1. Introduction
Pain is a vital warning signal for human survival and adaptation. It represents a multidimensional experience that extends beyond physical reactions; rather, it incorporates emotional, cognitive, and social factors [
1,
2]. Recent research has highlighted that pain is not solely a response to physiological stimuli; rather, it is closely intertwined with emotional states [
3]. For instance, negative emotions, such as anger or sadness, can increase pain sensitivity and influence one’s interpretation of pain experiences [
4]. This connection is attributed to the shared neural circuits of pain and emotion (for example, the prefrontal and anterior cingulate cortices), offering neurophysiological evidence that “emotional pain” elicits physiological responses similar to those observed in physical pain [
5,
6].
From this perspective, investigating the physiological signals of emotionally induced pain represents a critical extension of conventional pain research, which has primarily focused on physical pain elicited by mechanical pressure or thermal stimuli (
Table 1) [
7,
8,
9,
10,
11,
12]. Notably, most existing studies have sought to classify the presence or intensity of physical pain based on physiological signals [
13]. However, only a few investigations have objectively analyzed and classified subjective pain from emotional stimuli. This research gap likely stems from the inherently subjective nature of emotional distress, significant individual differences, and the complex interaction between emotional states and physiological responses. Consequently, a more comprehensive approach is needed to elucidate these complexities.
Skin conductance response (SCR) and skin conductance level (SCL) are particularly well suited for this research because they reflect autonomic nervous system (ANS) activity with sensitivity and are widely used to quantify changes in emotional states and pain responses [
14,
15,
16]. Psychological metrics—such as intensity, arousal, and valence—allow for the quantitative assessment of subjective experiences associated with emotion-inducing stimuli. Integrating these physiological and psychological data can enhance the accuracy of emotional pain recognition. In addition, machine learning techniques adequately capture complex nonlinear relationships and provide an effective framework for such studies [
17,
18].
Furthermore, many studies on pain classification using physiological signals have focused on physical pain; however, research on emotionally induced pain remains in its early stages. Previous studies have demonstrated the utility of physiological signals, such as electrocardiograms (ECG), electromyograms (EMG), electrodermal activity (EDA), and facial expressions, to detect physical pain, often employing machine learning algorithms to distinguish no pain from pain or categorize pain intensity (for example, low vs. high and low–medium–high) [
17,
19,
20,
21,
22]. In addition, these studies used distinct stimuli to evoke varying pain intensities. Instead of assessing the perceived pain from a single stimulus, these studies focused on responses to multiple stimuli designed to elicit different degrees of pain. However, efforts to integrate emotional pain or subjective psychological responses into physiological-signal-based analyses have been limited.
Therefore, we aim to examine whether emotional pain states could be quantitatively assessed and classified by integrating physiological signals and psychological indices, focusing on differences across perceived pain intensity levels. Specifically, an anger-inducing video stimulus was used to elicit emotional pain in participants, during which ECG, EDA, respiration (RESP), photoplethysmogram (PPG) readings, and finger temperature (FT) were measured, and self-reported emotional ratings (valence and arousal) were collected. We performed paired t-tests and one-way analysis of variance (ANOVA) to examine psychophysiological differences across different pain states. In addition, using the data collected, we classified the presence and intensity of pain based on a random forest (RF)-based machine learning model. Repeated nested cross-validation (CV) was adopted to mitigate the limitations imposed by the small sample size, and the class imbalance was handled using the synthetic minority oversampling technique (SMOTE). Finally, we used the permutation feature importance (PFI) to evaluate the contributions of key physiological and psychological features to the classification tasks to improve model interpretability. This study validates the effectiveness of physiological signals and subjective ratings for recognizing emotional pain states. We identify the key features that influence pain classification, laying the background for automated systems that integrate psychological and physiological indicators for pain recognition.
2. Materials and Methods
2.1. Participants
This study includes 112 adults (54 males, 23.21 ± 2.0 years; 58 females, 21.34 ± 2.1 years). All participants reported no history of medical conditions, neurological or psychiatric medication use, or use of any drugs that could affect the cardiovascular, respiratory, or central nervous systems. Before the experiment, participants were thoroughly informed about the study procedures and provided written informed consent. The Institutional Review Board of Chungnam National University approved this study (Approval No.: 201309-SB-041-01, An Integrated Cognitive-Affective Model for Human–Computer Interfaces). Each participant received USD 30 as compensation.
2.2. Painful and Non-Painful Stimuli
Emotional pain is a broader concept that encompasses social pain. It refers to psychological distress caused by experiences related to emotions, such as love, loss, rejection, betrayal, humiliation, and anger. Unlike physical pain, which is triggered by a direct sensory stimulus, emotional pain is processed within the central nervous system. Certain brain regions, such as the anterior cingulate cortex, show overlapping activation for both types of pain [
5]. In this study, two types of video stimuli were selected: (1) a non-painful stimulus designed to evoke a state without emotional pain, and (2) an anger stimulus intended to elicit emotional pain. Each video clip, between 1 and 3 min long, was selected from Korean films and television programs [
23].
Table 2 provides detailed descriptions of these stimuli. A preliminary psychological study involving 264 participants validated the effectiveness and appropriateness of both video stimuli for inducing the desired emotional states. In this preliminary study, participants indicated the emotions they experienced by selecting from a list (for example, anger, no emotion, joy, fear, and sadness). Over 90% of participants correctly identified the intended affective state (anger or no emotional response) for both video stimuli.
2.3. Psychological Assessment
After viewing each stimulus, participants rated their perceived pain intensity and emotional responses using a questionnaire. Pain intensity was assessed using a 7-point Likert scale, where one indicated low intensity and 7 indicated high intensity. Emotional responses were measured using 9-point Likert scales ranging from −4 (negative affect or low arousal) to +4 (positive affect or high arousal), for valence (unpleasant to pleasant) and arousal (relaxed to aroused).
2.4. Experimental Procedure
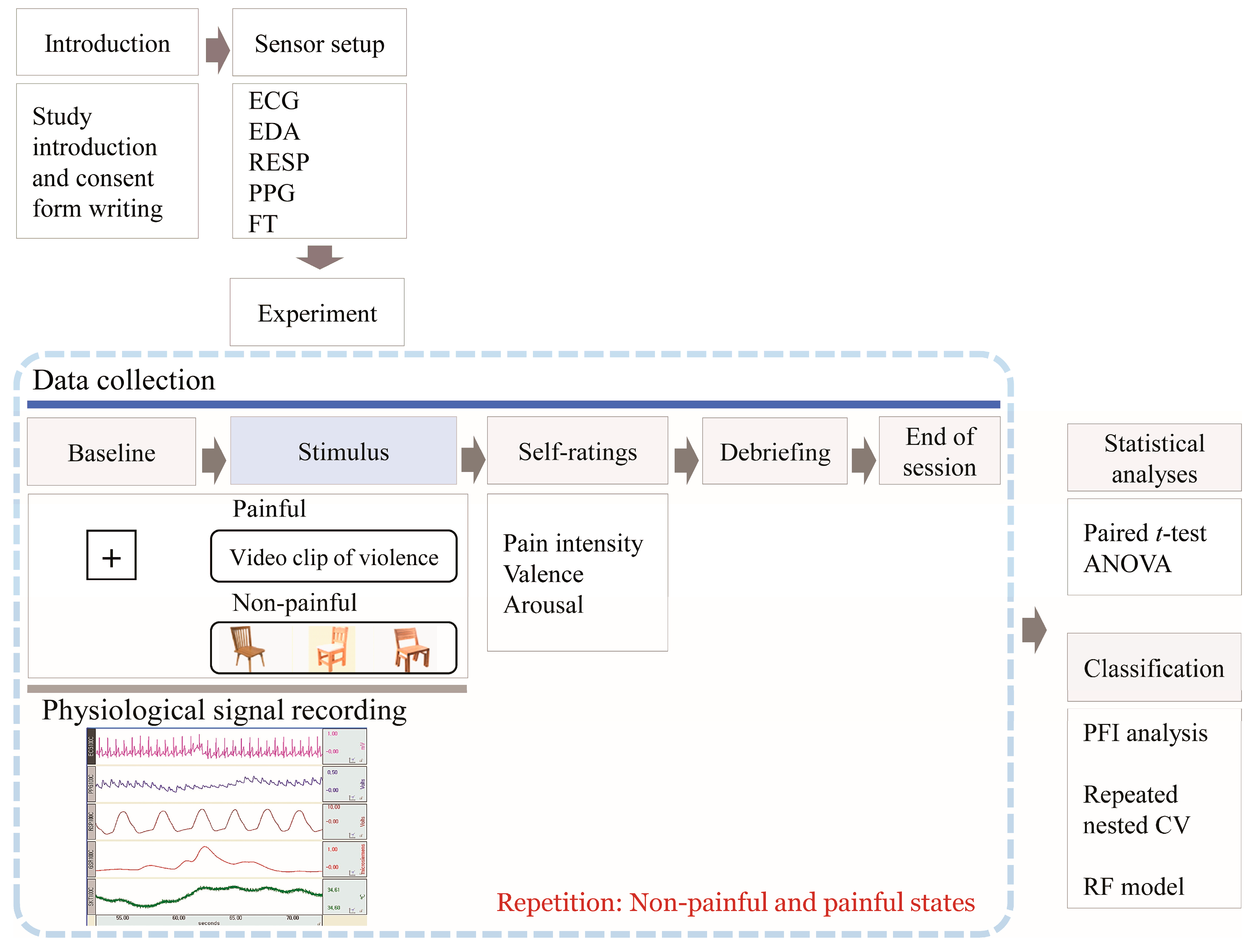
Figure 1 summarizes the experimental procedure. Before the main experiment, participants acclimated to the laboratory setting and received detailed instructions regarding the procedures and emotional rating scales. Electrodes were attached to the wrist, fingers, and ankle for physiological signal measurement. A 60 s baseline recording was taken to establish physiological baseline levels while participants watched a “+” sign on the monitor. Subsequently, participants viewed either a pain-inducing or a non-pain-inducing video clip lasting 1–3 min. Immediately after the stimulus, they rated the valence, arousal, and intensity of their pain. This was followed by a 2 min rest period to facilitate recovery. The order of stimulus presentation was counterbalanced across participants.
2.5. Physiological Signal Recording
Physiological responses were recorded using equipment from Biopac Systems Inc. (Goleta, CA, USA), including an MP100WS unit and AcqKnowledge software (ver. 3.7.1). All channels were sampled at 250 Hz with amplification and band-pass filtering. The ECG electrodes were placed on both wrists and the left ankle following the Lead-I configuration. To capture the EDA signal, AgCl electrodes with an 8 mm diameter were positioned on the volar surfaces of the index and middle fingers of the non-dominant hand. These electrodes were filled with 0.05 molar isotonic NaCl paste. A PPG sensor was placed on the first joint of the non-dominant thumb, while the FT electrode was attached to the first joint of the non-dominant ring finger. The RESP sensor was secured around the chest with a Velcro strap to monitor chest expansion based on the Hall effect.
2.6. Feature Extraction
For feature extraction, we collected a 30 s segment of baseline data (extracted from the final 30 s of the session) and 30 s segments during the painful and non-painful conditions (extracted from the middle of the session) from each patient.
