Unraveling the Impact of Copper Ions on Mineral Surfaces During Chalcopyrite–Molybdenite Flotation Separation Using Sodium Thioglycolate
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Flotation Experiments
2.3. Contact Angle Measurements
2.4. Zeta Potential Measurements
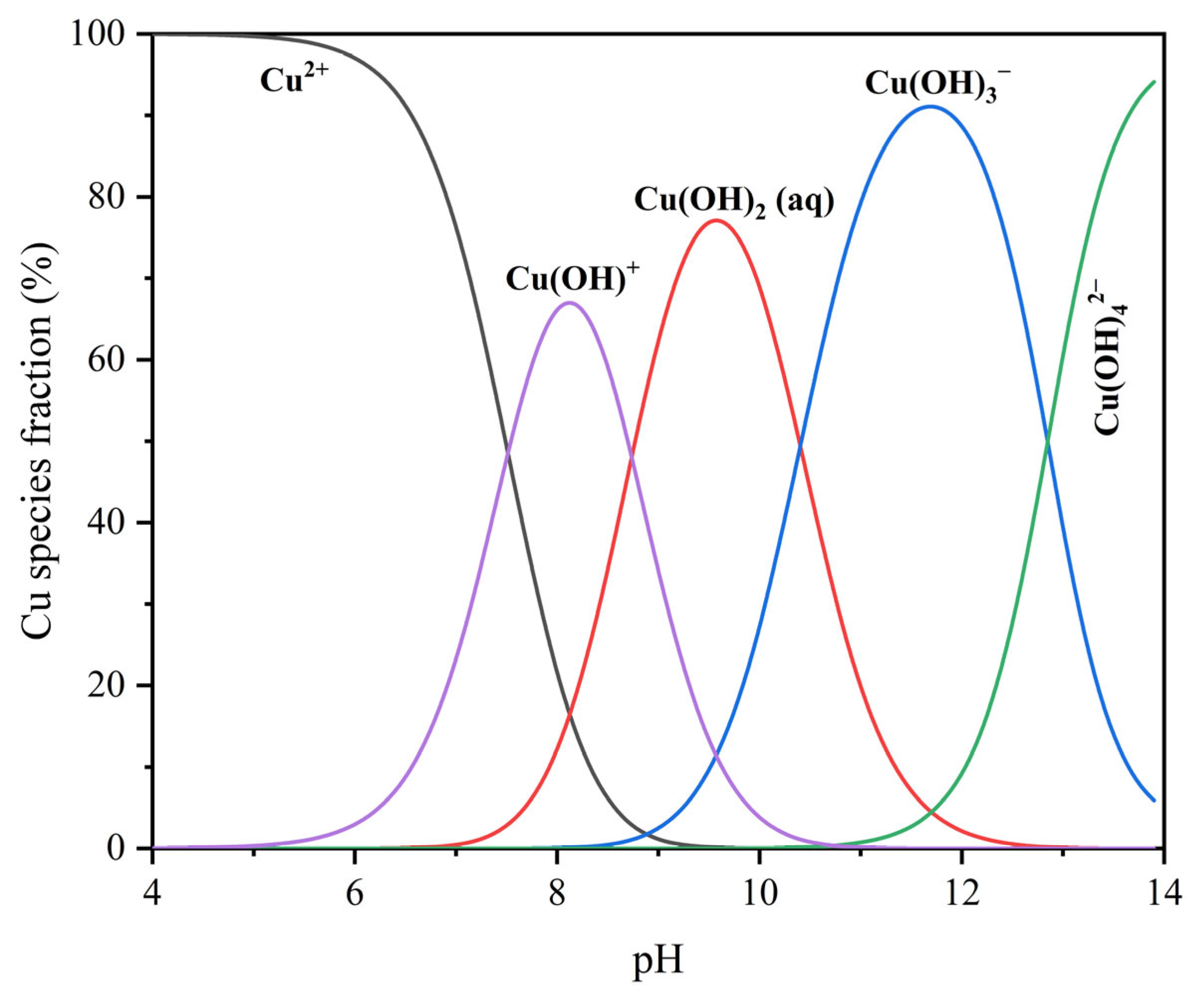
2.5. UV–Vis Spectrophotometer Measurement
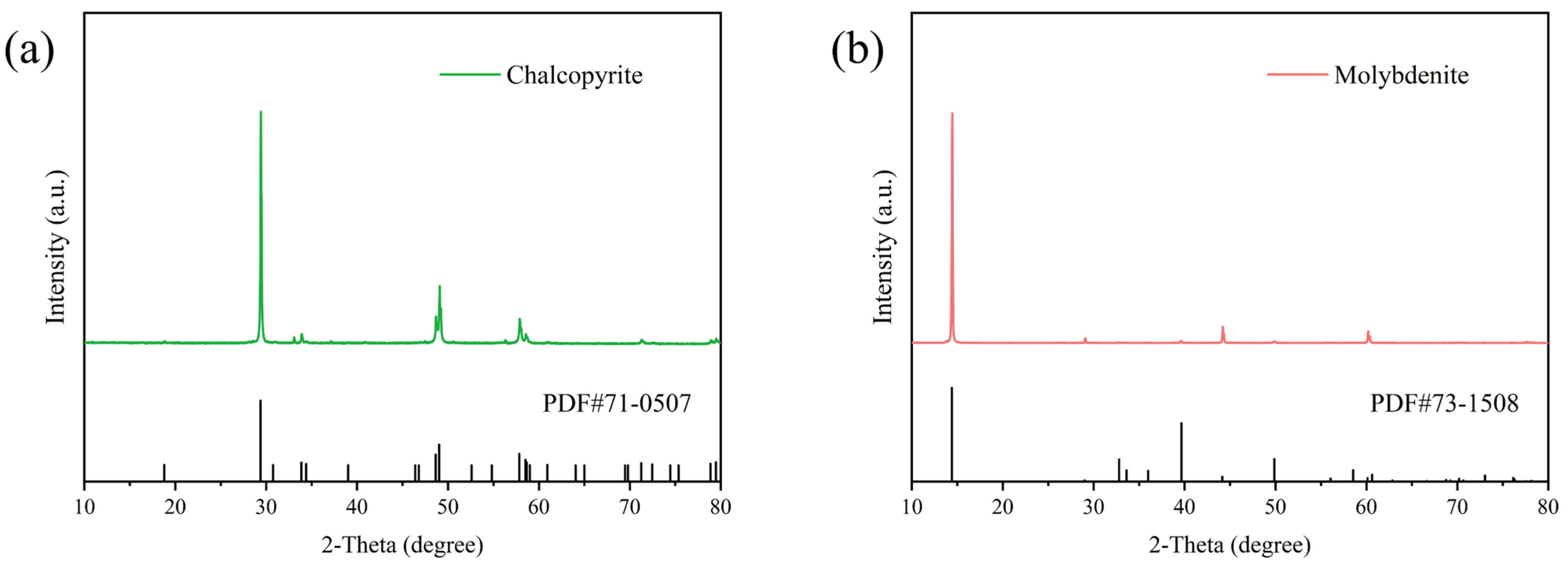
2.6. XPS Analysis
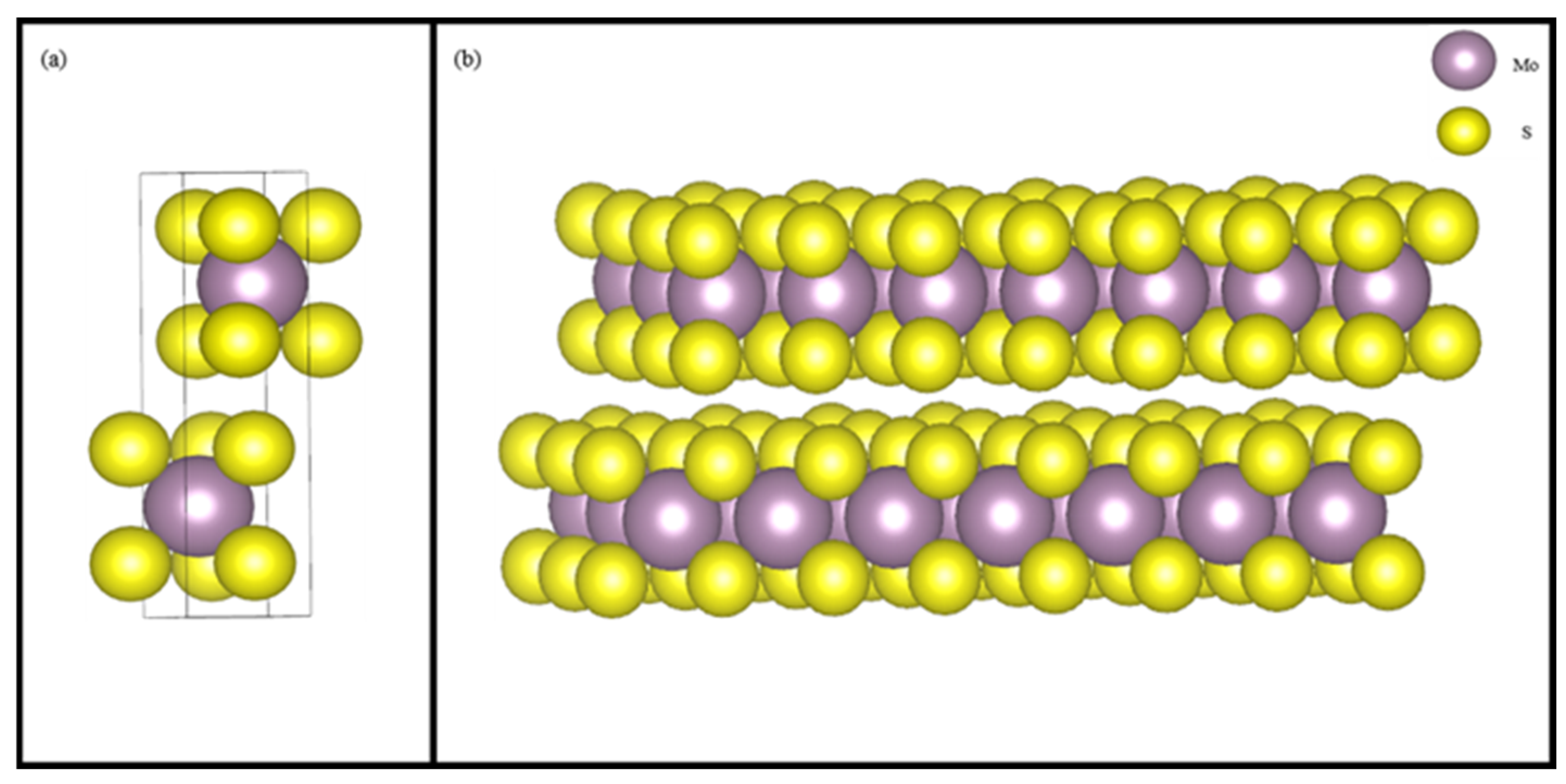
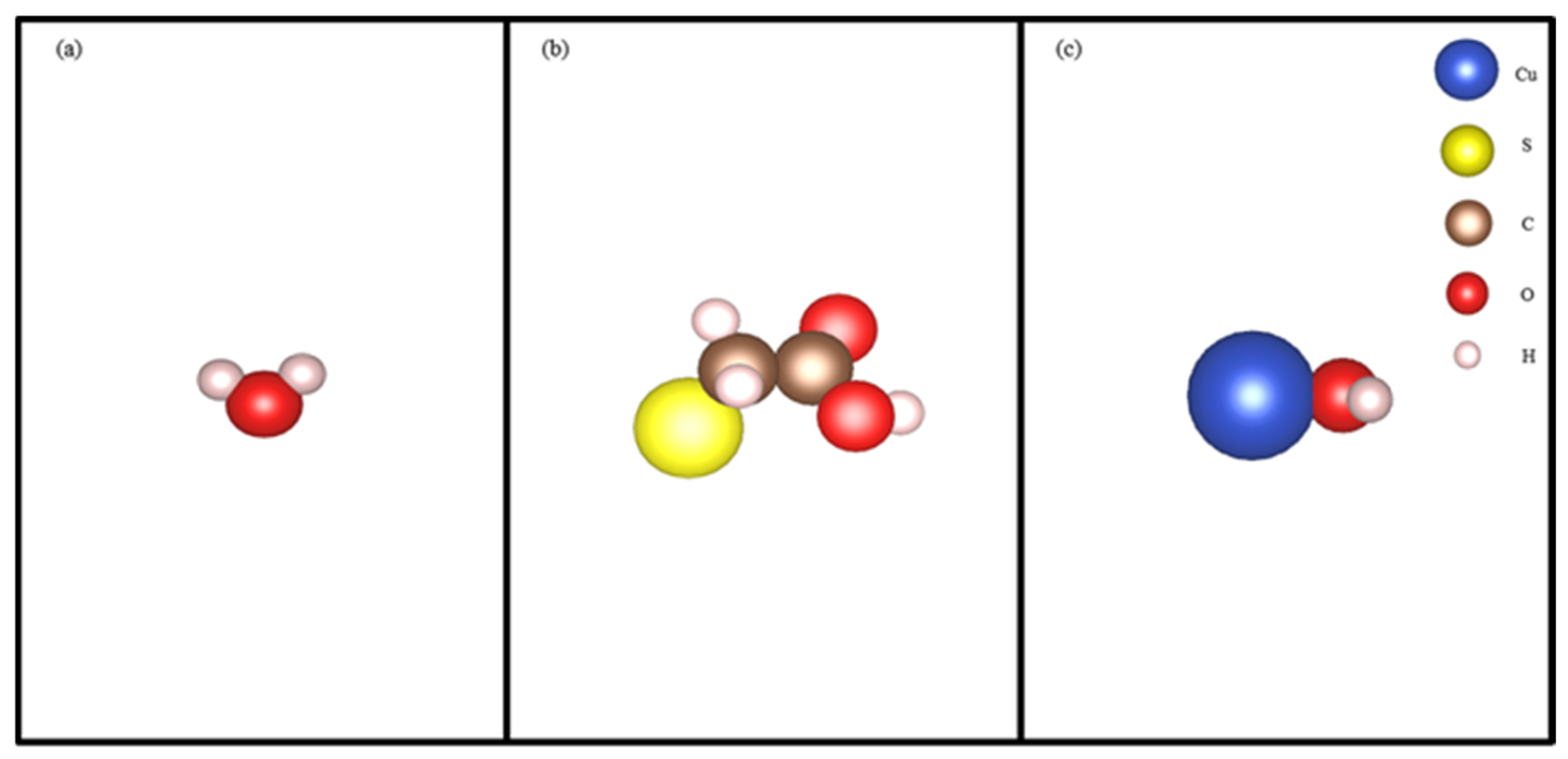
2.7. DFT Calculation
3. Results and Discussion
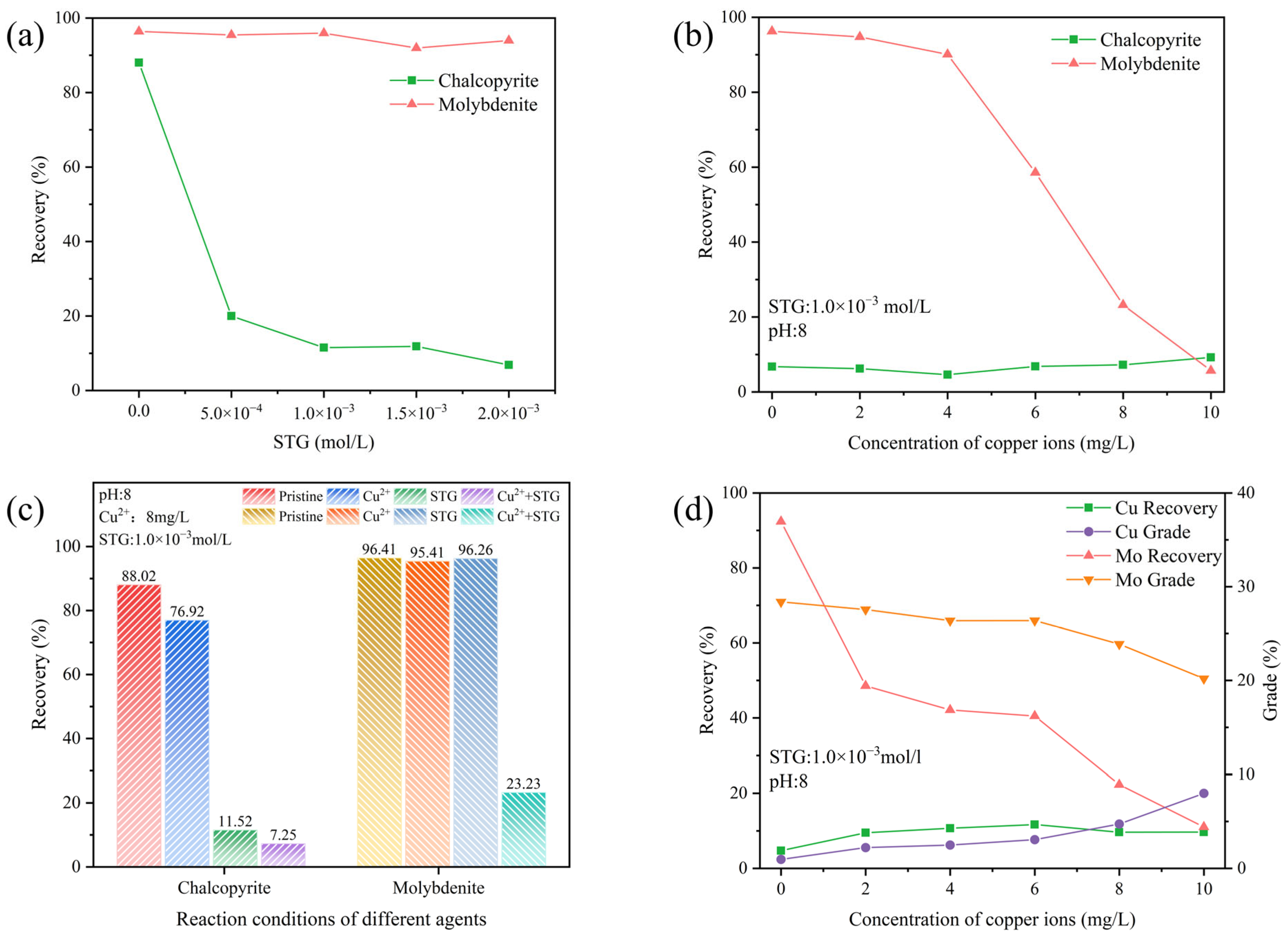
