On the Alkalinity of Solid Catalysts for Transesterification of Dimethyl Carbonate and Ethanol
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Catalyst Preparation
2.3. Reaction Test
2.4. Characterizations
3. Results
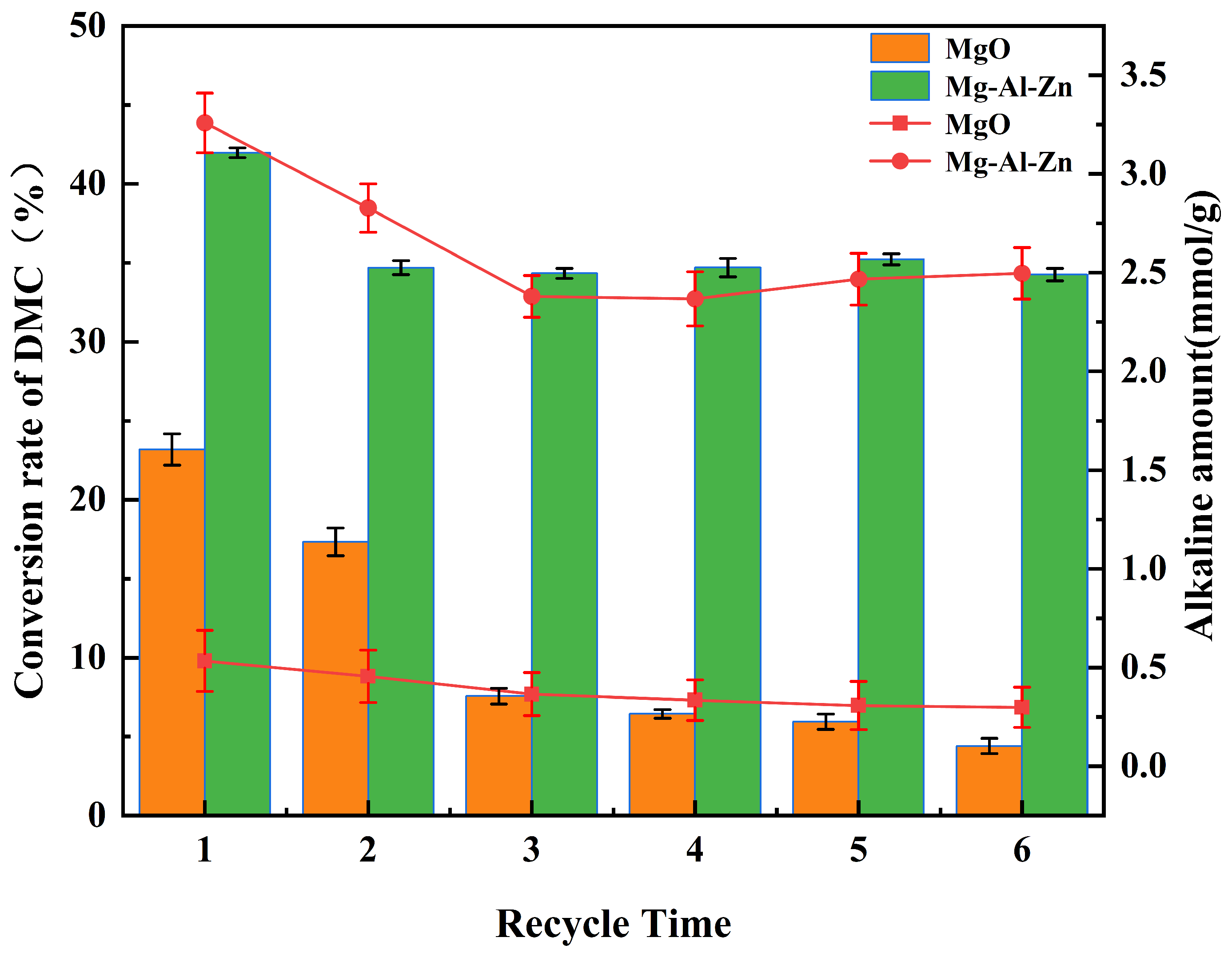
3.1. Catalysts Performance
3.2. The Morphology and Structure of Different Catalysts
3.3. Alkalinity of Different Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, R.; Cui, Z.; Yi, M.; Xie, Q.; Manthiram, A. Ethylene Carbonate-Free Electrolytes for Stable, Safer High-Nickel Lithium-Ion Batteries. Adv. Energy Mater. 2022, 12, 202103806. [Google Scholar] [CrossRef]
- Grégoire, C.M.; Cooper, S.P.; Khan-Ghauri, M.; Alturaifi, S.A.; Petersen, E.L.; Mathieu, O. Pyrolysis study of dimethyl carbonate, diethyl carbonate, and ethyl methyl carbonate using shock-tube spectroscopic CO measurements and chemical kinetics investigation. Combust. Flame 2023, 249, 112594. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.; Chen, Y.; Lao, B.; Qi, L.; Wang, H. Stability analysis for 5V high energy density pouch batteries of Si anode and SL/EMC electrolytes. J. Alloys Compd. 2019, 773, 105–111. [Google Scholar] [CrossRef]
- Fiorani, G.; Perosa, A.; Selva, M. Dimethyl carbonate: A versatile reagent for a sustainable valorization of renewables. Green Chem. 2018, 20, 288–322. [Google Scholar] [CrossRef]
- Huang, S.; Yan, B.; Wang, S.; Ma, X. Recent advances in dialkyl carbonates synthesis and applications. Chem. Soc. Rev. 2015, 44, 3079–3116. [Google Scholar] [CrossRef]