Table 3 lists the features used in this study. Time-domain features of heart rate variability (HRV) were derived from ECG signals and included heart rate (HR), standard deviation of NN intervals (SDNN), root mean square of successive differences between adjacent NNs (RMSSD), and the percentage of successive NNs differing by over 50 ms (pNN50). A frequency spectral analysis was used to evaluate normalized powers in the low-frequency band (LFnu, 0.04–0.15 Hz), high-frequency band (HFnu, 0.15–0.4 Hz), and the LF/HF ratio. HFnu represents the ratio of HF power to the total HF and LF power, whereas LFnu represents the ratio of LF power to the total HF and LF power. Thus, LFnu and HFnu always sum to 1. In this study, we used only HFnu and the LF/HF ratio, given the linear association between LFnu and HFnu. For EDA, SCL and SCR amplitudes were analyzed, as they indicate changes in skin electrical properties linked to sweat gland activity. SCL represents the average tonic component of EDA, while SCRs reflect the phasic waves of the EDA signal and were averaged over specific SCR events with amplitudes of ≥0.05 μS during a 30 s interval. The blood volume pulse (BVP), obtained by averaging the range of BVP, was defined as the difference between the highest and lowest values of each pulse wave over 30 s. The pulse transit time (PTT) was determined from the ECG and PPG signals by measuring the time from the R-peak of the ECG signal to the arrival of the pulse wave at the finger. Respiratory rate (RR) was calculated by counting the number of breaths. The FT was determined by averaging the FT values over 30 s.
2.7. Statistical Analysis
All statistical analyses were conducted using SPSS version 21.0 (IBM, Armonk, NY, USA). We assessed differences in ANS responses between no pain and pain conditions using paired t-tests. One-way ANOVA with the Bonferroni post hoc test was applied to examine psychological and ANS differences based on perceived pain levels.
2.8. Machine Learning in Pain Research
Machine learning approaches to pain research utilize the well-established autonomic response to noxious or emotionally stressful events, captured through ECG, PPG, EDA, EMG, and similar sensors. These data are then used to map physiological measures to pain labels [
24]. Framed as a supervised classification task, algorithms such as support vector machines (SVMs), gradient boosting, and RF learn nonlinear decision boundaries from feature vectors that encompass HRV, skin conductance (SC), muscle activity, and respiratory patterns [
24]. RFs are rooted in ensemble learning theory, employing techniques like bootstrap aggregation and random subspace selection, which produce low-variance, asymptotically consistent predictors that perform well on the small, noisy datasets commonly found in biomedical research [
25].
This machine learning framework for pain assessment offers three key advantages. Machine learning models can simultaneously integrate multiple physiological signals, enhancing multimodal assessments for improved robustness and accuracy [
26]. By fusing data from various physiological signals and even modalities such as facial expressions, these models increase information density and offer a comprehensive view of a patient’s pain experience. Analyses based on multi-sensor time-series data have been successfully applied across a wide range of domains beyond the biomedical field [
27]. Additionally, they can be customized to an individual’s baseline physiology, effectively addressing the inter-subject variability that often limits rule-based approaches [
28]. Furthermore, efficient algorithms, implemented in wearables or bedside monitors, provide continuous pain scores, enabling clinicians to adjust analgesic prescriptions, therapists to adapt rehabilitation exercises, and consumer apps to alert users before pain escalates [
28].
As summarized in
Table 1, machine learning models have demonstrated accuracies of 0.80–0.85 in detecting nociceptive heat and electrical pain. Recent narrative reviews indicate a shift in the field toward multimodal, explainable approaches, emphasizing the need to explore emotional or subjective pain domains [
26]. Our study addresses this need by focusing on the discrimination of perceived intensity levels of the same pain stimulus through a multimodal physiological signal and machine learning approach.
2.9. Machine Learning Pipeline
Our primary classification goals were to (i) separate baseline from painful states and (ii) discriminate perceived-intensity levels.
Section 2.9.1,
Section 2.9.2,
Section 2.9.3 and
Section 2.9.4 outline the complete machine learning pipeline devised to meet these objectives. In
Section 2.9.1, we compute PFI across physiological features for the baseline-versus-pain task.
Section 2.9.2 then trains and evaluates RF classifiers using the PFI-ranked features for baseline-versus-pain classification. In
Section 2.9.3, we repeat the PFI analysis for the pain intensity discrimination task. Finally,
Section 2.9.4 trains RF models on the top three ranked features to classify pain intensity.
2.9.1. PFI Analysis of 12 Physiological Features for Classifying Baseline and Painful States
We used an RF classifier to perform PFI analysis on 12 physiological features to distinguish between the baseline (negative class) and painful states (positive class). We utilized the PFI to establish an interpretable ranking of features within a small dataset. PFI assesses the significance of each feature by quantifying the decline in model accuracy when its values are randomly permuted, thereby directly linking the importance to predictive performance. Unlike Gini importance, which may favor features with multiple categories [
29], PFI provides more impartial rankings and mitigates the emphasis on misleading features that are sometimes found in methods, such as Shapley’s additive explanations [
30]. These attributes help to reduce overfitting artifacts and ensure that the selected features reflect meaningful physiological insights rather than noise.
We combined PFI with a repeated nested CV strategy involving subject-wise splitting to achieve stable rankings despite data scarcity. Participants’ data were grouped to prevent an overlap between the training and test sets. In each iteration, the dataset was randomly divided into five folds at the subject level; one set was designated as the outer test set, and the remaining four were designated as the outer training set. Then, optimal hyperparameters (scored by binary F1,
Table S1) were identified using a nested 4-fold CV within the outer training set. Subsequently, the model was retrained on the complete outer training set and evaluated using an outer test fold. This process was repeated 20 times using random seeds. We explored the following RF hyperparameters: the number of decision trees (30 or 50), the minimum number of samples required to split an internal node (15 or 20), and the minimum number of samples at a leaf node (6 or 12). The maximum depth of the tree was set to three, and the maximum number of features considered for splitting was set to “sqrt.”.
After selecting the hyperparameters, PFI was calculated for each outer test fold. First, we recorded the binary F1 score of the model under normal conditions (no permutations). Subsequently, each feature was permuted sequentially, with other features held constant, and the F1 score was recalculated to assess performance decline. Each permutation test was repeated 10 times per feature, and the results were averaged across folds and repeats to reduce variance from the limited sample size. This cross-validated, repeated methodology yields consistent estimates of feature importance, enhancing confidence that the top-ranked features are genuinely influential in distinguishing painful and baseline states.
2.9.2. Classification of Baseline and Painful States Based on Physiological Features
Based on the PFI results, we identified between two and five of the most important features and used these subsets to perform the classification tasks. Similar RF algorithm, CV structure, and hyperparameter settings as those used in the PFI analysis were employed. Specifically, a repeated nested CV protocol with subject-wise splitting was used, and the data were divided into five outer folds and four inner folds. This procedure was repeated 20 times with different random seeds. We used binary F1 as the optimization metric for hyperparameter tuning. After selecting the best combination of parameters, the RF was retrained on the entire outer training set and evaluated on the outer test set. We recorded the binary F1 (on the test and training sets), and accuracy, recall, and specificity (
Table S1). Finally, the metrics from all the outer folds and all repeats were averaged to reduce variance and yield a more reliable performance estimate, thereby minimizing overfitting. These performance metrics are reported as mean and standard deviation, calculated from 20 repeats.
We computed a confusion matrix for each outer fold of a repeat by comparing the predicted and true labels. Subsequently, this matrix was normalized row-wise by dividing each row by its total count. The normalized matrices from each fold were accumulated and averaged at the end of the repeat to produce a single mean-normalized confusion matrix. After completing all repeats, the repeat-level matrices were averaged again, resulting in a final mean-normalized confusion matrix that reflected the row-wise proportion of predicted classes across all folds and repetitions. This nested CV design with subject-wise splitting and repeated iterations ensures that the performance of the model is not overly dependent on any single data split, providing a more dependable assessment of how well the model generalizes to unseen samples.
2.9.3. PFI Analysis for Classifying Pain Intensity Levels
We evaluated two feature sets for the intensity-level classification. The first set comprised 12 physiological features (PHY_12), whereas the second included 2 self-reported measures of valence and arousal, resulting in 14 features altogether (PHY_12 + VAL_ARO). In line with our approach to PFI analysis for baseline versus pain classification, we used an RF classifier and calculated PFI using nested CV, which was repeated 20 times. Each repetition involved a 5-fold outer loop and a 4-fold inner loop, with the PFI scores being averaged across all folds and repetitions.
In addition, several adaptations were made for the intensity classification. First, we used stratified group splitting instead of subject-wise splitting to account for the varying sample sizes across the intensity categories. To address class imbalance, we applied the SMOTE-based oversampling within the outer and inner training folds, excluding it from the test and validation folds. The model’s performance was assessed using the macro F1 (
Table S1) score as an optimization metric during hyperparameter tuning. We explored several hyperparameters, including the number of decision trees (15 or 25), the minimum number of samples required to split a node (15 or 20), and the minimum number of samples at a leaf node (6 or 12). The maximum depth of the tree was set to two, and the maximum number of features considered for splitting was set to “sqrt.”.
2.9.4. Classification of Pain Intensity Levels
We assessed the impact of three different input datasets on intensity level classification according to the PFI ranking. The first dataset included only valence and arousal (VAL_ARO), the second consisted of the top-ranked features from the “PHY_12” feature set (PHY_Sel), and the third used the top-ranked features from the “PHY_12 + VAL_ARO” feature set (Combined_Sel). The same RF classifier, using a repeated nested CV design, stratified splitting, SMOTE-based oversampling, and hyperparameter settings consistent with the PFI analysis for intensity-level classification, was used for all classifications. Macro F1 was the optimization metric for hyperparameter tuning, and the best parameter set identified during the inner CV was retrained on the entire outer training fold. The resulting model was evaluated on the outer test fold, with the macro F1 (for the training and test sets) and balanced accuracy, recall, and specificity recorded (
Table S1). These outer-fold metrics were averaged over all folds and repetitions, and all performance measures are reported as means and standard deviations. We computed the normalized confusion matrix following the same procedure as used in the baseline-versus-pain classification, presenting the performance metrics and normalized confusion matrices for all three input datasets.
To benchmark the RF model, we repeated the intensity-level 6 vs. 7 classifications with two alternative algorithms: a linear-kernel SVM and k-nearest neighbors (k-NN). For the SVM, the regularization parameter C was tuned at three levels—0.01, 0.1, and 1—whereas the k-NN model was evaluated with neighborhood sizes of 13, 15, and 17. All remaining preprocessing was identical to that used in the RF pipeline.
To assess the impact of individual random forest hyperparameters, we conducted a one-factor-at-a-time (OFAT) sensitivity analysis. The grid search evaluated three parameters, the number of decision trees (15 or 25), the minimum samples needed to split a node (15 or 20), and the minimum samples required at a leaf node (6 or 12), resulting in eight unique combinations. These values were chosen a priori to mitigate overfitting risk. Each combination was passed through the identical preprocessing pipeline and evaluated with a repeated outer-loop CV (20 × 5-fold); all hyperparameter settings were tested directly in the outer folds, with no inner tuning loop. Test F1 scores were recorded for all eight configurations, with performance differences attributed to the varying parameter in each OFAT comparison.
