Featured Application
The automated mechatronic system guarantees the uniformity of drug formulations administered to patients via syringe pumps. This uniformity is achieved through biaxial mechanical agitation combined with photochemical treatment.
Abstract
Ensuring consistent drug concentration during intravenous (IV) administration is essential for patient safety in intensive care units (ICUs). Standard syringe pumps are prone to concentration variability due to sedimentation, molecular aggregation, and drug adsorption on plastic surfaces, especially during prolonged, low-rate infusions. We propose a novel mechatronic homogenization system integrated into syringe pumps, which combines dual-axis vibration, low-power eccentric motors, and a two-stage photonic treatment mechanism to maintain drug stability. The system is theoretically modeled and experimentally validated across multiple drug classes. Results show a significant reduction in concentration variability by over 90% compared to conventional syringe pumps, demonstrating strong potential for clinical impact. However, the study was conducted on a small class of drugs and requires a diversification of the classes of drugs that are subject to the experiment, especially in photonic treatment, where the effects on other classes of drugs administered intravenously are not fully known.
1. Introduction
The development of a mechatronic homogenization system represents a significant advance in the quest for maintaining drug concentration uniformity. This technology has the potential to revolutionize pharmaceutical formulations, particularly for drugs requiring precise dosage, such as insulin, furosemide, and gentamicin. However, while the innovation is promising, an in-depth analysis reveals several shortcomings that warrant further investigation. Unlike previous approaches, our system integrates dual-axis mechanical homogenization and photonic surface treatment into a compact, low-energy design that is scalable and feasible for clinical implementation.
Empirical observations have revealed temporal inconsistencies in patient physiological responses following the preparation and infusion of drugs using syringe-based delivery systems. These findings have initiated a targeted inquiry into the contributing factors, with particular focus on infusion duration and administered volume as key variables potentially affecting alterations in vital signs [1,2,3]. These parameters are increasingly recognized as contributing to hemodynamic instability and systemic physiological variability in critically ill patients. Intravenous administration of drugs via syringe pumps is foundational in intensive care unit (ICU) care. However, variability in drug concentration during infusion, especially for drugs with poor solubility or high adsorption potential (e.g., insulin, furosemide, chemotherapeutics, and dopamine), can compromise efficacy and safety [4]. These variations often arise from
- Sedimentation in the syringe [5].
- Surface adhesion to polypropylene walls [5].
- Chemical instability or aggregation [5].
Such phenomena contribute to hemodynamic instability, unintended overdosing/under-dosing, and cytotoxic effects, particularly during long infusions or when syringes are pre-filled hours before use.
We propose a low-energy, modular homogenization system embedded in syringe pumps that:
- Vibrates the syringe along dual axes to prevent sedimentation.
- Applies targeted photonic treatment (470 and 700 nm) [6] to modify syringe surface chemistry and stabilize the drug formulation.
- Operates within strict power, thermal, and safety limits for hospital environments.
The proposed electronic system operates through a dual mechanism: mechanical agitation via stirring of the drug solution and photonic activation through exposure to light within the syringe pump. Prior specialized studies have demonstrated that chemical compounds exposed to high-intensity light at a wavelength of 700 nm exhibit enhanced stability and maintain improved homogeneity. In the present study, a simulation of drug administration at low infusion rates (2–4 mL/h) was conducted. The objective is to evaluate and compare the degree of solution homogeneity achieved by conventional syringe pumps versus the newly developed model by our team [5,6,7].
Drug infusion devices are an important part of drug delivery in intensive care unit (ICU) patient care as well as in the operating rooms (OR). Drugs are delivered via these electromechanical systems with moderate precision [5,6,7]. The flow continuity of two brands of syringe pumps and four brands of syringes was studied as a possible cause of hemodynamic fluctuations observed in neonates. Syringe plunger force exhibited regular fluctuations, indicating plunger sticking or unexplained delivery irregularities [7].
Observational studies have identified a pattern of hemodynamic disturbances in patients occurring after a certain duration following the preparation of injectable drug solutions. Given the high incidence and variability in performance among syringe pump manufacturers, this phenomenon has prompted further investigation by the research team [8]. A leading hypothesis attributes these effects to temporal changes in solution homogeneity, potentially resulting in the inadvertent administration of drug concentrations that deviate from prescribed dosages [8]. These findings raise significant concerns regarding the precision and reliability of dose delivery by infusion devices, highlighting the need for detailed evaluation of potential discrepancies between intended and actual administered concentrations. Another reference shows that medication errors remain a common problem in clinical practice, with estimates suggesting that approximately one in five doses is affected in a typical hospital setting [8]. Critically ill patients in intensive care units (ICUs) are particularly susceptible to such errors, largely due to the simultaneous infusion of multiple high-potency intravenous drugs using syringe pumps [9]. The main findings show that there is a difference in concentration at the time of patient administration in in vitro tests. These differences range from 4 to 30% (compared to the desired concentration) for standard syringe pumps set at low infusion rates of 3–5 mL/h.
2. Materials and Methods
Previous studies addressed drug inhomogeneity using
- Manual agitation (labor-intensive, error-prone).
- Microfluidic mixers (costly and incompatible with bedside settings SBS-LAB-125, Steinberg Systems, Berlin, Germany).
- Ultrasonic or magnetic stirrers (bulky and impractical for syringe pumps).
- Piezoelectric/electric eccentric motor/actuators (limited adoption due to cost and integration challenges), model-JXBOVE = 6 mm-3 v.
None of these methods has been integrated into commercial syringe pumps nor validated in long-duration infusions typical of ICUs.
This work is built on
- Plasma/UV laser surface modification of polymers to reduce adsorption [8].
- Near-infrared stabilization of protein structures [9].
- Vibration-induced mixing for low Reynolds number fluids.
- Medcaptain HP-80 syringe pump (Medcaptain Medical Technology, Shenzhen, China), the syringe pump model in which the technology was implemented and tested.
We contribute a compact, affordable, and clinically feasible system combining these principles, with validation across multiple drug types.
Previous studies addressed drug inhomogeneity using various mechanical and optical means, but none have been successfully integrated into commercial syringe pumps. Our work combines compact piezo-driven vibration and photonic surface treatment into a practical, clinically compatible solution [9,10,11,12,13] (Figure 1).
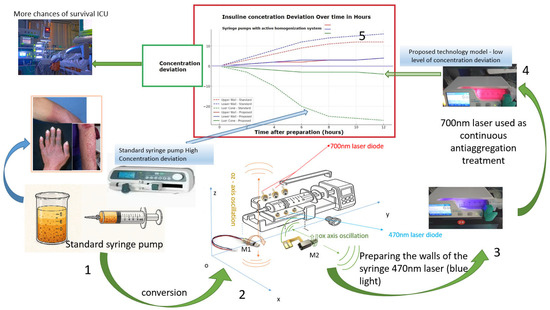
Figure 1.
Abstract graphic, technology comparison: current technology vs. technological proposal.