3.1. Flotation Experiments
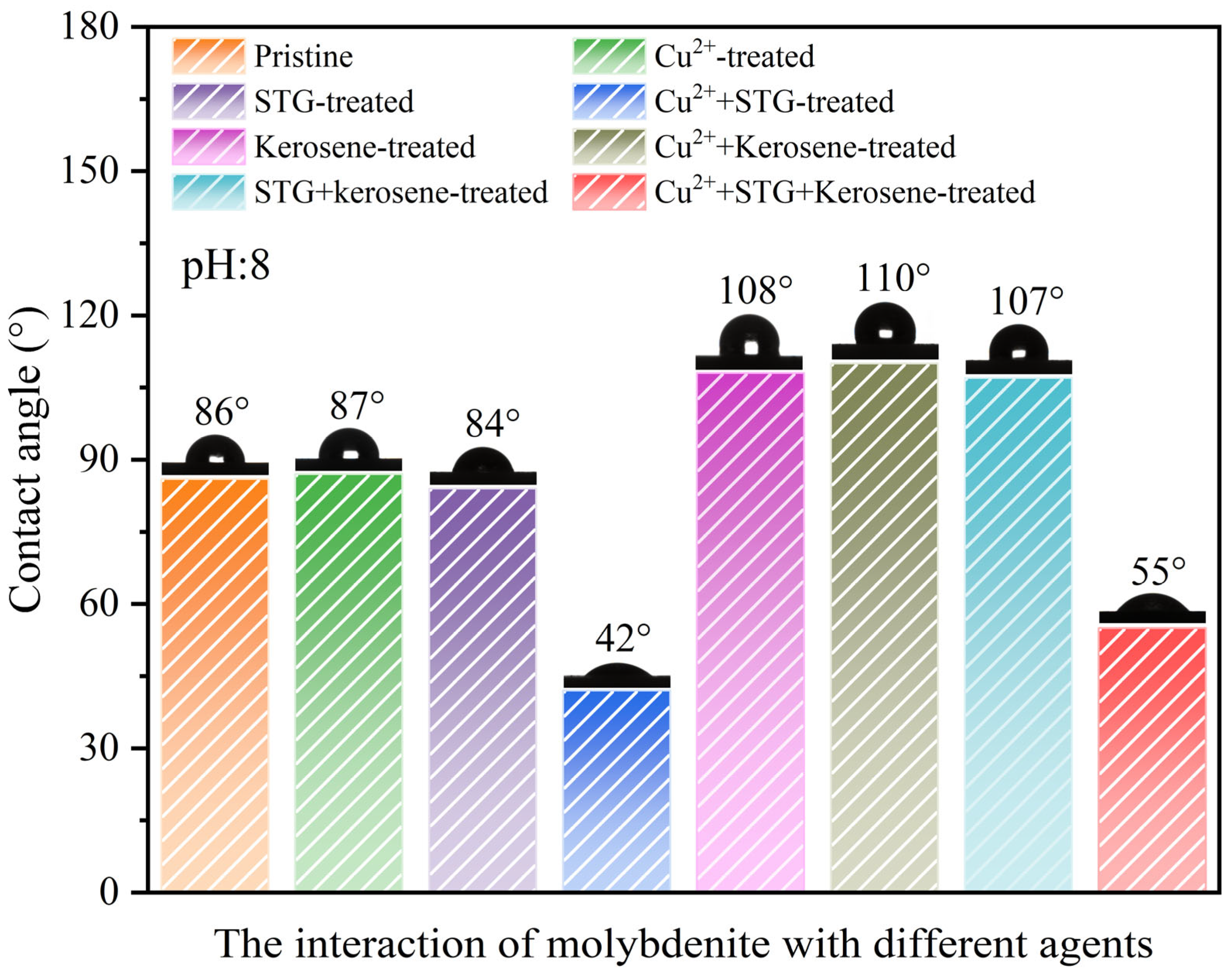
3.2. Contact Angle Test
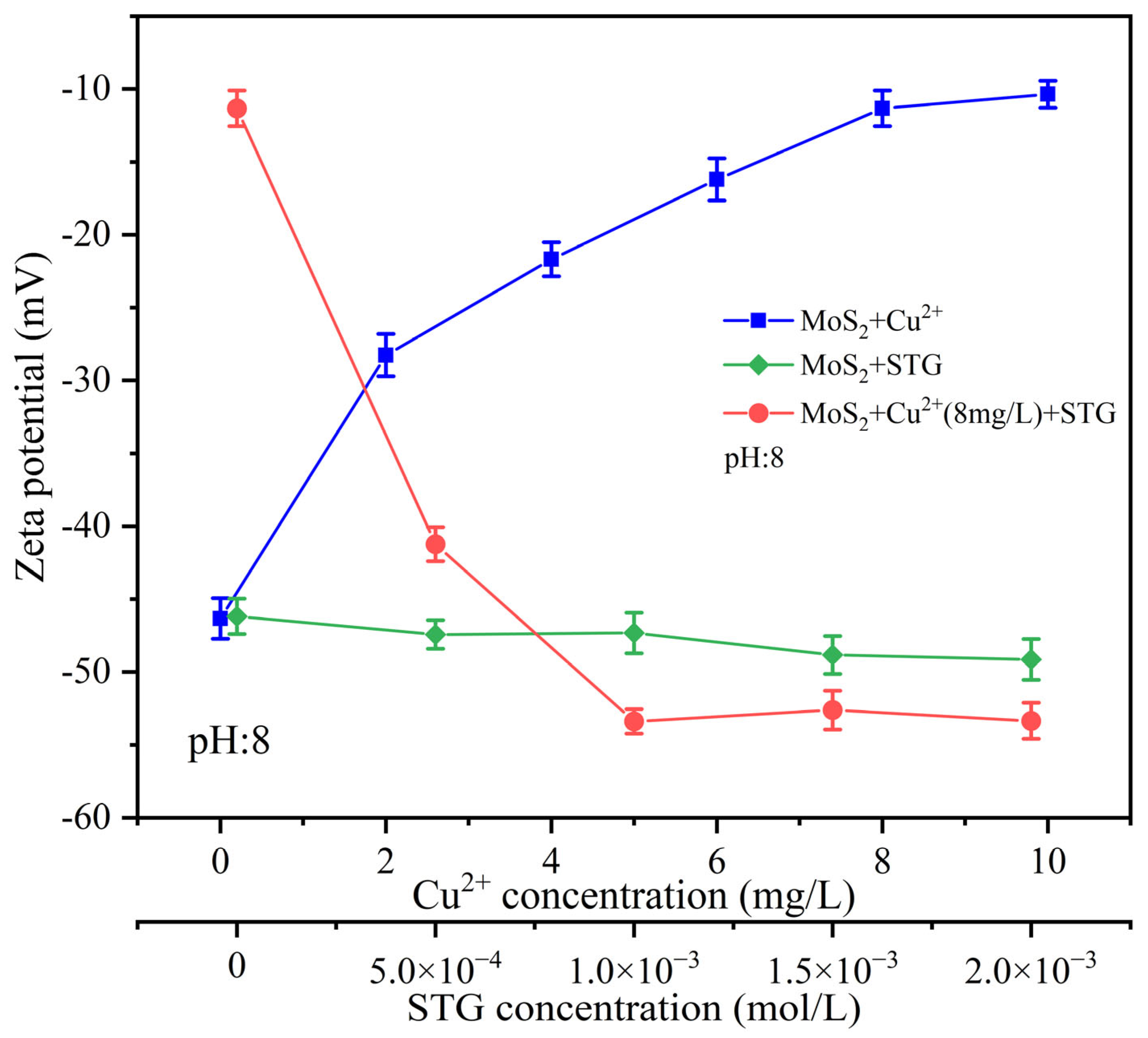
3.3. Zeta Potential Characterizations
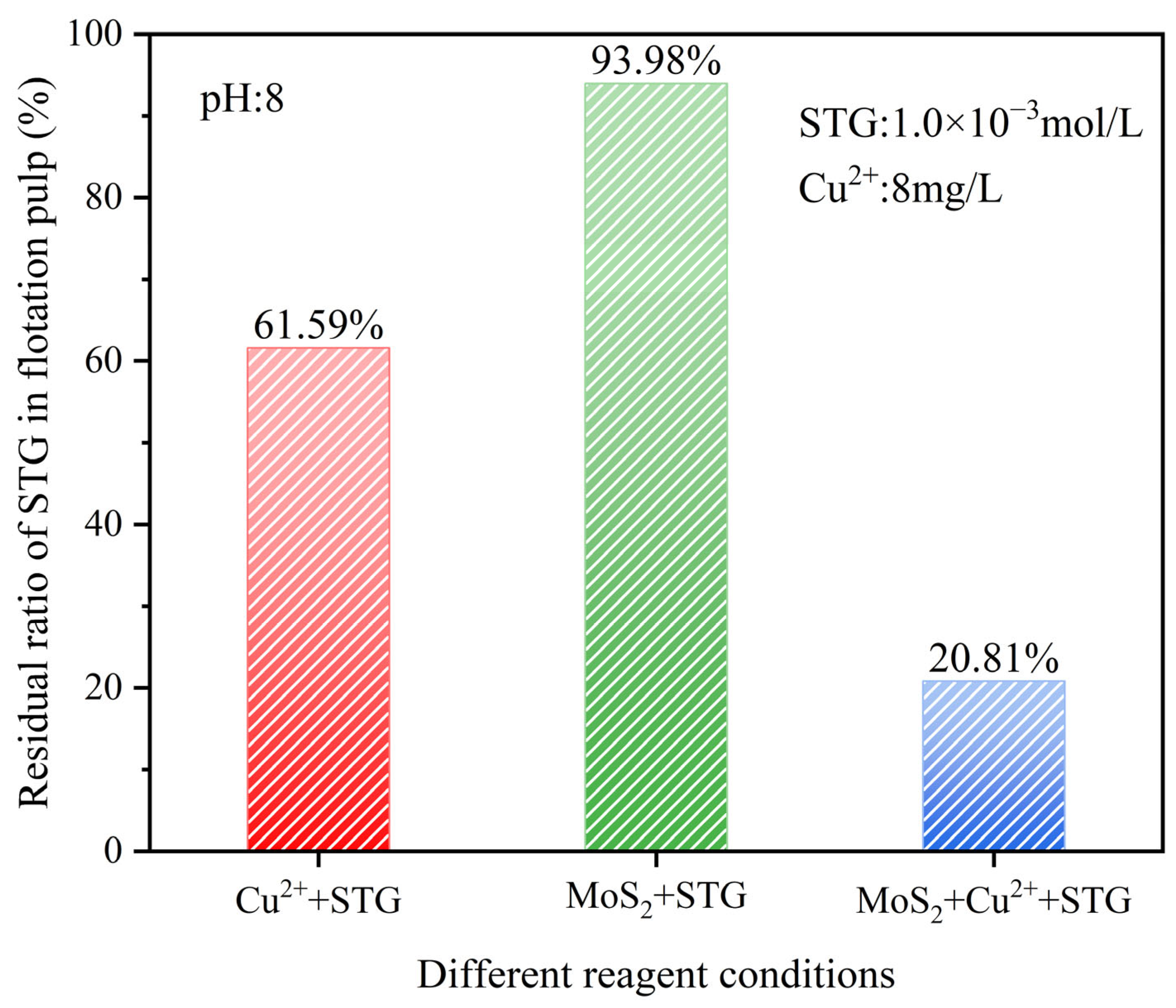
3.4. UV–Vis Spectrophotometer Analysis
3.5. XPS Analysis
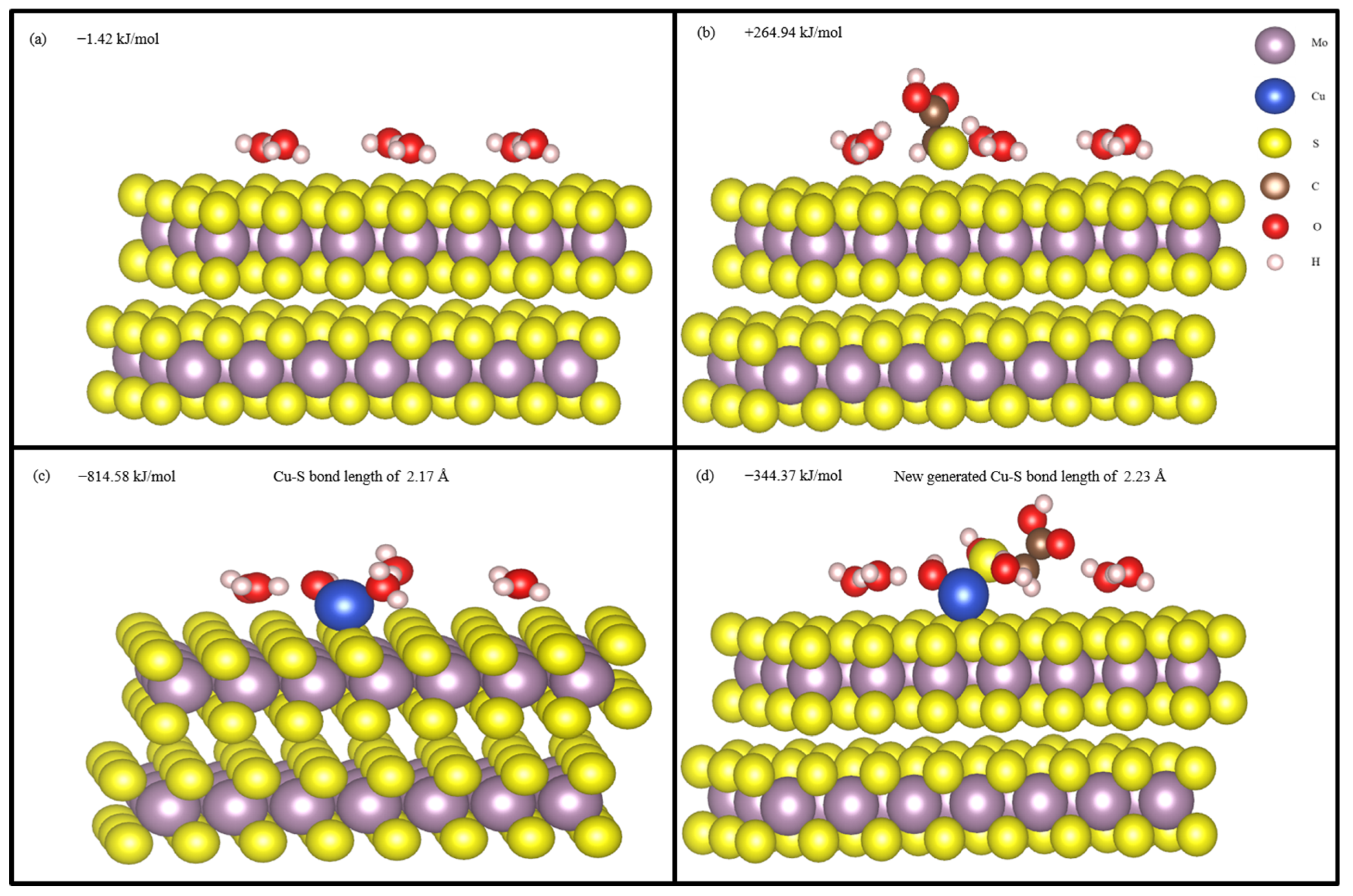
3.6. DFT Analysis
4. Conclusions
- (1)
- Flotation experiments showed that at pH 8.0, neither Cu2+ nor STG alone significantly affected molybdenite flotation. However, the combination of STG with Cu2+ strongly depressed molybdenite flotation, degenerating the separation efficiency.