3. Results
3.1. Validation of Emotional Pain: Analysis of Subjective and Affective Responses
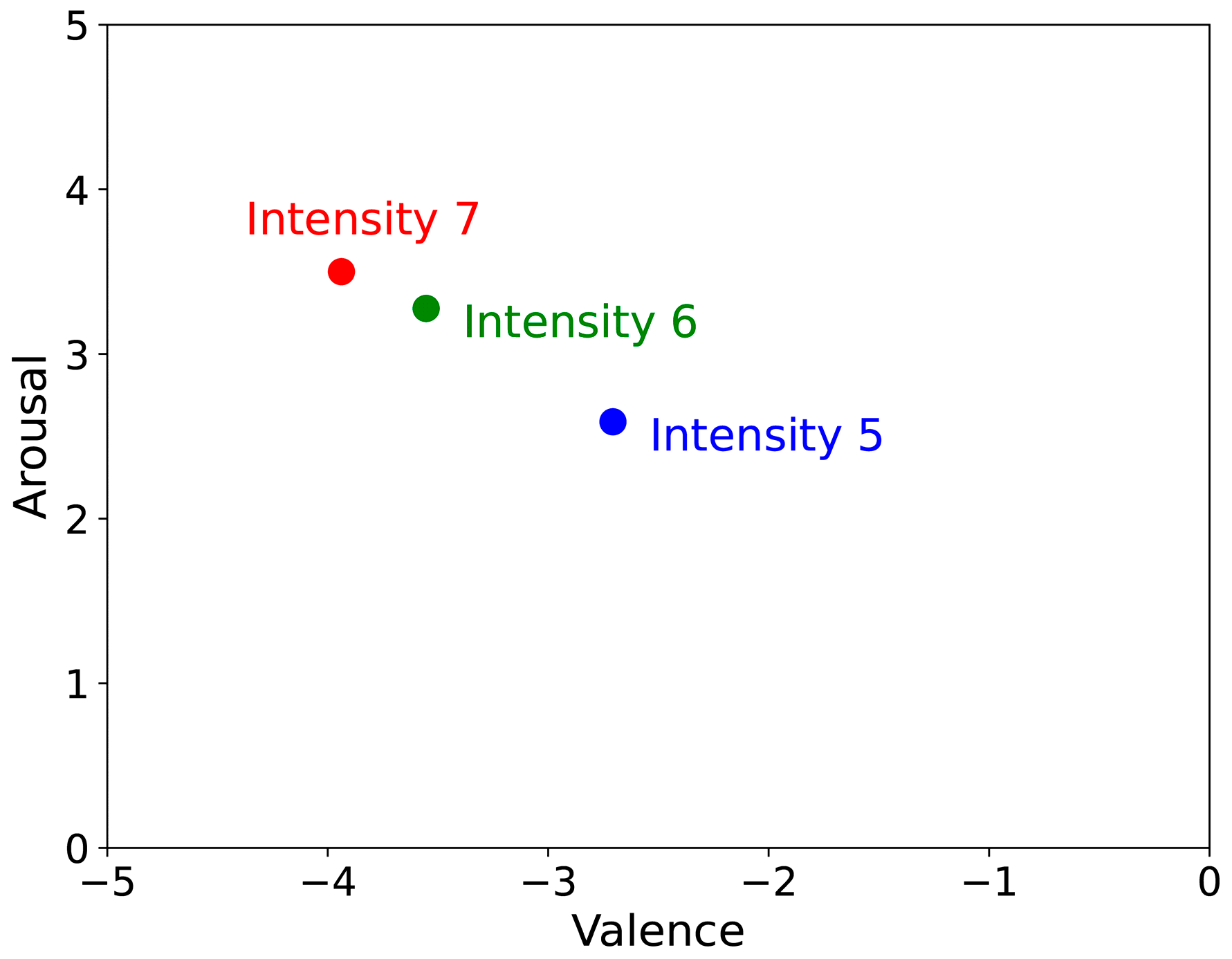
Participants reported an average pain intensity of 6.32 ± 0.72, reflecting a consistently high level of perceived pain. Of the 112 participants, the 7-point Likert scale revealed 17, 47, and 48 selected intensities of 5, 6, and 7, respectively. Most participants exhibited similar pain levels, indicating that the stimulus intensity was reliably controlled. Painful responses showed a mean valence and arousal of −3.60 ± 0.84, and 3.26 ± 0.79, respectively, suggesting that the stimulus induced a strongly negative affective state with high arousal. These results confirm the effectiveness of the experimental stimulus in eliciting emotional pain suitable for psychophysiological analyses. In contrast, the non-painful stimulus produced mean valence and arousal ratings of 0.49 ± 1.09 and −1.96 ± 1.21, respectively, indicating minimal emotional impact and low arousal. This outcome demonstrates the efficacy of non-painful stimulus in reducing emotional reactivity.
Figure 2 illustrates how each pain intensity level was positioned on the dimension of emotion.
3.2. Autonomic Physiological Responses to Emotional Pain
Physiological responses during the painful state were evaluated using paired
t-tests to compare the baseline and painful conditions (
Table 4), with Bonferroni corrections for multiple comparisons (
p < 0.0042). The results show significant differences in most physiological features between the two conditions, except for HR, SDNN, and FT. Notably, RMSSD, pNN50, HFnu, SCL, and SCR increased, whereas LF/HF, RR, BVP, and PTT decreased during painful conditions. These findings indicate that the painful stimulus elicited notable shifts in sympathetic and parasympathetic activities, as reflected in the HRVs and electrodermal responses. In addition, changes in peripheral blood flow and RR indicate a strong psychophysiological stress response to the painful stimulus.
3.3. Comparative Analysis of Physiological Features During Painful and Non-Painful States Based on Changes from Baseline
To compare physiological responses between painful and non-painful conditions, changes from baseline were calculated and analyzed using paired
t-tests (
Table 5), and Bonferroni corrections were applied (
p < 0.0042). The results show that SDNN, RMSSD, pNN50, SCR, and SCL were significantly higher under the painful condition, whereas BVP, PTT, and RR were significantly lower. SCR (t = −6.164,
p < 0.001) and BVP (t = 8.182,
p < 0.001) demonstrated the most significant changes, indicating marked autonomic activation and a decrease in peripheral blood flow in response to pain. FT decreased during the painful condition and increased during the non-painful condition (t = 3.471,
p = 0.001), reflecting the impact of emotional and pain-related stimuli on peripheral vascular regulation.
3.4. Psychophysiological Differences According to Pain Intensity
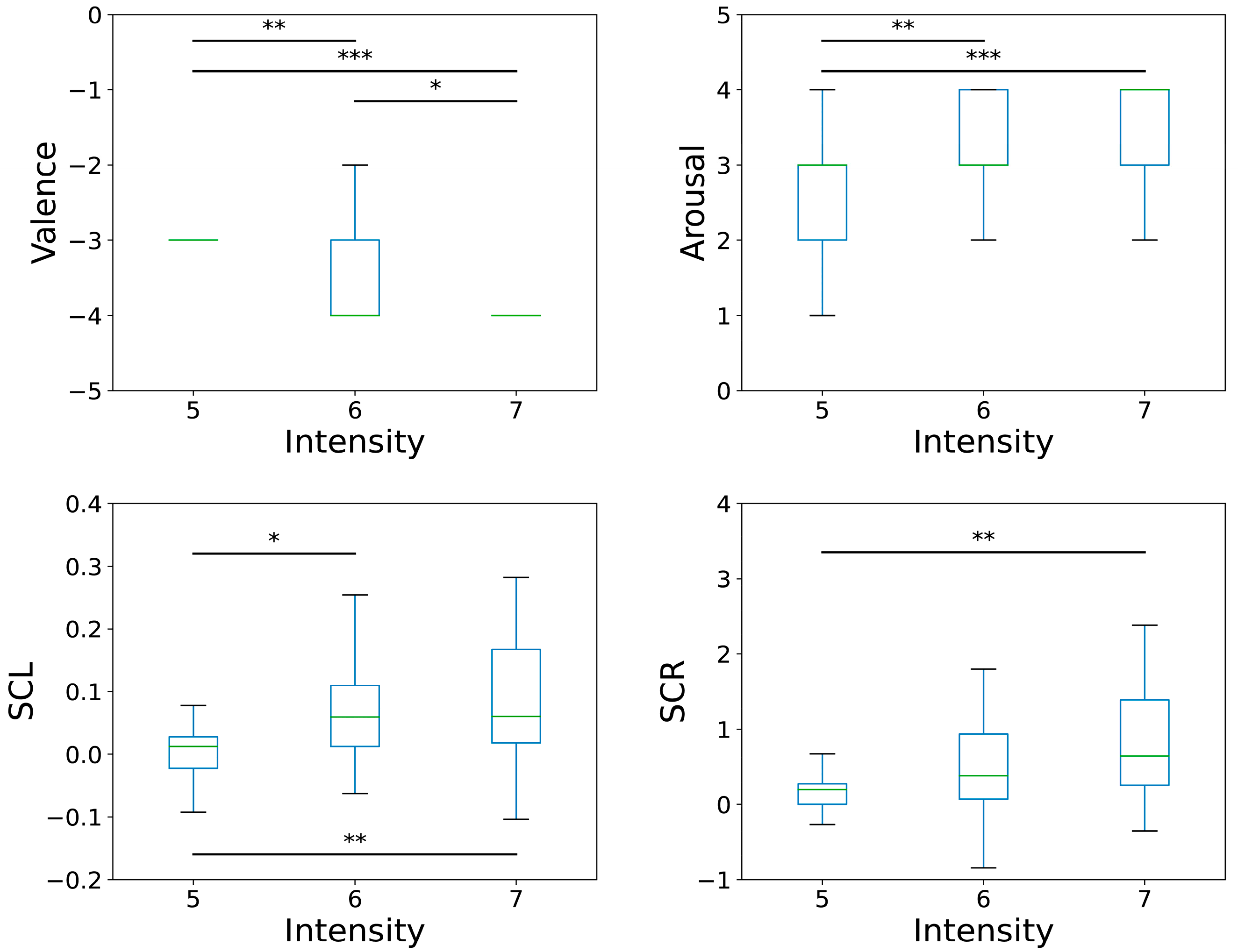
To examine the impact of pain intensity on psychophysiological responses, participants who selected pain levels of 5, 6, or 7 were categorized into three groups: intensity 5, intensity 6, and intensity 7. A one-way ANOVA was conducted to assess the effect of pain intensity on psychophysiological features, followed by a Bonferroni post hoc analysis (
Table 6). In the ANOVA, physiological features were represented by the difference between the painful state and baseline, whereas arousal and valence were used directly without transformation.
The ANOVA results show a significant difference in valence and arousal across the three groups (valence: F = 15.356, p < 0.001; arousal: F = 9.561, p < 0.001). Post hoc tests revealed that the intensity 5 group had significantly higher valence than the intensity 6 and 7 groups (5 > 6 = 7). In addition, the intensity 5 group exhibited significantly lower arousal than the intensity 6 and 7 groups (5 < 6 = 7), with no significant differences between the intensity 6 and 7 groups in both measures.
Regarding physiological features, SCR and SCL differed significantly across the pain intensity groups (SCR: F = 4.274,
p = 0.016; SCL: F = 3.669,
p = 0.029), suggesting greater sympathetic activation under higher pain intensities. SDNN and PTT showed marginal trends (
p < 0.10), but did not reach statistical significance after correction. Nonetheless, participants with intensity level 6 had lower SDNN than those with level 7, whereas those with intensity level 5 had lower PTT than those with level 7. No significant differences were observed in HR, RMSSD, pNN50, HFnu, LF/HF, RR, BVP, or FT (
p > 0.05). These findings illustrate that participants’ perceived pain intensity and emotional responses correspond to distinct patterns of ANS activation, highlighting the value of these physiological features in characterizing emotional pain states.
Figure 3 presents boxplots for four features, valence, arousal, SCL, and SCR, with significant differences identified among the groups in the ANOVA.
3.5. Classification of Baseline and Painful States
We investigated the classification of baseline and painful states using an RF model, employing 12 physiological features as input data. Before the classification analyses, we computed the PFI to identify the most influential features for distinguishing between the two states. The features were ranked in descending order of importance (
Figure S1). The top-ranked features were SCR, SCL, and RESP, followed by BVP, FT, and HR, all of which had positive mean PFI values. Notably, the SCR demonstrated greater significance than the other measures. However, the remaining six features yielded negative mean PFI values, indicating their limited utility.
