The Influence of Mixed Filter Materials on the Performance of Biological Slow Filtration in Rainwater Treatment
Abstract
1. Introduction
2. Materials and Methods
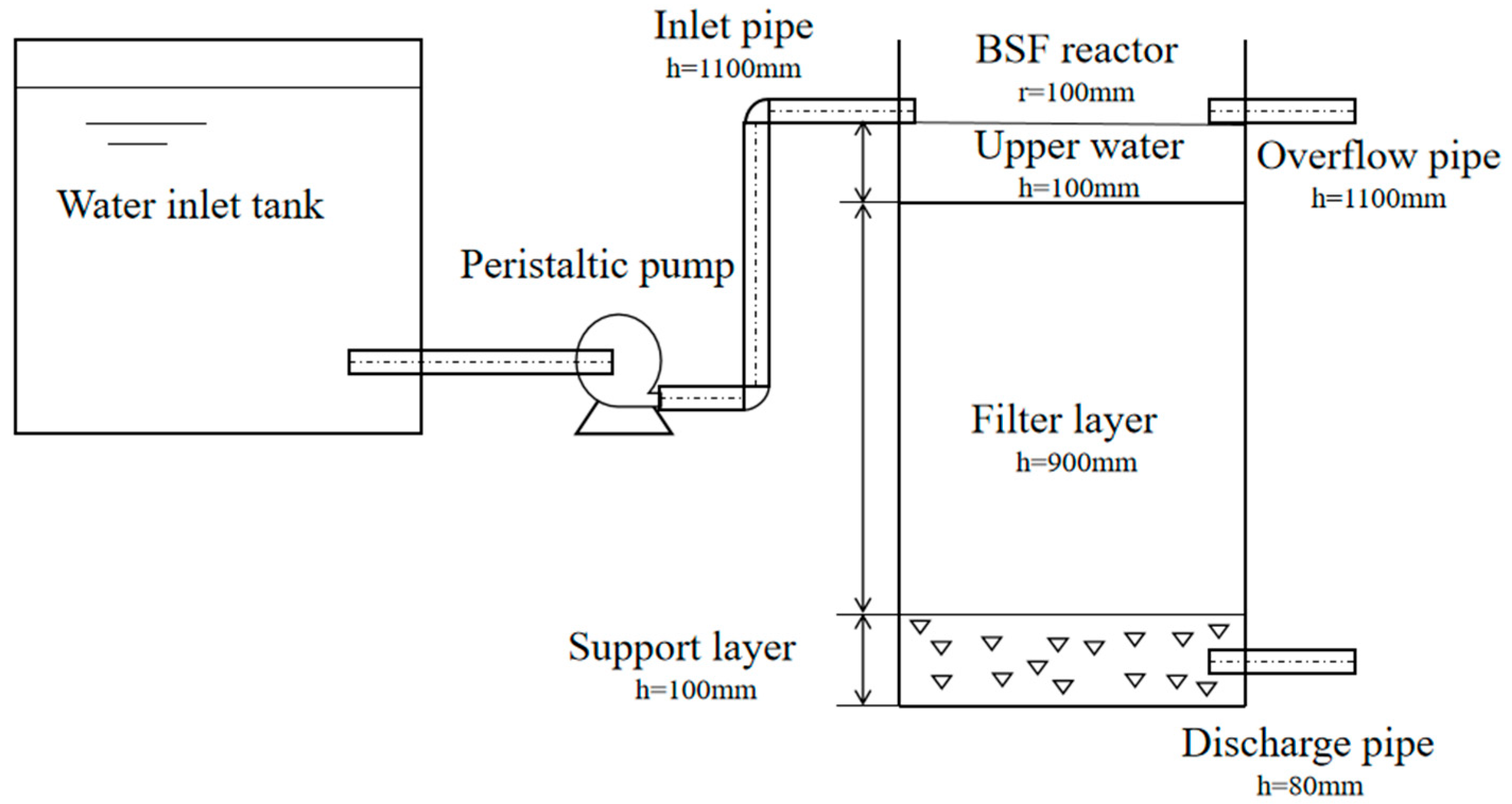
2.1. Test Device
2.2. Test Filter Material
2.3. Test Water
- (1)
- analytical-grade pure ammonium chloride (NH4Cl) to achieve an NH3-N concentration of 1.2–1.4 mg/L (calculated as N);
- (2)
- sieved (<0.1 mm) air-dried soil to achieve a turbidity of 14–15 NTU;
- (3)
- analytical-grade potassium dichromate (K2Cr2O7) solution to achieve a Cr(VI) concentration of 0.05–0.06 mg/L;
- (4)
- analytical-grade humic acid to achieve a TOC concentration of 15.0–16.0 mg/L.A magnetic stirrer was used to stir the mixture for 10 min every 2 h, taking the supernatant as the test water.
