024, 131, 205484. [Google Scholar] [CrossRef]
- Du, J.; Li, H.; Wang, L. Cooperative effect of surfactant addition and gas-inducing agitation on methane hydrate formation rate. Fuel 2018, 230, 134–137. [Google Scholar] [CrossRef]
- Keshavarz, M.; Ghaffarkhah, A.; Sadeghi, A. Effect of three representative surfactants on methane hydrate formation rate and induction time. Egypt. J. Pet. 2017, 26, 331–339. [Google Scholar] [CrossRef]
- Qin, Y.; Bao, R.; Zhou, L.; Yang, X.; Liu, C.; Wan, W.; Chen, Y.; Guo, Y. Enhanced formation kinetics of hydrates using 1, 3-dioxolane, l-leucine, and β-cyclodextrin. Fuel 2023, 332, 126165. [Google Scholar] [CrossRef]
- Shen, X.; Li, Y.; Li, Y.; Shen, L.; Maeda, N.; Zhang, Y.; Wang, H.; Wang, X. Ultrarapid formation and direct observation of methane hydrate in the presence of L-tryptophan. ACS Sustain. Chem. Eng. 2023, 11, 8774–8785. [Google Scholar] [CrossRef]
- Lee, W.; Kim, K.; Kang, D.W.; Ahn, Y.; Lee, J. Enhanced Hydrate Growth Kinetics with Hydrate Seeds and Promoter under a Static Reaction System. Energy Fuels 2025, 39, 2508–2520. [Google Scholar] [CrossRef]
- Govindaraj, V.; Mech, D.; Pandey, G.; Nagarajan, R.; Sangwai, J. Kinetics of methane hydrate formation in the presence of activated carbon and nano-silica suspensions in pure water. J. Nat. Gas Sci. Eng. 2015, 26, 810–818. [Google Scholar] [CrossRef]
- Qin, Y.; Shang, L.; Zhou, L.; Zhu, J.; Yuan, S.; Zang, C.; Ao, D.; Li, Z. Application of nanofluids in rapid methane hydrate formation: A review. Energy Fuels 2022, 36, 8995–9013. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, J.; Wang, Z.; Gao, Y.; Sun, B.; Liao, Y.; Linga, P. Effect of clay on methane hydrate formation and dissociation in sediment: Implications for energy recovery from clayey-sandy hydrate reservoirs. Appl. Energy 2023, 341, 121064. [Google Scholar] [CrossRef]
- Babu, P.; Yee, D.; Linga, P.; Palmer, A.; Khoo, B.; Tan, T.; Rangsunvigit, P. Morphology of methane hydrate formation in porous media. Energy Fuels 2013, 27, 3364–3372. [Google Scholar] [CrossRef]
- Ji, Y.; Hou, J.; Cui, G.; Lu, N.; Zhao, E.; Liu, Y.; Du, Q. Experimental study on methane hydrate formation in a partially saturated sandstone using low-field NMR technique. Fuel 2019, 251, 82–90. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, S.; Xu, N.; Liu, W.; Zhao, Y.; Zhao, J.; Jiang, L.; Zheng, J.-N. Quantitative analysis of methane hydrate formation in size-varied porous media for gas storage and transportation application. Fuel 2021, 301, 121021. [Google Scholar] [CrossRef]