Subsequently, we conducted classification experiments using only subsets of the top-ranked features identified by the PFI analysis. We compared subsets ranging from two to five features while monitoring changes in the F1 score (
Figure S2). Combining the top-three features (SCR, SCL, and RESP) yielded the highest F1 score of 0.83. Similar F1 values were observed across three-, four-, and five-feature subsets. The disparity between the training and test F1 scores was approximately 0.03, suggesting a consistent performance and minimal overfitting.
Table 7 summarizes the evaluation metrics derived from the classification model using these three key features, all exceeding 0.80. This underscores that even a minimal set of three physiological measures can effectively distinguish between the baseline and painful states.
Figure S3 presents the corresponding confusion matrix, which indicates that the majority of the samples were accurately classified. These findings illustrate that the selected physiological markers, particularly SCR, SCL, and RESP, effectively capture the transitions between the baseline and painful states.
3.6. Classification of Perceived Pain Intensity Levels
We classified self-reported pain intensity, measured on a 7-point Likert scale, using physiological signals or a combination of physiological signals with valence and arousal ratings. Participants could select any intensity from 1 to 7, but the final distribution across all 112 participants was comprised only of intensities 5 (17 participants), 6 (47 participants), and 7 (48 participants). Therefore, the feasible classification task was limited to three possible intensity levels. However, only 17 participants reported an intensity of 5; hence, the sample size was too small for a reliable machine learning-based classification. Consequently, our primary analyses focused on a more robust binary classification of intensities 6 and 7. Classifications involving intensity 5 (such as 5 vs. 6, 5 vs. 7, or the three-level classification of 5 vs. 6 vs. 7) are provided in the
Supplementary Materials.
We investigated three distinct feature sets following the PFI ranking (detailed in the Materials and Methods section) to assess the impacts of different input features. Specifically, we examined (1) valence and arousal-only (VAL_ARO), (2) top-ranked features from the PHY_12 feature set (PHY_Sel), and (3) top-ranked features from the combined PHY_12 + VAL_ARO set (Combined_Sel).
3.6.1. Classification Between Intensity Levels 6 and 7
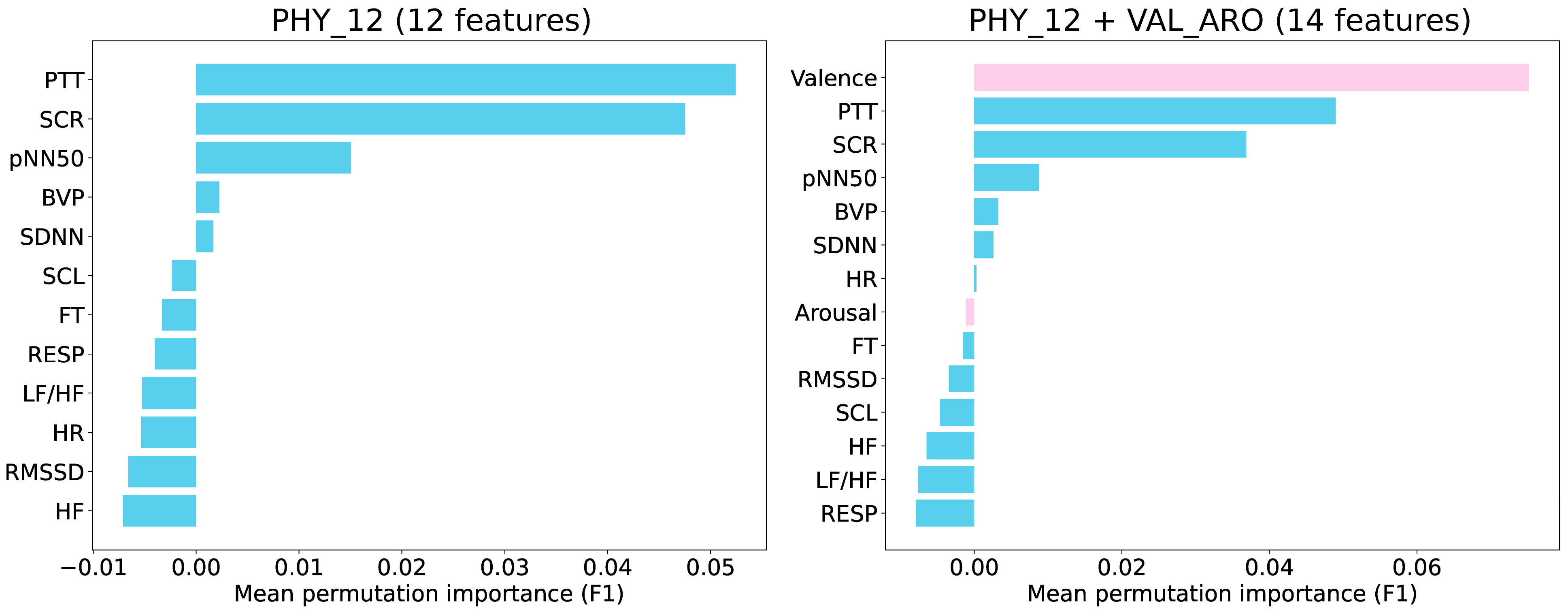
We began the analysis by calculating the PFI to identify the most influential features for classifying intensity levels 6 and 7 (
Figure 4). This was performed on two datasets: PHY_12 and PHY_12 + VAL_ARO. The results indicate that PTT, SCR, and pNN50 were the top three features in the PHY_12 dataset, whereas valence, PTT, and SCR ranked highest in the PHY_12 + VAL_ARO dataset.
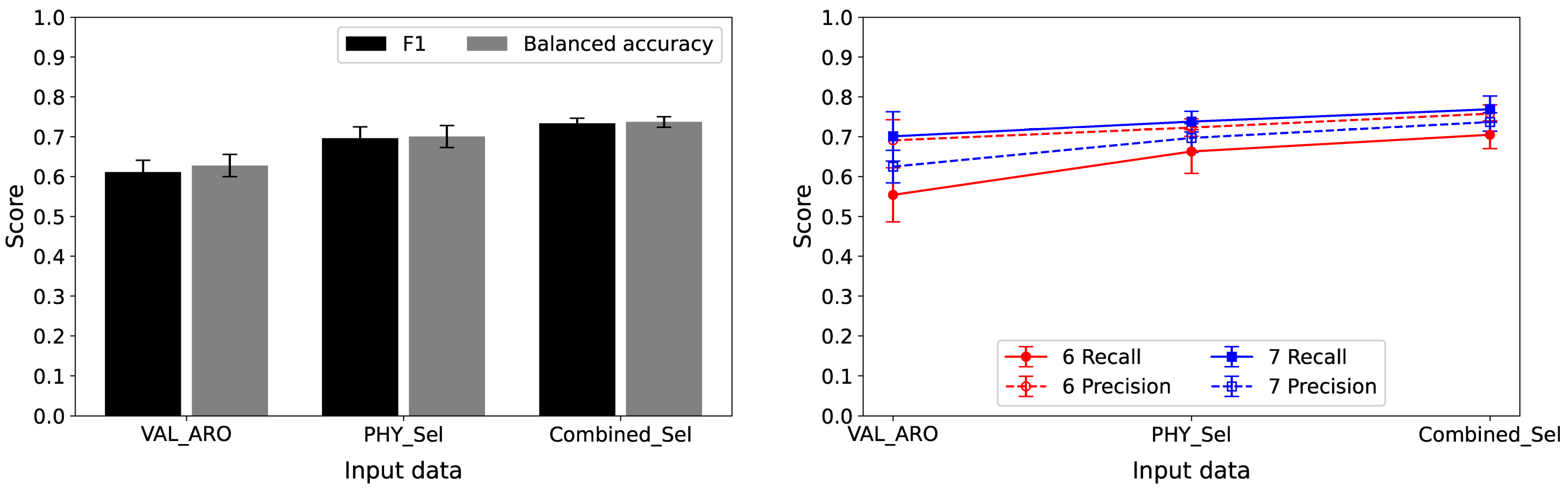
Table 8 and
Figure 5 summarize the classification performance for distinguishing intensity levels 6 and 7 across the three feature sets based on PFI analyses. The first set (VAL_ARO) included only valence and arousal; the second (PHY_Sel) featured the top three from PHY_12; and the third (Combined_Sel) incorporated the top three from PHY_12 + VAL_ARO. Combined_Sel (valence, PTT, and SCR) achieved the highest test performance, with an average F1 score of 0.733, surpassing PHY_Sel (0.696) and VAL_ARO (0.611). A similar trend was observed for balanced accuracy, where Combined_Sel had the highest balanced accuracy of 0.737. Regarding class-specific metrics, Combined_Sel provided superior recall and precision for intensity levels 6 and 7, indicating better overall performance. The training F1 scores were slightly higher than the test F1 scores, with differences ranging from 0.053 to 0.075. Normalized confusion matrices (
Figure S4) show that VAL_ARO correctly classified 55% of intensity 6 and 70% of intensity 7, whereas PHY_Sel improved these rates to 66% and 74%, respectively. Combined_Sel achieved 70% and 77%, aligning with its highest F1 score.
Despite using only the three highest-ranked physiological features, the classification accuracy was approximately 0.70, in contrast to the valence and arousal measures, which achieved an accuracy of approximately 0.61. These findings highlight the significant role of physiological measures in differentiating between intensity levels 6 and 7. In addition, they suggested that even a restricted selection of physiological signals could serve as an effective method for classifying pain intensity. In further analyses, we varied the number of features in PHY_Sel and Combined_Sel from two to four (
Figure S5). Our results demonstrate that using exactly three features produced the highest F1 scores for both sets. Thus, the results in
Table 8 represent the best classification performance for the given datasets and feature subsets.
We repeated the intensity-level 6 vs. 7 experiment with a linear-kernel SVM and k-NN, using identical preprocessing and CV settings (
Table S2). Both alternatives slightly outperformed the RF when VAL_ARO was used, but they yielded substantially lower performance measures than the RF for the feature sets that included physiological signals (PHY_Sel and Combined_Sel), underscoring RF’s superior performance when physiological data were incorporated.
We conducted an OFAT sensitivity analysis on three RF hyperparameters—number of decision trees, minimum samples required to split a node, and minimum samples at a leaf node—across each feature-selection scenario (VAL_ARO, PHY_Sel, and Combined_Sel). As shown in
Figure S6, only the minimum number of samples at a leaf node affected the test F1 score, while variations in the other two parameters had a minimal impact on model performance. These findings suggest that model accuracy is largely insensitive to the latter two parameters. However, we acknowledge that these conclusions are specific to the present grid. Future work will explore a finer range of minimum leaf node sample values to mitigate overfitting and enhance performance.
3.6.2. Classifications Involving Intensity Level 5
Classifications involving intensity level 5 (such as comparisons between levels 5 and 6, 5, and 7, or the three-level classification of levels 5, 6, and 7) were conducted using the same methodology applied for classifying intensities 6 and 7. The limited sample size of 17 participants for intensity level 5 posed significant challenges in achieving robust outcomes, despite employing SMOTE-based oversampling and repeated nested CV. Consequently, the results for intensity level 5 are considered supplementary and are detailed in the
Supplementary Materials. Below is a brief summary of these findings.