2.4. Water Sample Collection and Analysis
- (1)
- Turbidity: The scattering intensity of light by suspended particles in water was measured using spectrophotometry (680 nm) with a turbidity meter.
- (2)
- NH3-N: Nessler’s reagent spectrophotometric method was employed, wherein the reagent reacts with ammonia to form a yellow-brown complex, and colorimetric quantification was performed at 420 nm.
- (3)
- TOC: The spectral water quality detection method decomposes organic matter through high-temperature catalytic oxidation, detecting the generated CO2 concentration and converting it into the TOC value.
- (4)
- Cr(VI): The diphenylcarbazide spectrophotometric method was utilized, in which Cr(VI) reacts with diphenylcarbazide under acidic conditions to form a purple-red complex, with absorbance measured at 540 nm.
2.5. Test Scheme
- (1)
- Turbidity loading rate: 14–15 NTU × 0.2 m/h = 2.8–3.0 NTU·m/h
- (2)
- NH3-N loading rate: 1.2–1.4 mg/L × 0.2 m/h = 0.24–0.28 mg/(L·h)
- (3)
- TOC loading rate: 15.0–16.0 mg/L × 0.2 m/h = 3.0–3.2 mg/(L·h)
- (4)
- Cr(VI) loading rate: 0.05–0.06 mg/L × 0.2 m/h = 0.01–0.012 mg/(L·h)
3. Results and Discussion
3.1. The Removal Effect of a Single Filter Material
3.1.1. The Turbidity Removal Effect
3.1.2. The NH3-N Removal Effect
3.1.3. The TOC Removal Effect
3.1.4. The Cr(VI) Removal Effect
3.2. The Removal Effect of the Mixed Filter Material
3.2.1. The Turbidity Removal Effect
3.2.2. The NH3-N Removal Effect
3.2.3. The TOC Removal Effect
3.2.4. The Cr(VI) Removal Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, Q.; Zhang, G.; Fang, Q.; Qiu, D.; Zhou, T.; Su, X. Review of the United Nations World Water Development Report. Hydro-Sci. Eng. 2025, 1, 13. [Google Scholar]
- Raimondi, A.; Quinn, R.; Abhijith, G.R.; Becciu, G.; Ostfeld, A. Rainwater harvesting and treatment: State of the art and perspectives. Water 2023, 15, 1518. [Google Scholar] [CrossRef]
- Richards, S.; Rao, L.; Connelly, S.; Raj, A.; Raveendran, L.; Shirin, S.; Jamwal, P.; Helliwell, R. Sustainable water resources through harvesting rainwater and the effectiveness of a low-cost water treatment. J. Environ. Manag. 2021, 286, 112223. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, F.A.; Al-Shareef, A.W. Roof rainwater harvesting systems for household water supply in Jordan. Desalination 2009, 243, 195–207. [Google Scholar] [CrossRef]
- Deng, Y. Pollution in rainwater harvesting: A challenge for sustainability and resilience of urban agriculture. J. Hazard. Mater. Lett. 2021, 2, 100037. [Google Scholar] [CrossRef]
- Abdiyev, K.; Azat, S.; Kuldeyev, E.; Ybyraiymkul, D.; Kabdrakhmanova, S.; Berndtsson, R.; Khalkhabai, B.; Kabdrakhmanova, A.; Sultakhan, S. Review of slow sand filtration for raw water treatment with potential application in less-developed countries. Water 2023, 15, 2007. [Google Scholar] [CrossRef]
- Maiyo, J.K.; Dasika, S.; Jafvert, C.T. Slow sand filters for the 21st century: A review. Int. J. Environ. Res. Public Health 2023, 20, 1019. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Li, N. Application of bio-slow sand filters for drinking water production: Linking purification performance to bacterial community and metabolic functions. J. Water Process Eng. 2023, 53, 103622. [Google Scholar] [CrossRef]