- Shang, Y.; Zheng, M.; Zhang, H.; Zhou, X. The Guanidine-Promoted Direct Synthesis of Open-Chained Carbonates. Aust. J. Chem. 2019, 72, 933–938. [Google Scholar] [CrossRef]
- Song, Y.; He, X.; Yu, B.; Li, H.-R.; He, L.-N. Protic ionic liquid-promoted synthesis of dimethyl carbonate from ethylene carbonate and methanol. Chin. Chem. Lett. 2020, 31, 667–672. [Google Scholar] [CrossRef]
- Shi, J.; Wang, H.; Lin, Y.; Cui, Y.; Xue, X.; Hao, X.; Zhang, Z.; Liu, C. Mg and Al dual-metal functionalized mesoporous carbon as highly efficient heterogeneous catalysts for the synthesis of ethyl methyl carbonate. New J. Chem. 2021, 45, 21199–21205. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, C. Effect of Preparation Method on the Catalytic Property of Calcined Ca–Al Hydrotalcite for the Synthesis of Ethyl Methyl Carbonate. ACS Omega 2021, 6, 5056–5060. [Google Scholar] [CrossRef]
- Keller, T.; Holtbruegge, J.; Niesbach, A.; Górak, A. Transesterification of Dimethyl Carbonate with Ethanol to Form Ethyl Methyl Carbonate and Diethyl Carbonate: A Comprehensive Study on Chemical Equilibrium and Reaction Kinetics. Ind. Eng. Chem. Res. 2011, 50, 11073–11086. [Google Scholar] [CrossRef]
- Wang, H.; Liu, W.; Wang, Y.; Tao, N.; Cai, H.; Liu, J.; Lv, J. Mg–Al Mixed Oxide Derived from Hydrotalcites Prepared Using the Solvent-Free Method: A Stable Acid–Base Bifunctional Catalyst for Continuous-Flow Transesterification of Dimethyl Carbonate and Ethanol. Ind. Eng. Chem. Res. 2020, 59, 5591–5600. [Google Scholar] [CrossRef]