Similarly to the classification of levels 6 and 7, we performed a PFI analysis to identify important features, selecting the three top-ranked features from the PHY_12 and PHY_12 + VAL_ARO feature sets for each classification task (
Figures S7–S9). In the classification tasks involving intensity level 5, SCR, SCL, PTT, and SDNN were identified as the top-ranked features.
Next, we performed classification tasks involving intensity level 5 using three input datasets: VAL_ARO, PHY_Sel, and Combined_Sel. In the two-class tasks involving levels 5 and 6, and the three-class task of 5 vs. 6 vs. 7, combining valence–arousal scores with physiological signals, such as PTT and SCL, consistently outperformed the other datasets (
Tables S3 and S4, Figures S10 and S11). Confusion matrices demonstrate that integrating self-ratings with physiological metrics reduces misclassification rates. The highest F1 score in the 5 vs. 6 task was 0.705, indicating a promising performance level. In contrast, the three-class classification achieved a maximum F1 score of 0.506, with level 6 posing particular classification challenges.
However, in the 5 vs. 7 task, using valence–arousal scores alone achieved the highest performance: approximately 80% and >90% for levels 5 and 7 (
Table S5, Figure S12). These findings suggest that physiological data add limited value for well-separated intensity levels. However, the small sample size for level 5 highlights the need for cautious interpretation and further validation using larger datasets. We observed that, unlike in the 6 vs. 7 classification, tasks involving intensity 5 produced a training–test F1 gap exceeding 0.1 when PHY_Sel was used. In contrast, VAL_ARO and Combined_Sel maintained differences below 0.1, with most < 0.05.
4. Discussion
4.1. Autonomic Mechanisms of Emotional Pain
This study aims to clarify the relationship between subjective (psychological) and objective (physiological) indicators of emotional pain by examining psychophysiological responses to anger-induced pain across varying levels of perceived pain intensity. Our findings reveal significant increases in RMSSD, pNN50, HFnu, SCL, and SCR, which were associated with emotional pain, and significant decreases in LF/HF, RR, BVP, and PTT (
Table 4). These results align with our previous research that examined social pain induced by a social loss stimulus and revealed a similar pattern of physiological changes in SCL, SCR, RR, BVP, and PTT in response to the stimulus [
31].
RR is one of the most commonly used respiratory indices, in addition to respiratory period, respiratory depth, tidal volume, duty cycle, and respiratory variability [
32]. Negative affect can induce shallower and faster breathing, potentially lowering blood carbon dioxide levels [
33]. Decreases in RR have also been linked to anxious personality traits, particularly under mental stress or a physical load [
34,
35]. BVP reflects the volume of blood flowing through peripheral vessels such as those in the fingers and can also indicate vascular or blood pressure changes resulting from vasoconstriction or vasodilation [
36]. Reduced BVP suggests peripheral vasoconstriction in the fingers, and this is often associated with arousal triggered by emotional pain [
37]. PTT represents the duration from the ECG R-peak to the arrival of the pulse wave at the fingertip [
38] and is influenced by cardiac contractility and mean arterial pressure. The significant decrease in PTT observed during pain stimulation aligns with the findings of our previous social pain study [
31], indicating blood pressure elevation under stress. Increased SC activity signifies sympathetic nervous system (SNS) activation. SC reflects a psychogalvanic reflex wherein SNS excitation of eccrine sweat glands in the palms and soles occurs in response to stress or fear [
39]. When sympathetic activity intensifies, more sweat is secreted at the skin surface, leading to elevated EDA. Increases in SCL and SCR indicate sweat secretion attributable to SNS activation, suggesting a physiological response to emotional distress or social rejection.
Elevated HFnu, RMSSD, and pNN50 and lower LF/HF ratios during emotional pain indicate increased parasympathetic nervous system (PNS) activity. In this study, the LF/HF ratio, which reflects the balance between sympathetic and parasympathetic activation, decreased, suggesting a relative reduction in sympathetic influence and a shift towards parasympathetic dominance, as evidenced by the increase in other parasympathetic metrics. HRV indices represent the autonomic regulation of the HR and can serve as objective indicators of emotional states [
40]. RMSSD reflects short-term fluctuations driven predominantly by a vagal (parasympathetic) tone [
41], correlates with HF power [
36], and reflects self-regulatory capacity. Similarly, pNN50 is primarily governed by parasympathetic influence. Hence, the increased parasympathetic activity during emotional pain suggests that emotional engagement or recovery processes may be more pronounced than sympathetic arousal. Our findings suggest that emotional pain does not merely represent a simple stress response; rather, it entails a complex emotional state that prompts the coactivation of the PNS and SNS under heightened emotional arousal.
4.2. Intensity-Dependent Affective and Autonomic Modulation
Analysis of the psychophysiological differences associated with perceived pain intensity indicated that individuals experiencing more intense pain reported greater negative valence and higher arousal (
Table 6), suggesting that higher pain intensity corresponds to deeper negative emotional experiences. A study of chronic musculoskeletal pain showed a significant link between pain and negative affect, where increased pain predicted elevated negative emotions (sadness) [
42]. However, this study does not match our current work perfectly. Furthermore, individuals who frequently experience pain exhibit attentional biases toward negative emotional stimuli [
43,
44]. According to the four-stage pain processing model, increasing pain intensity elicits unpleasantness, encompassing negative emotions such as sadness or anger [
45]. This underscores that pain is not solely a physical sensation, but also an inherently emotional experience. For instance, while some individuals can tolerate pain of a certain intensity, others find the same level overwhelmingly distressing. These differences are likely due to emotional reactivity. Consequently, an individual’s mental state or capacity for emotion regulation can directly influence their pain experience. These observations suggest that psychological interventions, such as emotion regulation training or stress reduction programs, may be effective in pain management. They also highlight the importance of integrating physiological and emotional regulation strategies in pain interventions.
From a physiological perspective, SCR and SCL were significantly elevated at higher pain intensities, indicating intensified SNS activation among individuals who perceived pain more acutely. SCR emerged as the most sensitive index of pain intensity changes, underscoring its potential as a key biomarker for emotional pain detection. These observations are consistent with previous findings on automatic physical pain detection [
8], reinforcing the notion that emotional pain elicits robust autonomic responses, particularly within the SNS. Moreover, people reporting higher psychological pain tend to exhibit stronger emotional reactivity and perceive emotions more intensely. In practice, emotionally sensitive individuals may respond more vigorously to anger-inducing stimuli (such as unfair treatment and humiliating situations), leading to elevated SNS activity and stronger SC responses [
46]. Similarly, those experiencing greater social rejection or psychological distress show higher SC activity [
47]. These results reveal that individuals who perceived pain more intensely from the same stimulus exhibited heightened SNS activation, as evidenced by increased SCR and SCL. Thus, these results demonstrate that emotional pain elicits varying levels of physiological reactivity, depending on an individual’s psychological state or emotion regulation capacity.
Meanwhile, SDNN and PTT exhibited significant trends that may be linked to inhibition or delayed regulation of the PNS. Notably, the group reporting lower pain intensity tended to show higher PTT values, suggesting that reductions in peripheral blood flow may correlate with stimulus intensity. This finding also implies the potential influence of individual differences and psychological coping strategies. Furthermore, no distinctions were observed in HRV-related indices (RMSSD, HF, and pNN50) across varying pain intensities, indicating that the impact of emotional pain on HRV may be relatively minor or that large inter-individual variability hinders the attainment of statistical significance. The limited range of pain intensities in the experimental design and the possibility that subjective self-ratings and physiological responses may not perfectly align should be considered.
4.3. Machine Learning Performance
We explored the machine learning classification for baseline and pain states and different pain intensity levels. We addressed the challenge of using a relatively small dataset and minimized the risk of overfitting by implementing a classifier with repeated nested CV. In the PFI analysis for baseline versus painful states, SCR, SCL, and RESP were the most influential features, with SCR showing notably higher importance. Employing these three features yielded an F1 score of 0.83, indicating that even a minimal physiological set could reliably detect emotional pain. This high performance aligns with our findings, which demonstrate strong sympathetic involvement (as shown by SCR and SCL) during emotional pain. Moreover, these results demonstrate an accuracy comparable to that of the baseline versus high-pain classification reported in previous studies based on physical pain (
Table 1).
Regarding the classification of pain intensity levels, we evaluated physiological signals alone and in combination with the subjective ratings of valence and arousal. For the 6 vs. 7 binary classification, combining PTT, SCR, and valence yielded the highest F1 score (0.733). The accuracy for classifying intensity levels 6 and 7 was approximately 0.70, even when only the three highest-ranked physiological features were used. This value surpasses the ~0.61 accuracy obtained solely with valence and arousal. This demonstrates the pivotal role of physiological measures in differentiating closely spaced pain intensities and suggests that a subset of physiological signals can lead to effective pain intensity classification. The PFI rankings revealed that PTT and SCR were consistently informative, further corroborating our statistical findings.
However, only 17 participants classified pain intensity level as 5, and this posed significant challenges. Therefore, the classification results involving level 5 should be interpreted cautiously due to the small sample size. Despite using SMOTE, performance varied substantially, reflecting the inherent difficulty of modeling under-sampled classes. Notably, for well-separated intensities (5 vs. 7), subjective ratings alone could achieve relatively high accuracy, but more nuanced classifications (such as 5 vs. 6 or 6 vs. 7) benefited greatly from the inclusion of physiological features. These findings imply that self-ratings and physiological indicators each have distinct advantages, with subjective measures capturing broad emotional distinctions and physiological signals offering finer granularity when differentiating closely spaced intensity levels.
The classification results for differentiating pain intensity levels using physiological signals achieved an accuracy of 0.7, indicating feasibility, but falling short of the over 0.8 accuracy observed in the baseline versus the painful state classification. Previous studies on physical pain have reported higher accuracies, even with multiclass problems, but these studies typically relied on distinct stimuli for each intensity level (
Table 1) [
11,
12]. In contrast, our study focuses on classifying individually perceived intensities under a single standardized stimulus, which is a more challenging task. Therefore, our classification accuracy is expected to be lower than that observed in studies with varied stimuli. In addition, even in physical pain research, distinguishing very low-intensity stimuli from the baseline is difficult, with five-class classification results of approximately 40% (
Table 1) [
7,
9]. Given this context, our near-0.7 performance using selected physiological signals highlights that the perceived intensity can be classified from such signals, presenting a promising finding for further research into emotionally induced pain.
Overall, the machine learning results suggest that integrating physiological signals with self-reported states yielded the most robust models for classifying perceived pain intensity. Notably, the PFI analysis provides an interpretable ranking of feature importance, enabling the identification of the most influential physiological and psychological measures for classifying pain intensity. These outcomes align with our statistical findings, underscoring the prominent roles of SCL and SCR in the classification process.
5. Limitations
This study has some limitations. First, the small sample size for certain pain intensity levels, particularly the 17 participants that reported intensity 5, poses significant challenges to machine learning classification. Techniques such as SMOTE-based oversampling and repeated nested CV were used to mitigate the small sample size and data imbalance; however, the large difference (>0.1) between the training and test F1 scores in the intensity 5 classification indicated potential overfitting. SMOTE helped balance the classes during training, but did not fully resolve the fundamental issue of having too few samples for certain categories. These results underscore how the underrepresentation of certain intensities can reduce model reliability and interpretability.
Second, this study focuses on static snapshots of physiological responses rather than time-varying analyses, potentially overlooking the dynamic progression of emotional pain. In the present study, physiological features were extracted from a fixed 30 s epoch. This window was chosen to balance the requirements of multimodal data: rapidly changing signals such as SCR and RR benefit from shorter windows, whereas HRV requires at least ~30 s for reliable estimation. A 30 s segment therefore provided a practical compromise, and similar durations are commonly used in affective physiology research [
32]. Nonetheless, we acknowledge that a static window may fail to capture the full temporal dynamics of time-sensitive measures like SCR and RR, potentially obscuring discriminative information. In future work, we will employ time-series approaches—such as sliding-window analysis or sequence models—to track these rapid fluctuations more precisely and to test whether dynamic features improve classification performance.
