A Smart Rehabilitation Glove Based on Shape-Memory Alloys for Stroke Recovery
Abstract
1. Introduction
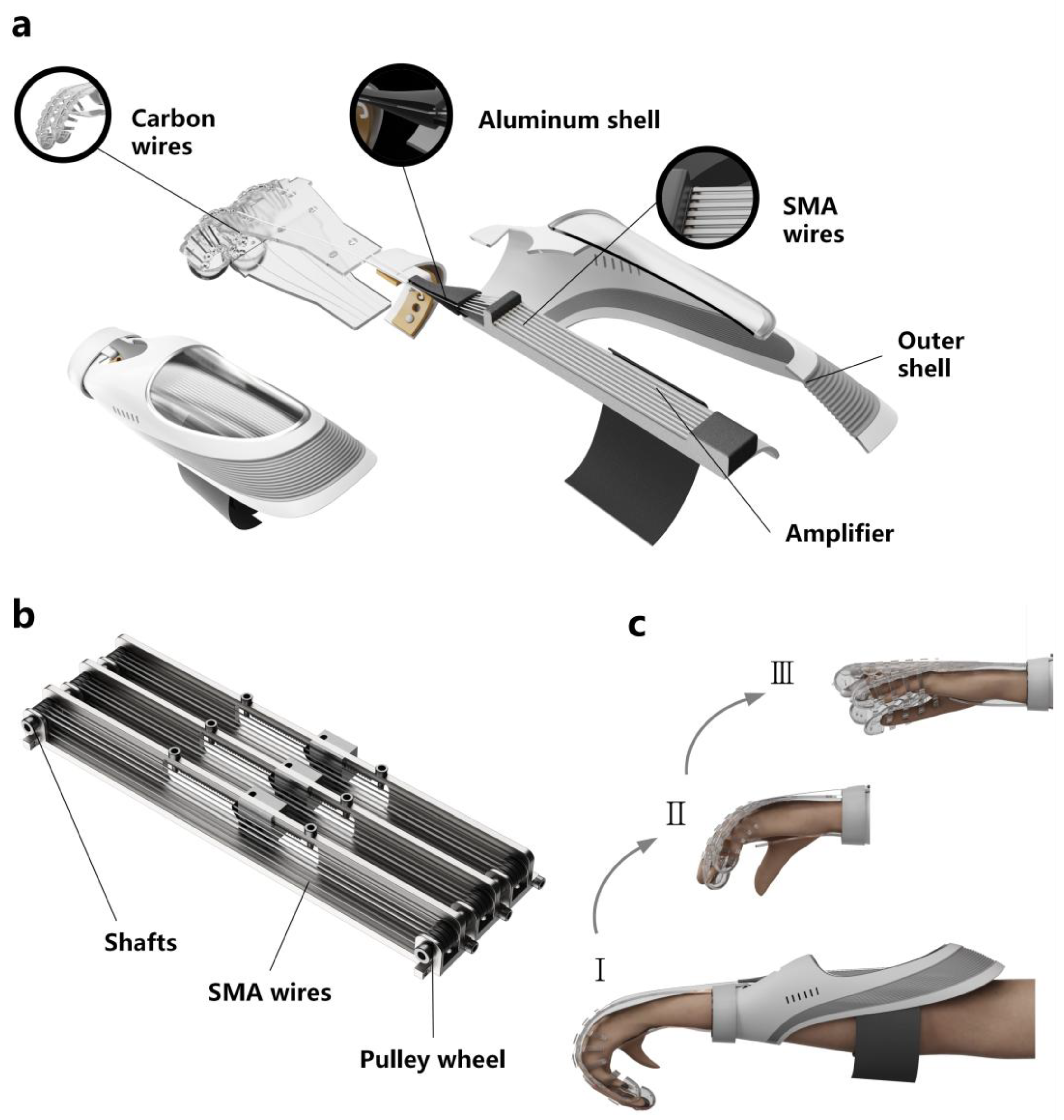
2. Design
2.1. Structural Design
2.2. Online Control in Mobile App
3. Method
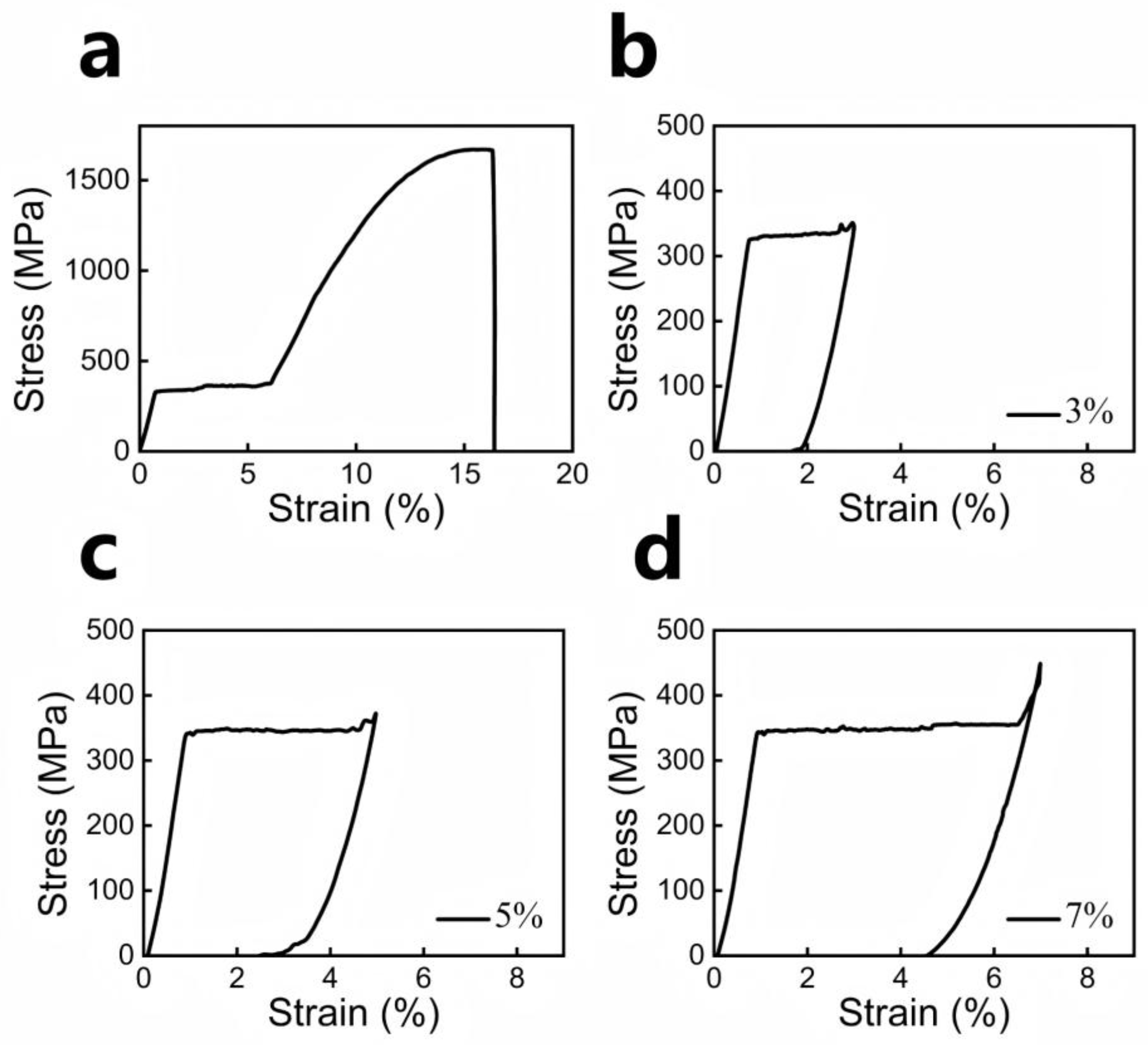
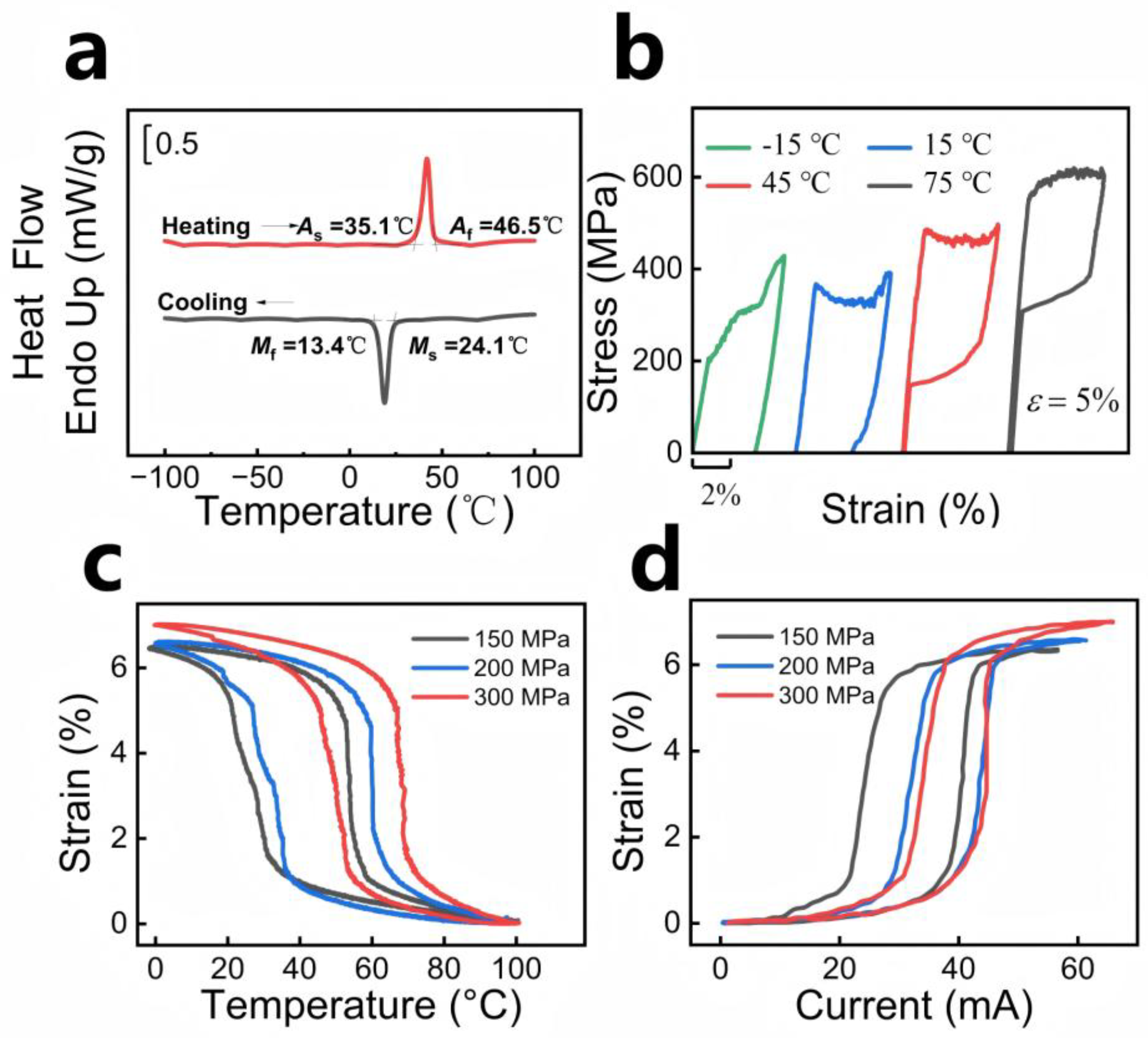
3.1. SMA Materials and Methods
3.2. Wireless Connection of Mobile and Rehabilitation Glove
4. Results and Discussion
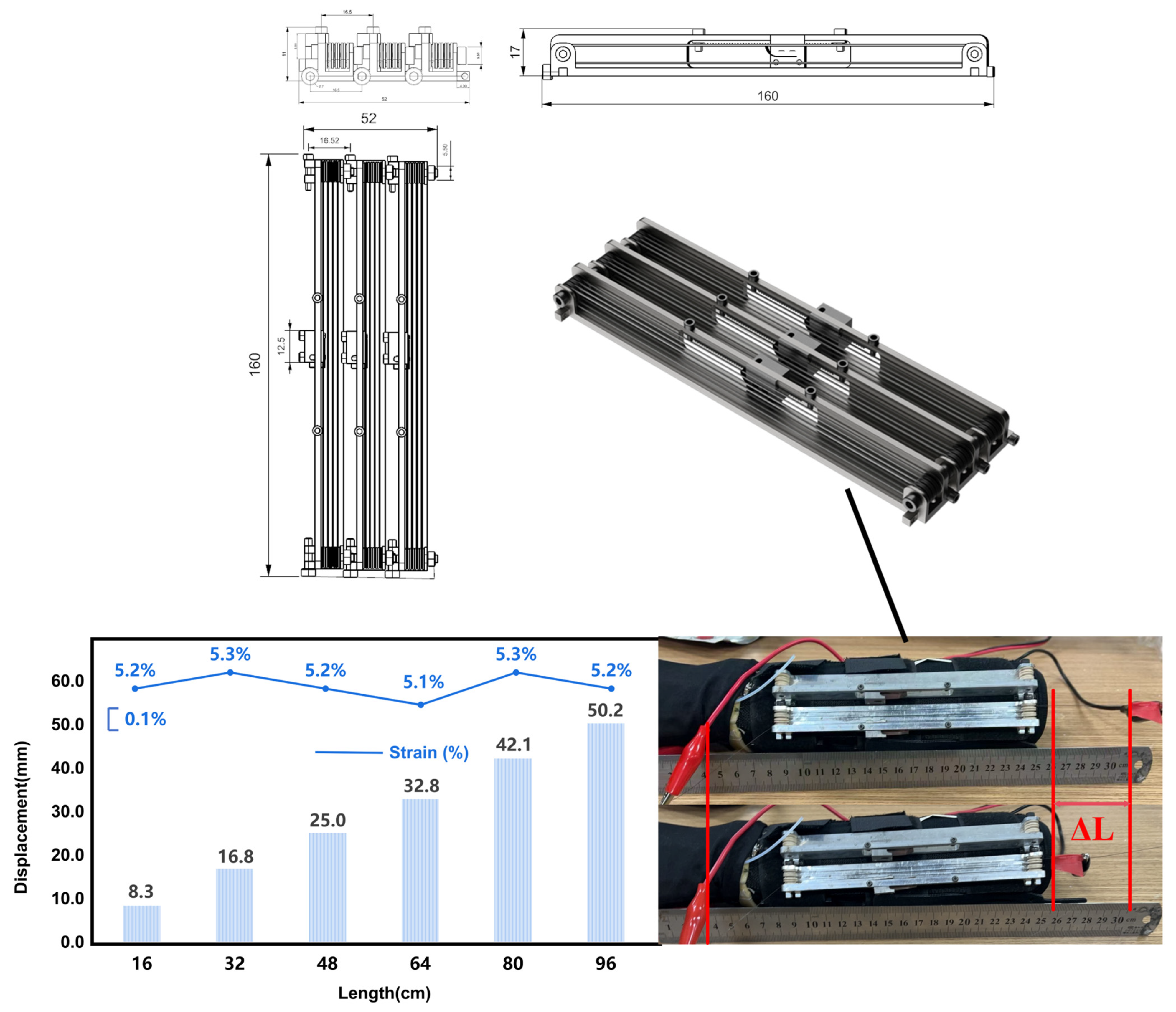
4.1. SMA Properties
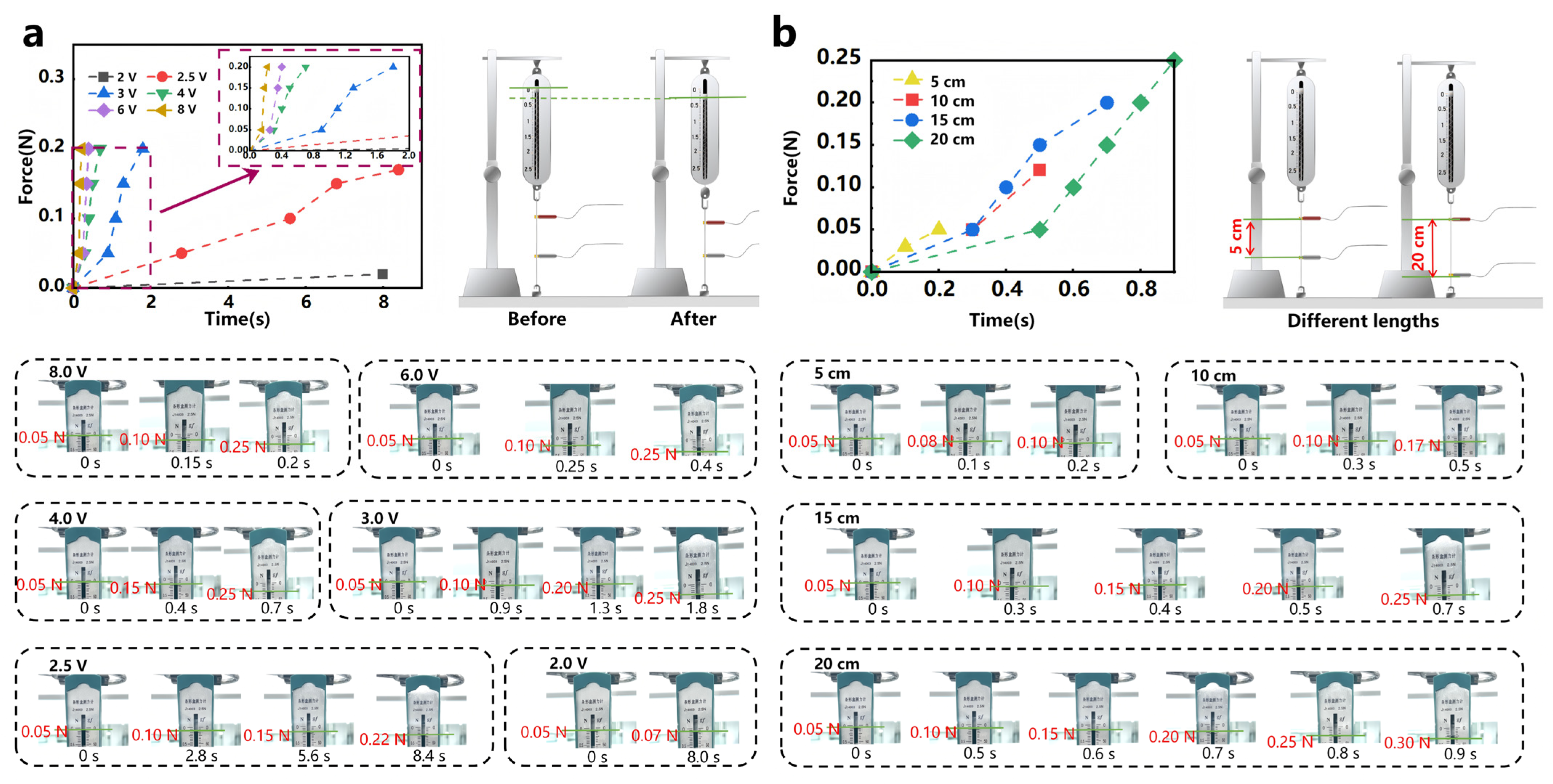
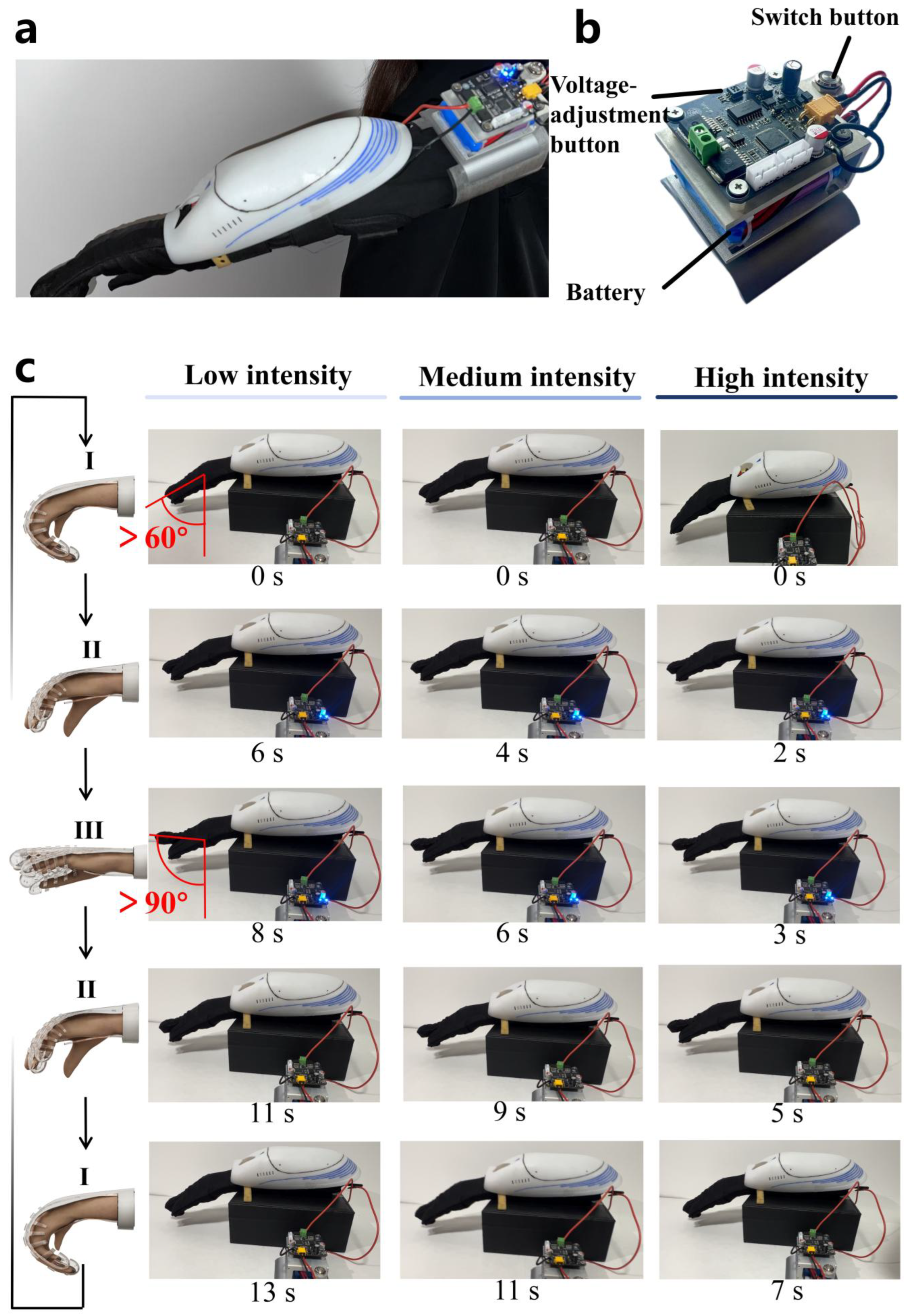
4.2. Training-Mode Modulation
4.3. Periodic Rehabilitation Training
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SMAs | Shape-Memory Alloys |
References
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Zhao, Z.; Yin, P.; Cao, L.; Zeng, J.; Chen, H.; Fan, D.; Fang, Q.; Gao, P.; Gu, Y. Estimated burden of stroke in China in 2020. JAMA Netw. Open 2023, 6, e231455. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Li, R.; Wang, L.; Yin, P.; Wang, Y.; Yan, C.; Ren, Y.; Qian, Z.; Vaughn, M.G.; McMillin, S.E. Temporal trend and attributable risk factors of stroke burden in China, 1990–2019: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2021, 6, e897–e906. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.F.; Prahm, C.; Kolbenschlag, J.; Oliveira, E.; Rodrigues, N.F. Application of AR and VR in hand rehabilitation: A systematic review. Biomed. Inform. 2020, 111, 103584. [Google Scholar] [CrossRef]
- Harvey, R.L. Improving poststroke recovery: Neuroplasticity and task-oriented training. Curr. Treat. Options Cardiovasc. Med. 2009, 11, 251–259. [Google Scholar] [CrossRef]
- Xie, Q.; Meng, Q.; Yu, W.; Wu, Z.; Xu, R.; Zeng, Q.; Zhou, Z.; Yang, T.; Yu, H. Design of a SMA-based soft composite structure for wearable rehabilitation gloves. Front. Neurorobot. 2023, 17, 1047493. [Google Scholar]
- Popov, D.; Gaponov, I.; Ryu, J.-H. Portable exoskeleton glove with soft structure for hand assistance in activities of daily living. IEEE/ASME Trans. Mechatron. 2016, 22, 865–875. [Google Scholar] [CrossRef]
- Keller, J.L.; Caro, C.M.; Dimick, M.P.; Landrieu, K.; Fullenwider, L.; Walsh, J.M. Thirty years of hand therapy: The 2014 practice analysis. J. Hand Ther. 2016, 29, 222–234. [Google Scholar] [CrossRef]