- Zhang, G.; Li, J.; Wu, F. Comparison experiment of biofilm formation with different filter materials in the process of bio-slow sand filtration treating micro-polluted cellar water. Technol. Water Treat. 2018, 44, 84–88, 109. [Google Scholar] [CrossRef]
- Rahmadyanti, E.; Palupi, A.E. Rainwater treatment with bio-slow sand filtration for sustainable water supply. J. Ecol. Eng. 2025, 26, 190–200. [Google Scholar] [CrossRef]
- Huang, Z. Research on the Application of Improved Biological Slow Filtration Technology in Drinking Northwest Cellar Water. Master’s Thesis, Beijing Jianzhu University, Beijing, China, 2019. [Google Scholar]
- Wang, W.; Xiong, N.; Yang, X.; He, J. Experimental study on bio-slow filtration system for treating micro-polluted water: A comparison of two fillers. J. Fudan Univ. 2024, 63, 276–290. [Google Scholar]
- Qian, S.; Hou, R.; Yuan, R.; Zhou, B.; Chen, Z.; Chen, H. Removal of Escherichia coli from domestic sewage using biological sand filters: Reduction effect and microbial community analysis. Environ. Res. 2022, 209, 112908. [Google Scholar] [CrossRef]
- Azis, K.; Mavriou, Z.; Karpouzas, D.G.; Ntougias, S.; Melidis, P. Evaluation of sand filtration and activated carbon adsorption for the post-treatment of a secondary biologically-treated fungicide-containing wastewater from fruit-packing industries. Processes 2021, 9, 1223. [Google Scholar] [CrossRef]
- Ma, J. Study on Zeolite Modification and Its Application in Treating Micro Polluted Cellar Water in Biological Slow Filtration. Master’s Thesis, Lanzhou Jiaotong University, Lanzhou, China, 2022. [Google Scholar]
- Zhou, T.; Zhai, T.; Ma, J.; Wu, F.; Zhang, G. Sodium chloride-activated polymeric ferric sulfate-modified natural zeolite (Z-Na-Fe): Application of bio-slow filtration for the removal of ammonia and phosphorus from micro-polluted cellar water. J. Water Process Eng. 2024, 61, 105367. [Google Scholar] [CrossRef]
- Widanarto, W.; Budianti, S.I.; Ghoshal, S.K.; Kurniawan, C.; Handoko, E.; Alaydrus, M. Improved microwave absorption traits of coconut shells-derived activated carbon. Diam. Relat. Mater. 2022, 126, 109059. [Google Scholar] [CrossRef]
- Pan, M.H. Analysis of Rapid Detection Methods of Iodine Adsorption Value of Wood Activated Carbon. Chem. Eng. Manag. 2021, 32, 68–69. [Google Scholar]
- GB/T 12496.8-2015; Test Methods of Wooden Activated Carbon—Determination of Iodine Number (TEXT OF DOCUMENT IS IN CHINESE). Available online: https://webstore.ansi.org/standards/spc/gb124962015?srsltid=AfmBOoqlEhb4Y_U418TAUJY4-QiPWEA1oAIkJO7a78vR8mxqPl4eWdYM (accessed on 27 June 2025).
- GB 5749-2022; Sanitary Standards for Drinking Water. Standards Press of China: Beijing, China, 2022.
- GB/T 5750.2-2023; Standard Test Methods for Drinking Water Part 2: Collection and Preservation of Water Samples. Standards Press of China: Beijing, China, 2023.
