Targeted Delivery Strategies for Hydrophilic Phytochemicals
Abstract
Featured Application
Abstract
1. Introduction
1.1. Background on Phytochemicals: Overview of the Importance and Therapeutic Potential of Plant-Derived Active Substances
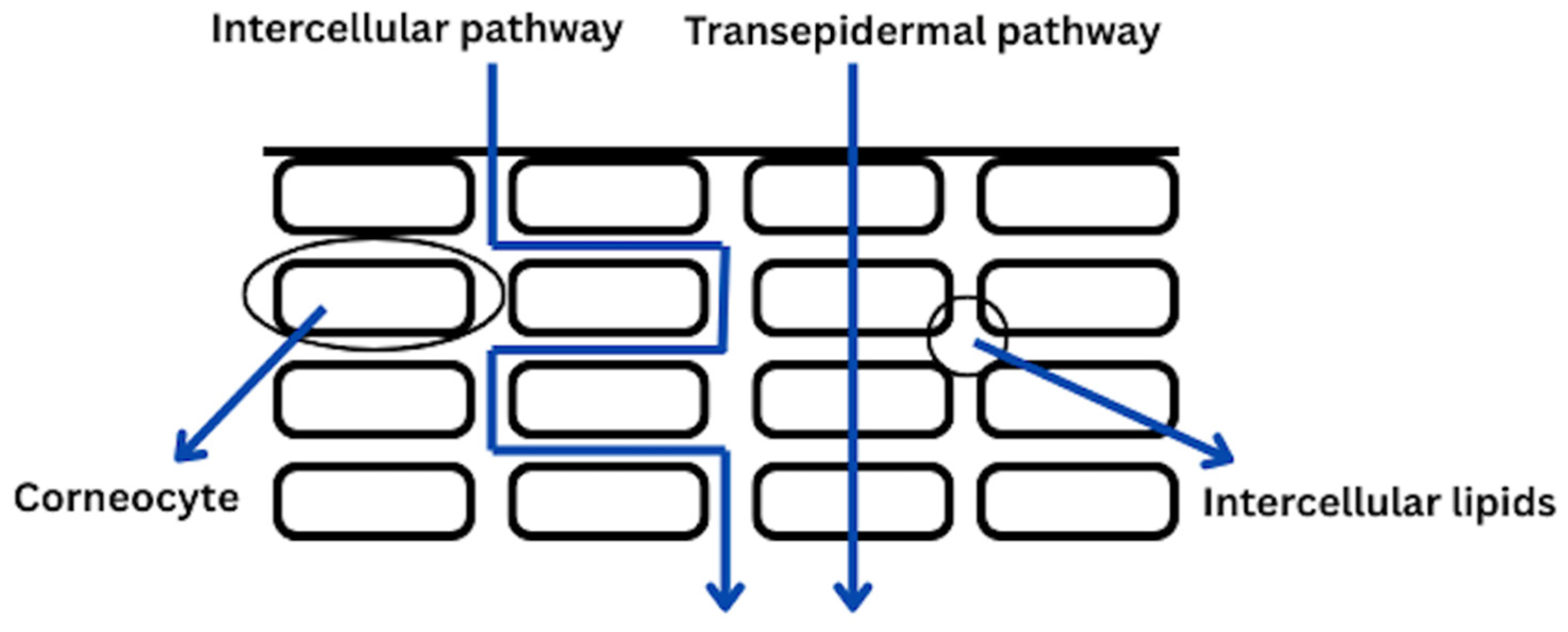
1.2. The Overview on the Phytochemicals Penetartions Rates: Discuss Factors Affecting Skin Penetration
1.3. Challenges of Hydrophilic Phytochemicals: Discuss Solubility, Stability, and Bioavailability Issues Associated with Hydrophilic Active Substances
2. Delivery Systems for Hydrophilic Phytochemicals
2.1. General Considerations
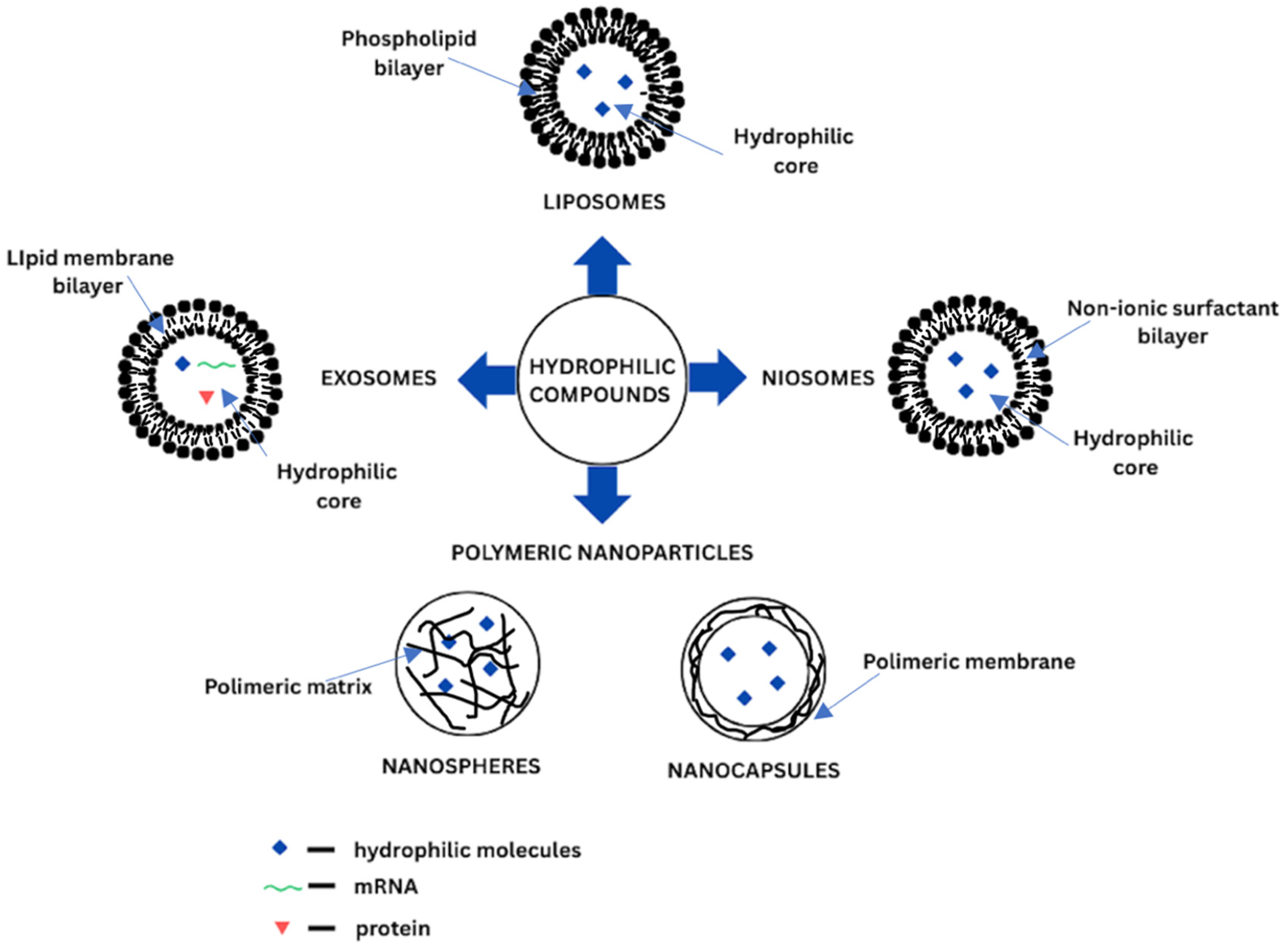
2.2. Liposomes
2.3. Niosomes
2.4. Exosomes
2.5. Polymeric Nanoparticles
| Group | Structure | Size | Preparation/Isolation Methods | Advantages | Limitations | References |
|---|---|---|---|---|---|---|
| Liposomes | Phospholipid bilayer (SUV, LUV, GUV) or several lipid bilayers (MLV, MVV), hydrophilic core | - SUV (20–200 nm) - LUV (200 nm–1 µm) - GUV (>1 µm) - OLV (100 nm–1 µm) - MLV (> 500 nm) - MVV (>1 µm) | - film hydratation - reverse phase evaporation - solvent injection - heating method - microfluidic channel - supercritical fluidic - freeze-thawing - freeze-drying - detergent removal - membrane extrusion - sonication - micro-emulsification - dual asymmetric centrifuging | - biocompatible - biodegradable - high bioavailability - low toxicity - low immunogenicity - both hydrophilic and hydrophobic compounds delivery - controlled release of the of active compounds - targeting delivery - extension of drug half-life | - high cost of production - low solubility - possible instability (e.g., during storage) - possible degradation via hydrolysis or oxidation - possible encapsulated drug leakage - special storage conditions are required | [32,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76] |
| Niosomes | Non-ionic surfactant bilayer in conjunction with cholesterol | - SUV (10–100 nm) - LUV (100–3000 nm) - MLV (>10 µm) | - thin film hydratation - reverse phase evaporation - ether injection (solvent) - emulsion method - lipid injection - bubble method - microfluidisation method - supercritical reverse phase evaporation - micelle solution and enzyme - trans membrane pH gradient drug uptake process - formation from proniosomes - sonication | - lower cost of production - biocompatible - biodegradable - low toxicity - low immunogenicity - both hydrophilic and hydrophobic compounds delivery - improved chemical stability - structurally flexible - controlled release of active compounds - targeting delivery - special storage conditions are not required | - low physical stability - possible degradation via hydrolysis - possible aggregation - possible encapsulated drug leakage - possible aggregation - limited shelf-life | [41,43,76,77,78,79] |
| Exosomes | Single lipid bilayer containing RNAs, proteins and lipids | −30–200 nm | - ultracentrifugation - ultrafiltration - size-exclusion chromatography - polymer precipitation - magnetic separation - acoustic fluid separation - immunological separation - dielectrophoretic separation | - biocompatible - biodegradable - low toxicity - low immunogenicity - innate stability - targeting delivery | - short-half life in circulation - special storage conditions are required | [47,48,50,51,80,81,82,83,84] |
| Polymeric nanoparticles | Solid core with polymeric matrix Inner liquid or solid core secured by polymeric shelf | - nanospheres (10–200 nm) - nanocapsules (50–300 nm) | - dialysis - emulsification diffusion - interfacial polymerization - nanoprecipitation - phase inversion temperature - salting out - super critical fluid technology - solvent evaporation | - biodegradable - low toxicity - high stability - both hydrophilic and hydrophobic compounds delivery - long shelf life - both hydrophilic and hydrophobic compounds delivery - controlled release of active compounds - high loading capacity - targeting delivery | - possible degradation - possible monomer aggregation | [53,85,86,87,88,89] |
3. Hydrophilic Phytochemicals
3.1. Flavonoids
3.1.1. Impact of Structural Modifications on Flavonoid Hydrophilicity and Bioavailability
3.1.2. Hydroxyl Groups, Glycosylation, and Polarity
3.1.3. Acetamide and Sulfate Modifications
3.1.4. Cyclodextrin Complexation
3.1.5. Membrane Localization and Hydrophilic Properties
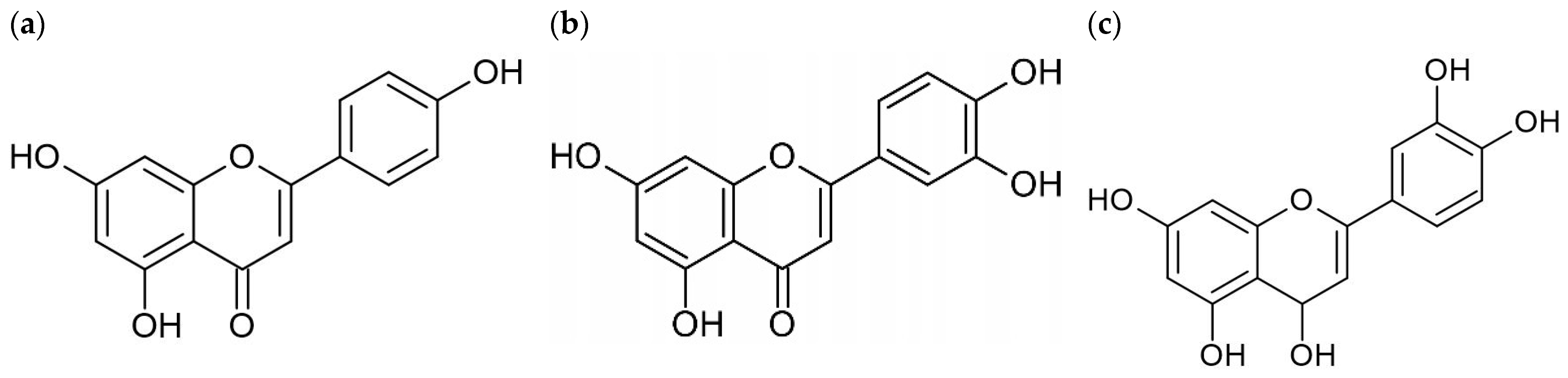
3.2. Quercetin
3.2.1. Structure and Solubility
3.2.2. Biological Activity
3.2.3. Delivery Barriers and Carrier-Based Strategies
3.3. Luteolin
3.3.1. Structural Properties and Biological Functions
3.3.2. Carrier-Based Delivery Strategies
3.4. Apigenin
3.4.1. Structure and Solubility
3.4.2. Biological Activity
3.5. Phenolic Acids
3.5.1. Ferulic Acid
3.5.2. Biological Activity
3.5.3. Carrier-Based Delivery Strategies
3.6. Caffeic Acid
3.6.1. Structure and Solubility
3.6.2. Biological Activity
3.6.3. Carrier-Based Delivery Strategies
3.7. Chlorogenic Acid
3.7.1. Structure and Solubility
3.7.2. Biological Activity
3.7.3. Carrier-Based Delivery Strategies
4. Applications of Delivery Systems for Hydrophilic Phytochemicals
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faccio, G. Plant Complexity and Cosmetic Innovation. iScience 2020, 23, 101358. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Xie, M.; Jiang, Z.; Lin, X.; Wei, X. Application of plant extracts cosmetics in the field of anti-aging. J. Dermat. Sci. Cosmet. Technol. 2024, 1, 100014. [Google Scholar] [CrossRef]
- Sławińska, N.; Olas, B. Selected Seeds as Sources of Bioactive Compounds with Diverse Biological Activities. Nutrients 2022, 15, 187. [Google Scholar] [CrossRef]
- Kumar, A.; Nirmal, P.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; Sneha, K.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Gonfa, Y.H.; Tessema, F.B.; Bachheti, A.; Rai, N.; Tadesse, M.G.; Singab, A.N.; Chaubey, K.K.; Bachheti, R.K. Anti-inflammatory activity of phytochemicals from medicinal plants and their nanoparticles: A review. Curr. Res. Biotechnol. 2023, 6, 100152. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Phenolic acids of plant origin-a review on their antioxidant activity in vitro (O/W emulsion systems) along with their in vivo health biochemical properties. Foods 2020, 9, 534. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Cho, H.E.; Moon, S.H.; Ahn, H.J.; Bae, S.; Cho, H.D.; An, S. Transdermal delivery systems in cosmetics. Biomed. Dermatol. 2020, 4, 10. [Google Scholar] [CrossRef]
- Cao, H.; Saroglu, O.; Karadag, A.; Diaconeasa, Z.; Zoccatelli, G.; Conte-Junior, C.A.; Gonzalez-Aguilar, G.A.; Ou, J.; Bai, W.; Zamarioli, C.M.; et al. Available technologies on improving the stability of polyphenols in food processing. Food Front. 2021, 2, 109–139. [Google Scholar] [CrossRef]