Third, pain intensity labels were derived solely from participants’ self-ratings, reflecting the study’s aim to capture subjective experience despite identical stimuli. While appropriate for this purpose, such ratings inevitably introduce interpretive challenges, owing to their inherent subjectivity. To enhance objectivity in future work, we plan to integrate additional modalities—automated facial expression analysis, clinician or observer ratings, and other behavioral cues—alongside physiological signals and self-reports. This multimodal approach should yield a more comprehensive and reliable assessment of pain intensity.
Finally, the scope of the emotional pain stimuli in this study was relatively narrow (primarily anger). This approach is limited in that it does not fully capture the multifaceted nature of emotional pain. Emotional pain encompasses a range of negative affective experiences, including sadness, fear, social rejection, shame, and loneliness, each of which may differentially influence ANS responses and pain perception. For example, social exclusion can heighten sympathetic activation, altering physiological measures such as SC, while feelings of helplessness may increase perceived pain sensitivity by evoking a sense of lost control over the stimulus. Such psychosocial variables may interact with physiological responses and subjective pain reports, thereby affecting the performance of our pain classification models. In future work, we plan to integrate a broader array of emotional stimuli and psychosocial factors to better reflect the heterogeneity and complexity of emotional pain. We will also employ validated instruments—such as the Orbach & Mikulincer Mental Pain Scale [
48]—to systematically assess emotional pain and combine these measures with physiological data, enabling the development of more robust and generalizable pain classification models.
Moving forward, we intend to recruit more participants, refine the stimulus protocol to capture multiple pain intensities more uniformly, and explore dynamic modeling approaches that account for the temporal evolution of emotional pain responses.
6. Conclusions
This study demonstrates that even emotionally induced pain exhibits sensitive variations in affective and autonomic responses according to perceived pain intensity. Valence, arousal, SCL, and SCR were effective indicators for distinguishing between the different intensity levels. In particular, SCR emerged as the most sensitive index of changes in pain intensity. This finding is consistent with previous research indicating that the SCR and SCL reflect heightened sympathetic activation in response to pain stimuli and respond sensitively to psychological stressors such as emotional distress or social rejection. Such physiological responses suggest that emotional pain involves the complex coactivation of the ANS, extending beyond simple stress reactions. Moreover, individual differences in emotional reactivity and emotion regulation capacity shape pain experience, emphasizing that subjective pain is not merely a sensory phenomenon, but also has an emotional dimension.
These findings suggest that autonomic measures can serve as objective metrics for assessing emotional pain, whereas subjective emotional evaluations (valence and arousal) complement physiological signals to enhance the discrimination of emotional intensity. This study effectively classifies both the presence and intensity of pain by combining physiological indicators with self-reported emotional ratings, offering key insights for machine learning-based pain recognition systems. Overall, this study deepens our understanding of pain by highlighting the intricate relationship between autonomic and emotional responses to emotional pain. It also lays the background for developing advanced pain recognition systems and healthcare interventions that consider psychological factors.
Supplementary Materials
The following supporting information can be downloaded at:
https://www.mdpi.com/article/10.3390/app15137149/s1, Table S1: Classification performance metrics; Table S2: Performance measures for classifying intensity levels 6 and 7 using SVM and k-NN; Table S3: Performance measures for classifying intensity levels 5, 6, and 7; Table S4: Performance measures for classifying intensity levels 5 and 6; Table S5: Performance measures for classifying intensity levels 5 and 7; Figure S1. Permutation importance analysis of the 12 physiological features for classifying baseline and emotional states; Figure S2: Test and training F1 scores for baseline vs. emotional state classification using the top 2–5 ranked physiological features; Figure S3: Normalized confusion matrix for classifying baseline and emotional states using top three ranked physiological features: SCR, SCL, and RESP; Figure S4. Normalized confusion matrices for classifying intensity levels 6 and 7. VAL_ARO represents valence and arousal, PHY_Sel denotes the top three ranked physiological features (PTT, SCR, and pNN50), and Combined_Sel refers to the top three ranked features from combined physiological and valence–arousal features (valence, PTT, and SCR); Figure S5: Test and training F1 scores for classifying intensity levels 6 and 7 using the top 2–4 ranked physiological features (PHY_Sel) and combined physiological and valence–arousal features (Combined_Sel); Figure S6: OFAT sensitivity of test F1 to RF hyperparameters across VAL_ARO, PHY_Sel, and Combined_Sel feature sets; Figure S7: Permutation importance analysis for classifying intensity levels 5, 6, and 7. (Left) Analysis using 12 physiological features; (Right) Analysis using physiological features combined with valence and arousal; Figure S8: Permutation importance analysis for classifying intensity levels 5 and 6. (Left) Analysis using 12 physiological features; (Right) Analysis using physiological features combined with valence and arousal; Figure S9: Permutation importance analysis for classifying intensity levels 5 and 7. (Left) Analysis using 12 physiological features; (Right) Analysis using physiological features combined with valence and arousal; Figure S10: Performance measures for classifying intensity levels 5, 6, and 7: (a) F1 score and balanced accuracy; (b) Recall and precision for each intensity; (c), (d), and (e) Normalized confusion matrices for classifications using VAL_ARO, PHY_Sel, and Combined_Sel feature sets. VAL_ARO represents valence and arousal, PHY_Sel denotes the top three ranked physiological features (SCR, PTT, and SCL), and Combined_Sel refers to the top three ranked features from combined physiological and valence–arousal features (valence, arousal, and PTT); Figure S11: Performance measures for classifying intensity levels 5 and 6: (a) F1 score and balanced accuracy; (b) Recall and precision for each intensity; (c), (d), and (e) Normalized confusion matrices for classifications using VAL_ARO, PHY_Sel, and Combined_Sel feature sets. VAL_ARO represents valence and arousal, PHY_Sel denotes the top three ranked physiological features (SCL, SDNN, and SCR), and Combined_Sel refers to the top three ranked features from combined physiological and valence–arousal features (arousal, valence, and SCL); Figure S12: Performance measures for classifying intensity levels 5 and 7: (a) F1 score and balanced accuracy; (b) Recall and precision for each intensity; (c), (d), and (e) Normalized confusion matrices for classifications using VAL_ARO, PHY_Sel, and Combined_Sel feature sets. VAL_ARO represents valence and arousal, PHY_Sel denotes the top three ranked physiological features (SCL, SCR, and PTT), and Combined_Sel refers to the top three ranked features from combined physiological and valence–arousal features (valence, arousal, and SCL).
Author Contributions
Conceptualization, E.-H.J. and Y.-J.E.; methodology, E.-H.J., Y.-J.E. and S.B.; software, E.-H.J., Y.-J.E. and S.B.; validation, E.-H.J., Y.-J.E. and S.B.; formal analysis, E.-H.J., Y.-J.E. and S.B.; investigation, E.-H.J., Y.-J.E. and S.B.; resources, E.-H.J. and Y.-J.E.; data curation, E.-H.J. and Y.-J.E.; writing—original draft preparation, E.-H.J., Y.-J.E. and S.B.; writing—review and editing, E.-H.J., Y.-J.E. and S.B.; visualization, E.-H.J., Y.-J.E. and S.B.; supervision, D.Y.; project administration, D.Y.; funding acquisition, D.Y. and S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Incheon National University Research Grant in 2022 and Korea Evaluation Institute of Industrial Technology grant funded by the Ministry of Trade, Industry and Energy, Korea (No. 20018248, Development of safety of the intended functionality from insufficiency of perception and decision making).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Chungnam National University (Approval No.: 201309-SB-041-01, An Integrated Cognitive-Affective Model for Human–Computer Interfaces).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy restriction.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ANS | Autonomic Nervous System |
| ANOVA | Analysis of Variance |
| BVP | Blood Volume Pulse |
| CV | Cross-Validation |
| ECG | Electrocardiogram |
| EDA | Electrodermal Activity |
| EMG | Electromyogram |
| FT | Finger Temperature |
| HR | Heart Rate |
| HRV | Heart Rate Variability |
| LF | Low Frequency |
| HF | High Frequency |
| k-NN | k-Nearest Neighbors |
| LFnu | Normalized Low Frequency |
| HFnu | Normalized High Frequency |
| LF/HF | Ratio of Low Frequency to High Frequency |
| MAE | Mean Absolute Error |
| NPV | Negative Predictive Value |
| NN | Normal-to-Normal intervals |
| OFAT | One-factor-at-a-time |
| PFI | Permutation Feature Importance |
| pNN50 | Percentage of successive NNs > 50 ms |
| PPG | Photoplethysmogram |
| PNS | Parasympathetic Nervous System |
| PPV | Positive Predictive Value |
| PTT | Pulse Transit Time |
| RESP | Respiration |
| RF | Random Forest |
| RMSE | Root Mean Squared Error |
| RMSSD | Root Mean Square of Successive Differences |
| RR | Respiratory Rate |
| RNN | Recurrent Neural Network |
| SCR | Skin Conductance Response |
| SCL | Skin Conductance Level |
| SC | Skin Conductance |
| SDNN | Standard Deviation of NN intervals |
| SMOTE | Synthetic Minority Oversampling Technique |
| SNS | Sympathetic Nervous System |
| SVM | Support Vector Machine |
References
- Raja, S.; Carr, D.; Cohen, M.; Finnerup, N.; Flor, H.; Gibson, S. The Revised IASP Definition of Pain: Concepts, Challenges, and Compromises. Pain [Revista En Internet] 2021 [Acceso 4 de Marzo de 2022]; 161(9): 1-16. Pain 2021, 161, 1976–1982. [Google Scholar] [CrossRef]
- Cao, B.; Xu, Q.; Shi, Y.; Zhao, R.; Li, H.; Zheng, J.; Liu, F.; Wan, Y.; Wei, B. Pathology of Pain and Its Implications for Therapeutic Interventions. Signal Transduct. Target. Ther. 2024, 9, 155. [Google Scholar] [CrossRef]