- Schäffner, B.; Schäffner, F.; Verevkin, S.P.; Börner, A. Organic Carbonates as Solvents in Synthesis and Catalysis. Chem. Rev. 2010, 110, 4554–4581. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Li, S.; Cai, Y.; Cui, R.; Chen, J.; Ye, C.; Qiu, T. DBU-assisted expeditious synthesis of highly stable IL@ZIFs intergrowth composite: A nanocatalyst for EMC production. Fuel 2023, 334, 126659. [Google Scholar] [CrossRef]
- Chen, J.; Huang, H.; Gong, W.; Chen, Y.; Dong, R.; Ren, L.; Qiu, T. Fine-Tuning Electron–Donor Capability in the Basic Anion of Poly(ionic liquid) Frameworks for Revolutionizing Catalytic Synthesis of Ethyl Methyl Carbonate with Both Ultrahigh Catalytic Activity and Selectivity. Langmuir 2024, 40, 9233–9243. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Jin, X.; Hu, Y.; Subramaniam, B.; Chaudhari, R.V. Intriguing Catalyst (CaO) Pretreatment Effects and Mechanistic Insights during Propylene Carbonate Transesterification with Methanol. ACS Sustain. Chem. Eng. 2017, 5, 4718–4729. [Google Scholar] [CrossRef]
- Sun, H.; Li, H.; Chang, X.; Miao, S.; Yuan, X.; Zhang, W.; Jia, M. Nitrogen-doped carbon supported ZnO as highly stable heterogeneous catalysts for transesterification synthesis of ethyl methyl carbonate. J. Colloid Interface Sci. 2021, 581, 126–134. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, M.; Wang, Y.; Yan, Z.; Xu, G.; Guo, J.; Shi, L. One-step embedding method for immobilized bifunctional and alkaline ionic liquids as effective catalysts applied in transesterification. React. Chem. Eng. 2023, 8, 1654–1664. [Google Scholar] [CrossRef]
- Ralphs, K.; Hardacre, C.; James, S.L. Application of heterogeneous catalysts prepared by mechanochemical synthesis. Chem. Soc. Rev. 2013, 42, 7701–7718. [Google Scholar] [CrossRef]
- Šepelák, V.; Düvel, A.; Wilkening, M.; Becker, K.-D.; Heitjans, P. Mechanochemical reactions and syntheses of oxides. Chem. Soc. Rev. 2013, 42, 7507–7520. [Google Scholar] [CrossRef]
- Shen, Z.L.; Jiang, X.Z.; Zhao, W.J. A New Catalytic Transesterification for the Synthesis of Ethyl Methyl Carbonate. Catal. Lett. 2003, 91, 63–67. [Google Scholar] [CrossRef]
- Hu, M.; Pu, J.; Qian, E.W.; Wang, H. Biodiesel Production Using MgO–CaO Catalysts via Transesterification of Soybean Oil: Effect of MgO Addition and Insights of Catalyst Deactivation. BioEnergy Res. 2023, 16, 2398–2410. [Google Scholar] [CrossRef]
- Praikaew, W.; Kiatkittipong, W.; Aiouache, F.; Najdanovic-Visak, V.; Termtanun, M.; Lim, J.W.; Lam, S.S.; Kiatkittipong, K.; Laosiripojana, N.; Boonyasuwat, S.; et al. Mechanism of CaO catalyst deactivation with unconventional monitoring method for glycerol carbonate production via transesterification of glycerol with dimethyl carbonate. Int. J. Energy Res. 2021, 46, 1646–1658. [Google Scholar] [CrossRef]