- (2)
- Contact angle, zeta potential, UV–Vis, and DFT calculation revealed that Cu2+ facilitated STG attachment onto molybdenite, enhancing its surface hydrophilicity and thereby diminishing its floatability.
- (3)
- XPS analysis revealed that Cu2+ predominantly adsorbed onto the molybdenite surface as Cu(I), providing reactive sites for the –SH or –COO− group in STG. The formation of a stable molybdenite–Cu(I)–STG complex effectively suppressed molybdenite flotation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Chen, L.; Xue, Z.; Yang, K.; Shao, Y.; Zeng, J.; Gao, Y. Performance evaluation of PHGMS technology for superfine chalcopyrite-molybdenite separation. Sep. Purif. Technol. 2024, 336, 126136. [Google Scholar] [CrossRef]
- Qin, X.; Liu, J.; Yu, Y.; Hao, J.; Gao, H.; Li, D.; Dai, L. Novel application of depressant sodium mercaptoacetate in flotation separation of chalcopyrite and pyrite. Adv. Powder Technol. 2023, 34, 104141. [Google Scholar] [CrossRef]
- Hlina, J.; Reboun, J.; Hamacek, A. Study of copper thick film metallization on aluminum nitride. Scr. Mater. 2020, 176, 23–27. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Song, X.-Y.; Huang, Y.-H.; Zhang, Z.; Wang, W.; Xu, L.-F. Selective flotation separation of molybdenite and chalcopyrite using O3 oxidation method. Trans. Nonferrous Met. Soc. China 2024, 34, 298–308. [Google Scholar] [CrossRef]
- Yi, G.; Macha, E.; Van Dyke, J.; Ed Macha, R.; McKay, T.; Free, M.L. Recent progress on research of molybdenite flotation: A review. Adv. Colloid. Interface Sci. 2021, 295, 102466. [Google Scholar] [CrossRef]
- Hao, J.; Liu, J.; Yu, Y.; Gao, H.; Qin, X.; Bai, X. Depressants for separation of chalcopyrite and molybdenite: Review and prospects. Miner. Eng. 2023, 201, 108209. [Google Scholar] [CrossRef]
- Tang, X.; Chen, Y.; Liu, K.; Zeng, G.; Peng, Q.; Li, Z. Selective flotation separation of molybdenite and chalcopyrite by thermal pretreatment under air atmosphere. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123958. [Google Scholar] [CrossRef]
- Liang, G.; Chimonyo, W.; Lv, J.; Peng, Y. Differential depression of calcium lignosulfonate on chalcopyrite and molybdenite flotation with collector kerosene. Miner. Eng. 2023, 201, 108192. [Google Scholar] [CrossRef]
- Qi, M.; Peng, W.; Wang, W.; Cao, Y.; Zhang, L.; Huang, Y. A novel molybdenite depressant for efficient selective flotation separation of chalcopyrite and molybdenite. Int. J. Min. Sci. Technol. 2024, 34, 1179–1196. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, Q.; Wen, S.; Xian, Y.; Liu, J.; Liang, G. Flotation separation of chalcopyrite from molybdenite with sodium thioglycolate: Mechanistic insights from experiments and MD simulations. Sep. Purif. Technol. 2024, 342, 126958. [Google Scholar] [CrossRef]
- Pan, C.-L.; Wei, X.-X.; Zhang, X.-G.; Xu, Y.-W.; Xu, P.-F.; Luo, Y.-C. 2-((5-Mercapto-1,3,4-thiadiazol-2-yl)thio)acetic acid as a novel chalcopyrite depressant for selective flotation separation of molybdenite from chalcopyrite. Miner. Eng. 2022, 183, 107625. [Google Scholar] [CrossRef]
- Cheng, D.; Ao, X.; Yuan, X.; Liu, Q. Effect of dissolved metal ions from mineral surfaces on the surface wettability of phosphate ore by flotation. Colloids Surf. A Physicochem. Eng. Asp. 2024, 701, 134995. [Google Scholar] [CrossRef]