- Ferretti, M.; Srinivasan, A.; Deschner, J.; Gassner, R.; Baliko, F.; Piesco, N.; Salter, R.; Agarwal, S. Anti-inflammatory effects of continuous passive motion on meniscal fibrocartilage. J. Orthop. Res. 2005, 23, 1165–1171. [Google Scholar] [CrossRef]
- Ada, L.; Dorsch, S.; Canning, C.G. Strengthening interventions increase strength and improve activity after stroke: A systematic review. Aust. J. Physiother. 2006, 52, 241–248. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Y.; Li, C.; Yang, X.; Chen, W. Flexible actuators for soft robotics. Adv. Intell. Syst. 2020, 2, 1900077. [Google Scholar] [CrossRef]
- Xia, J.; Li, Y.; Fu, S.; Xie, W.; Qu, J.; Li, Y.; Ren, T.; Yang, Y.; Liu, H. 3D-printed passive bellow actuator for portable soft wearable robots. Smart Mater. Struct. 2024, 33, 045018. [Google Scholar] [CrossRef]
- Liu, H.; Wu, C.; Lin, S.; Chen, Y. Finger joint aligned flat tube folding structure for robotic glove design. Smart Mater. Struct. 2023, 33, 015001. [Google Scholar] [CrossRef]
- Min, J.; Choi, T.; Cha, Y. Multiple Tendon-inspired Sensors for Hand Motion Detection. Smart Mater. Struct. 2023, 32, 035014. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, J.; Wang, H.; Zhou, C.; Chen, X. A single-layer less-wires stretchable wearable keyboard based on pressure switch conductive textile. Smart Mater. Struct. 2022, 31, 105008. [Google Scholar] [CrossRef]