- Li, J. Research on the Treatment of Micro Polluted Cellar Water by Biological Slow Filtration Columns with Different Filter Media. Master’s Thesis, Lanzhou Jiaotong University, Lanzhou, China, 2018. [Google Scholar]
- He, G. Research on Slow Biological Sand Filtration Technology and Its Application in Ensuring Rural Drinking Water Safety. Master’s Thesis, Chang’an University, Xi’an, China, 2016. [Google Scholar]
- Xiao, B.; Wang, R.; Jia, S. Effect of divalent metal ion on UASB granular sludge. China Water Wastewater 2002, 18, 26–28. [Google Scholar]
- Rožić, M.; Cerjan-Stefanović, Š.; Kurajica, S.; Vančina, V.; Hodžić, E. Ammoniacal nitrogen removal from water by treatment with clays and zeolites. Water Res. 2000, 34, 3675–3681. [Google Scholar] [CrossRef]
- Sun, S.P.; Nàcher, C.P.I.; Merkey, B.; Zhou, Q.; Xia, S.Q.; Yang, D.H.; Sun, J.H.; Smets, B.F. Effective biological nitrogen removal treatment processes for domestic wastewaters with low C/N ratios: A review. Environ. Eng. Sci. 2010, 27, 111–126. [Google Scholar] [CrossRef]
- Xiao, P.; Cao, D.; Li, H.; Wu, J.; Zheng, Z. Effects of three heavy metals on formation of aerobic granular sludge and related population control. J. Sichuan Agric. Univ. 2012, 30, 342–347. [Google Scholar]
- Dos Santos, P.R.; Daniel, L.A. A review: Organic matter and ammonia removal by biological activated carbon filtration for water and wastewater treatment. Int. J. Environ. Sci. Technol. 2020, 17, 591–606. [Google Scholar] [CrossRef]
- Jiang, H.; Ma, Y.; Liu, X.; Wang, H. The influence of Fe on microorganisms and process performance in sewage and sludge treatment processes. Environ. Eng. 2016, 34, 54. [Google Scholar]
- Tripathi, S.M.; Chaurasia, S.R. Detection, of Chromium in surface and groundwater and its bio-absorption using bio-wastes and vermiculite. Eng. Sci. Technol. Int. J. 2020, 23, 1153–1161. [Google Scholar] [CrossRef]
- Liu, L. Study on the Removal of Heavy Metals in Water by Functional Microbial Agents and Their Interaction Mechanisms. Ph.D. Thesis, Hunan University, Changsha, China, 2017. [Google Scholar]
- Zhang, G.; Zhao, K.; Wu, F.; Zhou, T.; Yang, G. The effect of hexavalent chromium ions on the treatment of slightly polluted raw water by biological slow filtration technology. China Water Wastewater 2023, 39, 60–65. [Google Scholar]









| Filter Media Characteristics | Quartz Sand | Volcanic Rock | Coconut Shell Activated Carbon |
|---|---|---|---|
| Particle size | 0.5–1 mm | 0.5–1 mm | 0.5–1 mm |
| Specific surface area | - | 7.0 m2/g | 712.1 m2/g |
| Average aperture | - | 6.06 nm | 3.45 nm |
| Iodine adsorption value | - | - | 700 mg/g |
| Element wt% | O | Si | C | Ca | Fe | Mg |
|---|---|---|---|---|---|---|
| Quartz sand | 23.06 | 76.94 | - | - | - | - |
| Volcanic rock | 36.68 | 21.81 | - | 9.34 | 27.28 | 4.89 |
| Coconut shell activated carbon | 7.52 | 0.05 | 85.3 | 4.41 | 1.1 | 1.62 |
| Water Quality Indicator | Unit | Rainwater Concentration | Drinking Water Standards [20] | Detection Method |
|---|---|---|---|---|
| Turbidity | NTU | 9.79–15.09 | 1 | Spectrophotometry |
| pH | - | 6.98–7.61 | 6.5–8.5 | Acid–base indicator method |
| TDS | mg/L | 63–109 | 1000 | Electrical conductivity method |
| NH3-N | mg/L | 0.8–1.46 | 0.5 | Nessler’s reagent spectrophotometry |
| Cu | mg/L | <0.05 | 1 | Diacetyl dioxime spectrophotometry |
| Fe | mg/L | <0.1 | 0.3 | Phenanthroline spectrophotometry |
| Ni | mg/L | <0.02 | 0.02 | Dimethylglyoxime spectrophotometry |
| Mn | mg/L | <0.02 | 0.1 | Formaldehyde oxime spectrophotometry |
| TOC | mg/L | 3.11–16.04 | 5 | Spectroscopic water quality detection method |
| As | mg/L | <0.01 | 0.01 | Atomic fluorescence spectrometry |