Several studies have investigated the effects of sedimentation in drug infusion systems, highlighting that flow interruptions and prolonged infusion times exacerbate the risk of concentration variability [11,14,15,16,17]. For example, research on lipid emulsions and protein-based drugs has demonstrated that separation occurs over time, leading to inconsistent dosing. Agitation-based solutions, such as magnetic stirrers and ultrasonic mixing, have been proposed, but these methods are often impractical for bedside applications due to their energy requirements and potential interference with infusion dynamics [1,3,7,12,18].
In contrast to previous approaches, our system uses a dual-axis vibration homogenizer, minimizing sedimentation effects and ensuring a stable concentration profile throughout the infusion process. By incorporating this functionality into a standard syringe pump design, we improve usability while maintaining compatibility with existing clinical protocols. Our approach builds on previous research in fluid dynamics and mechatronics, integrating vibration-assisted mixing into a practical and energy-efficient solution suitable for intensive care environments. Another issue addressed is the adhesion of chemicals to the walls of plastic syringes such as polypropylene. This adhesion is inhibited by our system by applying photonic technology to the syringe walls during the pre-fill process. Another technology approach is the application of pulsed photonic technology to maintain homogeneity by preventing the formation of aggregates (700 nm IR) [7].
2.1. Mechanical Design
The core system includes
- Two eccentric micro-vibration motors, oriented 90° apart (X and Y axes).
- A microcontroller-based astable multivibrator circuit using NPN BLC547 transistors to alternate motor activation.
- Piezoelectric drivers for energy-efficient oscillation.
- Plastic-compatible laser diodes for photonic treatment.
- A total of 470 nm/(400 mW): Syringe wall pre-treatment to prevent adsorption.
- A total of 700 nm/(500 mW): Continuous illumination to prevent aggregation during infusion.
The total mechanical energy required for each vibration-induced agitation cycle is estimated to be approximately 0.3951 mJ per axis. Considering actuation along two orthogonal axes, the total energy consumption per cycle is approximately 0.7902 mJ. Agitation is applied intermittently to minimize interference with the primary forces exerted during the delivery process. The system includes two eccentric micro-vibration motors mounted perpendicularly, a custom transistor-based multivibrator circuit, and dual-wavelength laser diodes. Total power consumption is kept under 1.6 W. Photonic treatment helps reduce surface adsorption, while vibration ensures consistent drug dispersion.
The core innovation of our approach lies in the integration of a two-axis vibration mechanism within the syringe pump. This system consists of (Figure 2):
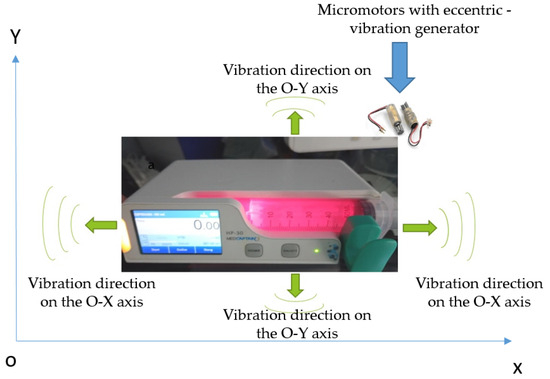
Figure 2.
Proposed model of syringe pump with mechatronic homogenization system dual-axis vibration system.
The 470 nm laser driver has the role of powering the laser diodes in the pre-filling phase of the syringe. This wavelength ensures a treatment of the polypropylene surfaces for 2 min. The automatic system announces by sound signal that the syringe is ready for the next stage, filling the syringe with the medicinal treatment. This diode automatically stops when fluids are present in the syringe so as not to distort the chemical structure of the drug by the presence of ultraviolet rays. The ultraviolet treatment of the syringe walls has the role of reducing the adhesion of chemical substances such as insulin to the syringe walls. The lower the adhesion to the syringe walls, the greater the degree of homogeneity of the substance injected into the patient.
The 700 nm driver remains permanently on during the entire duration of the drug distribution; its role is to anti-aggregate the prepared drug.
2.2. Theoretical Modeling
Homogeneity is quantified using
- Homogeneity Index (H) based on density standard deviation.
- Density gradient (∇ρ) for vertical stratification.
- Fick’s second law for diffusion-based mixing.
- Kinetic energy and viscous resistance for vibration modeling.
Assuming a 50 mL aqueous solution:
- Vibration-induced energy: ~0.39 mJ per cycle.
- Power requirement: ~3.95 mW per axis.
- IR laser power requirement: ~1.4 W (realistic with 3 × 500 mW NIR diodes).
To validate our hypothesis, we developed a mathematical model describing the diffusion and mixing dynamics of injectable drugs under continuous vibration. Our model accounts for:
The interplay between Brownian motion and forced mechanical agitation.
The relationship between vibration frequency, amplitude, and homogeneity over time.
Drug-specific properties, such as viscosity and molecular weight, influence the effectiveness of the homogenization process.
Next, we will present the calculation methods for homogenizing solutions depending on the viscosity and volume gradient.
Homogeneity Index (H) Based on Density Variation A solution is considered homogeneous if the density (ρ) is uniform throughout the volume. The homogeneity index can be defined as
- H = homogeneity index (0 to 1, where 1 is perfectly homogeneous)
- σρ = standard deviation of density in different regions of the solution
- ρ− = mean density of the solution
If H ≈ 1
H ≈ 1, the administered drug solution is highly homogeneous; if H < 1H < 1, the solution is heterogeneous.
Density gradient (∇ρ) as a measure of drug solution mixing in the intensive care unit (ICU)
Another way to assess homogeneity is to analyze the spatial density gradient in a drug solution formed by
The author’s interest is in the law of Diffusion-Based Homogeneity Over Time. If diffusion is the primary mixing mechanism, the density variation follows Fick’s second law:
2.2.1. Kinetic Energy Theory of Fluid Motion
The kinetic energy required to move the fluid inside the syringe due to vibration can be estimated as
The team analyzes kinetic power to determine what energy is required for the homogeneous mixture of two substances in the 50 mL syringe pump.
m = ρV is the mass of the liquid (V = 50 mL = 0.050 L)ν = 2πfA
Thus,
Viscosity resistance
The work conducted per cycle to overcome viscous damping is given by
where
η\etaη = dynamic viscosity (e.g., for water, 10−3 Pa·s).
d = diameter of the syringe (assumed ~ 20 mm = 0.02 m)
2.2.2. Total Energy per Second (Power Requirement)
The total power required is the sum of kinetic and viscous dissipation contributions per unit time.