- Chen, X.; Gong, L.; Wei, L.; Yeh, S.-C.; Da Xu, L.; Zheng, L.; Zou, Z. A wearable hand rehabilitation system with soft gloves. IEEE Trans. Ind. Inform. 2020, 17, 943–952. [Google Scholar] [CrossRef]
- Polygerinos, P.; Galloway, K.C.; Savage, E.; Herman, M.; O’Donnell, K.; Walsh, C.J. Soft robotic glove for hand rehabilitation and task specific training. In Proceedings of the 2015 IEEE International Conference on Robotics and Automation (ICRA), Seattle, WA, USA, 26–30 May 2015; pp. 2913–2919. [Google Scholar]
- Kauffman, G.B.; Mayo, I. The story of nitinol: The serendipitous discovery of the memory metal and its applications. Chem. Educ. 1997, 2, 1–21. [Google Scholar] [CrossRef]
- Raju, S.; Hollis, K.; Neglen, P. Use of compression stockings in chronic venous disease: Patient compliance and efficacy. Ann. Vasc. Surg. 2007, 21, 790–795. [Google Scholar] [CrossRef]
- Kang, W.-R.; Kim, E.-H.; Jeong, M.-S.; Lee, I.; Ahn, S.-M. Morphing wing mechanism using an SMA wire actuator. Int. J. Aeronaut. Space Sci. 2012, 13, 58–63. [Google Scholar] [CrossRef]