- Neubert, R.H.H. Mechanisms of penetration and diffusion of drugs and cosmetic actives across the human Stratum corneum. Eur. J. Pharm. Biopharm. 2024, 202, 114394. [Google Scholar] [CrossRef]
- Trommer, H.; Neubert, R.H.H. Overcoming the stratum corneum: The modulation of skin penetration. A review. Ski. Pharmacol. Physiol. 2006, 19, 106–121. [Google Scholar] [CrossRef]
- Boncheva, M. The physical chemistry of the stratum corneum lipids. Int. J. Cosmet. Sci. 2014, 36, 505–515. [Google Scholar] [CrossRef]
- Dayan, N. Pathways for Skin Penetration. Cosmet. Toilet. 2005, 120, 67–76. [Google Scholar]
- Śliwowska, A. Penetration of active subtsnces through the skin. Factors affecting this phenomenon and methods for its modification. Aesthetic Cosmetol. Med. 2020, 9, 399–405. [Google Scholar]
- Souto, E.B.; Fangueiro, J.F.; Fernandes, A.R.; Cano, A.; Sanchez-Lopez, E.; Garcia, M.L.; Severino, P.; Paganelli, M.O.; Chaud, M.V.; Silva, A.M. Physicochemical and biopharmaceutical aspects influencing skin permeation and role of SLN and NLC for skin drug delivery. Heliyon 2022, 8, e08938. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef]
- Barry, B.W. Mode of action of penetration enhancers in human skin. J. Control Release 1987, 6, 85–97. [Google Scholar] [CrossRef]
- Sikora, E.; Miastkowska, M.; Lasoń, E. Selected Skin Delivery Systems; Wydawnictwo PK: Cracov, Poland, 2020; pp. 1–118. [Google Scholar]
- Vitorino, C.; Sousa, J.; Pais, A. Overcoming the Skin Permeation Barrier: Challenges and Opportunities. Curr. Pharm. Des. 2015, 21, 2698–2712. [Google Scholar] [CrossRef]
- Adachi, K.; Shimizu, M.; Shono, F.; Funatsu, K.; Yamazaki, H. Octanol/water partition coefficients estimated using retention times in reverse-phase liquid chromatography and calculated in silico as one of the determinant factors for pharmacokinetic parameter estimations of general chemical substances. J. Toxicol. Sci. 2024, 49, 127–137. [Google Scholar] [CrossRef]
- Tiwari, N.; Osorio-Blanco, E.R.; Sonzogni, A.; Esporrín-Ubieto, D.; Wang, H.; Calderón, M. Nanocarriers for Skin Applications: Where Do We Stand? Angew Chem. Int. Ed. Engl. 2022, 61, e202107960. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.L. Strategies to Modify the Drug Release from Pharmaceutical Systems, 1st ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 87–194. [Google Scholar]
- Mojumdar, E.H.; Gooris, G.S.; Groen, D.; Barlow, D.J.; Lawrence, M.J.; Demé, B.; Bouwstra, J.A. Stratum corneum lipid matrix: Location of acyl ceramide and cholesterol in the unit cell of the long periodicity phase. Biochim. Biophys. Acta Biomembr. 2016, 1858, 1926–1934. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yang, H.; Capanoglu, E.; Cao, H.; Xiao, J. Technological aspects and stability of polyphenols. In Polyphenols: Properties, Recovery, and Applications, 1st ed.; Galanakis, C.M., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 295–323. [Google Scholar]
- Parisi, O.I.; Puoci, F.; Restuccia, D.; Farina, G.; Iemma, F.; Picci, N. Polyphenols and Their Formulations: Different Strategies to Overcome the Drawbacks Associated with Their Poor Stability and Bioavailability. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 1, pp. 29–45. [Google Scholar]
- Wahyuningsih, S.; Wulandari, L.; Wartono, M.W.; Munawaroh, H.; Ramelan, A.H. The Effect of pH and Color Stability of Anthocyanin on Food Colorant. IOP Conf. Ser. Mater. Sci. Eng. 2017, 193, 012047. [Google Scholar] [CrossRef]
- Chua, L.S.; Thong, H.Y.; Soo, J. Effect of pH on the extraction and stability of anthocyanins from jaboticaba berries. Food Chem. Adv. 2024, 5, 100835. [Google Scholar] [CrossRef]
- Pina, F.; Oliveira, J.; De Freitas, V. Anthocyanins and derivatives are more than flavylium cations. Tetrahedron 2015, 71, 3107–3114. [Google Scholar] [CrossRef]
- Farhan, M. The Promising Role of Polyphenols in Skin Disorders. Molecules 2024, 29, 865. [Google Scholar] [CrossRef]
- Soni, V.; Chandel, S.; Jain, P.; Asati, S. Role of liposomal drug-delivery system in cosmetics. In Nanobiomaterials in Galenic Formulations and Cosmetics; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 93–120. [Google Scholar]
- Rahimpour, Y.; Hamishehkar, H. Liposomes in cosmeceutics. Expert Opin. Drug Deliv. 2012, 9, 443–455. [Google Scholar] [CrossRef]
- Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Iqbal, Z. Nanotechnology in Cosmetics and Cosmeceuticals—A Review. Gels 2022, 8, 173. [Google Scholar] [CrossRef]
- Salvioni, L.; Morelli, L.; Ochoa, E.; Labra, M.; Fiandra, L.; Palugan, L.; Prosperi, D.; Colombo, M. The emerging role of nanotechnology in skincare. Adv. Colloid Interface Sci. 2021, 293, 102437. [Google Scholar] [CrossRef]
- Trucillo, P. Drug Carriers: Classification, Administration, Release Profiles, and Industrial Approach. Processes 2021, 9, 470. [Google Scholar] [CrossRef]
- Ahmadi Ashtiani, H.R.; Bishe, P.; Lashgari, N.A.; Nilforoushzadeh, M.A.; Zare, S. Liposomes in Cosmetics. J. Ski. Stem Cell 2016, 3, e65815. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Šturm, L.; Ulrih, N.P. Basic methods for preparation of liposomes and studying their interactions with different compounds, with the emphasis on polyphenols. Int. J. Mol. Sci. 2021, 22, 6547. [Google Scholar] [CrossRef]
- Mawazi, S.M.; Ann, T.J.; Widodo, R.T. Application of Niosomes in Cosmetics: A Systematic Review. Cosmetics 2022, 9, 127. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C.; Moacă, E.-A.; Péter, F. Niosomes: Composition, Formulation Techniques, and Recent Progress as Delivery Systems in Cancer Therapy various. Pharmaceutics 2024, 16, 223. [Google Scholar] [CrossRef]
- Lens, M. Niosomes as Vesicular Nanocarriers in Cosmetics: Characterisation, Development and Efficacy. Pharmaceutics 2025, 17, 287. [Google Scholar] [CrossRef] [PubMed]
- Karim, K.; Mandal, A.; Biswas, N.; Guha, A.; Chatterjee, S.; Behera, M.; Kuotsu, K. Niosome: A future of targeted drug delivery systems. J. Adv. Pharm. Technol. Res. 2010, 1, 374–380. [Google Scholar]
- Hazira, R.M.N.; Reddy, M.S. Niosomes: A nanocarrier drug delivery system. GSC Biol. Pharm. Sci. 2023, 22, 120–127. [Google Scholar] [CrossRef]
- Chen, Y.F.; Luh, F.; Ho, Y.S.; Yen, Y. Exosomes: A review of biologic function, diagnostic and targeted therapy applications, and clinical trials. J. Biomed. Sci. 2024, 31, 67. [Google Scholar] [CrossRef]
- Frydrychowicz, M.; Kolecka-Bednarczyk, A.; Madejczyk, M.; Yasar, S.; Dworacki, G. Exosomes-structure, biogenesis and biological role in non-small-cell lung cancer. Scand. J. Immunol. 2015, 81, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Varshney, A.; Bajaj, R.; Pokharkar, V. Exosomes as New Generation Vehicles for Drug Delivery: Biomedical Applications and Future Perspectives. Molecules 2022, 27, 7289. [Google Scholar] [CrossRef]