2.2.3. Numerical Calculation
Typical values:
ρ ≈ 1000\rho\approx 1000 ρ ≈ 1000 kg/m³ (for aqueous solution)
V = 50V = 50V = 50 mL = 0.00005 m³
f = 10f = 10f = 10 Hz
A = 2A = 2A = 2 mm = 0.002 m
d = 0.02d = 0.02d = 0.02 m
η ≈ 10−3 Pa·s
V = 50V = 50V = 50 mL = 0.00005 m³
f = 10f = 10f = 10 Hz
A = 2A = 2A = 2 mm = 0.002 m
d = 0.02d = 0.02d = 0.02 m
η ≈ 10−3 Pa·s
2.2.4. Results of the Calculation
Kinetic Energy (KE): 0.00039 J (~0.39 mJ)
Work Against Viscosity (W): 3.95 × 10 − 83.95/times 10−8 J (~39.5 nJ)
Total Power Required (P): 3.95 mW
2.2.5. Interpretation
The energy required per cycle to homogenize the solution is ~0.39 mJ.
The work lost due to viscosity is negligible, meaning the mixing is efficient.
The power requirement for continuous homogenization is ~3.95 mW, which is very low and easily achievable with a small motorized vibration system.
The calculations result in a required power of 3.95 mW per oscillation axis x = 2.
3.95 mW × (X) = 3.95 mw × 2 = 7.9 mW (Power required to achieve a homogeneous solution)
The proposed circuit is an astable circuit, which ensures an alternative start of the microvibration-generating motors on the two axes XOY.
The choice of axes was determined due to the hypotheses regarding the sedimentation of the administered drugs. These axial movements at the previously calculated forces ensure the homogeneity of the drugs.
The proposed circuit is based on two transistors in an astable circuit. The role of the transistors is to control the eccentric motors intermittently so as not to interfere with the movements.
The proposed circuit is based on two transistors in an astable circuit (Figure 3, astable circuit with eccentric motors).
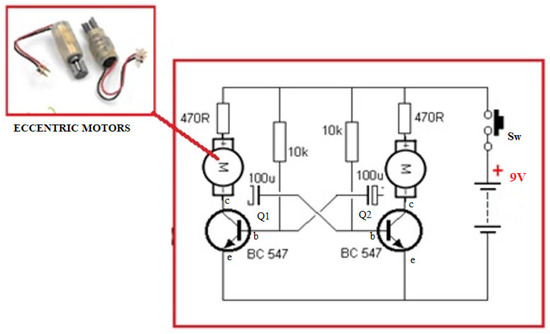
Figure 3.
Astable circuit with eccentric motors—vibration generators on two axes.
The role of the transistors is to control the eccentric motors intermittently so as not to interfere with the movements. Two NPN transistors with a positive base were chosen in order to be able to make this device at a minimum price. The NPN BLC 547 transistors support a thermal dissipation power of 625 mW, with a power of 100 mA. The supply voltage of 9 v with 100 mA can easily provide power to the eccentric motor of 48 mW. Thus:
- 9 v × 100 mA = 900 mW
- calculating the safety index
- 900 mW/500 mW = 1.8 safety index
- In the attached circuit, we have a safety index = 1.8.
The assessment of quality and safety measures in medical practice has become increasingly relevant and essential in modern healthcare. In the intravenous administration of injectable treatments, the dynamic interplay between injection quality and patient safety presents challenges in independently quantifying either parameter. By developing a standardized metric that integrates both quality and safety—referred to as the Quality-Safety Index—a unified method for evaluating these factors can be theoretically established. This approach would allow for a more comprehensive analysis within the context of individual patient profiles, clinical evaluations, and personalized treatment protocols.
The safety rate refers both to the rate of component overload during operation and to the potential risks associated with medical malpractice.
The two vibration generators are located on different axes of symmetry at 90 degrees to each other. The role is to ensure the homogeneity of the drugs inserted into the patient.
Certain wavelengths of light could influence the surface interactions of drugs such as insulin and propofol with plastic syringe materials. This effect would probably work through photoexcitation, modification of the surface charge, or breaking of molecular bonds responsible for adhesion.
The proposed system is distinguished by two different wavelengths, 470 nm and 700 nm (500 mW), respectively. The first wavelength of 470 nm (400 mw) is the one that treats the walls of the syringe. This wavelength, generated by the laser diode, has the role of impressing the plastic material by behavioral changes on the chemical substances. Following laser treatment of the syringe walls, chemicals no longer adhere to the plastic surface for 12 h (the investigation period chosen was 12 h). The UV wavelength of 470 nm is applied to the empty syringe, without treatment as in the image below (Figure 4).
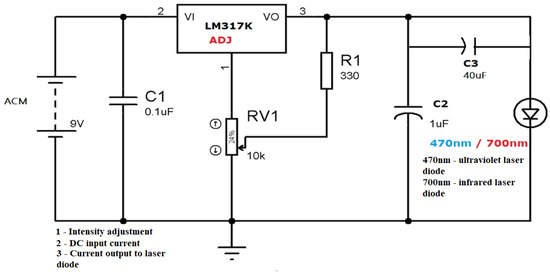
Figure 4.
Laser circuit: 470nm—ultraviolet laser diode/700nm—infrared laser diode Common power supply circuit.
Certain wavelengths of light can modulate the interaction between drugs like insulin and propofol and the plastic surfaces of syringes. This effect is primarily driven by photoexcitation, which alters surface charge properties or disrupts molecular adhesion mechanisms.
The proposed system utilizes two distinct wavelengths—470 and 780 nm, each serving a specific function in maintaining drug homogeneity.
The blue light is used only to treat the walls of the syringe; there should be no drugs during exposure to the 400–500 nm wavelength laser. Erroneous exposure can destroy and precipitate the treatment. A photodiode can be integrated inside the device, which detects whether there is medication or not in the syringe. If liquid is detected, then the processor does not activate the UV treatment of the syringe walls, and activates the alarm (medication present in the syringe—UV treatment impossible). The duration of exposure of the syringe was 2 min and 30 s. Notably, 470 nm 400 mW (Blue Light Treatment): This wavelength is applied before drug administration to modify the syringe’s inner surface, preventing chemical adhesion for up to 12 h. Generated by a laser diode, it induces structural modifications in the plastic, altering its interaction with the drug molecules.
The treatment is applied to empty syringes before use, ensuring long-term stability. Notably, 700 nm 500 mW (Near-Infrared Light for Dynamic Stability): This wavelength is actively applied throughout the infusion process, acting as an antiplatelet for the drug solution. Research suggests that NIR light between 700 and 980 nm can stabilize protein structures, preventing unfolding and surface adhesion. Continuous exposure ensures homogeneous drug delivery and reduces the risk of concentration fluctuations. By combining these two light-based interventions, the system significantly improves the stability and bioavailability of intravenously administered medications, minimizing drug loss due to adsorption and aggregation.
The authors go on to cite other recent additional studies such as [6,19,20,21], by which I cite the following:
The distributional homogeneity indexes of one active ingredient image were then computed and compared. The results show that both techniques achieved similar conclusions. However, the analysis times were drastically different. While Raman imaging required a total analysis time of 4 h per tablet to obtain the distribution map of acetylsalicylic acid with a step size of 100 µm, it only took 7.5 min to achieve the same result with LDIR. The results obtained in the present study show that LDIR is a promising technique for the analysis of pharmaceutical formulations and that it could be a valuable tool when developing new pharmaceutical formulations [6,21].
