Investigation of the Electrodialysis of Sodium Tungstate Solutions for the Production of Tungstic Acid
Abstract
1. Introduction
- −
- Sequential removal of sodium salt impurities, such as silicon, phosphorus, molybdenum, arsenic, and additional silicon;
- −
- Precipitation of calcium tungstate (CaWO4, synthetic scheelite) [12], followed by acid decomposition of the precipitate to produce H2WO4;
- −
- −
- The final stage of the process involves the calcination of ammonium paratungstate to obtain high-purity tungsten trioxide.
2. Materials and Methods
3. Results and Discussion
- −
- Sequential removal of sodium salt impurities, such as silicon, phosphorus, molybdenum, arsenic, and additional silicon;
- −
- In alkaline solutions up to pH ≈ 8, normal (monomeric) tungstate ions predominate;
- −
- In the pH range from 8 to approximately 6, hexatungstate ions (HW6O215−) are formed;
- −
- With a further decrease in pH, particularly in highly dilute solutions, the monohydrogen hexatungstate ion (HW6O215−) is converted into the trihydrogen form (H3W6O213−);
- −
- Upon the gradual neutralization of alkaline sodium tungstate solutions to pH ≈ 5, a precipitate of sodium paratungstate (Na10W12O41·28H2O) is formed;
- −
- Normal alkaline tungstates are highly soluble, whereas paratungstates exhibit significantly lower solubility;
- −
- At pH < 4, metatungstate aquapolyions are formed, typically with a Me2O:WO3 ratio of 1:4 (e.g., Na2W2O7). The number of coordinated water molecules in isopoly and heteropoly tungstate species varies depending on temperature and concentration, which in turn influences the physicochemical properties of the resulting salts.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, L.; Wang, P.; Graedel, T.E.; Pauliuk, S.; Xianga, K.; Ren, Y.; Chen, W.-Q. Refining the understanding of China’s tungsten dominance with dynamic material cycle analysis. Resour. Conserv. Recycl. 2020, 158, 104829. [Google Scholar] [CrossRef]
- Wang, X.; Qin, W.; Jiao, F.; Dong, L.; Guo, J.; Zhang, J.; Yang, C. Review of tungsten resource reserves, tungsten concentrate production and tungsten beneficiation technology in China. Trans. Nonferrous Met. Soc. China 2022, 32, 2318–2338. [Google Scholar] [CrossRef]
- Khatkov, V.Y.; Boyarko, G.Y. The current state of the Russian tungsten industry. Proceedings of Tomsk Polytechnic University. Georesource Eng. 2019, 330, 124–137. [Google Scholar] [CrossRef]
- Kraydenko, R.I.; Perederin, Y.V.; Filatov, D.S.; Manucharyan, A.B.; Karpov, A.G.; Vasilishin, M.S. Tungsten mining technology: The current state of technology. Polzunovsky Bull. 2015, 2, 135–139. [Google Scholar]
- Tungsten–2020 U.S. Geological Survey Minerals Yearbook. Available online: https://pubs.usgs.gov/myb/vol1/2020/myb1-2020-tungsten.pdf (accessed on 5 May 2025).