- Isola, A.L.; Chen, S. Exosomes: The Messengers of Health and Disease. Curr. Neuropharmacol. 2017, 15, 157–165. [Google Scholar] [CrossRef]
- Fan, T.; Sun, N.; He, J. Exosome-Derived LncRNAs in Lung Cancer. Front. Oncol. 2020, 10, 1728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Chen, Z.; Xiong, M.; Tian, J.; Song, D.; Duan, S.; Zhang, L. Encapsulation and assessment of therapeutic cargo in engineered exosomes: A systematic review. J. Nanobiotechnol. 2024, 22, 18. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Elkomy, M.H.; Alsaidan, O.A.; Gomaa, H.A.M.; Abdelgawad, M.A.; Mostafa, E.M. Polymeric Nanoparticles for Delivery of Natural Bioactive Agents: Recent Advances and Challenges. Polymers 2023, 15, 1123. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Nagasamy Venkatesh, D.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Deng, S.; Gigliobianco, M.R.; Censi, R.; Di Martino, P. Polymeric nanocapsules as nanotechnological alternative for drug delivery system: Current status, challenges and opportunities. Nanomaterials 2020, 10, 847. [Google Scholar] [CrossRef]
- Thakur, R.; Sharma, A.; Arora, V. Nanoparticles Methods for Hydrophobic Drugs—A Novel Approach: Graphical Abstract. Mater. Open 2023, 1, 2350002. [Google Scholar] [CrossRef]
- Kothamasu, P.; Kanumur, H.; Ravur, N.; Maddu, C.; Parasuramrajam, R.; Thangavel, S. Nanocapsules: The weapons for novel drug delivery systems. BioImpacts 2012, 2, 71–81. [Google Scholar]
- Arpicco, S.; Battaglia, L.; Brusa, P.; Cavalli, R.; Chirio, D.; Dosio, F. Recent studies on the delivery of hydrophilic drugs in nanoparticulate systems. J. Drug Deliv. Sci. Technol. 2016, 32, 298–312. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Constantinou, A.I. Drug Delivery Innovations for Enhancing the Anticancer Potential of Vitamin E Isoforms and Their Derivatives. BioMed Res. Int. 2015, 2015, 584862. [Google Scholar] [CrossRef] [PubMed]
- Dymek, M.; Sikora, E. Liposomes as biocompatible and smart delivery systems–the current state. Adv. Colloid Interface Sci. 2022, 309, 102757. [Google Scholar] [CrossRef] [PubMed]
- Sforzi, J.; Palagi, L.; Aime, S. Liposome-based bioassays. Biology 2020, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.A.; Ferreira, L.S.; Baldino, L.; Pinho, S.C.; Reverchon, E. Current Applications of Liposomes for the Delivery of Bioactives: A Review on the Encapsulation of Vitamins. Nanomaterials 2023, 13, 1557. [Google Scholar] [CrossRef]
- Luiz, H.; Oliveira Pinho, J.; Gaspar, M.M. Advancing Medicine with Lipid-Based Nanosystems—The Successful Case of Liposomes. Biomedicines 2023, 11, 435. [Google Scholar] [CrossRef]
- Barba, A.A.; Bochicchio, S.; Dalmoro, A.; Lamberti, G. Lipid delivery systems for nucleic-acid-based-drugs: From production to clinical applications. Pharmaceutics 2019, 11, 360. [Google Scholar] [CrossRef]
- Riccardi, D.; Baldino, L.; Reverchon, E. Liposomes, transfersomes and niosomes: Production methods and their applications in the vaccinal field. J. Transl. Med. 2024, 22, 339. [Google Scholar] [CrossRef]
- Effiong, D.E.; Uwah, T.O.-O.; Udofa, E. Nanotechnology in Cosmetics: Basics, Current Trends and Safety Concerns—A Review. Adv. Nanopart. 2019, 9, 1–22. [Google Scholar]
- Azadi, Y.; Ahmadpour, E.; Ahmadi, A. Targeting Strategies in Therapeutic Applications of Toxoplasmosis: Recent Advances in Liposomal Vaccine Delivery Systems. Curr. Drug Targets 2019, 21, 541–558. [Google Scholar] [CrossRef]
- Falciani, C.; Accardo, A.; Brunetti, J.; Tesauro, D.; Lelli, B.; Pini, A.; Bracci, L.; Morelli, G. Target-Selective Drug Delivery through Liposomes Labeled with Oligobranched Neurotensin Peptides. ChemMedChem 2011, 6, 678–685. [Google Scholar] [CrossRef]
- Giofrè, S.; Renda, A.; Sesana, S.; Formicola, B.; Vergani, B.; Leone, B.E.; Denti, V.; Paglia, G.; Gropusso, S.; Romeo, V.; et al. Dual Functionalized Liposomes for Selective Delivery of Poorly Soluble Drugs to Inflamed Brain Regions. Pharmaceutics 2022, 14, 2402. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.P.; Lloyd, A.W. Basic Principles of Liposomes for Drug Use. In Liposome Dermatics; Braun-Falco, I., Korting, H.C., Maibach, H.I., Eds.; Griesbach Conference; Springer: Berlin/Heidelberg, Germany, 1992; pp. 20–26. [Google Scholar]
- Honda, M.; Asai, T.; Oku, N.; Araki, Y.; Tanaka, M.; Ebihara, N. Liposomes and nanotechnology in drug development: Focus on ocular targets. Int. J. Nanomed. 2013, 8, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Maja, L.; Željko, K.; Mateja, P. Sustainable technologies for liposome preparation. J. Supercrit. Fluids 2020, 165, 104984. [Google Scholar] [CrossRef]
- Gatto, M.S.; Johnson, M.P.; Najahi-Missaoui, W. Targeted Liposomal Drug Delivery: Overview of the Current Applications and Challenges. Life 2024, 14, 672. [Google Scholar] [CrossRef]
- Enaru, B.; Fărcaş, A.; Stănilă, A.; Socaci, S.; Diaconeasa, Z. Novel Methods for Liposome Formulation: Advancements and Innovations. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2023, 80, 1–13. [Google Scholar] [CrossRef]
- Andra, V.V.S.N.L.; Pammi, S.V.N.; Bhatraju, L.V.K.P.; Ruddaraju, L.K. A Comprehensive Review on Novel Liposomal Methodologies, Commercial Formulations, Clinical Trials and Patents. BioNanoScience 2022, 12, 274–291. [Google Scholar] [CrossRef]
- Gunda, R.K.; Kumar, J.N.S.; Bhargavi, G.; Bhavani, S.P.; Sandhya, B.; Padmaja, K.; Praveen, S. A Review on Formulation and Evaluation of Liposomal Drugs. Br. J. Multidiscip. Adv. Stud. 2023, 4, 31–44. [Google Scholar] [CrossRef]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 2018, 3420204. [Google Scholar] [CrossRef]
- Moammeri, A.; Chegeni, M.M.; Sahrayi, H.; Ghafelehbashi, R.; Memarzadeh, F.; Mansouri, A.; Akbarzadeh, I.; Abtahi, M.S.; Hejabi, F.; Ren, Q. Current advances in niosomes applications for drug delivery and cancer treatment. Mater. Today Bio 2023, 23, 100837. [Google Scholar] [CrossRef] [PubMed]
- Kauslya, A.; Borawake, P.D.; Shinde, J.V.; Chavan, R.S. Niosomes: A Novel Carrier Drug Delivery System. J. Drug Deliv. Ther. 2021, 11, 162–170. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, D.; Thakur, P.; Devi, P.; Sharma, M.D. A Comprehensive Review on Niosomes; Targeted Drug Delivery System. YMER 2024, 22, 1969–1987. [Google Scholar]
- Bai, G.; Truong, T.M.; Pathak, G.N.; Benoit, L.; Rao, B. Clinical applications of exosomes in cosmetic dermatology. Skin Health Dis. 2024, 4, e348. [Google Scholar] [CrossRef]