- Lv, J.; Cai, H.; Guo, Y.; Liu, W.; Tao, N.; Wang, H.; Liu, J. Selective Synthesis of Ethyl Methyl Carbonate via Catalytic Reactive Distillation over Heterogeneous MgO/HZSM-5. ChemistrySelect 2019, 4, 7366–7370. [Google Scholar] [CrossRef]
- Miao, Y.-N.; Wang, Y.; Pan, D.-H.; Song, X.-H.; Xu, S.-Q.; Gao, L.-J.; Xiao, G.-M. Zn-Co@N-Doped Carbon Derived from ZIFs for High-Efficiency Synthesis of Ethyl Methyl Carbonate: The Formation of ZnO and the Interaction between Co and Zn. Catalysts 2019, 9, 94. [Google Scholar] [CrossRef]
- Liu, P.; Li, H.; Zhou, X. Clean synthesis of ethyl methyl carbonate using Mg-Al mixed oxide as catalyst. IOP Conf. Ser. Earth Environ. Sci. 2021, 687, 012063. [Google Scholar] [CrossRef]
- Bing, W.; Zheng, L.; He, S.; Rao, D.; Xu, M.; Zheng, L.; Wang, B.; Wang, Y.; Wei, M. Insights on Active Sites of CaAl-Hydrotalcite as a High-Performance Solid Base Catalyst toward Aldol Condensation. ACS Catal. 2017, 8, 656–664. [Google Scholar] [CrossRef]
- Liao, Y.; Li, F.; Dai, X.; Zhao, N.; Xiao, F. Solid base catalysts derived from Ca-M-Al (M = Mg, La, Ce, Y) layered double hydroxides for dimethyl carbonate synthesis by transesterification of methanol with propylene carbonate. Chin. J. Catal. 2017, 38, 1860–1869. [Google Scholar] [CrossRef]
- Li, F.; Liao, Y.-h.; Zhao, N.; Xiao, F.-k. The effect of NaF amount on solid base catalysts derived from F-Ca-Mg-Al layered double hydroxides and dimethyl carbonate synthesis. J. Fuel Chem. Technol. 2022, 50, 80–88. [Google Scholar] [CrossRef]