2.2.6. IR Irradiation System Functionality
Some drugs can crystallize or phase separate over time, especially if the temperature fluctuates. Controlled IR exposure stabilizes the local temperature of the solution, prevents the formation of crystal nuclei, and maintains the solution in a meta-stable state.
2.2.7. Activation of Stabilizing Excipients
In some formulations, excipients (e.g., polymers, surfactants) can change their behavior under IR, becoming more effective in stabilizing the solution. For example, poloxamers can become more mobile, and surfactants can form micelles more efficiently.
2.2.8. Localized Molecular Agitation (Focused IR)
Some perfusion systems may include IR diodes that emit in a controlled manner to maintain particle movement at a microscopic level without mechanically disturbing the solution (unlike physical agitation).
Nucleation is the process by which the molecules of a substance begin to arrange themselves in an ordered network, forming small crystal “embryos” (called crystal nuclei). If these nuclei become stable enough, they grow and lead to the complete crystallization of the substance.
There are two main types of nucleation:
- Homogeneous nucleation occurs spontaneously throughout the solution, without impurities or surfaces.
- Heterogeneous nucleation occurs on impurities, on the walls of the container, or on suspended particles.
- Infrared light is a form of electromagnetic radiation, i.e., a stream of photons with a certain energy. Each photon has an energy defined by the following equation:E = photon energy,h = Planck’s constant (~6.626 × 10−34 J·s),ν = wave frequency,λ = wavelength (700 nm–1 mm for IR),c = speed of light.
Molecules have chemical bonds that act as mass-spring systems: the atoms are the masses, and the chemical bond is the elastic spring. They have natural frequencies of vibration (like a swing or a guitar string).
When IR radiation has exactly the natural frequency of vibration of a chemical bond, the photons are absorbed, and their energy is converted into molecular vibration.
Resonance: the key to molecular agitation.
It is like when you push a child in a swing at the right time, each small impulse adds energy, and the movement increases.
The phenomenon of resonance: if the frequency of IR radiation corresponds to the natural frequency of a molecular vibration (e.g., stretching of the C–H bond (covalent bond between a carbon (C) atom and a hydrogen (H) atom), that bond will vibrate more intensely, thus the agitation occurs.
IR radiation causes various types of intramolecular vibrations: vibration type Description, Stretching Bond stretching/compression, bending. Change in angle between bonds, twisting around the molecule’s axis. The increase in diffusion can be quantitatively described by the Einstein–Stokes equation:
- D is the diffusion coefficient.
- K b is the Boltzmann constant.
- T is the absolute temperature.
- η is the dynamic viscosity of the medium.
- r is the hydrodynamic radius of the diffusing particle.
According to this relation, a rise in temperature T results in a proportional increase in the diffusion coefficient D, provided that viscosity and particle size remain relatively constant. Consequently, IR irradiation indirectly promotes the homogenization of multi-component systems by facilitating the redistribution of molecular species through enhanced diffusional dynamics.
2.2.9. Matters in IR Spectroscopy
The stretching vibration of the C–H bond absorbs IR radiation in a specific frequency range: Typically, 2800–3100 cm−1, depending on the type of C–H bond (sp3, sp2, or sp hybridized carbon). This makes it a diagnostic peak in IR spectra for identifying the presence and type of C–H bonds in a substance (C–H bonds = covalent bond between a carbon (C) atom and a hydrogen (H) atom). In the context of infrared-induced molecular excitation, when the frequency of incident IR radiation coincides with the fundamental vibrational mode of the C–H bond, resonant absorption occurs. This results in an amplification of the bond’s vibrational amplitude, a phenomenon known as vibrational resonance, which enhances intramolecular energy and contributes to increased molecular mobility and diffusion.
Rocking/Scissoring More complex oscillatory movements.
The 700 nm wavelength is used throughout the treatment period, with the role of antiplatelet (Figure 5).

Figure 5.
Laser syringe treatment 470–700 nm.
The proposed prototype operates in two distinct phases. In the initial phase, the empty syringe undergoes surface treatment using 470 nm (blue light) wavelength light for a duration of two minutes. Upon completion, an audible alarm signals the nurse to proceed with syringe filling. During the subsequent drug administration phase, the system continuously emits 700 nm (red/magenta light) wavelength light in parallel with the activation of the syringe pump, ensuring consistent treatment delivery.
Caution: Personnel are required to wear ultraviolet (UV) protective eyewear during the installation of the syringe onto the syringe pump, due to the exposure involved in the surface treatment process intended to reduce drug adhesion to the syringe walls. As an alternative safety measure, a retractable UV filter may be integrated into the syringe pump housing to shield the operator from direct exposure. Failure to implement appropriate protective measures may result in irreversible ocular damage. Today’s polypropylene and polyamide plastics are inherently hydrophobic, with low surface energy, and therefore do not adhere well to other materials. Adhesion enhancement is the most common application, but other surface characteristics such as wettability, water and chemical resistance, non-fouling, tribological behavior, and oxygen and moisture transmission are also addressed. It has been estimated that 70% of the total production of plastics requires surface treatment prior to processing. Methods range from vacuum to atmospheric pressure, wet to dry, simple to sophisticated, and inexpensive to very expensive to achieve the required functional characteristics of the plastics. Most methods used today are dry and environmentally friendly. The methods presented are roughly divided into surface activation techniques (e.g., plasma, corona, flame, and UV laser) and surface coating techniques (e.g., plasma polymerization, chemical vapor deposition, Parylene, and physical vapor deposition). UV treatment of plastics such as polypropylene allows chemicals to be less adherent to the walls of plastic syringes [9].
Reactive oxidative species generated by plasma or laser treatments interact with polymer substrates, inducing the formation of surface functional groups that enhance adhesion. On plasma-treated polyethylene (PE) and polypropylene (PP) surfaces, functional groups such as hydroxyl, carbonyl, carboxyl, and amide have been identified. The improved wettability and adhesive properties of low-density polyethylene (LDPE) and PP are primarily attributed to the oxidation of the surface layer, typically confined to depths of less than 10 nanometers. In contrast, flame treatment can result in deeper oxidation, reaching depths of approximately 300–400 nanometers with prolonged exposure. Additionally, such surface modifications contribute to a reduced permeability to various chemical agents [9,10,11,12,13,14,15,16,17,18,19].
Mathematical calculations for a 50 mL syringe.