- Thakur, A.; Shah, D.; Rai, D.; Parra, D.C.; Pathikonda, S.; Kurilova, S.; Cili, A. Therapeutic Values of Exosomes in Cosmetics, Skin Care, Tissue Regeneration, and Dermatological Diseases. Cosmetics 2023, 10, 65. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Patil, S.M.; Sawant, S.S.; Kunda, N.K. Exosomes as drug delivery systems: A brief overview and progress update. Eur. J. Pharm. Biopharm. 2020, 154, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Palakurthi, S.S.; Shah, B.; Kapre, S.; Charbe, N.; Immanuel, S.; Pasham, S.; Thalla, M.; Jain, A.; Palakurthi, S. A comprehensive review of challenges and advances in exosome-based drug delivery systems. Nanoscale Adv. 2024, 6, 5803–5826. [Google Scholar] [CrossRef]
- Verma, G.; Rajagopalan, M.D.; Valluru, R.; Sridhar, K.A. Chapter 7—Nanoparticles: A Novel Approach to Target Tumors. In Nano- and Microscale Drug Delivery Systems; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 113–129. [Google Scholar]
- Yadav, H.K.S.; Almokdad, A.A.; Shaluf, S.I.M.; Debe, M.S. Polymer-Based Nanomaterials for Drug-Delivery Carriers. In Nanocarriers for Drug Delivery: Nanoscience and Nanotechnology in Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 531–556. [Google Scholar]
- Maqbool, I.; Noreen, S. A Review of Novel Techniques for Nanoparticles Preparation. Glob. Drug Des. Dev. Rev. 2019, 4, 41–50. [Google Scholar] [CrossRef]
- Pereira, A.S.; Daniel-da-Silva, A.L.; Trindade, T. From nanoparticles to nanocomposites: A brief overview. In Nanocomposite Particles for Bio-Applications: Materials and Bio-Interfaces; Trindade, T., Daniel da Silva, A.L., Eds.; Jenny Stanford Publishing: Singapore, 2011; pp. 1–20. [Google Scholar]
- Pisal, M.; Barbade, P.; Dudhal, S. Nanocapsule. Int. J. Pharm. Sci. Rev. Res. 2020, 60, 53–62. [Google Scholar]
- Ozkan, G.; Ceyhan, T.; Çatalkaya, G.; Rajan, L.; Ullah, H.; Daglia, M.; Capanoglu, E. Encapsulated phenolic compounds: Clinical efficacy of a novel delivery method. Phytochem. Rev. 2024, 23, 781–819. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Flavonoids and phenolic acids from Oregano: Occurrence, biological activity and health benefits. Plants 2018, 7, 2. [Google Scholar] [CrossRef]
- Pandey, R.; Bhairam, M.; Shukla, S.S.; Gidwani, B. Colloidal and vesicular delivery system for herbal bioactive constituents. DARU J. Pharm. Sci. 2021, 29, 415–438. [Google Scholar] [CrossRef]
- Gugleva, V.; Ivanova, N.; Sotirova, Y.; Andonova, V. Dermal drug delivery of phytochemicals with phenolic structure via lipid-based nanotechnologies. Pharmaceuticals 2021, 14, 837. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Kather, F.S.; Boddu, S.H.S.; Rao, R.; Nair, A.B. Vesicular carriers for phytochemical delivery: A comprehensive review of techniques and applications. Pharmaceutics 2025, 17, 464. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Decker, E.A.; Weiss, J. Emulsion-based delivery systems for lipophilic bioactive components. J. Food Sci. 2007, 72, 109–124. [Google Scholar] [CrossRef]
- Mouhid, L.; Corzo-Martínez, M.; Torres, C.; Vázquez, L.; Reglero, G.; Fornari, T.; Ramírez de Molina, A. Improving in vivo efficacy of bioactive molecules: An overview of potentially antitumor phytochemicals and currently available lipid-based delivery systems. J. Oncol. 2017, 2017, 7351976. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: A review. Adv. Colloid Interface Sci. 2018, 253, 1–22. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Nagarajan, J.; Heng, W.W.; Galanakis, C.M.; Nagasundara Ramanan, R.; Raghunandan, M.E.; Sun, J.; Ismail, A.; Beng-Ti, T.; Prasad, K.N. Extraction of phytochemicals using hydrotropic solvents. Sep. Sci. Technol. 2016, 51, 1151–1165. [Google Scholar] [CrossRef]
- Zhang, L.; McClements, D.J.; Wei, Z.; Wang, G.; Liu, X.; Liu, F. Delivery of synergistic polyphenol combinations using biopolymer-based systems: Advances in physicochemical properties, stability and bioavailability. Crit. Rev. Food Sci. Nutr. 2020, 60, 2083–2097. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, H.; Xu, C.; Gu, L. A review: Using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocoll. 2015, 43, 153–164. [Google Scholar] [CrossRef]
- Vieira da Silva, B.; Barreira, J.C.M.; Oliveira, M.B.P.P. Natural phytochemicals and probiotics as bioactive ingredients for functional foods: Extraction, biochemistry and protected-delivery technologies. Trends Food Sci. Technol. 2016, 50, 144–158. [Google Scholar] [CrossRef]
- Rahman, N.R.A.; Yunus, N.A.; Mustaffa, A.A. Selection of optimum ionic liquid solvents for flavonoid and phenolic acids extraction. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 012061. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Frezza, C.; Venditti, A.; Serafini, M.; Bianco, A. Chapter 4—Phytochemistry, Chemotaxonomy, Ethnopharmacology, and Nutraceutics of Lamiaceae. In Studies in Natural Products Chemistry, 1st ed.; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 62, pp. 125–178. [Google Scholar]
- Roy, M.K.; Juneja, L.R.; Isobe, S.; Tsushida, T. Steam processed broccoli (Brassica oleracea) has higher antioxidant activity in chemical and cellular assay systems. Food Chem. 2009, 114, 263–269. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Tang, Y.; Tsao, R. Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory, and potential health beneficial effects: A review. Mol. Nutr. Food Res. 2017, 61, 1600767. [Google Scholar] [CrossRef]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Isika, D.K.; Sadik, O.A. Selective Structural Derivatization of Flavonoid Acetamides Significantly Impacts Their Bioavailability and Antioxidant Properties. Molecules 2022, 27, 8133. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Wu, X.; Li, B.; He, B. Efficient glucosylation of flavonoids by organic solvent-tolerant Staphylococcus saprophyticus CQ16 in aqueous hydrophilic media. J. Mol. Catal. B Enzym. 2014, 99, 8–13. [Google Scholar] [CrossRef]
- Park, S. Cyclic glucans enhance solubility of bioavailable flavonoids. Molecules 2016, 21, 1556. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Plaza, M.; Pozzo, T.; Liu, J.; Gulshan Ara, K.Z.; Turner, C.; Nordberg Karlsson, E. Substituent effects on in vitro antioxidizing properties, stability, and solubility in flavonoids. J. Agric. Food Chem. 2014, 62, 3321–3333. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Erlejman, A.G.; Verstraeten, S.V.; Keen, C.L.; Fraga, C.G. Flavonoid-membrane interactions: A protective role of flavonoids at the membrane surface? Clin. Dev. Immunol. 2005, 12, 19–27. [Google Scholar] [CrossRef]
- Xie, L.; Deng, Z.; Zhang, J.; Dong, H.; Wang, W.; Xing, B.; Liu, X. Comparison of Flavonoid O-Glycoside, C-Glycoside and Their Aglycones on Antioxidant Capacity and Metabolism during In Vitro Digestion and In Vivo. Foods 2022, 11, 882. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Han, F.; Xiao, Y.; Lee, I.S. Microbial transformation of prenylquercetins by Mucor hiemalis. Molecules 2020, 25, 528. [Google Scholar] [CrossRef]