- Hao, J.; Liu, J.; Lai, H.; Liao, R.; Gao, H.; Bai, X. A new application of Cu2+ on differential modification to promote copper–molybdenum separation with a novel chalcopyrite depressant amidinothiourea. Sep. Purif. Technol. 2025, 353, 128282. [Google Scholar] [CrossRef]
- Yang, W.; Qiu, X.; Liu, C.; Zhao, G.; Yan, H.; He, X.; Ding, K.; Jiao, Q.; Qiu, T. Flotation separation of chalcopyrite and molybdenite in advanced oxidation systems: Experimental and mechanism study. Powder Technol. 2025, 457, 120876. [Google Scholar] [CrossRef]
- Cai, J.; Jia, X.; Ma, Y.; Pei, B.; Ibrahim, A.M.; Su, C.; Shen, P.; Liu, D. Separation of copper-sulfur using sodium polyacrylate as pyrite depressant in acidic pulp: Floatability and adsorption studies. Miner. Eng. 2022, 188, 107815. [Google Scholar] [CrossRef]
- Chandra, A.P.; Gerson, A.R. A review of the fundamental studies of the copper activation mechanisms for selective flotation of the sulfide minerals, sphalerite and pyrite. Adv. Colloid. Interface Sci. 2009, 145, 97–110. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, C.; Wang, L.; Qiu, Z.; Liu, S.; Chen, W.; Liu, G. The green separation of sphalerite from pyrite: Triazine-dithione collector and its flotation mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2025, 718, 136947. [Google Scholar] [CrossRef]
- Hirajima, T.; Suyantara, G.P.W.; Ichikawa, O.; Elmahdy, A.M.; Miki, H.; Sasaki, K. Effect of Mg2+ and Ca2+ as divalent seawater cations on the floatability of molybdenite and chalcopyrite. Miner. Eng. 2016, 96, 83–93. [Google Scholar] [CrossRef]
- Özçelik, S.; Ekmekçi, Z. Surface Chemistry and Flotation of Gold-Bearing Pyrite. Minerals 2024, 14, 914. [Google Scholar] [CrossRef]
- Feng, Q.; Zhao, G.; Zhang, G.; Zhao, W.; Han, G. Degradation mechanism of surface hydrophobicity by ferrous ions in the sulfidization flotation system of smithsonite. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129119. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, H.; Yang, B.; Jia, F.; Yan, H.; Zeng, M.; Qu, H. Effect of Pb2+ on the flotation of molybdenite in the presence of sulfide ion. Results Phys. 2019, 14, 102361. [Google Scholar] [CrossRef]
- Yuanjia Luo, W.S.; Han, H.; Peng, J.; Jiang, F. Uncovering the oxidation mechanism of sphalerite (ZnS) in the absence and presence of water: A first-principles investigation. Int. J. Min. Sci. Technol. 2025, 35, 149–157. [Google Scholar]
- Zhong, J.; Zhang, Q.; Xing, S.; Sun, W.; Tang, H.; Zhang, L.; Jiang, F.; Luo, Y. A new attempt for low-alkalinity flotation separation of chalcopyrite/molybdenite from pyrite using nonpolar collectors. J. Mol. Liq. 2025, 422, 126898. [Google Scholar] [CrossRef]
- Luo, Y.; Xia, Y.; Wang, C.; Chen, J.; Ou, L. Application of calcium lignosulphonate as an environmentally friendly depressant in the Cu–As separation by froth flotation at low alkalinity. J. Clean. Prod. 2023, 406, 137073. [Google Scholar] [CrossRef]
- Qiu, T.; Ding, K.; Yan, H.; Yang, L.; Wu, H.; Zhao, G.; Qiu, X. Electrochemistry and DFT study of galvanic interaction on the surface of monoclinic pyrrhotite (001) and galena (100). Int. J. Min. Sci. Technol. 2024, 34, 1151–1162. [Google Scholar] [CrossRef]