- Nishida, M.; Wayman, C.; Honma, T. Electron microscopy studies of the Ti11Ni14 phase in an aged Ti-52.0 at% Ni shape memory alloy. Scr. Metall. 1985, 19, 983–987. [Google Scholar] [CrossRef]
- Sinha, A.; Mondal, B.; Chattopadhyay, P.P. Mechanical properties of Ti–(~49 at%) Ni shape memory alloy, part II: Effect of ageing treatment. Mater. Sci. Eng. A 2013, 561, 344–351. [Google Scholar] [CrossRef]
- Yan, X.; Ge, Y. Influence of post-weld annealing on transformation behavior and mechanical properties of laser-welded NiTi alloy wires. J. Mater. Eng. Perform. 2014, 23, 3474–3479. [Google Scholar] [CrossRef]
- Heidari, B.; Kadkhodaei, M.; Barati, M.; Karimzadeh, F. Fabrication and modeling of shape memory alloy springs. Smart Mater. Struct. 2016, 25, 125003. [Google Scholar] [CrossRef]
- Cappello, L.; Meyer, J.T.; Galloway, K.C.; Peisner, J.D.; Granberry, R.; Wagner, D.A.; Engelhardt, S.; Paganoni, S.; Walsh, C.J. Assisting hand function after spinal cord injury with a fabric-based soft robotic glove. J. Neuroeng. Rehabil. 2018, 15, 1–10. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Wang, J.; Qin, N.; Yang, H.; Sun, K.; Hao, J.; Shu, L.; Liu, J.; Chen, Q. A star-nose-like tactile-olfactory bionic sensing array for robust object recognition in non-visual environments. Nat. Commun. 2022, 13, 79. [Google Scholar] [CrossRef]
- Liu, Q.; Ghodrat, S.; Huisman, G.; Jansen, K.M. Shape memory alloy actuators for haptic wearables: A review. Mater. Des. 2023, 233, 112264. [Google Scholar] [CrossRef]
- Sui, M.; Ouyang, Y.; Jin, H.; Chai, Z.; Wei, C.; Li, J.; Xu, M.; Li, W.; Wang, L.; Zhang, S. A soft-packaged and portable rehabilitation glove capable of closed-loop fine motor skills. Nat. Mach. Intell. 2023, 5, 1149–1160. [Google Scholar] [CrossRef]
- Hua, S.; Liu, Y.; Jiang, J.; Xiao, F.; Hou, R.; Li, A.; Cai, X.; Hu, X.; Ding, X.; Jin, X. Shape memory alloy based smart compression stocking and real-time health monitoring app for deep venous thrombosis. Smart Mater. Struct. 2023, 32, 075024. [Google Scholar] [CrossRef]
- Ding, X.; He, S.; Xiao, F.; Wu, S. A Shape Memory Alloy Rehabilitation Glove. CN 216908523 U, 8 July 2022. [Google Scholar]