- Dantuluri, M.; Gunnarsson, G.T.; Riaz, M.; Nguyen, H.; Desai, U.R. Capillary electrophoresis of highly sulfated flavanoids and flavonoids. Anal. Biochem. 2005, 336, 316–322. [Google Scholar] [CrossRef]
- Tripoli, E.; Giammanco, M.; Tabacchi, G.; Di Majo, D.; Giammanco, S.; La Guardia, M. The phenolic compounds of olive oil: Structure, biological activity and beneficial effects on human health. Nutr. Res. Rev. 2005, 18, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Jiang, Y.; Zheng, X.; Wang, T.; Li, J.; Zhang, B.; Zhu, J.; Wei, X.; Huang, R.; et al. Quercetin Promotes Apoptosis of Gastric Cancer Cells through the EGFR-ERK Signaling Pathway. J. Food Biochem. 2024, 2024, 9945178. [Google Scholar] [CrossRef]
- Hatahet, T.; Morille, M.; Hommoss, A.; Devoisselle, J.M.; Müller, R.H.; Bégu, S. Quercetin topical application, from conventional dosage forms to nanodosage forms. Eur. J. Pharm. Biopharm. 2016, 108, 41–53. [Google Scholar] [CrossRef]
- Vicentini, F.T.M.C.; Simi, T.R.M.; Del Ciampo, J.O.; Wolga, N.O.; Pitol, D.L.; Iyomasa, M.M.; Bentley, M.V.L.B.; Fonseca, M.J.V. Quercetin in w/o microemulsion: In vitro and in vivo skin penetration and efficacy against UVB-induced skin damages evaluated in vivo. Eur. J. Pharm. Biopharm. 2008, 69, 948–957. [Google Scholar] [CrossRef]
- Rajhard, S.; Hladnik, L.; Vicente, F.A.; Srčič, S.; Grilc, M.; Likozar, B. Solubility of luteolin and other polyphenolic compounds in water, nonpolar, polar aprotic and protic solvents by applying FTIR/HPLC. Processes 2021, 9, 1952. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef]
- Halevas, E.G.; Avgoulas, D.I.; Katsipis, G.; Pantazaki, A.A. Flavonoid-liposomes formulations: Physico-chemical characteristics, biological activities and therapeutic applications. Eur. J. Med. Chem. Rep. 2022, 5, 100059. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Al-Sanea, M.M.; Hendawy, O.M.; Salama, A.; Ibrahim, M.F.; Ghoneim, M.M. Influence of stabilizer on the development of luteolin nanosuspension for cutaneous delivery: An in vitro and in vivo evaluation. Pharmaceutics 2021, 13, 1812. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Ma, H.; Chen, H.; Zhang, X.; Ye, M. Luteolin Prevents UVB-Induced Skin Photoaging Damage by Modulating SIRT3/ROS/MAPK Signaling: An in vitro and in vivo Studies. Front. Pharmacol. 2021, 12, 728261. [Google Scholar] [CrossRef] [PubMed]
- Karak, P. Biological Activities of Flavonoids: An Overview. Int. J. Pharm. Sci. Res. 2019, 10, 1567–1574. [Google Scholar]
- Hou, M.; Sun, R.; Hupe, M.; Kim, P.; Park, K.; Crumrine, D.; Lin, T.-K.; Santiago, J.; Mauro, T.; Elias, P.; et al. Topical Apigenin Improves Epidermal Permeability Barrier Homeostasis in Normal Murine Skin by Divergent Mechanisms. Exp. Dermatol. 2014, 22, 210–215. [Google Scholar] [CrossRef]
- Singh, H.; Mishra, A.K.; Mohanto, S.; Kumar, A.; Mishra, A.; Amin, R.; Darwin, C.R.; Emran, T.B. A recent update on the connection between dietary phytochemicals and skin cancer: Emerging understanding of the molecular mechanism. Ann. Med. Surg. 2024, 85, 104790. [Google Scholar] [CrossRef]
- Abd-El-Aziz, N.M.; Hifnawy, M.S.; Lotfy, R.A.; Younis, I.Y. LC/MS/MS and GC/MS/MS metabolic profiling of Leontodon hispidulus, in vitro and in silico anticancer activity evaluation targeting hexokinase 2 enzyme. Sci. Rep. 2024, 14, 6872. [Google Scholar] [CrossRef]
- Materska, M. Evaluation of the Lipophilicity and Stability. Acta Sci. Pol. Technol. Aliment 2010, 9, 61–69. [Google Scholar]
- Petruczynik, A.; Waksmundzka-Hajnos, M. Application of hydrophilic interaction chromatography in phytochemical analysis. Acta Chromatogr. 2013, 25, 1–25. [Google Scholar] [CrossRef]
- Avinash, G.; Sharma, N.; Prasad, K.R.; Kaur, R.; Singh, G.; Pagidipala, N.; Thulasinathan, T. Unveiling the distribution of free and bound phenolic acids, flavonoids, anthocyanins, and proanthocyanidins in pigmented and non-pigmented rice genotypes. Front. Plant Sci. 2024, 15, 1324825. [Google Scholar] [CrossRef]
- Casadey, R.; Broglia, M.; Barbero, C.; Criado, S.; Rivarola, C. Controlled release systems of natural phenolic antioxidants encapsulated inside biocompatible hydrogels. React. Funct. Polym. 2020, 156, 104729. [Google Scholar] [CrossRef]
- Konczak, I.; Zabaras, D.; Dunstan, M.; Aguas, P. Antioxidant capacity and hydrophilic phytochemicals in commercially grown native Australian fruits. Food Chem. 2010, 123, 1048–1054. [Google Scholar] [CrossRef]
- Adam, A.; Crespy, V.; Levrat-Verny, M.A.; Leenhardt, F.; Leuillet, M.; Demigné, C.; Rémésy, C. The bioavailability of ferulic acid is governed primarily by the food matrix rather than its metabolism in intestine and liver in rats. J. Nutr. 2002, 132, 1962–1968. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.; Frainay, C.; Jourdan, F.; Naake, T.; Neumann, S.; Novoa-del-Toro, E.M.; Salek, R.M.; Salzer, L.; Scharfenberg, S.; Witting, M. Networks and Graphs Discovery in Metabolomics Data Analysis and Interpretation. Front. Mol. Biosci. 2022, 9, 841373. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Moghadasian, M.H. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chem. 2008, 109, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Jaswal, V.S.; Choudhary, S.; Sonika; Sharma, A.; Beniwal, V.; Tuli, H.S.; Sharma, S. Ferulic Acid: A Promising Therapeutic Phytochemical and Recent Patents Advances. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 115–123. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Ski. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Janus, E.; Pinheiro, L.R.; Nowak, A.; Kucharska, E.; Świątek, E.; Podolak, N.; Perużyńska, M.; Piotrowska, K.; Duchnik, W.; Kucharski, Ł.; et al. New Ferulic Acid and Amino Acid Derivatives with Increased Cosmeceutical and Pharmaceutical Potential. Pharmaceutics 2023, 15, 117. [Google Scholar] [CrossRef]
- Keshavarz, F.; Dorfaki, M.; Bardania, H.; Khosravani, F.; Nazari, P.; Ghalamfarsa, G. Quercetin-loaded Liposomes Effectively Induced Apoptosis and Decreased the Epidermal Growth Factor Receptor Expression in Colorectal Cancer Cells: An In Vitro Study. Iran. J. Med. Sci. 2023, 48, 321–328. [Google Scholar]
- Shukla, D.; Nandi, N.; Singh, B.; Singh, A.; Kumar, B.; Narang, R.; Singh, C. Ferulic acid-loaded drug delivery systems for biomedical applications. J. Drug Deliv. Sci. Technol. 2022, 75, 103621. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Majhi, S.; Saha, B.P.; Mukherjee, P.K. Chlorogenic acid-phospholipid complex improve protection against UVA induced oxidative stress. J. Photochem. Photobiol. B 2014, 130, 293–298. [Google Scholar] [CrossRef]