- Zaitsev, V.P.; Markin, O.V.; Kholkin, A.I.; Chudinov, E.G.; Bobkov, A.V.; Kuznetsov, I.P. The Method of Processing Solutions of Sodium Tungstate. RF Patent No. 96123057/25, 20 January 1998. [Google Scholar]
- Belsky, S.S. Processing of scheelite concentrate to produce tungsten trioxide. Bulletin 2015, 12, 204–208. [Google Scholar]
- Peganov, V.A.; Shatalov, V.V.; Molchanov, T.V.; Medvedev, A.S.; Molchanov, S.A.; Adosik, G.M.; Kursinov, I.; Popov, G.; Mikhailovsky, V.G. Method of Hydrometallurgical Processing of Tungsten Concentrates. RF Patent No. 98123867/02, 28 December 1998. [Google Scholar]
- Bogacheva, L.M.; Akbarov, U.; Ismatov, H.R.; Pirmatov, E.A.; Sidyakin, V.V.; Edunov, I.A. Method of Purification of Sodium Tungstate Solutions from Impurities. USSR Patent No. 4722840/02, 30 January 1992. [Google Scholar]
- Baimbetov, B.; Moldabayeva; Yeleuliyeva, A.; Jumankulova, S.; Taimassova, A.; Adilzhan, Z.; Baisultanov, R.; Yakob, E.; Serikbayev, V. Prospects of Processing Tungsten Ores from the Akchatau Deposit. Processes 2024, 12, 77. [Google Scholar] [CrossRef]
- Zelikman, A.N.; Korshunov, B.G. Metallurgy of rare metals. In Textbook for Universities, 2nd Edition, Revised and Expanded; Metallurgiya: Moscow, Russia, 1991; 432p, Available online: https://reallib.org/reader?file=412937 (accessed on 10 March 2025).
- Sipenko, A.V.; Sotnikov, A.A. Research on the decomposition of artificial scheelite by sintering with ammonium chloride. Notes Min. Inst. 2009, 181, 158–160. [Google Scholar]
- Verevkin, G.V.; Kulmukhamedov, G.K. Method of Obtaining Ammonium Paravolframate. RF Patent No. 96122224/02, 9 October 1998. [Google Scholar]
- Doronin, A.V. Method of Obtaining Pure Tungstic Acid. Patent of the Russian Federation No. 2014135710/02, 9 February 2014. [Google Scholar]
- Zhang, L.; Shen, L.; Zhou, Q.; Qi, T.; Peng, Z.; Liu, G.; Li, X. Green and low-carbon preparation of ammonium paratungstate by adding ammonia to ammonium metatungstate solution. Hydrometallurgy 2023, 2221, 106196. [Google Scholar] [CrossRef]
- Erdoğan, M.; Karakaya, İ. Electrochemical Reduction of Tungsten Compounds to Produce Tungsten Powder. Metall. Mater. Trans. B 2010, 41, 798–804. [Google Scholar] [CrossRef]
- Orefice, M.; Nguyen, V.T.; Raiguel, S.; Jones, P.T.; Binnemans, K. Solvometallurgical Process for the Recovery of Tungsten from Scheelite. Ind. Eng. Chem. Res. 2022, 61, 754–764. [Google Scholar] [CrossRef]
- Shoinbaev, A.T. Processing of sodium tungstate solutions obtained after leaching of sintering products of tungstite concentrate with sodium carbonate. Izvestiya NAS RK 2006, 4, 72–77. [Google Scholar]
- Mirzaev, D.; Parmanova, S.T.; Ulugova, D.G. Processing of Tungsten-About a Year of Waste to Equalize the Content of Obtaining Tungsten. Universum: Technical Sciences: Electronic. Scientific Chide. 2023, Volume 10, pp. 19–21. Available online: https://7universum.com/ru/tech/archive/item/16175 (accessed on 10 March 2025).
- Chegrintsev, S.N.; Dyachenko, A.N.; Kraydenko, R.I. Sorption extraction of tungsten from a solution of sodium tungstate. Chem. Interests Sustain. Dev. 2013, 21, 345–348. Available online: https://www.elibrary.ru/title_about_new.asp?id=7582 (accessed on 13 March 2025).