General Formula (Vibration-Stabilizing IR Power Model):
- PIR = required IR laser power (in watts, W)
- Ereq_ = total energy required to maintain homogeneity (in joules, J)
- t = duration of administration or homogenization (in seconds, s)
Ereq = m⋅c⋅ΔT + Evib
- m = mass of the solution (kg)
- c = specific heat capacity of the solution (J/kg·K), typically close to water, ≈4180 J/kg\cdotpK\approx 4180 \, \text{J/kg·K} ≈ 4180 J/kg\cdotpK
- ΔT = permissible temperature increase (°C) caused by IR absorption
2.2.10. Evib Additional Energy Needed for Molecular Agitation (J) (This Can Be Experimentally Determined Based on Solution Viscosity and Density)
For a 50 mL infusion pump syringe with aqueous solution (≈0.05 kg), allowing a temperature increase of 2 °C in 10 min:
Ereq = 0.05⋅4180⋅2 = 418 J
This would be the minimum power needed from the IR laser, assuming 100% absorption efficiency (which is idealized). In practice, you must also account for laser-material absorption efficiency (η\etaη):
Following mathematical calculations, 3 × 500 mW/700 nm laser diodes with separate drivers for better dispersion of the laser light and three other 470 nm laser diodes at 400 mW power were installed in the proposed prototype (Figure 6).

Figure 6.
A 700 nm Laser diode.
Experimental Validation
- Drugs tested: Insulin (1.09 g/cm3), Furosemide (1.606 g/cm3), and Gentamicin (40 mg/mL).
- Infusion simulation: 2–5 mL/h over 12 h in horizontal syringe position.
- Sampling: From the top, middle, and bottom of the syringe at 2 h intervals.
- Analysis method: UV-Vis spectrophotometry (280 nm for insulin, others adjusted).
Drugs tested: insulin, furosemide, and gentamicin. Using UV-Vis analysis, we measured concentration variation at the top, middle, and bottom of syringe samples over a 12 h infusion simulation. Our system reduced variability by over 90% compared to standard syringe pumps. (Figure 5 Sampling methodology).
We conducted a series of controlled experiments to evaluate the performance of our system in maintaining drug homogeneity. Key parameters measured included
- Real-time concentration monitoring using spectrophotometric analysis.
- Sedimentation rate assessment under different infusion conditions.
- Comparison with conventional syringe pumps, highlighting the reduction in concentration variability achieved through our approach (Table 1).
 Table 1. Comparison of conventional syringe pump/syringe pump with mechatronic homogenization system.
Table 1. Comparison of conventional syringe pump/syringe pump with mechatronic homogenization system.
2.2.11. Experimental Investigation of Drug Homogeneity in Syringe Pumps
The experimental study evaluates the stability and distribution of various intensive care unit (ICU) drugs during infusion. Simulated drug injection conditions were established based on clinical protocols, such as furosemide/insulin administered at 2 mL/h. The research team measured the concentration from the three collection points in the syringe body to determine the relative homogeneity of the total mass of drugs administered. Sample collection was performed at the three points of the syringe, top, middle, and bottom, at different time intervals: 2/4/6/8/10/12 h (Figure 7 and Figure 8).

Figure 7.
Sample collection.

Figure 8.
Sampling methodology.
Samples collected from the middle of the syringe represent the effective drug concentration delivered to the patient. In contrast, samples taken from the top and bottom of the syringe serve to assess the degree of sedimentation and potential concentration gradients within the solution. The sampling needle was inserted carefully so as not to generate homogenization factors by swinging the solution in the syringe. The sample volume was 1 mL from the three parts mentioned above.
2.2.12. Drug Stability and Loss Analysis
- Gentamicin (40 mg/mL)
- Plastic Syringe: After 12 h, an average drug loss of 9.7% was observed, with the formation of a light brown precipitate at 25 °C.
- Glass Syringe: After 12 h, an average drug loss of 7% was noted, with precipitate formation occurring at 12 h (at 25 °C).
Method used to determine insulin concentration: ELISA (Enzyme-Linked Immunosorbent Assay).
Principle:
Gentamicin does not contain strong chromophores, but can be chemically derivatized (with FMOC-Cl (Fluorenylmethyloxycarbonyl chloride is a chemical reagent derived from fluorene, mainly used for: derivatization of compounds containing amino groups (–NH2) labeling of compounds for fluorescent or UV detection in chromatography (e.g., HPLC)) to form fluorescent or UV (ultraviolet) detectable compounds, which can be analyzed by high-performance liquid chromatography (HPLC).
HPLC-Fluorescence = HPLC with fluorescence detection.
A fluorescence detector is used, which measures the light emitted by a fluorescent compound after it has been excited with a certain wavelength. It is much more sensitive than UV (can detect lower concentrations).
It is used when the compound is fluorescent or can be derivatized to become fluorescent.
Insulin (Density of Insulin 1.09 g/cm3).
- Plastic Syringe: After 12 h, an average drug loss of 27.2% was observed, with the formation of a light brown precipitate at 25 °C [1].
- Glass Syringe: After 12 h, an average drug loss of 11% was noted, with precipitate formation occurring at 12 h (at 25 °C) [1].
Method used to determine insulin concentration:
ELISA (Enzyme-Linked Immunosorbent Assay).
It is the most used method for determining insulin in biological or pharmaceutical solutions, due to its excellent sensitivity and specificity.
ELISA uses specific anti-insulin antibodies fixed in a microtiter plate (96 wells). Insulin in the sample binds to these antibodies. Then, an enzyme-labeled secondary antibody is added, which generates a signal (colorimetric) proportional to the insulin concentration.
Experimental steps (Sandwich ELISA):
Plate coated with anti-insulin antibody: binds insulin in the sample.
Adding a sample or standard (known concentrations of insulin).
Washing: removes unbound material.
Adding a secondary antibody linked to an enzyme (e.g., peroxidase).
Washing again.
Adding enzyme substrate (e.g., TMB—tetramethylbenzidine), develops a color.
Reading absorbance at 450 nm with a microplate reader.
Comparing absorbance with a standard curve: determining the unknown concentration.
Furosemide (Density of furosemide 1.606 g/cm3)
- Plastic Syringe: After 12 h, an average drug loss of 26.1% was observed, with the formation of a light brown precipitate at 25 °C.
- Glass Syringe: After 12 h, an average drug loss of 5% was noted, with precipitate formation occurring at 12 h (at 25 °C).
The observed loss correlates with prolonged injection times, particularly when the solution was prepared 6 h before administration.
Drug Concentration Variability in Syringe
- Increased attention was paid to insulin, where a (+16.3%) increase in drug concentration was detected in the lower section of the syringe when mounted in the syringe pump after 12 h.
- Within the proposed pump, a mechatronic homogenization system before sample collection significantly reduced concentration discrepancies across the syringe.
The measured concentration differences between the upper, middle, and lower sections were reduced to 0.7–1.1%.
Potential measurement errors were estimated at 0.3–0.4%, which is within an acceptable accuracy range.
Furosemide concentration detection method: Furosemide absorbs UV light, especially around 230–270 nm, due to the aromatic and sulfonamide groups in the structure.