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.F.; Morozzi, G. Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspects of production that affect their occurrence in the oil. J. Chromatogr. A 2004, 1054, 113–127. [Google Scholar] [CrossRef]
- Sun, T.; Simon, P.W.; Tanumihardjo, S.A. Antioxidant phytochemicals and antioxidant capacity of biofortified carrots (Daucus carota L.) of various colors. J. Agric. Food Chem. 2009, 57, 4142–4147. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Fu, Y.; Malakhova, M.; Kurinov, I.; Zhu, F.; Yao, K.; Li, H.; Chen, H.; Li, W.; Lim, D.Y.; et al. Caffeic acid directly targets ERK1/2 to attenuate solar UV-induced skin carcinogenesis. Cancer Prev. Res. 2014, 7, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Scalia, S.; Franceschinis, E.; Bertelli, D.; Iannuccelli, V. Comparative evaluation of the effect of permeation enhancers, lipid nanoparticles and colloidal silica on in vivo human skin penetration of quercetin. Ski. Pharmacol. Physiol. 2013, 26, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Nagula, R.L.; Wairkar, S. Recent advances in topical delivery of flavonoids: A review. J. Control Release 2019, 296, 190–201. [Google Scholar] [CrossRef]
- dos Santos, C.O.L.; Spagnol, C.M.; Guillot, A.J.; Melero, A.; Corrêa, M.A. Caffeic acid skin absorption: Delivery of microparticles to hair follicles. Saudi Pharm. J. 2019, 27, 791–797. [Google Scholar] [CrossRef]
- Trivedi, H.R.; Puranik, P.K. Chlorogenic acid-optimized nanophytovesicles: A novel approach for enhanced permeability and oral bioavailability. Future J. Pharm. Sci. 2023, 9, 116. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Spanidi, E.; Mourtzinos, I.; Gardikis, K. Innovative delivery systems loaded with plant bioactive ingredients: Formulation approaches and applications. Plants 2021, 10, 1238. [Google Scholar] [CrossRef]
- Costa, R.; Costa Lima, S.A.; Gameiro, P.; Reis, S. On the development of a cutaneous flavonoid delivery system: Advances and limitations. Antioxidants 2021, 10, 1376. [Google Scholar] [CrossRef]
- Alharbi, W.S.; Almughem, F.A.; Almehmady, A.M.; Jarallah, S.J.; Alsharif, W.K.; Alzahrani, N.M.; Alshehri, A.A. Phytosomes as an emerging nanotechnology platform for the topical delivery of bioactive phytochemicals. Pharmaceutics 2021, 13, 1475. [Google Scholar] [CrossRef]
- Dwivedi, K.; Mandal, A.K.; Afzal, O.; Altamimi, A.S.A.; Sahoo, A.; Alossaimi, M.A.; Almalki, W.H.; Alzahrani, A.; Barkat, M.A.; Almeleebia, T.M.; et al. Emergence of Nano-Based Formulations for Effective Delivery of Flavonoids against Topical Infectious Disorders. Gels 2023, 9, 671. [Google Scholar] [CrossRef]
- Kazi, M.; Alhajri, A.; Alshehri, S.M.; Elzayat, E.M.; Al Meanazel, O.T.; Shakeel, F.; Noman, O.; Altamimi, M.A.; Alanazi, F.K. Enhancing oral bioavailability of apigenin using a bioactive self-nanoemulsifying drug delivery system (Bio-SNEDDS): In vitro, in vivo and stability evaluations. Pharmaceutics 2020, 12, 749. [Google Scholar] [CrossRef]
- Chavda, V.P.; Patel, A.B.; Mistry, K.J.; Suthar, S.F.; Wu, Z.X.; Chen, Z.S.; Hou, K. Nano-Drug Delivery Systems Entrapping Natural Bioactive Compounds for Cancer: Recent Progress and Future Challenges. Front. Oncol. 2022, 12, 867655. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; OS, B. Plant Latex: A Rich Source of Haemostatic Proteases, Herbal Medicine in India, 1st ed.; Springer: Singapore, 2020; pp. 143–153. [Google Scholar]
- Panda, S.; Vijayalakshmi, S.; Pattnaik, S.; Swain, R.P. Nanosponges: A novel carrier for targeted drug delivery. Int. J. PharmTech Res. 2015, 8, 213–224. [Google Scholar]
- Aizik, G.; Ostertag-Hill, C.A.; Chakraborty, P.; Choi, W.; Pan, M.; Mankus, D.V.; Lytton-Jean, A.K.R.; Kohane, D.S. Injectable hydrogel based on liposome self-assembly for controlled release of small hydrophilic molecules. Acta Biomater. 2024, 183, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Abd, E.; Namjoshi, S.; Mohammed, Y.H.; Roberts, M.S.; Grice, J.E. Synergistic skin penetration enhancer and nanoemulsion formulations promote the human epidermal permeation of caffeine and naproxen. J. Pharm. Sci. 2016, 105, 212–220. [Google Scholar] [CrossRef]
- Rathee, J.; Malhotra, S.; Pandey, M.; Jain, N.; Kaul, S.; Gupta, G.; Nagaich, U. Recent Update on Nanoemulsion Impregnated Hydrogel: A Gleam into the Revolutionary Strategy for Diffusion-Controlled Delivery of Therapeutics. AAPS PharmSciTech 2023, 24, 151. [Google Scholar] [CrossRef]
- Tokudome, Y.; Nakamura, K.; Itaya, Y.; Hashimoto, F. Enhancement of skin penetration of hydrophilic and lipophilic compounds by pH-sensitive liposomes. J. Pharm. Pharm. Sci. 2015, 18, 249–257. [Google Scholar] [CrossRef]
- Lee, S.; Woo, H.; Lee, Y.; Kwon, Y.N.; Choi, Y.W. Skin Penetration and Localization Characteristics of Lipogel Containing Ascorbyl Palmitate. J. Pharm. Investig. 2001, 31, 225–232. [Google Scholar]
- Rangsimawong, W.; Obata, Y.; Opanasopit, P.; Takayama, K.; Ngawhirunpat, T. Investigation of skin penetration enhancing mechanism of limonene-containing liposome entrapped hydrophilic compound. Isan J. Pharm. Sci. 2017, 13, 10–17. [Google Scholar]
- Verma, D.D.; Verma, S.; Blume, G.; Fahr, A. Liposomes increase skin penetration of entrapped and non-entrapped hydrophilic substances into human skin: A skin penetration and confocal laser scanning microscopy study. Eur. J. Pharm. Biopharm. 2003, 55, 271–277. [Google Scholar] [CrossRef]
- Ban, J.; Mo, Z.; Cui, X.; Xu, Y.; Lyu, Z. Comparative study of liposomes and liposomes-in-polymer hydrogel as transdermal carriers for improving the topical delivery of imperatorin. J. Holist. Integr. Pharm. 2021, 2, 32–41. [Google Scholar] [CrossRef]
- Mengesha, Y. Nanotechnology-Enhanced Controlled-Release Systems in Topical Therapeutics. Precis. Nanomed. 2024, 7, 1365–1385. [Google Scholar]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes-novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Honeywell-Nguyen, P.L.; Bouwstra, J.A. Vesicles as a tool for transdermal and dermal delivery. Drug Discov. Today Technol. 2005, 2, 67–74. [Google Scholar] [CrossRef]
- Elsayed, M.M.A.; Abdallah, O.Y.; Naggar, V.F.; Khalafallah, N.M. Lipid vesicles for skin delivery of drugs: Reviewing three decades of research. Int. J. Pharm. 2007, 332, 1–16. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, 131–155. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Sharifi, S.; Mahmoud, N.N.; Voke, E.; Landry, M.P.; Mahmoudi, M. Importance of Standardizing Analytical Characterization Methodology for Improved Reliability of the Nanomedicine Literature. Nano-Micro Lett. 2022, 14, 172. [Google Scholar] [CrossRef]