- Luo, A.; Chen, J. Effect of hydration and hydroxylation on the adsorption of metal ions on quartz surfaces: DFT study. Appl. Surf. Sci. 2022, 595, 153553. [Google Scholar] [CrossRef]
- Luo, Y.; Ou, L.; Chen, J. Experimental and computational study of the differential sulfidization mechanism of smithsonite and calcite. Miner. Eng. 2023, 198, 108102. [Google Scholar] [CrossRef]
- Luo, Y.; Sun, W.; He, S.; Peng, J.; Jiang, F. Innovative discovery on the impact of hydration on the alternative flotation of smithsonite and calcite under benzohydroxamic acid system. J. Mol. Liq. 2024, 415, 126433. [Google Scholar] [CrossRef]
- Luo, Y.; Ou, L.; Chen, J.; Zhang, G.; Xia, Y.; Zhu, B.; Zhou, H. Study on the enhancement mechanism of Pb ion on smithsonite sulfidation with coordination chemistry and first-principles calculations. Appl. Surf. Sci. 2022, 597, 153672. [Google Scholar] [CrossRef]
- Butt, H.-J.; Liu, J.; Koynov, K.; Straub, B.; Hinduja, C.; Roismann, I.; Berger, R.; Li, X.; Vollmer, D.; Steffen, W.; et al. Contact angle hysteresis. Curr. Opin. Colloid. Interface Sci. 2022, 59, 101574. [Google Scholar] [CrossRef]
- Chau, T.T.; Bruckard, W.J.; Koh, P.T.; Nguyen, A.V. A review of factors that affect contact angle and implications for flotation practice. Adv. Colloid. Interface Sci. 2009, 150, 106–115. [Google Scholar] [CrossRef]
- Zhao, L.; Zhuang, L.; Zhang, Z. The direct observation and distribution regularity of contact angle for spherical particle attached to air bubble in static three-phase system. Miner. Eng. 2023, 201, 108191. [Google Scholar] [CrossRef]
- Kruszelnicki, M.; Polowczyk, I.; Kowalczuk, P.B. Insight into the influence of surface wettability on flotation properties of solid particles–Critical contact angle in flotation. Powder Technol. 2024, 431, 119056. [Google Scholar] [CrossRef]
- Feng, Q.; Zhao, W.; Wen, S.; Cao, Q. Activation mechanism of lead ions in cassiterite flotation with salicylhydroxamic acid as collector. Sep. Purif. Technol. 2017, 178, 193–199. [Google Scholar] [CrossRef]
- Salmani Nuri, O.; Irannajad, M.; Mehdilo, A. Effect of surface dissolution by oxalic acid on flotation behavior of minerals. J. Mater. Res. Technol. 2019, 8, 2336–2349. [Google Scholar] [CrossRef]
- Zhang, Q.; Wen, S.; Feng, Q.; Zhang, S. Surface characterization of azurite modified with sodium sulfide and its response to flotation mechanism. Sep. Purif. Technol. 2020, 242, 116760. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Q. Depression mechanism of acid for flotation separation of fluorapatite and dolomite using ToF-SIMS and XPS. J. Mol. Liq. 2024, 394, 123584. [Google Scholar] [CrossRef]
- Liu, R.; Liu, D.; Li, J.; Liu, S.; Liu, Z.; Gao, L.; Jia, X.; Ao, S. Improved understanding of the sulfidization mechanism in cerussite flotation: An XPS, ToF-SIMS and FESEM investigation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124508. [Google Scholar] [CrossRef]
- Zhang, N.; Ejtemaei, M.; Nguyen, A.V.; Zhou, C. XPS analysis of the surface chemistry of sulfuric acid-treated kaolinite and diaspore minerals with flotation reagents. Miner. Eng. 2019, 136, 1–7. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, W.; Wei, D.; Wang, W.; Cui, B.; Liu, W. Effect of copper ions on the flotation separation of chalcopyrite and molybdenite using sodium sulfide as a depressant. Miner. Eng. 2018, 115, 44–52. [Google Scholar] [CrossRef]