- Bushnell, M.C.; Čeko, M.; Low, L.A. Cognitive and Emotional Control of Pain and Its Disruption in Chronic Pain. Nat. Rev. Neurosci. 2013, 14, 502–511. [Google Scholar] [CrossRef]
- Villemure, C.; Bushnell, M.C. Mood Influences Supraspinal Pain Processing Separately from Attention. J. Neurosci. 2009, 29, 705–715. [Google Scholar] [CrossRef]
- Eisenberger, N.I.; Lieberman, M.D. Why Rejection Hurts: A Common Neural Alarm System for Physical and Social Pain. Trends Cogn. Sci. 2004, 8, 294–300. [Google Scholar] [CrossRef]
- Kross, E.; Berman, M.G.; Mischel, W.; Smith, E.E.; Wager, T.D. Social Rejection Shares Somatosensory Representations with Physical Pain. Proc. Natl. Acad. Sci. USA 2011, 108, 6270–6275. [Google Scholar] [CrossRef]
- Kächele, M.; Thiam, P.; Amirian, M.; Schwenker, F.; Palm, G. Methods for Person-Centered Continuous Pain Intensity Assessment from Bio-Physiological Channels. IEEE J. Sel. Top. Signal Process. 2016, 10, 854–864. [Google Scholar] [CrossRef]
- Lopez-Martinez, D.; Picard, R. Continuous Pain Intensity Estimation from Autonomic Signals with Recurrent Neural Networks. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 5624–5627. [Google Scholar] [CrossRef]
- Wang, R.; Xu, K.; Feng, H.; Chen, W. Hybrid RNN-ANN Based Deep Physiological Network for Pain Recognition. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 5584–5587. [Google Scholar] [CrossRef]
- Tsai, P.F.; Wang, C.H.; Zhou, Y.; Ren, J.; Jones, A.; Watts, S.O.; Chou, C.; Ku, W.S. A Classification Algorithm to Predict Chronic Pain Using Both Regression and Machine Learning—A Stepwise Approach. Appl. Nurs. Res. 2021, 62, 151504. [Google Scholar] [CrossRef]
- Othman, E.; Werner, P.; Saxen, F.; Fiedler, M.-A.; Al-Hamadi, A. An Automatic System for Continuous Pain Intensity Monitoring Based on Analyzing Data from Uni-, Bi-, and Multi-Modality. Sensors 2022, 22, 4992. [Google Scholar] [CrossRef] [PubMed]
- Pouromran, F.; Lin, Y.; Kamarthi, S. Automatic Pain Recognition from Blood Volume Pulse (BVP) Signal Using Machine Learning Techniques. arXiv 2023, arXiv:2303.10607. [Google Scholar]
- Gkikas, S.; Tsiknakis, M. Automatic Assessment of Pain Based on Deep Learning Methods: A Systematic Review. Comput. Methods Programs Biomed. 2023, 231, 107365. [Google Scholar] [CrossRef] [PubMed]
- Storm, H. Changes in Skin Conductance as a Tool to Monitor Nociceptive Stimulation and Pain. Curr. Opin. Anaesthesiol. 2008, 21, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Sugimine, S.; Saito, S.; Takazawa, T. Normalized Skin Conductance Level Could Differentiate Physical Pain Stimuli from Other Sympathetic Stimuli. Sci. Rep. 2020, 10, 10950. [Google Scholar] [CrossRef]
- Treister, R.; Kliger, M.; Zuckerman, G.; Goor, I.; Eisenberg, E. Differentiating between Heat Pain Intensities: The Combined Effect of Multiple Autonomic Parameters. Pain 2012, 153, 1807–1814. [Google Scholar] [CrossRef]
- Walter, S.; Gruss, S.; Limbrecht-Ecklundt, K.; Traue, H.C.; Werner, P.; Al-Hamadi, A.; Diniz, N.; da Silva, G.M.; Andrade, A.O. Automatic Pain Quantification Using Autonomic Parameters. Psychol. Neurosci. 2014, 7, 363–380. [Google Scholar] [CrossRef]
- Werner, P.; Lopez-Martinez, D.; Walter, S.; Al-Hamadi, A.; Gruss, S.; Picard, R.W. Automatic Recognition Methods Supporting Pain Assessment: A Survey. IEEE Trans. Affect. Comput. 2022, 13, 530–552. [Google Scholar] [CrossRef]
- Chu, Y.; Zhao, X.; Han, J.; Su, Y. Physiological Signal-Based Method for Measurement of Pain Intensity. Front. Neurosci. 2017, 11, 279. [Google Scholar] [CrossRef]
- Patil, M.S.; Patil, H.D. Logistic Regression Based Model for Pain Intensity Level Detection from Biomedical Signal. Int. Res. J. Multidiscip. Scope 2024, 5, 652–662. [Google Scholar] [CrossRef]
- Pouromran, F.; Radhakrishnan, S.; Id, S.K. Exploration of Physiological Sensors, Features, and Machine Learning Models for Pain Intensity Estimation. PLoS ONE 2021, 16, e0254108. [Google Scholar] [CrossRef]
- Pouromran, F.; Lin, Y.; Kamarthi, S. Personalized Deep Bi-LSTM RNN Based Model for Pain Intensity Classification Using EDA Signal. Sensors 2022, 22, 8087. [Google Scholar] [CrossRef]
- Jang, E.H.; Eom, E.; Cheong, C.; Sohn, J.H. Characteristics of Psychological and Physiological Responses While Anger Experiencing in Individuals with Depression. Int. J. Psychophysiol. 2018, 131, S164. [Google Scholar] [CrossRef]
- Fernandez Rojas, R.; Brown, N.; Waddington, G.; Goecke, R. A Systematic Review of Neurophysiological Sensing for the Assessment of Acute Pain. NPJ Digit. Med. 2023, 6, 76. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Fang, R.; Hosseini, E.; Zhang, R.; Fang, C.; Rafatirad, S.; Homayoun, H. Survey on Pain Detection Using Machine Learning Models (Preprint). JMIR AI 2023, 4, e53026. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Han, T.; Wang, L.; Zhu, Z.; Huang, H.; Ding, J.; Wu, Z. Pipeline Deformation Prediction Based on Multi-Source Monitoring Information and Novel Data-Driven Model. Eng. Struct. 2025, 337, 120461. [Google Scholar] [CrossRef]
- Ayena, J.C.; Bouayed, A.; Ben Arous, M.; Ouakrim, Y.; Loulou, K.; Ameyed, D. Predicting Chronic Pain Using Wearable Devices: A Scoping Review of Sensor Capabilities, Data Security, and Standards Compliance. Front. Digit. Health 2025, 7, 1581285. [Google Scholar] [CrossRef]
- Strobl, C.; Boulesteix, A.L.; Zeileis, A.; Hothorn, T. Bias in Random Forest Variable Importance Measures: Illustrations, Sources and a Solution. BMC Bioinform. 2007, 8, 25. [Google Scholar] [CrossRef]
- Molnar, C.; König, G.; Bischl, B.; Casalicchio, G. Model-Agnostic Feature Importance and Effects with Dependent Features: A Conditional Subgroup Approach. Data Min. Knowl. Discov. 2024, 38, 2903–2941. [Google Scholar] [CrossRef]
- Jang, E.; Eum, Y.; Yoon, D.; Sohn, J.; Byun, S. Comparing Multimodal Physiological Responses to Social and Physical Pain in Healthy Participants. Front. Public Health 2024, 12, 1387056. [Google Scholar] [CrossRef]
- Kreibig, S.D. Autonomic Nervous System Activity in Emotion: A Review. Biol. Psychol. 2010, 84, 394–421. [Google Scholar] [CrossRef]
- Jafari, H.; Courtois, I.; Van den Bergh, O.; Vlaeyen, J.W.S.S.; Van Diest, I. Pain and Respiration: A Systematic Review. Pain 2017, 158, 995–1006. [Google Scholar] [CrossRef]
- Boiten, F.A. The Effects of Emotional Behaviour on Components of the Respiratory Cycle. Biol. Psychol. 1998, 49, 29–51. [Google Scholar] [CrossRef]
- Masaoka, Y.; Homma, I. Anxiety and Respiratory Patterns: Their Relationship during Mental Stress and Physical Load. Int. J. Psychophysiol. 1997, 27, 153–159. [Google Scholar] [CrossRef]
- Peper, E.; Harvey, R.; Lin, I.; Tylova, H.; Moss, D. Is There More to Blood Volume Pulse Than Heart Rate Variability, Respiratory Sinus Arrhythmia, and Cardiorespiratory Synchrony ? Biofedback 2007, 35, 54–61. [Google Scholar]
- MacDonald, G. Social Pain and Hurt Feelings. In The Cambridge Handbook of Personality Psychology; Matthews, G., Corr, P.J., Eds.; Cambridge Handbooks in Psychology; Cambridge University Press: Cambridge, UK, 2009; pp. 541–555. ISBN 9780511596544. [Google Scholar]
- Takahashi, K. Remarks on Emotion Recognition from Bio-Potential Signals. In Proceedings of the 2nd International Conference on Autonomous Robots and Agents, Palmerston North, New Zealand, 8–10 December 2004; pp. 1148–1153. [Google Scholar]
- Bach, D.R.; Friston, K.J.; Dolan, R.J. Analytic Measures for Quantification of Arousal from Spontaneous Skin Conductance Fluctuations. Int. J. Psychophysiol. 2010, 76, 52–55. [Google Scholar] [CrossRef]
- Appelhans, B.M.; Luecken, L.J. Heart Rate Variability and Pain: Associations of Two Interrelated Homeostatic Processes. Biol. Psychol. 2008, 77, 174–182. [Google Scholar] [CrossRef]
- Zygmunt, A.; Stanczyk, J. Heart Rate Variability in Children with Neurocardiogenic Syncope. Clin. Auton. Res. 2004, 14, 99–106. [Google Scholar] [CrossRef]
- Naylor, M.R.; Krauthamer, G.M.; Naud, S.; Keefe, F.J.; Helzer, J.E. Predictive Relationships between Chronic Pain and Negative Emotions: A 4-Month Daily Process Study Using Therapeutic Interactive Voice Response (TIVR). Compr. Psychiatry 2011, 52, 731–736. [Google Scholar] [CrossRef][Green Version]
- Li, W.; Liu, P.; Hu, Y.; Meng, J. Pain Modulates Responses to Emotional Stimuli. Front. Psychol. 2020, 11, 595987. [Google Scholar] [CrossRef]
- Duschek, S.; Werner, N.S.; Limbert, N.; Winkelmann, A.; Montoya, P. Attentional Bias toward Negative Information in Patients with Fibromyalgia Syndrome. Pain Med. 2014, 15, 603–612. [Google Scholar] [CrossRef]
- Wade, J.B.; Dougherty, L.M.; Archer, C.R.; Price, D.D. Assessing the Stages of Pain Processing: A Multivariate Analytical Approach. Pain 1996, 68, 157–167. [Google Scholar] [CrossRef]
- Ben-Shakhar, G. Standardization within Individuals: A Simple Method to Neutralize Individual Differences in Skin Conductance. Psychophysiology 1985, 22, 292–299. [Google Scholar] [CrossRef]
- Eisenberger, N.I.; Lieberman, M.D.; Williams, K.D. Does Rejection Hurt? An FMRI Study of Social Exclusion. Science 2003, 302, 290–292. [Google Scholar] [CrossRef]
- Orbach, I.; Mikulincer, M.; Sirota, P.; Gilboa-Schechtman, E. Mental Pain: A Multidimensional Operationalization and Definition. Suicide Life-Threat. Behav. 2003, 33, 219–230. [Google Scholar] [CrossRef]
Figure 1.
Overview of the experimental procedure.
Figure 1.
Overview of the experimental procedure.
Figure 2.
Positions of each pain intensity level on the dimension of emotion.
Figure 2.
Positions of each pain intensity level on the dimension of emotion.
Figure 3.
Boxplots of valence, arousal, SCL, and SCR across three pain intensity groups (* p < 0.05, ** p < 0.01, *** p < 0.001).
Figure 3.
Boxplots of valence, arousal, SCL, and SCR across three pain intensity groups (* p < 0.05, ** p < 0.01, *** p < 0.001).
Figure 4.
Permutation importance analysis for classifying intensity levels 6 and 7. (Left) Analysis using 12 physiological features. (Right) Analysis using physiological features combined with valence and arousal. The pink bars indicate arousal and valence.
Figure 4.
Permutation importance analysis for classifying intensity levels 6 and 7. (Left) Analysis using 12 physiological features. (Right) Analysis using physiological features combined with valence and arousal. The pink bars indicate arousal and valence.
Figure 5.
Performance measures for classifying intensity levels 6 and 7: (left) F1 score and balanced accuracy; (right) recall and precision for each intensity. VAL_ARO represents valence and arousal, PHY_Sel denotes the top three ranked physiological features (PTT, SCR, and pNN50), and Combined_Sel refers to the top three ranked features from combined physiological and valence–arousal features (valence, PTT, and SCR).