- Xu, L.; Zhao, B. A Fundamental Study on the Preparation of Sodium Tungstate from Wolframite via the Smelting Process. Metals 2024, 14, 299. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, W.; Zhang, Q.; Gong, B.; Cao, J. Electrode selection of electrolysis with membrane for sodium tungstate solution. J. Cent. South Univ. Technol. 1999, 6, 107–110. [Google Scholar] [CrossRef]
- Vasiunina, N.; Dubova, I.; Druzhinin, K.; Ghilmanshina, T. Processing of Sludge Water from Alumina Production by Electrodialysis. Ecol. Ind. Russia 2024, 28, 28–32. [Google Scholar] [CrossRef]
- Melnikov, S.S.; Mugtamov, O.A.; Zabolotsky, V.I. Study of electrodialysis concentration process of inorganic acids and salts for the two-stage conversion of salts into acids utilizing bipolar electrodialysis. Sep. Purif. Technol. 2020, 235, 116198. [Google Scholar] [CrossRef]
- Kemerovo, M.A. Electrodialysis of a binary solution containing sodium and zinc ions using MK-100 M membranes. Electron. Process. Mater. 2019, 55, 73–78. [Google Scholar] [CrossRef]
- Sadyrbaeva, T.J. Electrodialysis extraction of zinc (II) by liquid membranes based on di-(2-ethylhexyl) phosphoric acid. Electrochemistry 2019, 55, 609–618. [Google Scholar] [CrossRef]
- Dzhubari, M.K.; Alekseeva, N.V. Efficiency of electrodialysis in industrial wastewater treatment. Bull. Technol. Univ. 2020, 23, 33–39. [Google Scholar]
- Krasnova, T.A. The Experience of Using Electrodialysis for Processing Wastewater from Organic Industries. Sorpt. Chromatogr. Process. 2012, 12, 419–427. Available online: http://www.chem.vsu.ru/sorbcr/images/pdf/2012/3/2012_03_12.pdf (accessed on 13 March 2025).
- Gonova, V. Experimental investigation of the purification of solutions from nickel ions by electrodialysis. Modern high-tech technologies. Reg. Appl. 2023, 73, 37–41. [Google Scholar] [CrossRef]
- Niftaliev, S.I.; Kozaderova, O.A.; Kim, K.B.; Velho, F. The use of electrodialysis to produce acid and alkali from a concentrated solution of sodium sulfate. Bull. Voronezh State Univ. Eng. Technol. 2014, 4, 175–178. [Google Scholar] [CrossRef]
- Islamov, K.B. Research and Modeling of the Electrodialysis Process of Sodium Tungstate Solutions: Dis. Mr. of Technical Sciences. 06/08/2022/Islamov K.B., Almaty, 2022; 88p. Available online: https://official.satbayev.university/download/document/25720/2022%20%D0%9C%D0%90%D0%93%20%D0%98%D1%81%D0%BB%D0%B0%D0%BC%D0%BE%D0%B2%20%D0%9A.%D0%91..pdf (accessed on 4 April 2025).
- Standard 18289-78; The State Standard of the USSR. Reagents. Sodium Tungstate Is 2-Aqueous. Publishing House of Standards: Moscow, Russia, 1979. Available online: https://files.stroyinf.ru/Data2/1/4294834/4294834827.pdf (accessed on 4 April 2025).
- Zefirov, N.S.; Knunyants, I.L.; Kulov, N.N. (Eds.) Chemical Encyclopedia; Soviet Encyclopedia: Moscow, Russia, 1988; Volume 1, 625p, Available online: https://www.geokniga.org/books/10851 (accessed on 22 April 2025).
- Shokhakimova, A.A. Study of heterogeneous cation exchange membranes based on inert polymers Universum: Technical sciences: Electron. Sci. J. 2022, 1, 39–42. Available online: https://7universum.com/ru/tech/archive/item/12989 (accessed on 22 April 2025).
- Wang, W.; Zhang, Y.; Yang, X.; Sun, H.; Wu, Y.; Shao, L. Monovalent Cation Exchange Membranes with Janus Charged Structure for Ion Separation. Engineering 2023, 25, 204–213. [Google Scholar] [CrossRef]
- Malyshev, V.P. Probabilistic Deterministic Experiment Planning; Nauka: Almaty, Kazakhstan, 1981; p. 115. [Google Scholar]
- Komarova, L.F.; Somin, V.A.; Lapshin, D.A. Desalination of groundwater using membrane technologies. Polzunovskiy Vestnik. 2023, 1, 200–206. [Google Scholar] [CrossRef]
- Chemistry and Technology of Rare and Scattered Elements. Part III. Edited by K.A. Bolshakov. Textbook for Universities. 2nd Edition, Revised. and Additional M., “Higher School”. 1976; 320p. Available online: https://djvu.online/file/MtJa8NuNRaVvc (accessed on 22 April 2025).