Laboratory steps: preparation of a standard solution of furosemide, measurement of absorption at a specific wavelength, and application of Beer–Lambert’s law to determine the following concentration:
A = ε × C × L
A = Absorbance (unitless)
It is the measure of the amount of light absorbed by a substance at a specific wavelength.
It is read directly from the spectrophotometer.
ε = Molar absorption coefficient (or molar absorptivity)
Unit: L·mol−1·cm−1
Represents the ability of a substance to absorb light at a certain wavelength.
It is a constant specific to each substance and depends on the wavelength.
Its value must be known or determined experimentally.
c = Concentration of the solution
Unit: mol/L (molarity)
This is the unknown you want to know in most experiments.
l = Cuvette length (length of light travel through solution)
Unit: cm—1 cm, as standard cuvettes in spectrophotometers have this length.
2.2.13. Methodology of Drug Concentration Sampling (In Vitro)
- Samples were collected from the top, middle, and bottom sections of the syringe at 2, 4, 6, 8, 10, and 12 h post-preparation (positioning the syringe horizontally).
- A syringe pump simulation was used, administering small drug volumes (3–5 mL/h) to replicate clinical infusion conditions.
- Sampling was conducted according to Figure 6, ensuring a systematic evaluation of concentration variations over time.
- Samples were collected at two-hour intervals throughout the drug delivery process until the syringe was nearly depleted. At the time of the final sampling point, between 80% and 95% of the syringe contents had been administered, depending on the infusion rate settings of the syringe pump (4–5 mL/h). This substantiates the relatively short duration of the homogeneity assessment, as the majority of the drug volume had already been delivered by the end of the 12 h observation period. Furthermore, it is important to note that many pharmaceutical manufacturers recommend discarding medications that have been exposed to ambient room temperature for more than 12 h, which further supports the selected timeframe for evaluation. The research team measured the concentration from the three collection points in the syringe body to determine the relative homogeneity of the total mass of drugs administered. Sampling was conducted from syringe pumps with a homogenization system and classic syringe pumps.
2.2.14. Analysis Methods—Spectrophotometry
The method used to identify the concentration of insulin, furosemide was performed by UV-Vis spectrophotometry. In insulin, it absorbs the wavelength of 280 nm due to the presence of aromatic amino acids; thus, the percentage of effective concentration can be determined. The author notes that the different density of the drugs in the mixture generates a relative instability from one drug to another administered intravenously.
The proposed system aligns with the principles outlined in ISO 13485 [22] regarding medical device quality management, ensuring traceability, thermal safety, and performance consistency. The modular nature of the design facilitates integration into current production and compliance workflows.
3. Results
The values in Table 1 represent the difference in concentration from the concentration prepared for in vitro administration. The first value represents the sample taken from the top of the syringe, the second value (middle) represents the concentration taken from the luer cone (the concentration actually administered to the virtual patient), and the third value represents the drug concentration at the bottom of the syringe. All samples are taken with the syringe on the syringe pump in a horizontal position (Table 1).
The sedimentation rate is represented in the last parameter in the percentage ratio of the initial concentration, to determine the difference in heterogeneity through sedimentation or attraction to the polyethylene walls of the syringe as in the case of insulin (Figure 9).
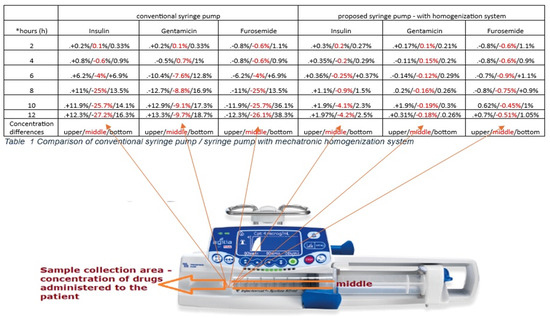
Figure 9.
Sampling model Informative graphic representation, Informative graphic representation, please read the data in table no. 1 above. This figure only identifies that the data in the middle of each column represents the concentration of the delivered solution, data marked in red.
Looking at the subsequent chemical reactions from exposure to high-intensity 700 nm light, no imbalances in the chemical structure were found during the administration interval (12 h). No alterations in chemical structure were observed during the 12 h administration period as a result of exposure to high-intensity light at a wavelength of 700 nm. Microscopic analysis confirmed the preservation of both molecular integrity and solution homogeneity throughout the duration of administration, supporting the efficacy of the proposed system in maintaining drug stability (Figure 7).
Future investigations will extend this analysis to additional pharmaceutical compounds subjected to 700 nm photonic exposure. These forthcoming studies, to be presented in subsequent publications within the field, will require further financial resources and experimental infrastructure. Figure 7 illustrates the variation in insulin concentration levels with and without the homogenization system integrated into the syringe pump proposed by our team. The solid green line represents the concentration profile achieved using the enhanced syringe pump with the homogenization system, while the dashed green line indicates the concentration deviation observed with a conventional syringe pump lacking homogenization capabilities. The test was repeated 5 times/on three levels, top, middle and bottom, if the analysis value did not deviate more than +/−1.5%, the initial value, V1, was validated, in order not to overload the analyzed data, at two-hour intervals (2, 4, 6, 8, 10, 12 h).
4. Discussion
The combination of vibration and photonic treatment effectively mitigates drug stratification and surface adhesion. Our approach is energy-efficient and easily integrable into existing devices.
Our findings validate that dual-axis vibration + photonic intervention significantly enhances homogeneity across diverse drug types. The energy consumption is minimal, ensuring integration feasibility into existing pump platforms.
However, drug-specific tuning (frequency, exposure time) is essential. The effectiveness of the system depends on viscosity, molecular weight, and drug-light interaction profiles.
The system also indirectly supports dose reproducibility, reducing reliance on manual mixing or pump recalibration.
Current investigations reveal that standard syringe pumps, particularly those used for insulin delivery, exhibit a significant degree of molecular adhesion to the polypropylene walls of the syringe. Analytical sampling indicates a concentration increase of up to 12% along the upper syringe walls and up to 16% along the lower walls, relative to the intended concentration. Additionally, measurements taken at the luer cone after 12 h show a notable concentration decrease of 27.2% compared to the initially prepared solution. These discrepancies become pronounced between 6 and 8 h following syringe placement in the pump, indicating substantial concentration variability over time.
A similar distribution pattern is observed with furosemide, though its behavior differs in that it does not adhere to the syringe walls. Instead, the drug exhibits gravitational sedimentation, accumulating at the bottom of the syringe. This results in a localized concentration increase of approximately 38.3% at the syringe base and a decrease of 26.1% at the luer cone—the site corresponding to the concentration actually delivered to the simulated patient.
In the case of the proposed system, which integrates a dual-axis vibration generator along with a dual-wavelength photonic module (470 nm during the pre-fill phase and 700 nm during administration), a significant improvement in drug solution homogeneity is observed. In the case of insulin, concentration discrepancies are reduced from −27% to −4%, while for furosemide, deviations decrease from −26.1% to just 0.5%. These findings highlight a substantial advancement in the precision of drug delivery, ensuring not only accurate volumetric administration but also improved consistency in the concentration of infusible compounds.