- Mao, S.; Dong, E.; Jin, H.; Xu, M.; Zhang, S.; Yang, J.; Low, K.H. Gait study and pattern generation of a starfish-like soft robot with flexible rays actuated by SMAs. J. Bionic Eng. 2014, 11, 400–411. [Google Scholar] [CrossRef]
- ISO 13482; Robots and Robotic Devices—Safety Requirements for Personal Care Robots. ISO: Geneva, Switzerland, 2014.
- Pavon, J.M.; Adam, S.S.; Razouki, Z.A.; McDuffie, J.R.; Lachiewicz, P.F.; Kosinski, A.S.; Beadles, C.A.; Ortel, T.L.; Nagi, A.; Williams, J.W., Jr. Effectiveness of intermittent pneumatic compression devices for venous thromboembolism prophylaxis in high-risk surgical patients: A systematic review. J. Arthroplast 2016, 31, 524–532. [Google Scholar] [CrossRef]
- Chen, P.; Liu, Y.; Min, N.; Wang, M.; Cai, X.; Jin, M.; Jin, X. Enhanced Two Way Shape Memory Effect in Nanocrystalline NiTi Shape Memory Alloy Wires. Scripta Mater. 2023, 236, 115669. [Google Scholar] [CrossRef]
- Mandanka, K.; Mistry, S.; Tank, B. Design and development of wearable device using bluetooth low energy. In Proceedings of the 2017 International Conference on Computing Methodologies and Communication (ICCMC), Erode, India, 18–19 July 2017; pp. 762–766. [Google Scholar]