- Yu, Y.; Shi, L.; Guo, J.; Li, X.; Yang, W.; Zhang, Z.; Xu, G. In-depth understanding of soluble base deactivation during the carbonate transesterification process. Fuel 2021, 285, 119201. [Google Scholar] [CrossRef]
- Hernández-Giménez, A.M.; Hernando, H.; Danisi, R.M.; Vogt, E.T.C.; Houben, K.; Baldus, M.; Serrano, D.P.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Deactivation and regeneration of solid acid and base catalyst bodies used in cascade for bio-oil synthesis and upgrading. J. Catal. 2022, 405, 641–651. [Google Scholar] [CrossRef]
- Xu, M.-X.; Hu, Z.; Zhou, J.-L.; Cai, Q.; Zhang, X.-Y.; Li, M.-X.; Wu, Y.-W.; Wang, T.-P.; Lu, Q. Synergistic poisoning of KCl and PbCl2 on commercial V2O5-MoO3/TiO2 catalysts for MSW incineration flue gas denitrification. Catal. Commun. 2022, 172, 106545. [Google Scholar] [CrossRef]
- Shan, R.; Shi, J.; Yan, B.; Chen, G.; Yao, J.; Liu, C. Transesterification of palm oil to fatty acids methyl ester using K2CO3 /palygorskite catalyst. Energy Convers. Manag. 2016, 116, 142–149. [Google Scholar] [CrossRef]
- Almerindo, G.I.; Probst, L.F.D.; Campos, C.E.M.; de Almeida, R.M.; Meneghetti, S.M.P.; Meneghetti, M.R.; Clacens, J.-M.; Fajardo, H.V. Magnesium oxide prepared via metal–chitosan complexation method: Application as catalyst for transesterification of soybean oil and catalyst deactivation studies. J. Power Sources 2011, 196, 8057–8063. [Google Scholar] [CrossRef]