Similar findings on drug heterogeneity over time.
The heterogeneity of intravenously administered drug solutions can contribute to hemodynamic instability in patients, manifesting as fluctuations in glucose levels, alterations in heart rate, and inconsistent delivery of critical medications such as antibiotics in cases of sepsis. Chemotherapeutic agents, in particular, are known to exhibit significant physicochemical instability, leading to increased heterogeneity over time. This instability may result in adverse reactions at specific intervals following administration, including allergic responses, cutaneous necrosis, and other complications.
In several publications, several high-risk agents are particularly prone to sedimentation and concentration variability, thus posing a risk to patient safety. These include, but are not limited to, antineoplastic agents such as Vinca alkaloids, cytarabine, bleomycin, etoposide, doxorubicin, cisplatin, and fluorouracil [4,5,7]. Therefore, ensuring homogeneity throughout the infusion period is essential to minimize adverse events and ensure therapeutic efficacy.
It has been observed that the intravenous administration of treatment at a reduced concentration, influenced by sedimentation, may initially lead to a diminished therapeutic effect in chemotherapy. However, this can result in a negative feedback effect on the patient, as the subsequent administration of a highly concentrated drug—due to precipitation within the syringe pump—may cause unintended toxicity. Excessively high local concentrations can lead to tissue denaturation until dilution occurs via systemic circulation, with clinical evidence of necrosis in the limbs where chemotherapy agents have been infused [4,5,6,7,11] (Figure 10).
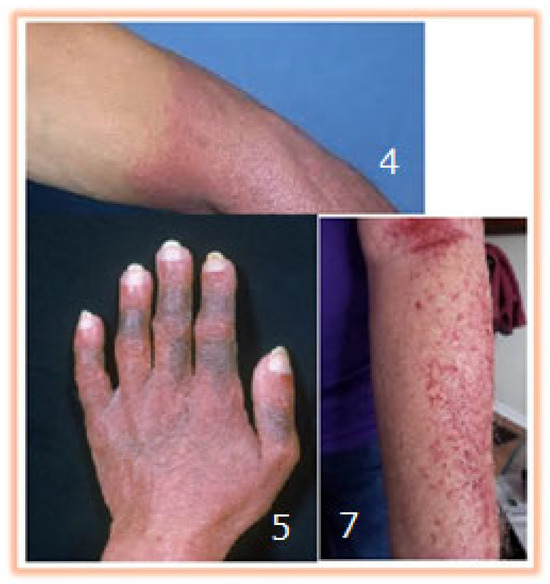
Figure 10.
Adverse effects visible only in the limbs where the chemotherapy Representation of adverse effects from other publications [4] Dutta, C, 2015; [5] Zahid, N,2008; [7] Capes, D.F,1995.
Simulating drug administration in syringe pumps offers us a new perspective to understand the necrosis and allergic condition of the skin adjacent to the venous branch (Figure 11).
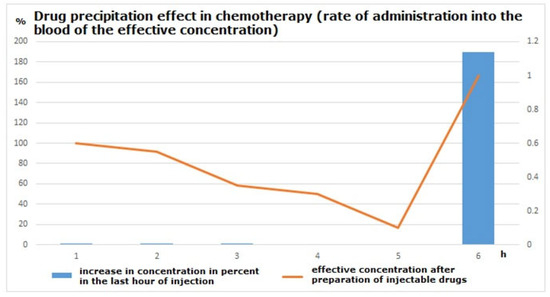
Figure 11.
Blood concentration of administered drugs (orange line) [5,6,7,14,23].
Existing literature acknowledges the issue of concentration variability in administered drugs; however, no established technical solution has been proposed to address this challenge. Therefore, the research team considers ensuring the homogeneity of all administered medications to be a significant advancement, benefiting both oncology patients and critically ill individuals in intensive care units [6,7,10,18,19,20,21].
One limitation of the present study is the relatively narrow range of substances tested. While results for insulin, furosemide, and gentamicin are promising, further validation across broader drug classes, particularly chemotherapeutics and unstable protein-based formulations is essential [5,22,24,25,26,27,28,29,30]
5. Conclusions
We present a novel, low-cost, and clinically viable mechatronic homogenization system for syringe pumps. Our dual-axis vibration and photonic surface treatment approach significantly improves drug concentration stability during long-term infusions. This system addresses a critical safety issue in ICUs and oncology and could be widely adopted due to its simplicity and compatibility with existing devices. As performance parameters, we found an increase in the homogeneity of the drugs taken from the luer cone for insulin and furosemide. In the case of insulin, concentration discrepancies are reduced from −27% to −4%, while for furosemide, deviations decrease from −26.1% to just 0.5%. These findings highlight a substantial advancement in the precision of drug delivery, ensuring not only accurate volumetric administration but also improved consistency in the concentration of infusible compounds.
Other substances must be investigated further to determine the reliability of the proposed system.
The analysis was limited to three substances administered intravenously; therefore, the authors specify the need to extend the research to more categories of drugs administered intravenously using syringe pumps.
Future research will focus on implementing the proposed system in real clinical settings, to evaluate its performance under standard working conditions in ICUs and oncology wards. Particular attention will be paid to its impact on dosing precision, patient safety, and reduction in medication errors over extended infusion durations.
Author Contributions
Conceptualization, D.A.D.; methodology, D.A.D.; validation, D.A.D.; formal analysis, D.A.D. and A.R.; investigation, D.A.D.; writing—original draft preparation, D.A.D. and A.R.; writing—review and editing, D.A.D. and A.R.; visualization, D.A.D.; supervision, A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The proposed system aims to enhance patient safety with no identifiable negative societal or ethical concerns. No patient data was used. As the study exclusively involved the use of medical devices and pharmaceutical substances, with no human subjects or personal data collection, ethical approval and informed consent were not required. The primary objective of this investigation was to evaluate the stability and homogeneity of drug solutions prepared in syringe pumps over a 12 h administration period. All testing was conducted on laboratory-prepared samples under controlled conditions, without involving patient participation.
Informed Consent Statement
Not applicable.
Data Availability Statement
All analyzed data and values are present in the present article. It is imperative to extend the study to more drugs analyzed in administration through automatic syringes such as dopamine.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ICU | Intensive care units |
| h | Hours |
| TLA | Three-letter acronym |
References
- Poulsen, C.; Jacobsen, D.; Palm, L. Effect of ethylenediamine on chemical degradation of insulin aspart in pharmaceutical solutions. Pharm. Res. 2008, 25, 2534–2544. [Google Scholar] [CrossRef] [PubMed]
- Tariq, R.A.; Vashisht, R.; Sinha, A.; Scherbak, Y. Medication Dispensing Errors and Prevention. [Updated 12 February 2024]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online:https://www.ncbi.nlm.nih.gov/books/NBK519065/.