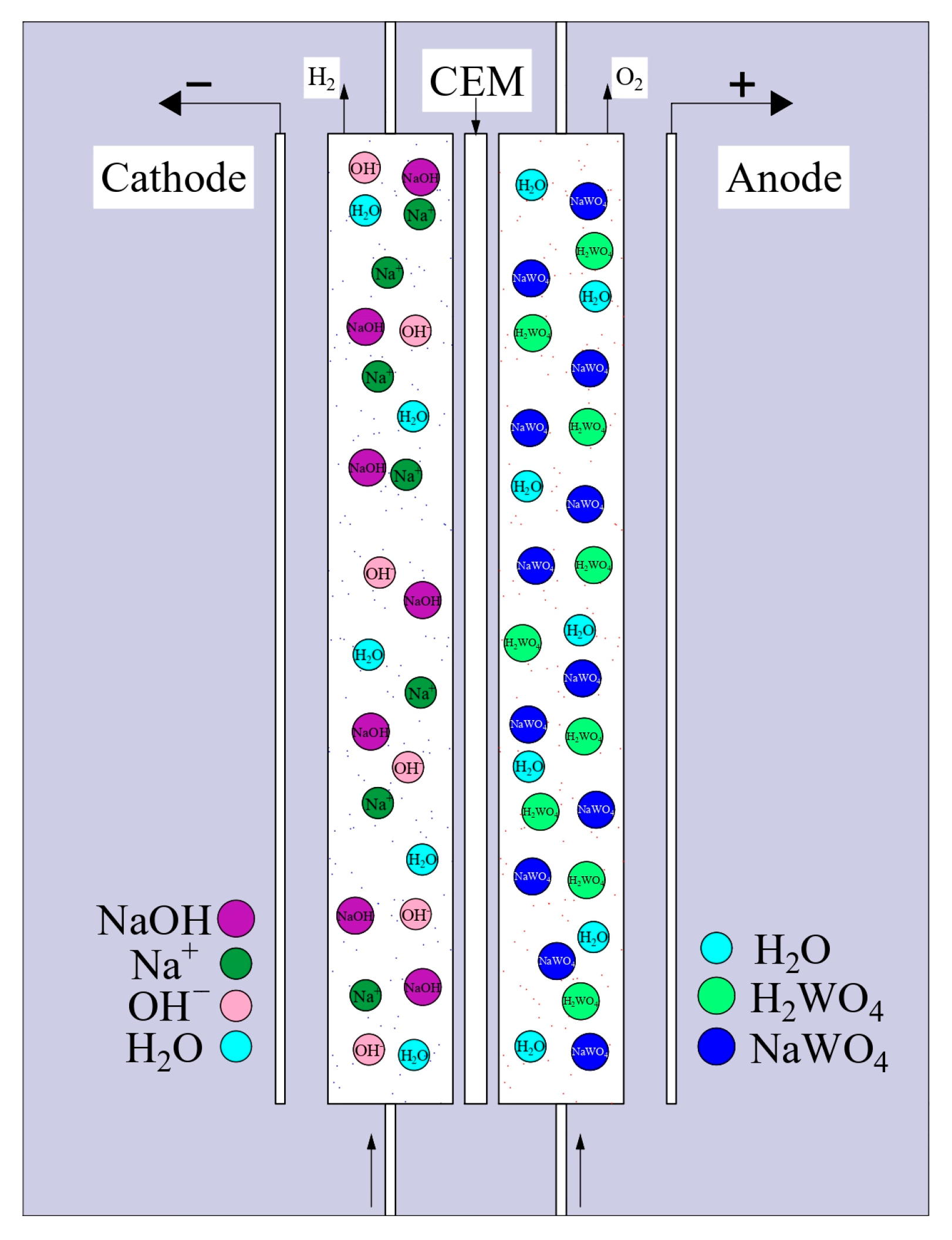