Figure 5.
Performance measures for classifying intensity levels 6 and 7: (left) F1 score and balanced accuracy; (right) recall and precision for each intensity. VAL_ARO represents valence and arousal, PHY_Sel denotes the top three ranked physiological features (PTT, SCR, and pNN50), and Combined_Sel refers to the top three ranked features from combined physiological and valence–arousal features (valence, PTT, and SCR).
Table 1.
Examples of previous studies on pain intensity classification.
Table 1.
Examples of previous studies on pain intensity classification.
| Authors | Physiological Signals | Number of Participants | Pain Type | Intensity Levels | Algorithms | Accuracy |
|---|
| Kächele et al., 2016 [7] | EMG, ECG, EDA | 87 (BioVid) | Heat | 5 classes: Baseline + level 1–4 | Random forest, SVM | Baseline vs. level 4: ~85%; 5-class: ~40% |
| Lopez-Martinez & Picard, 2018 [8] | ECG, EDA | 87 (BioVid) | Heat | 5 classes: Baseline + level 1–4 | LSTM-based RNN | Regression: MAE = 1.05; RMSE = 1.29; R2 = 0.24 |
| Wang et al., 2020 [9] | EMG, ECG, EDA | 87 (BioVid) | Heat | 5 classes: Baseline + level 1–4 | Hybrid biLSTM-RNN + ANN | Baseline vs. level 1: 59%; baseline vs. level 4: 83% |
| Tsai et al., 2021 [10] | Step count, sleep | 77 | Chronic pain | Binary: No/mild vs. moderate/higher | LSTM-based DNN | Pain intensity: 75% (F1 = 0.58); pain interference: 82% (F1 = 0.59) |
| Othman et al., 2022 [11] | EMG, ECG, EDA, video, audio | 127 | Heat, electrical | 4-class: None, low, moderate, severe | RF, LSTM | Heat pain classification: ~80%; electrical pain regression: MSE ~0.03, ICC ~0.88 |
| Pouromran et al., 2023 [12] | BVP | 32 | Cold pressor test | 4 levels: None, low, medium, high | XGBoost | 3-class (low, medium, high): F1 ~0.8 |
Table 2.
Descriptions of the video stimuli for each state.
Table 2.
Descriptions of the video stimuli for each state.
| State | Stimulus Protocol | Validity |
|---|
| Baseline | A plus sign (+) presented on the monitor | Not applicable |
| Painful | A video depicting an indiscriminate assault on a bus driver | 92.8% (245/264) |
| Non-painful | A video displaying various images of chairs | 94.8% (253/264) |
Table 3.
Description of the physiological features.
Table 3.
Description of the physiological features.
| Signals | Features | Definition |
|---|
| ECG | HR (beat/min) | Average of HR |
| SDNN (ms) | Standard deviation of NN intervals |
| RMSSD (ms) | Root mean square of successive differences between adjacent NNs |
| pNN50 (%) | Percentage of successive NNs that differed by more than 50 ms |
| HFnu | HFnu = HF power/(LF power + HF power), where LF power and HF power are the absolute powers in the LF (0.04–0.15 Hz) and HF (0.15–0.4 Hz) bands, respectively. |
| LF/HF | Ratio of LFnu to HFnu |
| RESP | RR (breath/min) | Number of breaths per minute |
| PPG | BVP (V) | Average of BVP range |
| ECG, PPG | PTT (ms) | Elapsed time between the R-peak of the ECG and the arrival of the pulse wave at the finger |
| EDA | SCL (µS) | Tonic level of electrical conductivity of skin |
| SCR (µS) | Average of the SCR amplitude (0.05 µS or greater) of all the specific SCR events |
| Temperature | FT (°C) | Average of FT |
Table 4.
Statistical comparison of physiological features between baseline and painful states with Bonferroni corrections for multiple comparisons (p < 0.0042).
Table 4.
Statistical comparison of physiological features between baseline and painful states with Bonferroni corrections for multiple comparisons (p < 0.0042).
| Feature | State | Statistics |
|---|
| Baseline | Painful | t | p |
|---|
| HR (beat/min) | 78.85 ± 8.93 | 77.98 ± 10.36 | 1.613 | 0.109 |
| SDNN (ms) | 36.59 ± 15.63 | 40.74 ± 16.29 | −2.478 | 0.015 |
| RMSSD (ms) | 30.16 ± 13.16 | 35.18 ± 17.83 | −4.120 | <0.001 |
| pNN50 (%) | 12.02 ± 14.79 | 16.04 ± 17.63 | −3.773 | <0.001 |
| HFnu | 0.55 ± 0.15 | 0.63 ± 0.13 | 5.688 | <0.001 |
| LF/HF | 0.99 ± 0.76 | 0.66 ± 0.40 | 5.156 | <0.001 |
| RR (breath/min) | 3.80 ± 1.10 | 3.13 ± 0.48 | 7.597 | <0.001 |
| BVP (V) | 0.18 ± 0.11 | 0.11 ± 0.09 | 10.750 | <0.001 |
| PTT (ms) | 276.44 ± 24.58 | 270.53 ± 26.11 | 5.644 | <0.001 |
| SCL (µS) | 0.25 ± 0.18 | 0.33 ± 0.19 | −6.055 | <0.001 |
| SCR (µS) | 0.13 ± 0.31 | 0.84 ± 0.96 | −8.423 | <0.001 |
| FT (°C) | 32.55 ± 2.37 | 32.52 ± 2.30 | 1.638 | 0.104 |
Table 5.
Comparative analysis of physiological features during painful and non-painful states based on changes from baseline with Bonferroni corrections (p < 0.0042).
Table 5.
Comparative analysis of physiological features during painful and non-painful states based on changes from baseline with Bonferroni corrections (p < 0.0042).
| ΔFeature | Pain condition | Statistics |
|---|
| Non-Painful-Baseline | Painful-Baseline | t | p |
|---|
| ΔHR (beat/min) | 4.90 ± 3.47 | −0.87 ± 6.14 | 2.132 | 0.034 |
| ΔSDNN (ms) | −3.02 ± 13.06 | 4.15 ± 19.11 | −3.400 | 0.001 |
| ΔRMSSD (ms) | −1.02 ± 6.45 | 5.02 ± 13.89 | −4.402 | <0.001 |
| ΔpNN50 (%) | −0.51 ± 7.86 | 4.03 ± 12.17 | −3.445 | 0.001 |
| ΔHFnu | 0.05 ± 0.16 | 0.08 ± 0.16 | −1.304 | 0.194 |
| ΔLF/HF | −0.26 ± 0.72 | −0.33 ± 0.74 | 0.726 | 0.467 |
| ΔRR (breath/min) | −0.28 ± 0.75 | −0.68 ± 1.02 | 3.426 | 0.001 |
| ΔBVP (V) | −0.01 ± 0.04 | −0.07 ± 0.07 | 8.182 | <0.001 |
| ΔPTT (ms) | −1.17 ± 7.08 | −5.91 ± 11.93 | 3.774 | <0.001 |
| ΔSCL (µS) | 0.01 ± 0.08 | 0.08 ± 0.14 | −4.145 | <0.001 |
| ΔSCR (µS) | 0.10 ± 0.52 | 0.71 ± 0.96 | −6.164 | <0.001 |
| ΔFT (°C) | 0.08 ± 0.23 | −0.04 ± 0.27 | 3.471 | 0.001 |
Table 6.
One-way ANOVA and Bonferroni post hoc comparison of psychophysiological responses at different pain intensities.
Table 6.
One-way ANOVA and Bonferroni post hoc comparison of psychophysiological responses at different pain intensities.
| Feature | Intensity 5 | Intensity 6 | Intensity 7 | F | p | Post hoc |
|---|
| Valence | −2.71 ± 1.795 | −3.55 ± 0.544 | −3.94 ± 0.245 | 15.356 | <0.001 | 5 > 6 = 7 |
| Arousal | 2.59 ± 0.795 | 3.28 ± 0.649 | 3.50 ± 0.799 | 9.561 | <0.001 | 5 < 6 = 7 |
| ΔHR (beat/min) | −1.31 ± 5.795 | −0.81 ± 6.479 | −0.08 ± 6.288 | 0.297 | 0.744 | - |
| ΔSDNN (ms) | 8.58 ± 15.909 | −0.49 ± 20.376 | 7.11 ± 19.414 | 2.356 | 0.100 | 6 < 7 |
| ΔRMSSD (ms) | 5.41 ± 10.529 | 2.87 ± 14.404 | 7.25 ± 16.165 | 1.056 | 0.351 | - |
| ΔpNN50 (%) | 4.07 ± 12.176 | 2.07 ± 13.583 | 6.24 ± 11.447 | 1.326 | 0.270 | - |
| ΔHFnu | 0.06 ± 0.168 | 0.08 ± 0.155 | 0.08 ± 0.154 | 0.100 | 0.905 | - |
| ΔLF/HF | −0.30 ± 1.024 | −0.36 ± 0.667 | −0.28 ± 0.564 | 0.150 | 0.861 | - |
| ΔBVP (V) | −0.06 ± 0.059 | −0.06 ± 0.079 | −0.07 ± 0.069 | 0.349 | 0.706 | - |
| ΔPTT (ms) | −3.45 ± 11.566 | −4.64 ± 12.394 | −9.07 ± 10.793 | 2.361 | 0.099 | 5 > 7 |
| ΔRR (breath/min) | −0.62 ± 0.824 | −0.78 ± 1.105 | −0.53 ± 0.751 | 0.877 | 0.419 | - |
| ΔSCL (µS) | 0.00 ± 0.060 | 0.08 ± 0.146 | 0.11 ± 0.171 | 3.669 | 0.029 | 5 < 7 |
| ΔSCR (µS) | 0.26 ± 0.388 | 0.66 ± 0.905 | 1.02 ± 1.135 | 4.274 | 0.016 | 5 < 7 |
| ΔFT (°C) | −0.00 ± 0.369 | −0.00 ± 0.319 | −0.06 ± 0.226 | 0.691 | 0.503 | - |
Table 7.
Performance measures for classifying baseline and emotional states using top three ranked physiological features: SCR, SCL, and RESP.
Table 7.
Performance measures for classifying baseline and emotional states using top three ranked physiological features: SCR, SCL, and RESP.
| F1 (Test) | Accuracy | Recall | Specificity | F1 (Training) |
|---|
| 0.834 ± 0.010 | 0.830 ± 0.011 | 0.855 ± 0.014 | 0.805 ± 0.020 | 0.867 ± 0.003 |
Table 8.
Performance measures for classifying intensity levels 6 and 7.
Table 8.
Performance measures for classifying intensity levels 6 and 7.
| Input Data | F1 (Test) | Balanced Accuracy | Recall (6) | Precision (6) | Recall (7) | Precision (7) | F1 (Training) |
|---|
| VAL_ARO | 0.611 ± 0.030 | 0.628 ± 0.028 | 0.554 ± 0.068 | 0.691 ± 0.052 | 0.701 ± 0.062 | 0.625 ± 0.041 | 0.666 ± 0.005 |
| PHY_Sel | 0.696 ± 0.029 | 0.700 ± 0.027 | 0.663 ± 0.055 | 0.723 ± 0.022 | 0.738 ± 0.026 | 0.697 ± 0.036 | 0.771 ± 0.012 |
| Combined_Sel | 0.733 ± 0.013 | 0.737 ± 0.014 | 0.705 ± 0.035 | 0.758 ± 0.022 | 0.769 ± 0.033 | 0.737 ± 0.023 | 0.786 ± 0.006 |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).