- Boz, N.; Kara, M. Solid Base Catalyzed Transesterification of Canola Oil. Chem. Eng. Commun. 2008, 196, 80–92. [Google Scholar] [CrossRef]
- Hao, Q. Synthesis of Dimethyl Carbonate via Transesterification Catalyzed by Solid Base. Master’s Dissertation, Dalian University of Technology, Dalian, China, 2023. [Google Scholar]
- Almusaiteer, K.A.; Al-Mayman, S.I.; Mamedov, A.; Al-Zeghayer, Y.S. In Situ IR Studies on the Mechanism of Dimethyl Carbonate Synthesis from Methanol and Carbon Dioxide. Catalysts 2021, 11, 517. [Google Scholar] [CrossRef]
- Guo, Y.; Mei, S.; Yuan, K.; Wang, D.-J.; Liu, H.-C.; Yan, C.-H.; Zhang, Y.-W. Low-Temperature CO2 Methanation over CeO2-Supported Ru Single Atoms, Nanoclusters, and Nanoparticles Competitively Tuned by Strong Metal–Support Interactions and H-Spillover Effect. ACS Catal. 2018, 8, 6203–6215. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Li, H.; Xue, W.; Wang, Y.; Zhao, X. Facile and Green Preparation of Zinc Oxyacetate. Chem. Lett. 2017, 46, 1151–1154. [Google Scholar] [CrossRef]
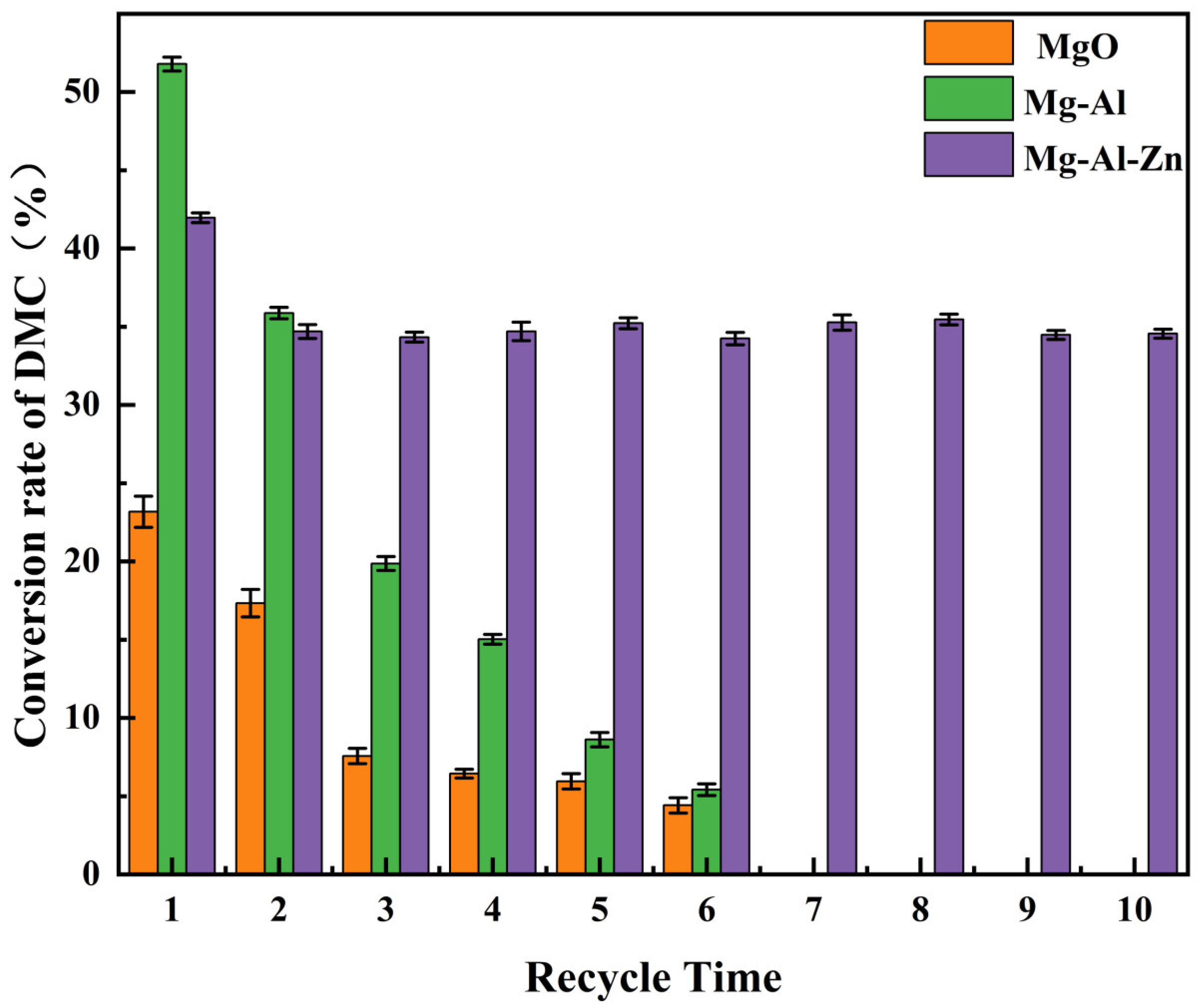
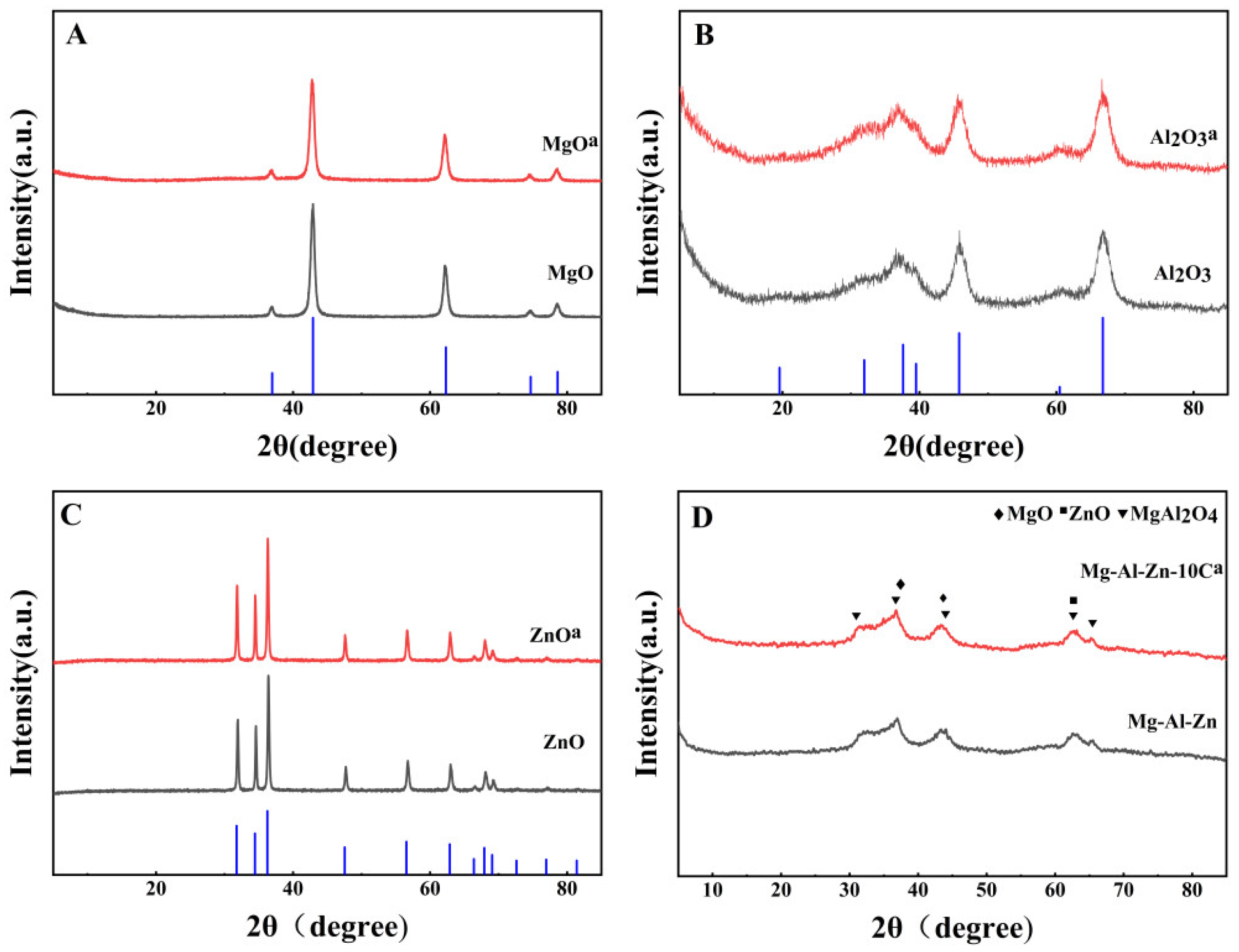

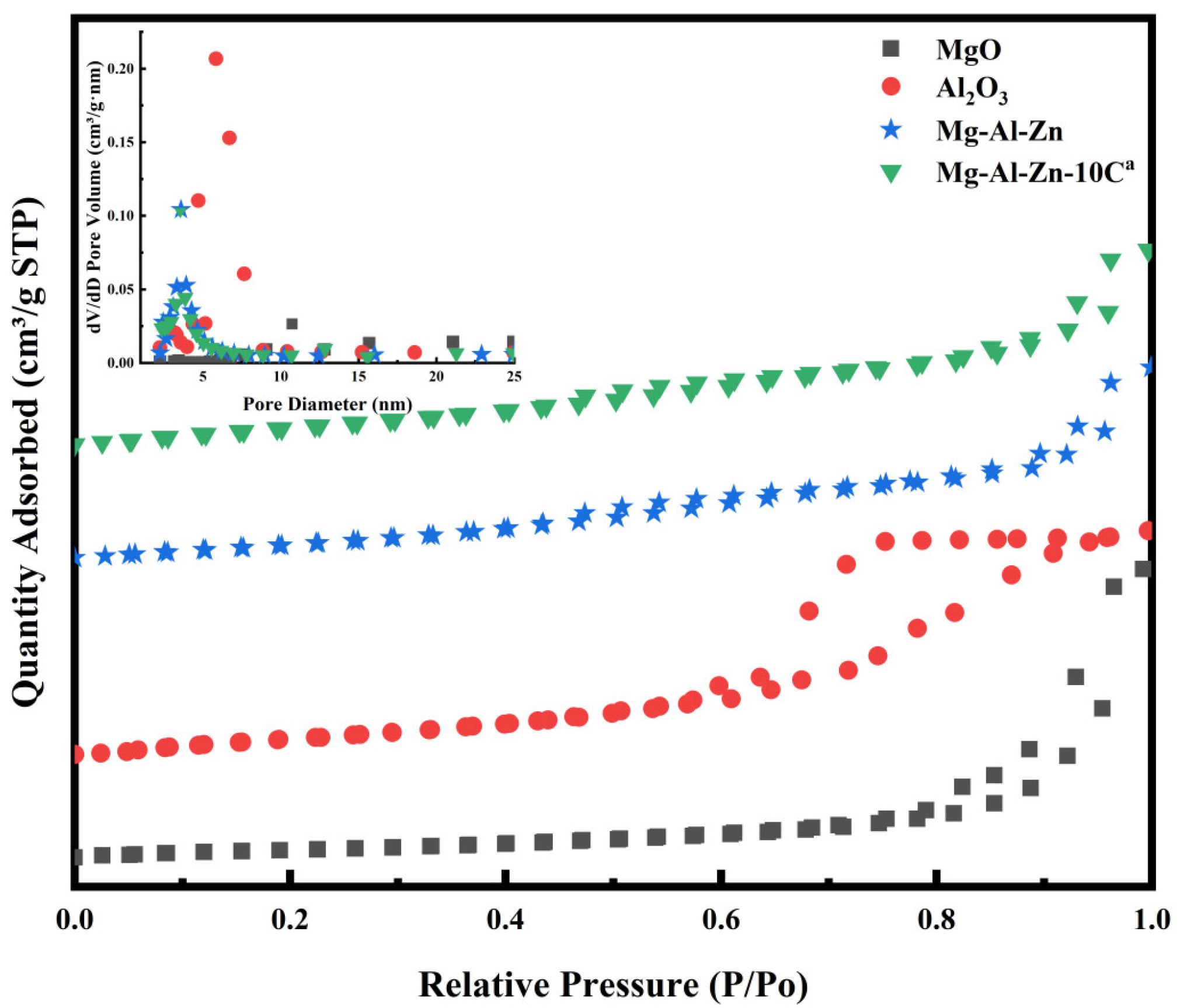

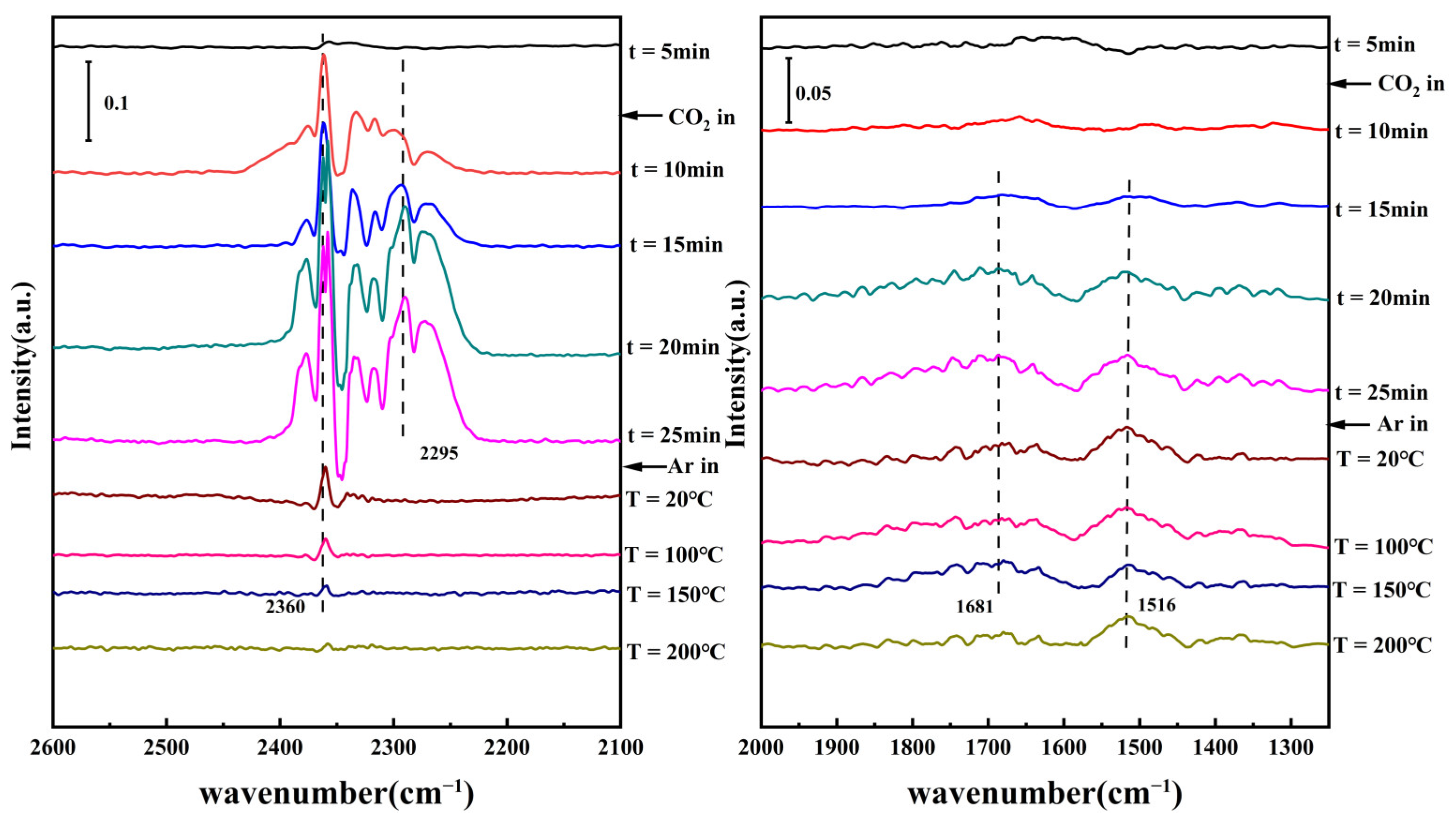
| Catalyst | DMC Con. (%) | EMC Sel. (%) | DEC Sel. (%) |
|---|---|---|---|
| Blank | 0.57 | - | - |
| MgO | 23.49 | 95.25 | 4.67 |
| Al2O3 | 7.08 | 96.42 | 3.45 |
| ZnO | 5.25 | 95.02 | 4.86 |