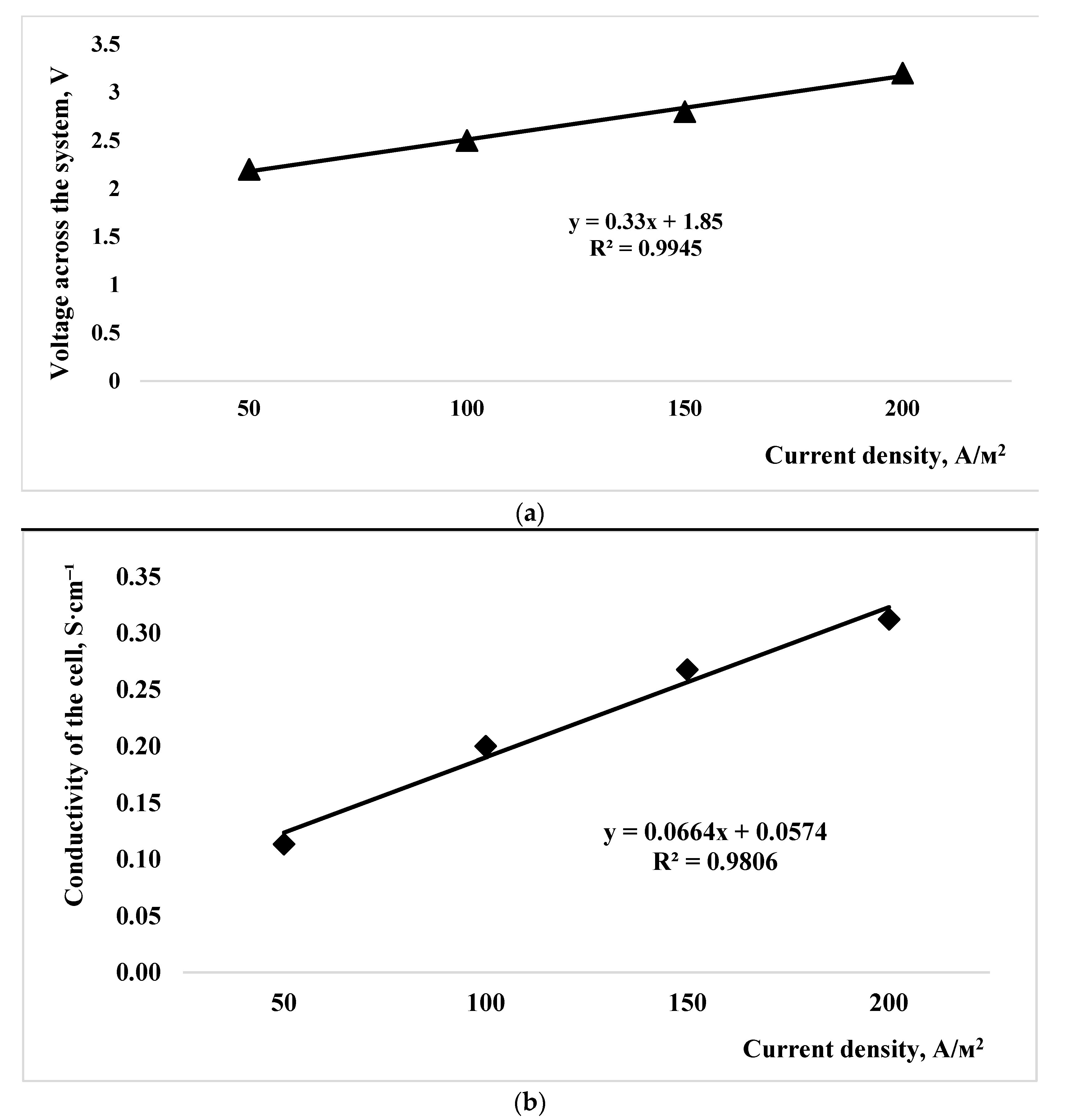