- Yang, B.; Wang, D.; Wang, T.; Zhang, H.; Jia, F.; Song, S. Effect of Cu2+ and Fe3+ on the depression of molybdenite in flotation. Miner. Eng. 2019, 130, 101–109. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Wu, X.; Wang, Z.; Wei, Z. Pyrite activation in seawater flotation by copper and lead ions: XPS and in-situ electrochemical investigation. Appl. Surf. Sci. 2025, 680, 161363. [Google Scholar] [CrossRef]
- Huang, W.; Liu, R.; Jiang, F.; Tang, H.; Wang, L.; Sun, W. Adsorption mechanism of 3-mercaptopropionic acid as a chalcopyrite depressant in chalcopyrite and galena separation flotation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128063. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, X.; Luo, A.; Chen, J.; Meng, Y. A modified co-polyacrylamide to depress molybdenite in Cu/Mo separation and its competitive adsorption mechanism between reagents. Sep. Purif. Technol. 2024, 337, 126239. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, L.; Yue, T.; Tang, H.; Wang, L.; Sun, W.; Zhang, C.; Chen, J. Defect-boosted molybdenite-based co-catalytic Fenton reaction. Inorg. Chem. Front. 2021, 8, 3440–3449. [Google Scholar] [CrossRef]
- Liu, S.; Xie, L.; Liu, G.; Zhong, H.; Wang, Y.; Zeng, H. Hetero-difunctional Reagent with Superior Flotation Performance to Chalcopyrite and the Associated Surface Interaction Mechanism. Langmuir 2019, 35, 4353–4363. [Google Scholar] [CrossRef]
- Pan, W.; Li, S.; Zhu, Y.; Gao, L.; Ma, Z.; Cao, Y.; Du, S. Hydration mechanism of molybdenite affected by surface oxidation: New insights from DFT and MD simulations. Colloids Surf. A Physicochem. Eng. Asp. 2024, 698, 134599. [Google Scholar] [CrossRef]
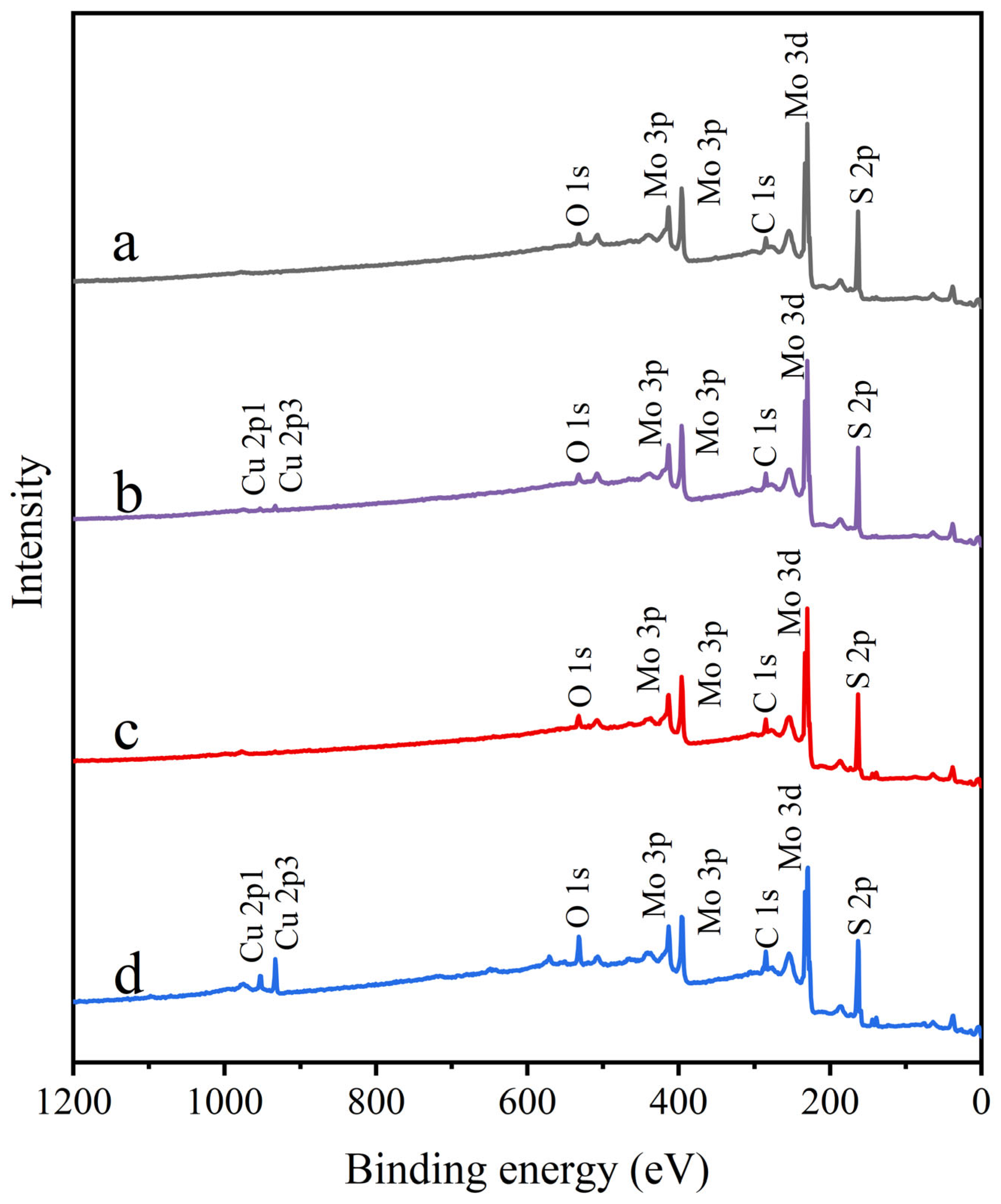
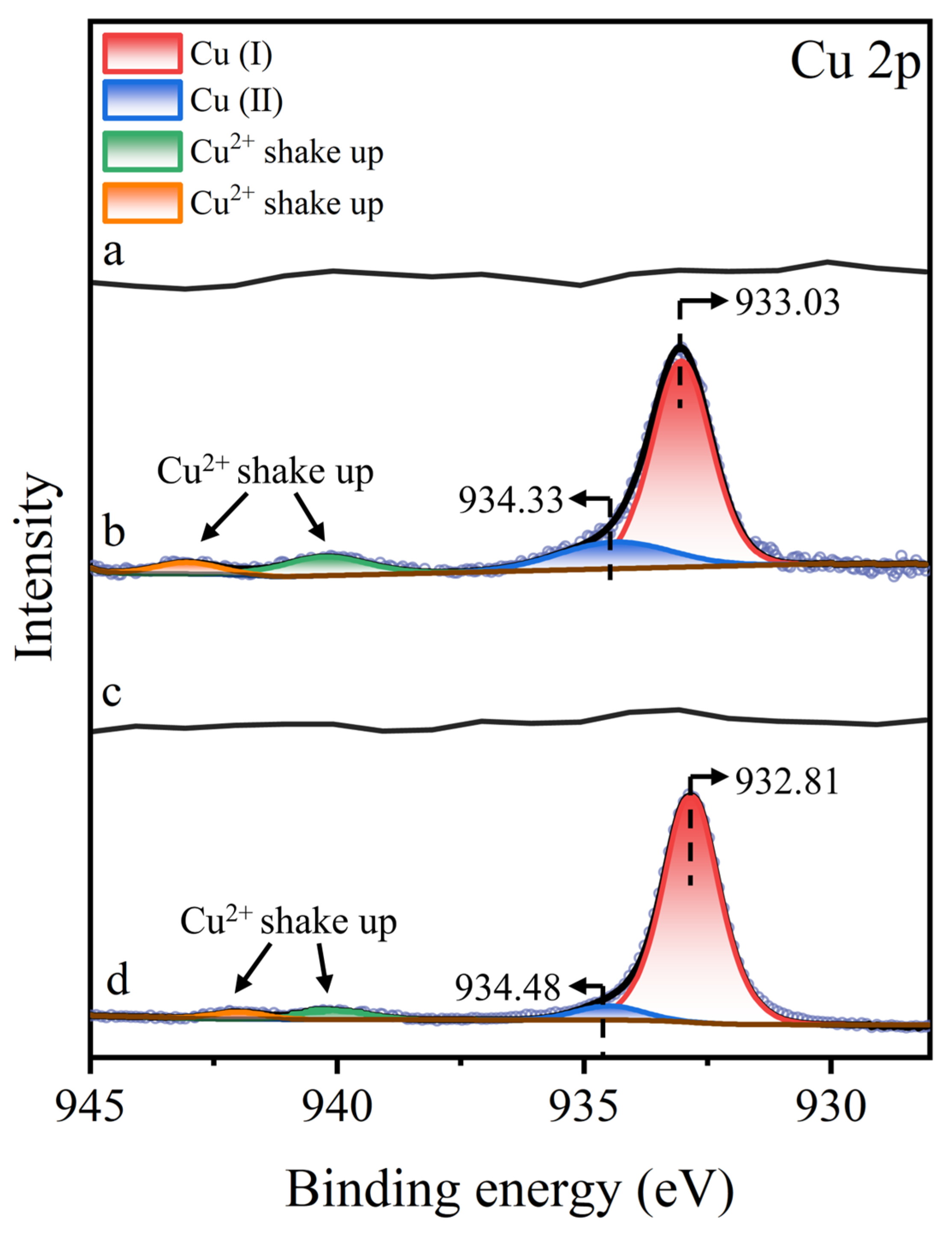
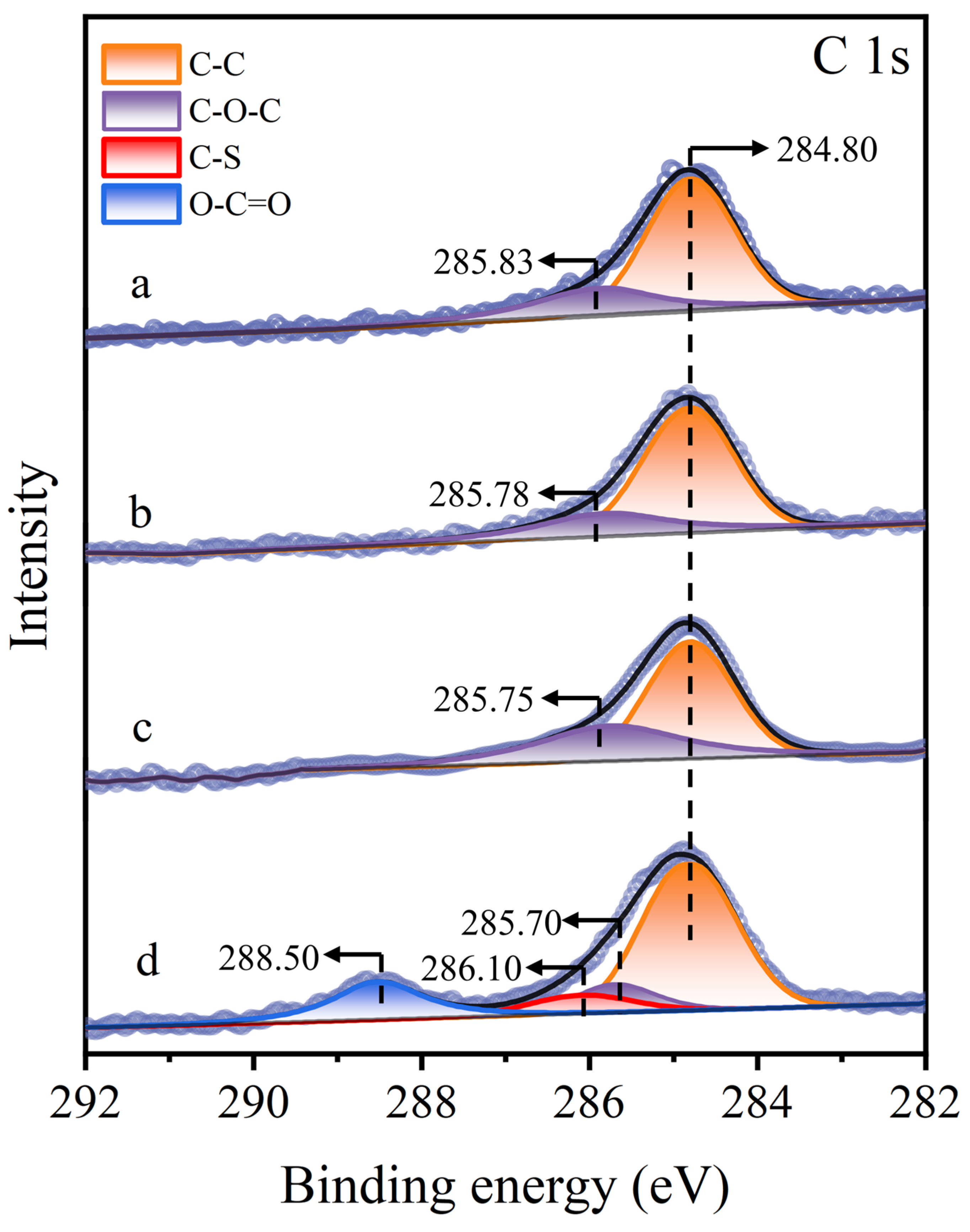

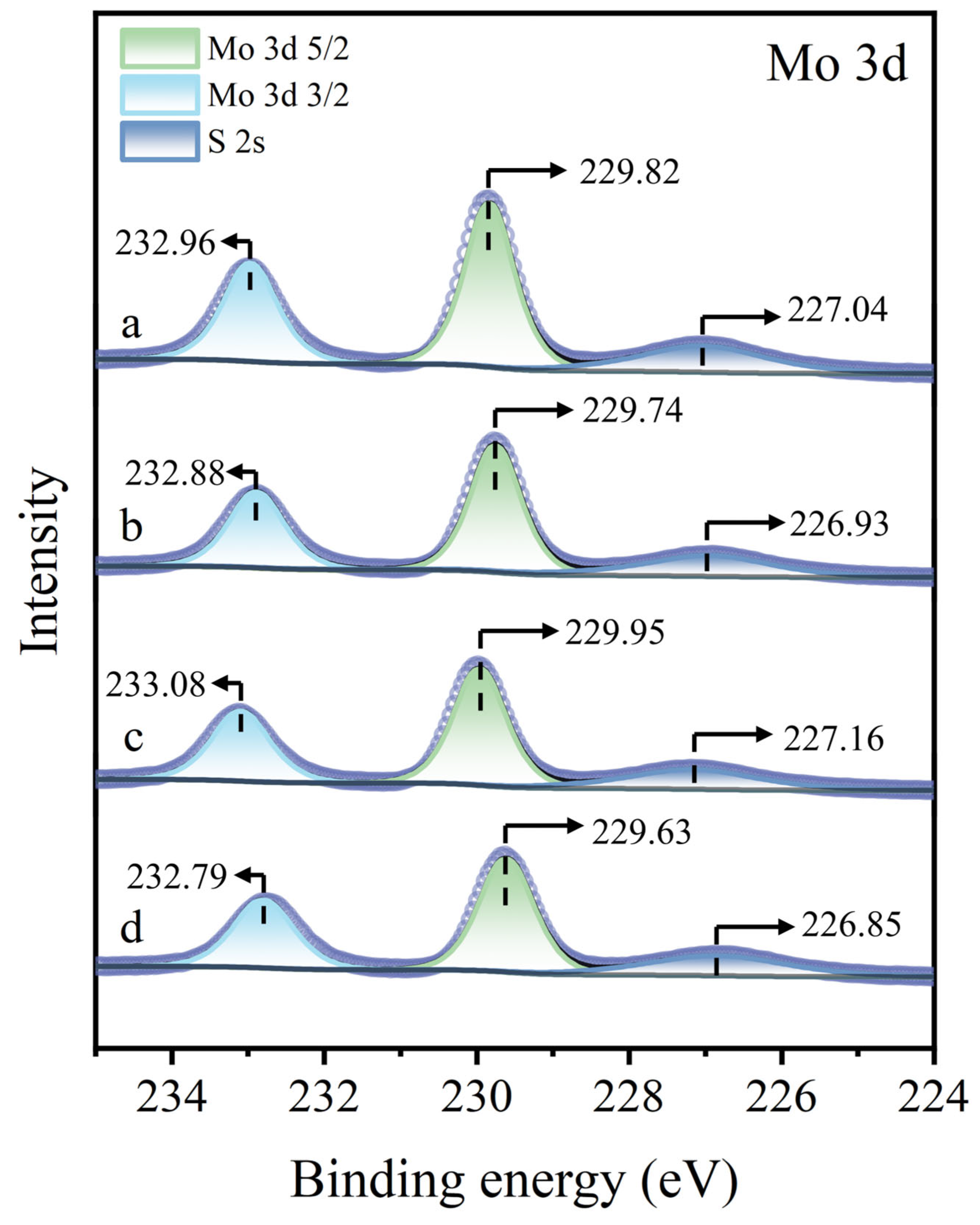
| Samples | Elements (Atomic%) | ||||
|---|---|---|---|---|---|
| Cu | Mo | S | C | O | |
| Untreated molybdenite | 0.19 | 26.83 | 50.88 | 12.90 | 9.19 |
| Cu2++ molybdenite | 0.91 | 27.11 | 45.19 | 18.48 | 8.31 |
| STG+ molybdenite | 0.22 | 23.80 | 47.63 | 17.21 | 11.14 |
| Cu2++STG+ molybdenite | 3.65 | 18.78 | 44.85 | 18.50 | 14.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, F.; He, S.; Qi, J.; Luo, Y.; Tang, H. Unraveling the Impact of Copper Ions on Mineral Surfaces During Chalcopyrite–Molybdenite Flotation Separation Using Sodium Thioglycolate. Appl. Sci. 2025, 15, 7293. https://doi.org/10.3390/app15137293
Jiang F, He S, Qi J, Luo Y, Tang H. Unraveling the Impact of Copper Ions on Mineral Surfaces During Chalcopyrite–Molybdenite Flotation Separation Using Sodium Thioglycolate. Applied Sciences. 2025; 15(13):7293. https://doi.org/10.3390/app15137293
Chicago/Turabian StyleJiang, Feng, Shuai He, Jiaxing Qi, Yuanjia Luo, and Honghu Tang. 2025. "Unraveling the Impact of Copper Ions on Mineral Surfaces During Chalcopyrite–Molybdenite Flotation Separation Using Sodium Thioglycolate" Applied Sciences 15, no. 13: 7293. https://doi.org/10.3390/app15137293
APA StyleJiang, F., He, S., Qi, J., Luo, Y., & Tang, H. (2025). Unraveling the Impact of Copper Ions on Mineral Surfaces During Chalcopyrite–Molybdenite Flotation Separation Using Sodium Thioglycolate. Applied Sciences, 15(13), 7293. https://doi.org/10.3390/app15137293