- Li, B.; Meng, X.Z.; Cheng, T.; Wan, Q. Affecting mechanism of natural sandstone porous media on the formation and phase equilibrium of methane hydrate. Energy Fuels 2024, 38, 4091–4101. [Google Scholar] [CrossRef]
- Xie, Y.; Cheng, L.; Feng, J.; Zheng, T.; Zhu, Y.; Zeng, X.; Sun, C.; Chen, G. Kinetics behaviors of CH4 hydrate formation in porous sediments: Non-unidirectional influence of sediment particle size on hydrate formation. Energy 2024, 289, 130021. [Google Scholar] [CrossRef]
- Chen, X.; Espinoza, D.N. Surface area controls gas hydrate dissociation kinetics in porous media. Fuel 2018, 234, 358–363. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Wang, Y.; Li, G.; Zhang, Y.; Hu, H.; Wan, K.; Zeng, H.-P. Influence of particle size on the heat and mass transfer characteristics of methane hydrate formation and decomposition in porous media. Energy Fuels 2021, 35, 2153–2164. [Google Scholar] [CrossRef]
- Wu, C.; Fan, S.; Wang, Y.; Lang, X. Morphology observation on formation and dissociation cycles of methane hydrate in stacked quartz sandy sediments. J. Nat. Gas Sci. Eng. 2022, 98, 104382. [Google Scholar] [CrossRef]
- Li, X.; Huang, W.; Sun, J.; Wang, Z.; Wang, J.; Liu, Y. NMR Investigation of Methane Hydrate Formation and Dissociation Behavior Induced by Heat Flow in Sandy Porous Media. Energy Fuels 2024, 38, 5834–5846. [Google Scholar] [CrossRef]
- Weinberger, J.L.; Brown, K.M. Fracture networks and hydrate distribution at Hydrate Ridge, Oregon. Earth Planet. Sci. Lett. 2006, 245, 123–136. [Google Scholar] [CrossRef]
- Cook, A.E.; Goldberg, D.; Kleinberg, R.L. Fracture-controlled gas hydrate systems in the northern Gulf of Mexico. Mar. Pet. Geol. 2008, 25, 932–941. [Google Scholar] [CrossRef]
- Lee, M.W.; Collett, T.S. Characteristics and interpretation of fracture-filled gas hydrate–An example from the Ulleung Basin, East Sea of Korea. Mar. Pet. Geol. 2013, 47, 168–181. [Google Scholar] [CrossRef]
- Holland, M.; Schultheiss, P. Comparison of methane mass balance and X-ray computed tomographic methods for calculation of gas hydrate content of pressure cores. Mar. Pet. Geol. 2014, 58, 168–177. [Google Scholar] [CrossRef]
- Pan, H.; Li, H.; Chen, J.; Zhang, Y.; Cai, S.; Huang, Y.; Hao, Y.; Deng, J. A unified contact cementation theory for gas hydrate morphology detection and saturation estimation from elastic-wave velocities. Mar. Pet. Geol. 2020, 113, 104146. [Google Scholar] [CrossRef]
- Jin, Z.H.; Johnson, S.E.; Cook, A.E. Crack extension induced by dissociation of fracture-hosted methane gas hydrate. Geophys. Res. Lett. 2015, 42, 8522–8529. [Google Scholar] [CrossRef]