| Mg-Al | 51.79 | 91.23 | 8.47 |
| Mg-Al-Zn | 41.84 | 91.58 | 8.32 |
| Catalyst | SBET (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| MgO | 81.76 | 0.51 | 14.92 |
| Al2O3 | 186.90 | 0.43 | 6.08 |
| ZnO | 0.61 | - | - |
| Mg-Al-Zn | 160.97 | 0.26 | 7.51 |
| Mg-Al-Zn-10Ca | 163.94 | 0.25 | 7.87 |
| Catalyst | Brb | Phe | AyR | Din | Alkali Strength (H0) |
|---|---|---|---|---|---|
| MgO | √ | √ | √ | × | 11.0–15.0 |
| Al2O3 | √ | × | × | × | 7.2–9.3 |
| ZnO | √ | × | × | × | 7.2–9.3 |
| Mg-Al-Zn | √ | √ | × | × | 9.3–11.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Wu, S.; Shen, W.; Fang, Y. On the Alkalinity of Solid Catalysts for Transesterification of Dimethyl Carbonate and Ethanol. Appl. Sci. 2025, 15, 7225. https://doi.org/10.3390/app15137225
Zhang T, Wu S, Shen W, Fang Y. On the Alkalinity of Solid Catalysts for Transesterification of Dimethyl Carbonate and Ethanol. Applied Sciences. 2025; 15(13):7225. https://doi.org/10.3390/app15137225
Chicago/Turabian StyleZhang, Tianyu, Shun Wu, Weihua Shen, and Yunjin Fang. 2025. "On the Alkalinity of Solid Catalysts for Transesterification of Dimethyl Carbonate and Ethanol" Applied Sciences 15, no. 13: 7225. https://doi.org/10.3390/app15137225
APA StyleZhang, T., Wu, S., Shen, W., & Fang, Y. (2025). On the Alkalinity of Solid Catalysts for Transesterification of Dimethyl Carbonate and Ethanol. Applied Sciences, 15(13), 7225. https://doi.org/10.3390/app15137225