- Saleh, A.H.; Borhan, G.; Goujon, F.; Devémy, J.; Dequidt, A.; Malfreyt, P.; Sahihi, M. Molecular and energetic descriptions of the plasma protein adsorption onto the PVC surface: Implications for biocompatibility in medical devices. ACS Omega 2024, 9, 38054–38065. [Google Scholar] [CrossRef] [PubMed]
- Dutta, C.; Yang, M.; Long, F.; Shahbazian-Yassar, R.; Tiwari, A. Preformed Seeds Modulate Native Insulin Aggregation Kinetics. J. Phys. Chem. B 2015, 119, 15089–15099. [Google Scholar] [CrossRef] [PubMed]
- Zahid, N.; Taylor, K.M.G.; Gill, H.; Maguire, F.; Shulman, R. Adsorption of insulin onto infusion sets used in adult intensive care unit and neonatal care settings. Diabetes Res. Clin. Pract. 2008, 80, e11–e13. [Google Scholar] [CrossRef] [PubMed]
- Sacré, P.Y.; Alaoui Mansouri, M.; De Bleye, C.; Coïc, L.; Hubert, P.; Ziemons, E. Evaluation of distributional homogeneity of pharmaceutical formulation using laser direct infrared imaging. Int. J. Pharm. 2022, 612, 121373. [Google Scholar] [CrossRef] [PubMed]
- Capes, D.F.; Dunster, K.R.; Sunderland, V.B.; McMillan, D.; Colditz, P.B.; McDonald, C. Fluctuations in syringe-pump infusions: Association with blood pressure variations in infants. Am. J. Health Syst. Pharm. 1995, 52, 1646–1653. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.; Ipema, H.; Hartke, P.; Krueger, C.; Rodriguez, R.; Gross, A.E.; Gabay, M. Intravenous Push Administration of Antibiotics: Literature and Considerations. Hosp. Pharm. 2018, 53, 157–169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kastrup, M.; Balzer, F.; Volk, T.; Spies, C. Analysis of event logs from syringe pumps: A retrospective pilot study to assess possible effects of syringe pumps on safety in a university hospital critical care unit in Germany. Drug Saf. 2012, 35, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Johansson, K.S. Surface Modification of Plastics. In Plastics Design Library, Applied Plastics Engineering Handbook, 2nd ed.; Kutz, M., Ed.; William Andrew Publishing: Stockholm, Sweden, 2017; ISBN 9780323390408. [Google Scholar]
- Spencer, S.; Ipema, H.; Hartke, P.; Krueger, C.; Rodriguez, R.; Gross, A.E.; Gabay, M. Amikacin Sulfate: Full Prescribing Information; West-Ward: Eatontown, NJ, USA, 2016. [Google Scholar]
- Silva, M.D.S.; Araújo, J.L.; Nunes, G.A.D.A.; Rosa, M.F.F.; Luz, G.V.D.S.; Rosa, S.D.S.; Piratelli-Filho, A. Precision and reliability study of hospital infusion pumps: A systematic review. Biomed. Eng. Online 2023, 22, 26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sravani, J.; Panda, C.; Agha, M.; Vijapurkar, S.; Sandeep, G. Soundless Trouble: Syringe Pump Malfunction and the Hypotension Threat. Cureus 2024, 16, e56996. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Groh, V.; Wu, J.; Yee, C.; Spies, T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 2002, 419, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Gentamicin Sulfate: Full Prescribing Information; Hospira Inc.: Lake Forest, IL, USA, 2017.
- Malinzi, J. Mathematical Analysis of a Mathematical Model of Chemovirotherapy: Effect of Drug Infusion Method. Comput. Math. Methods Med. 2019, 2019, 7576591. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Invanz (Ertapenem): Full Prescribing Information; Merck Sharp & Dohme Corp: Whitehouse Station, NJ, USA, 2017.
- Draghici, D.A.; Pantea, I.; Roman, N.; Drugus, D.; Repanovici, A. In vitro mechanism whit pulsative laser on thrombolysis. Acta Teh. Napoc. 2022, 65. [Google Scholar]
- Rocha, P.; Rocha, M.; Andrade, I.; Mota, M. Assessment of nurses’ knowledge about the importance of continuous infusion of catecholamines in intensive care unit. Rev. Min. Enferm. 2010, 14, 459–464. [Google Scholar]
- Pharmaceuticals, A. Merrem (Meropenem): Full Prescribing Information; Astrazeneca Pharmaceuticals LP: Wilmington, DE, USA, 2016. [Google Scholar]
- Roman, N.A.; Tuchel, V.I.; Nicolau, C.; Grigorescu, O.-D.; Necula, R. Functional electrostimulation in patients affected by the most frequent central motor neuron disorders—A scoping review. Appl. Sci. 2023, 13, 3732. [Google Scholar] [CrossRef]
- ISO 13485; Medical Devices—Quality Management Systems—Requirements for Regulatory Purposes. ISO: Geneva, Switzerland, 2016.
- Escobedo, R.; Miranda, R.; Martínez, J. Infrared Irradiation: Toward Green Chemistry, a Review. Int. J. Mol. Sci. 2016, 17, 453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Claves, J.; Bourrel, G.; Favreau, P.; Burgalé, J.; Redureau, E. Comparative study of sorption phenomena between three medications and syringes made of cyclic olefin copolymer or polypropylene. Pharm. Res. 2023, 40, 505–516. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmed, S.; Anwar, Z.; Sheraz, M.A.; Khaskheli, A.R. Photostability and photostabilization of drugs and drug products. Int. J. Photoenergy 2016, 2016, 8135608. [Google Scholar] [CrossRef]
- ICH Q1B Guideline. Photostability Testing of New Active Substances and Medicinal Products. EMA Scientific Guideline. 2008. Available online: https://www.ema.europa.eu/en/ich-q1b-photostability-testing-new-active-substances-medicinal-products-scientific-guideline (accessed on 4 June 2025).
- Kurien, B.T.; Everds, N.E.; Scofield, R.H. Mechanized syringe homogenization of human and animal tissues. Assay Drug Dev. Technol. 2004, 2, 395–401. [Google Scholar] [CrossRef]
- Vlase, S.; Itu, C.; Marin, M.; Scutaru, M.L.; Sabou, F.; Necula, R. Vibration analysis of the Gamma-Ray element in the ELI-NP interaction chamber. J. Comput. Appl. Mech. 2024, 55, 275–288. [Google Scholar] [CrossRef]
- Bolla, B.; Grillot-Courvalin, C.; Lepeule, R. Understanding IV antimicrobial drug losses: The importance of flushing infusion administration sets. JAC Antimicrob. Resist. 2020, 2, dlaa061. [Google Scholar] [CrossRef]
- Rout, J.; Patel, T.; Smith, K. Management of residual volumes in intermittent medication infusions in hospitals: A scoping review protocol. Br. J. Nurs. 2024, 33, 278–284. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).