- Hu, G.; Zhang, Z.; Zhao, Y.; Bu, Q.; Li, A.; Zhao, W.; Wang, Z.; Qiu, X.; Liu, T.; Liu, S.; et al. Formation mechanism and geophysical properties of fracture-filling gas hydrate in the host sediments: A comprehensive review. Earth Sci. Rev. 2024, 258, 104917. [Google Scholar] [CrossRef]
- Yang, H.; Xue, X.; Chen, X.; Xie, J.; Zheng, Q. A study on the electrical characteristics of eractured gas hydrate reservoirs based on digital rock technology. Lithosphere 2021, 2021, 1365284. [Google Scholar] [CrossRef]
- Liu, S.; Hu, G.; Bu, Q.; Zhao, Y.; Sun, Z. Frequency-dependent anisotropic electromagnetic responses of fractured reservoirs with various hydrate distributions based on numerical simulation. J. Mar. Sci. Eng. 2025, 13, 48. [Google Scholar] [CrossRef]
- Han, T.; Liu, S.; Fu, L. Effects of hydrate distribution on the anisotropic electrical properties of fractured reservoirs through effective medium modeling. Geophysics 2025, 90, 1–49. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, J.; Zheng, J.; Song, Y. Hydrate reformation characteristics in natural gas hydrate dissociation process: A review. Appl. Energy 2019, 256, 113878. [Google Scholar] [CrossRef]
- Li, B.; Sun, Y.; Jiang, S.; Shen, Y.; Qi, Y.; Zhang, G. Investigating the influence of Joule-Thomson cooling on hydrate reformation near the wellbore. Gas Sci. Eng. 2024, 124, 205288. [Google Scholar] [CrossRef]
- Kou, X.; Zhang, H.; Li, X.; Chen, Z.; Wang, Y. Interfacial heat and mass transfer effects on secondary hydrate formation under different dissociation conditions. Langmuir 2024, 40, 3617–3627. [Google Scholar] [CrossRef]
- Wen, Z.; Yao, Y.; Sun, X.; Lei, X.; Cui, C. The Impact of Gas Flow on Hydrate Reformation and Reservoir Seepage Characteristics in Clayey Silt Sediments. Energy Fuels 2024, 38, 5149–5158. [Google Scholar] [CrossRef]
- Shi, B.; Liao, Q.; Song, S.; Zhu, Y.; Gong, J.; Chen, G. Microscopic study on the memory effect of methane hydrate in the presence of silica nanoparticles: I. Dissociation mechanism & guest supersaturated hypothesis. Sep. Purif. Technol. 2025, 357, 130100. [Google Scholar] [CrossRef]
- Liao, Q.; Shi, B.; Song, S.; Zhu, Y.; Gong, J.; Chen, G. Microscopic study on the memory effect of methane hydrate in the presence of silica nanoparticles: II. Residual structure hypothesis & impurity imprinting hypothesis. Sep. Purif. Technol. 2025, 357, 130101. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, Y.; Li, Q.; Li, L.; Huang, H.; Wang, S.; Wang, W. CO2 hydrate formation promoted by a natural amino acid l-methionine for possible application to CO2 capture and storage. Energy Technol. 2017, 5, 1195–1199. [Google Scholar] [CrossRef]