| Pb | mg/L | <0.01 | 0.01 | Atomic absorption spectrophotometer flame method |
| NO3− | mg/L | 0.021–0.091 | 10 | Phenol–disulfonic acid spectrophotometry |
| Cr(VI) | mg/L | 0.021–0.067 | 0.05 | Diphenylcarbazide spectrophotometry |
| Total Colony Count | CFU/mL | 64–77 | 100 | Luciferase luminescence assay |
| Escherichia coli | CFU/100 mL | Not detected | Not detected | Multiple-tube fermentation method |
| Water Quality Indicator | Unit | Rainwater Concentration | Test Water Concentration |
| Turbidity | NTU | 9.79–15.09 | 14–15 |
| NH3-N | mg/L | 0.8–1.46 | 1.2–1.4 |
| TOC | mg/L | 15.21–16.04 | 15.0–16.0 |
| Cr(VI) | mg/L | 0.052–0.067 | 0.05–0.06 |
| Water Quality Indicators | Detection Method | Detection Instrument | Manufacturer |
| Turbidity | Spectrophotometry | AE86065 turbidity meter | Dongguan Frank Technology Co., Ltd. (Guangdong, China) |
| TOC | Spectral water quality detection | Water Detective Type 3 | Shenzhen Bit Atom Technology Co., Ltd. (Shenzhen, China) |
| NH3-N | Nessler’s reagent spectrophotometry | PT-001B multi-parameter water quality detector | Xiamen Pantian Biotechnology Co., Ltd. (Xiamen, China) |
| Cr(VI) | Diphenylcarbazide spectrophotometric method |
| Slow Filtration Reactor Number | Filter Material | Mixing Ratio |
|---|---|---|
| 1# | Single filter material: Quartz sand | - |
| 2# | Single filter material: Volcanic rock | - |
| 3# | Single filter material: coconut shell activated carbon | - |
| 4# | Mixed filter material: volcanic rock–coconut shell activated carbon | 1:1 |
| 5# | Mixed filter material: volcanic rock–coconut shell activated carbon | 1:3 |
| 6# | Mixed filter material: volcanic rock–coconut shell activated carbon | 3:1 |
| Early Stages of Operation | Remove the Stable Period | |||||||
|---|---|---|---|---|---|---|---|---|
| Turbidity | NH3-N | TOC | Cr(VI) | Turbidity | NH3-N | TOC | Cr(VI) | |
| 1 | 83.87 ± 1.11% | 69.07 ± 1.43% | 29.81 ± 0.32% | 14.56 ± 6.09% | 93.28 ± 0.25% | 97.55 ± 1.25% | 49.62 ± 0.70% | 68.46 ± 2.89% |
| 2 | 91.46 ± 0.99% | 80.10 ± 2.63% | 43.34 ± 1.25% | 42.29 ± 5.00% | 97.36 ± 0.08% | 98.77 ± 0.99% | 70.55 ± 0.42% | 93.39 ± 1.69% |
| 3 | 86.47 ± 0.36% | 86.82 ± 2.53% | 51.80 ± 0.21% | 61.30 ± 5.37% | 94.93 ± 0.26% | 98.90 ± 0.42% | 68.60 ± 0.42% | 90.60 ± 2.08% |
| 4 | 88.19 ± 0.65% | 86.81 ± 1.10% | 53.49 ± 0.65% | 68.40 ± 3.99% | 94.50 ± 0.25% | 99.15 ± 0.59% | 70.97 ± 1.00% | 91.42 ± 1.26% |
| 5 | 87.94 ± 0.61% | 88.97 ± 1.11% | 54.97 ± 1.08% | 72.19 ± 4.93% | 94.37 ± 0.33% | 99.27 ± 0.03% | 71.51 ± 0.64% | 93.63 ± 1.83% |
| 6 | 92.71 ± 0.42% | 88.25 ± 1.11% | 52.86 ± 0.64% | 63.99 ± 6.36% | 97.45 ± 0.12% | 99.14 ± 0.62% | 70.87 ± 0.67% | 90.86 ± 1.33% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, D.; Meng, X.; Zhang, H.; Luo, Z. The Influence of Mixed Filter Materials on the Performance of Biological Slow Filtration in Rainwater Treatment. Appl. Sci. 2025, 15, 7394. https://doi.org/10.3390/app15137394
Mu D, Meng X, Zhang H, Luo Z. The Influence of Mixed Filter Materials on the Performance of Biological Slow Filtration in Rainwater Treatment. Applied Sciences. 2025; 15(13):7394. https://doi.org/10.3390/app15137394
Chicago/Turabian StyleMu, Dawei, Xiangzhen Meng, Huali Zhang, and Zhi Luo. 2025. "The Influence of Mixed Filter Materials on the Performance of Biological Slow Filtration in Rainwater Treatment" Applied Sciences 15, no. 13: 7394. https://doi.org/10.3390/app15137394
APA StyleMu, D., Meng, X., Zhang, H., & Luo, Z. (2025). The Influence of Mixed Filter Materials on the Performance of Biological Slow Filtration in Rainwater Treatment. Applied Sciences, 15(13), 7394. https://doi.org/10.3390/app15137394