- Penmatsa, P.L.; Reddy, D.R.K. Smart detection and transmission of abnormalities in ECG via Bluetooth. In Proceedings of the 2016 IEEE International Conference on Smart Cloud (SmartCloud), New York, NY, USA, 18–20 November 2016; pp. 41–44. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Sun, S.; Liu, Y.; Xiao, F.; Li, W.; Wu, S.; Cai, X.; Ding, X.; Jin, X. A Smart Rehabilitation Glove Based on Shape-Memory Alloys for Stroke Recovery. Appl. Sci. 2025, 15, 7266. https://doi.org/10.3390/app15137266
Xie Y, Sun S, Liu Y, Xiao F, Li W, Wu S, Cai X, Ding X, Jin X. A Smart Rehabilitation Glove Based on Shape-Memory Alloys for Stroke Recovery. Applied Sciences. 2025; 15(13):7266. https://doi.org/10.3390/app15137266
Chicago/Turabian StyleXie, Yutong, Songrhon Sun, Yiwen Liu, Fei Xiao, Weijie Li, Shukun Wu, Xiaorong Cai, Xifan Ding, and Xuejun Jin. 2025. "A Smart Rehabilitation Glove Based on Shape-Memory Alloys for Stroke Recovery" Applied Sciences 15, no. 13: 7266. https://doi.org/10.3390/app15137266
APA StyleXie, Y., Sun, S., Liu, Y., Xiao, F., Li, W., Wu, S., Cai, X., Ding, X., & Jin, X. (2025). A Smart Rehabilitation Glove Based on Shape-Memory Alloys for Stroke Recovery. Applied Sciences, 15(13), 7266. https://doi.org/10.3390/app15137266