| Chemical Compound Encapsulated in the Carrier | Type of Carrier | Role of Carrier | Method of Encapsulation | Pharmacokinetics | Therapeutic Effect | References |
|---|---|---|---|---|---|---|
| Apigenin | Transfersomes, phytosomes, ethosomes, liposomes, hydrogels | Enhanced solubility and stability; improved skin penetration; controlled and prolonged release; protection from oxidative degradation | Lipid hydration, sonication, hydrogel encapsulation, phospholipid complexation | Prolonged release; improved retention in the stratum corneum; enhanced skin distribution | Anti-inflammatory and antioxidant effects; targeted delivery to inflamed skin areas | [148,158,159,160,161,162,163] |
| Quercetin | Liposomes, niosomes, nanoliposomes, phytosomes, polymeric micelles, nanocrystals, lipid nanoparticles (SLNs, PEVs), nanoemulsions, nanofibers, hydrogels | Enhanced solubility and stability; protection from enzymatic and oxidative degradation; improved penetration through stratum corneum | Lipid hydration, sonication, micro-/nanoemulsion, phospholipid complexation, polymer/lipid encapsulation | Slower elimination; prolonged and controlled release; improved metabolic and structural stability in physiological conditions | Increased transdermal bioavailability; targeted anti-inflammatory, antioxidant, and anticancer activity (e.g., EGFR (Epidermal Growth Factor Receptor) inhibition, deeper skin layer delivery) | [93,129,148,155,159,160,161,163] |
| Luteolin | Niosomes, transfersomes, phytosomes, nanoemulsions, liposomes, SLNs, hydrogels | Improved physicochemical stability, enhanced skin penetration, anti-inflammatory and antioxidant support | Encapsulation in invasomes, transfersomal/nanosystemic formulations, phospholipid complexation | Prolonged release, deeper diffusion, better skin distribution, improved structural retention | Increased transdermal bioavailability; targeted anti-inflammatory and antioxidant effects | [148,155,159,160,161,163] |
| Ferulic Acid | Phytosomes, SLNs, NLCs, nanoemulsions, liposomes, hydrogels | Enhanced stability, solubility, and skin penetration; protection from oxidative degradation; prolonged release | Lipid matrix encapsulation (SLN, NLC), phospholipid complexation, hydrogel and nanoemulsion integration | Slower release, improved structural integrity, protection in physiological conditions | Increased bioavailability via deeper skin penetration and resistance to metabolic degradation; targeted antioxidant and anticancer effects | [93,148,149,161] |
| Chlorogenic Acid | Phytosomes, liposomes, nanocapsules, nanoemulsions, hydrogels, NPVs (Phospholipon® 90H, LIPOID® S100), CA–HSPC complex | Improved solubility, stability, and skin penetration; protection from oxidation; enhanced SC retention | Lipid hydration, phospholipid complexation (HSPC, NPVs), hydrogel embedding | Prolonged release, enhanced SC retention, improved structural stability in physiological conditions | Increased transdermal bioavailability; targeted antioxidant action and UV protection in inflammatory sites | [148,150,157,160,161] |
| Caffeic Acid | Ethosomes, liposomes, phytosomes, SLNs, hydrogels, polymeric/lipid nanoparticles, chitosan microparticles | Enhanced stability, solubility, and skin penetration; targeted release in skin and follicles; antioxidant protection | Lipid hydration, phospholipid complexation, hydrogel encapsulation, biodegradable nanoparticle formation, spray-drying (microparticles) | Slower or prolonged release, better distribution and penetration through stratum corneum and follicles | Improved bioavailability and retention; antioxidant action; potential for folliculitis treatment | [93,148,155,156,160,161] |
| Curcumin | Transferosomes, nanosponges, liposomes, liposome-in-hydrogel | Enhanced penetration through SC, protection from oxidation, prolonged release, increased bioavailability | High-pressure technique, solvent diffusion, lipid hydration, chitosan hydrogel formation | Prolonged action, reduced degradation, improved structural stability | Higher transdermal bioavailability; targeted delivery to deep skin layers; applications in inflammation, endodontics, and periodontics | [155,164,165,166] |
| Genistein | Nanoemulsion, Liposomes, Polymeric Micelles | Enhanced skin permeability, protection from degradation, increased stability and bioavailability | Encapsulation in lipid and polymeric nanoparticles or micelles | Faster absorption, prolonged release, stability in physiological environment | Improved transdermal bioavailability; targeted antioxidant activity through the skin | [160,163] |
| Rutin | Nanoemulsion, Liposomes, Niosomes | Improved skin permeability, stability, solubility; protection from oxidation | Encapsulation in liposomes and niosomes using lipid hydration | Faster absorption, improved stability, prolonged action | Enhanced bioavailability; effective in anti-inflammatory therapy and skin delivery | [160,163] |
| Morusin | Niosome | Enhanced stability and skin penetration | Lipid-based encapsulation in niosomes | Increased dermal penetration and bioavailability | Targeted delivery through the skin | [163] |
| Capsaicin | Transferosome | Enhanced skin penetration, reduced systemic side effects | High-pressure encapsulation technique | Improved dermal absorption compared to conventional formulations | Targeted delivery to pain receptors and peripheral nerves | [164] |
| Vincristine sulfate | Transferosome | Site-specific delivery with minimized systemic toxicity | High-pressure encapsulation technique | Enhanced skin penetration | Targeted anticancer delivery with reduced effect on healthy tissue | [164] |
| Cannabidiol | Ethosome | Enhanced skin permeability and localized delivery | Ethosomal formulation | Increased accumulation in stratum corneum | Improved therapeutic targeting of cutaneous endocannabinoid system | [164] |
| Caffeine | Nanoemulsion | Improved solubility and enhanced penetration through the stratum corneum | Nanoemulsion with eucalyptus/oleic oil and Volpo-N10 emulsification system | Better diffusion through SC, increased solubility, enhanced skin retention | Effective delivery to deeper skin layers | [167,168] |
| Naproxen | Nanoemulsion | Improved solubility and deeper skin penetration | Nanoemulsion using Volpo-N10, ethanol, eucalyptus/oleic oil, and PBS (Phosphate-Buffered Saline) buffer | Increased solubility in the stratum corneum, enhanced diffusion | Enhanced transdermal bioavailability and targeted delivery to deeper skin layers | [167,168] |
| Kaempferol | Submicron Emulsion | Enhanced solubility and skin penetration | Submicron emulsion using PEG-400 (Polyethylene glycol) or eucalyptus oil | Improved penetration through the stratum corneum | Increased skin bioavailability with targeted action in the stratum corneum | [159] |