- GB/T 17747.3-2011; Natural gas-Calculation of compression factor-Part 3: Calculation using physical properties. Standards Press of China: Beijing, China, 2011.
- Lei, L.; Park, T.; Jarvis, K.; Pan, L.; Tepecik, I.; Zhao, Y.; Ge, Z.; Choi, J.-H.; Gai, X.; Galindo-Torres, S.A.; et al. Pore-scale observations of natural hydrate-bearing sediments via pressure core sub-coring and micro-CT scanning. Sci. Rep. 2022, 12, 3471. [Google Scholar] [CrossRef]
- Wang, S.; Yang, M.; Liu, W.; Zhao, J.; Song, Y. Investigation on the induction time of methane hydrate formation in porous media under quiescent conditions. J. Pet. Sci. Eng. 2016, 145, 565–572. [Google Scholar] [CrossRef]
- Liu, T.; Liu, X. Identification of morphologies of gas hydrate distribution based on amplitude variation with angle analysis. Geophysics 2018, 83, B143–B154. [Google Scholar] [CrossRef]
- Mu, Z.; Yan, Z.; Qiu, Z.; Zhang, P.; Zhang, Y.; Tian, S.; Zhang, B. Impact of fracture orientation on gas hydrate extraction using horizontal well depressurization and hydraulic fracturing. Energy 2025, 315, 134367. [Google Scholar] [CrossRef]


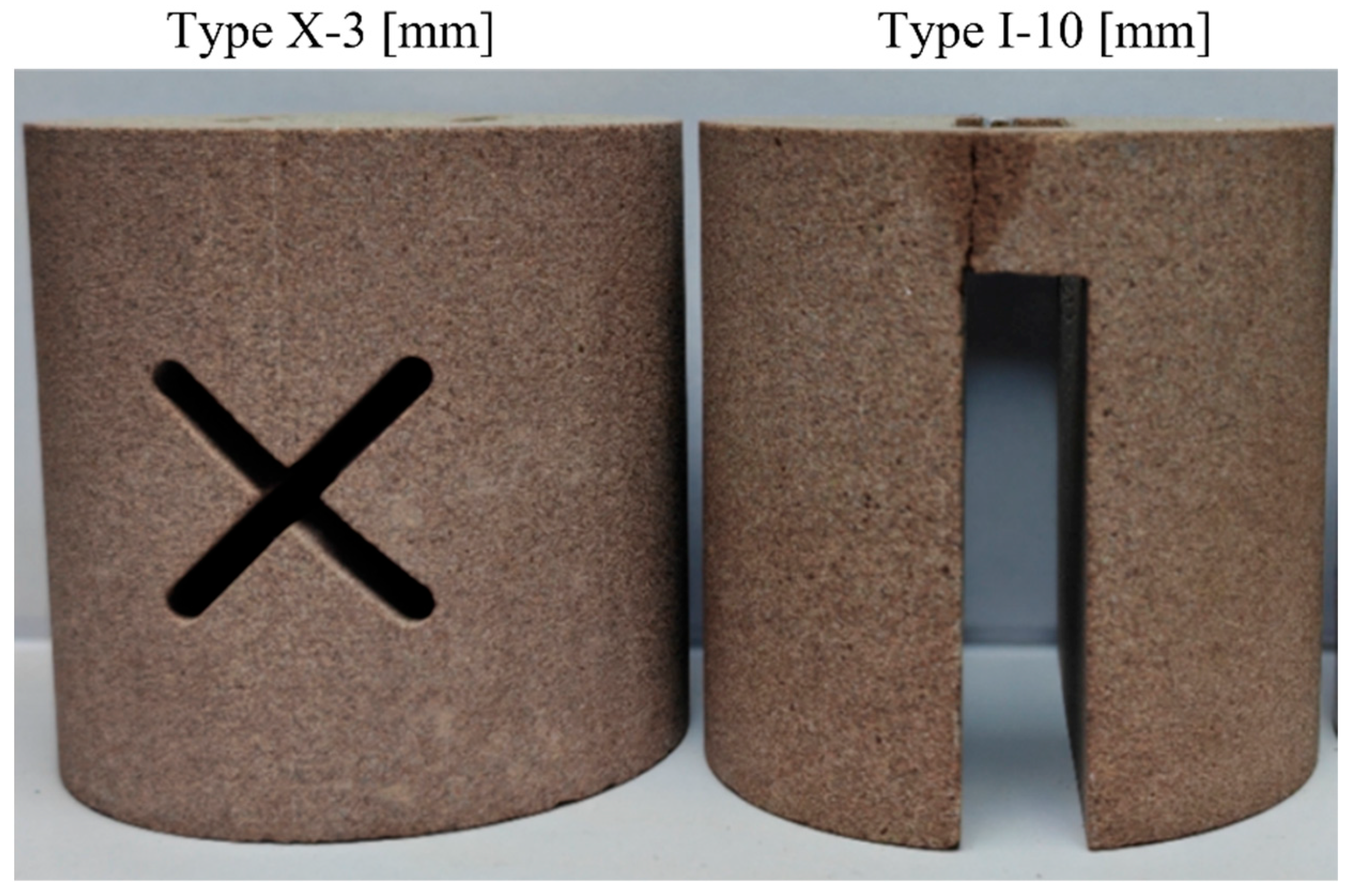






| Number | Pressure [MPa] | Fracture Shape | Fracture Width [mm] | Roughness [mm] | Note |
|---|---|---|---|---|---|
| 1 | 5 | I | 3/6/10 | / | 3 groups |
| 2 | X | 3/6/10 | / | 3 groups | |
| 3 | I | 10 | 2.5/3.5 | 2 groups | |
| 4 | X | 10 | 2.5/3.5 | 2 groups |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Hu, X.; Si, H.; Wang, J.; Pan, J.; Luo, T.; Han, T.; Wang, A. Formation Kinetics and Morphology Characteristics of Natural Gas Hydrates in Sandstone Fractures. Appl. Sci. 2025, 15, 7399. https://doi.org/10.3390/app15137399
Ma C, Hu X, Si H, Wang J, Pan J, Luo T, Han T, Wang A. Formation Kinetics and Morphology Characteristics of Natural Gas Hydrates in Sandstone Fractures. Applied Sciences. 2025; 15(13):7399. https://doi.org/10.3390/app15137399
Chicago/Turabian StyleMa, Chaozheng, Xiaoxu Hu, Hongxiang Si, Jiyao Wang, Juntao Pan, Tingting Luo, Tao Han, and Aowang Wang. 2025. "Formation Kinetics and Morphology Characteristics of Natural Gas Hydrates in Sandstone Fractures" Applied Sciences 15, no. 13: 7399. https://doi.org/10.3390/app15137399
APA StyleMa, C., Hu, X., Si, H., Wang, J., Pan, J., Luo, T., Han, T., & Wang, A. (2025). Formation Kinetics and Morphology Characteristics of Natural Gas Hydrates in Sandstone Fractures. Applied Sciences, 15(13), 7399. https://doi.org/10.3390/app15137399