| Sulbutamol Sulfate | Ethosome | Enhanced skin penetration for systemic and localized delivery | Ethosomal formulation | Improved penetration through the stratum corneum | Targeted delivery to respiratory and cutaneous receptors | [164] |
| Ammonium Glycyrrhizinate | Ethosome | Anti-inflammatory action on the skin | In vitro percutaneous permeation through human skin | Improved availability in deeper skin layers | Improved therapeutic effectiveness | [164] |
| Cyclodextrins | Cyclodextrins | Stabilization against oxidation and improved bioavailability | Complexation with β-cyclodextrin | Stabilization against oxidation | Higher bioavailability with β-CD application | [164] |
| Econazole Nitrate | Polymeric Nanosponge | Improved skin penetration, therapeutic stabilization | Ultrasonic technique | Increased stability and prolonged action | Enhanced transdermal bioavailability | [165] |
| Resveratrol | PEGylated liposome | Enhanced stability, prolonged presence in tissues | PEGylation of liposomes | Prolonged action in tissues | Enhanced bioavailability and protection from degradation | [155] |
| Calcein | pH-sensitive Liposome | Enhanced penetration through the SC depending on pH | Lipid layer technique and freeze-thaw cycles | Faster penetration at pH 5.0 | Targeted delivery to the stratum corneum at pH 5.0 | [169] |
| NBD-PE (N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)phosphatidylethanolamine) | pH-sensitive Liposome | Facilitated skin penetration in acidic conditions | Lipid layer technique and freeze-thaw cycles | Increased penetration at pH 5.0 | Targeted delivery to the stratum corneum at pH 5.0 | [169] |
| Ascorbyl Palmitate (AsP) | Ethosome | Anti-inflammatory action, assessed by in vitro skin permeation | Ethosomal formulation | Improved availability in deeper skin layers | Enhanced therapeutic effectiveness, targeted dermal action | [170] |
| Sodium Fluorescein (NaFI) | Limonene-containing Liposome | Enhanced skin penetration due to limonene, modification of stratum corneum | Thin-layer lipid hydration technique, sonication, addition of limonene | Increased fluidization of the lipid membrane, improved penetration | Improved transdermal bioavailability | [171] |
| Carboxyfluorescein (CF) | Liposome | Enhanced skin penetration through liposomal carriers | Rotational evaporation technique, formation of thin lipid layer, hydration | Improved distribution to the stratum corneum and deeper skin layers | Improved transdermal bioavailability | [172] |
| Tetracaine | Liposome | Local skin anesthesia | Thin lipid layer hydration, sonication method | Faster penetration through the stratum corneum | Improved bioavailability in the stratum corneum | [172] |
| Betamethasone dipropionate | Liposome | Improved effectiveness in eczema treatment | Hydration and sonication technique | Improved penetration and retention in deeper skin layers | Higher bioavailability and therapeutic effectiveness | [172] |
| Hesperidin | Lipid-Polymer Hybrid Nanoparticles (LPHNPs), Microemulsion-based ointment | Enhanced skin penetration, controlled release, protection from degradation | Encapsulation in lipid-polymer hybrid nanoparticles; eucalyptus oil-based water emulsion | Initial burst followed by prolonged release; improved diffusion through the skin | Improved bioavailability in deeper skin layers, with targeted action and enhanced stability | [159,172] |
| Hesperetin | Microemulsion, Topical Film | Improved bioavailability, enhanced skin penetration | Microemulsion with eucalyptus oil, film matrix | Faster penetration through the stratum corneum | Enhanced transdermal bioavailability | [159] |
| Naringenin | Ubmicron Emulsion, Gel, Elastic Liposome | Enhanced stability, improved bioavailability, better skin penetration | Encapsulation in submicron emulsion and elastic liposomes | Increased skin diffusion, controlled release | Targeted antioxidant and anti-inflammatory action | [159] |
| Catechins | Nanotransfersomes, Grape Seed Extract Cream, Multilamellar phosphatidylcholine liposomes, Ethanol-enriched liposomes | Improved skin absorption and penetration, protection from degradation | Nanotransfersomes with hyaluronic acid, grape seed extract cream; multilamellar and ethanol-based liposomes | Longer retention in the skin, increased penetration, slower release | Higher bioavailability through the stratum corneum; targeted antioxidant and protective action in skin layers | [159] |
| Myricetin | Lipid Nanoparticles, Liposomes | Protection from degradation, increased stability | Multilamellar liposomes, lipid nanoparticles | Improved stability, prolonged action | Enhanced bioavailability in skin layers, targeted anti-inflammatory action | [160] |
| Imperatorin | Lipid-Polymer Hybrid Nanoparticles (LPHNPs) | Prolonged action and controlled release | Encapsulation in lipid-polymer hybrid nanoparticles | Initial burst followed by sustained release | Enhanced skin penetration | [173] |
| Norfloxacillin | Lipid-Polymer Hybrid Nanoparticles (LPHNPs) | Prolonged action and controlled release | Encapsulation in lipid-polymer hybrid nanoparticles | Initial burst release and prolonged release | Enhanced skin penetration | [174] |
| Indomethacin | Polymeric Nanoparticles | Enhanced penetration, controlled release | Encapsulation in polymeric nanocapsules and nanospheres | Diffusion-based release (Higuchi model) | Increased bioavailability via deeper skin layers | [174] |
| Amphotericin B | Polycaprolactone (PCL) Nanoparticles | Skin penetration and controlled release | Encapsulation in PCL polymeric nanoparticles | pH-dependent release (faster at pH 7.4) | Enhanced delivery to deeper skin layers | [174] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharafan, M.; Dziki, A.; Malinowska, M.A.; Sikora, E.; Szopa, A. Targeted Delivery Strategies for Hydrophilic Phytochemicals. Appl. Sci. 2025, 15, 7101. https://doi.org/10.3390/app15137101
Sharafan M, Dziki A, Malinowska MA, Sikora E, Szopa A. Targeted Delivery Strategies for Hydrophilic Phytochemicals. Applied Sciences. 2025; 15(13):7101. https://doi.org/10.3390/app15137101
Chicago/Turabian StyleSharafan, Marta, Anna Dziki, Magdalena Anna Malinowska, Elżbieta Sikora, and Agnieszka Szopa. 2025. "Targeted Delivery Strategies for Hydrophilic Phytochemicals" Applied Sciences 15, no. 13: 7101. https://doi.org/10.3390/app15137101
APA StyleSharafan, M., Dziki, A., Malinowska, M. A., Sikora, E., & Szopa, A. (2025). Targeted Delivery Strategies for Hydrophilic Phytochemicals. Applied Sciences, 15(13), 7101. https://doi.org/10.3390/app15137101