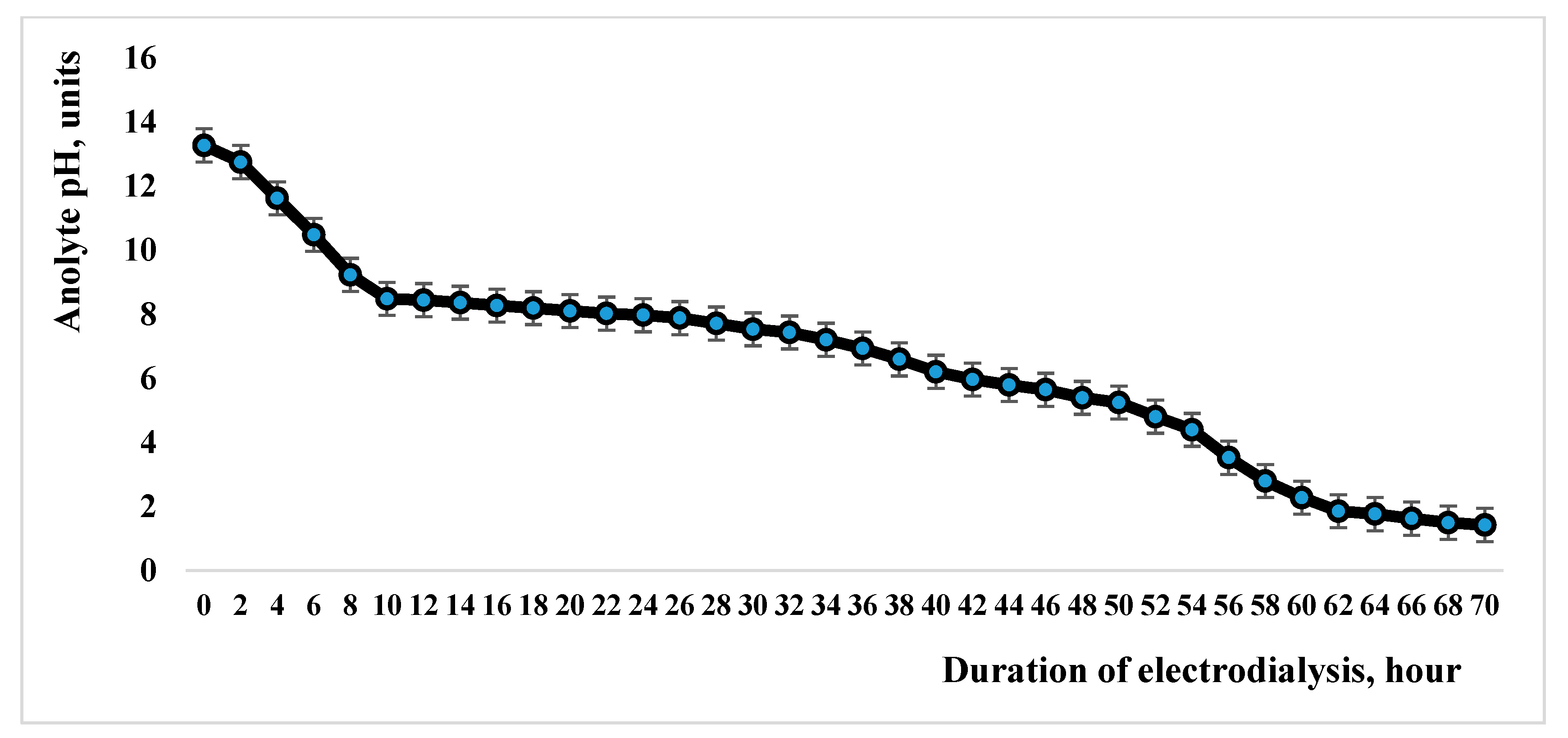
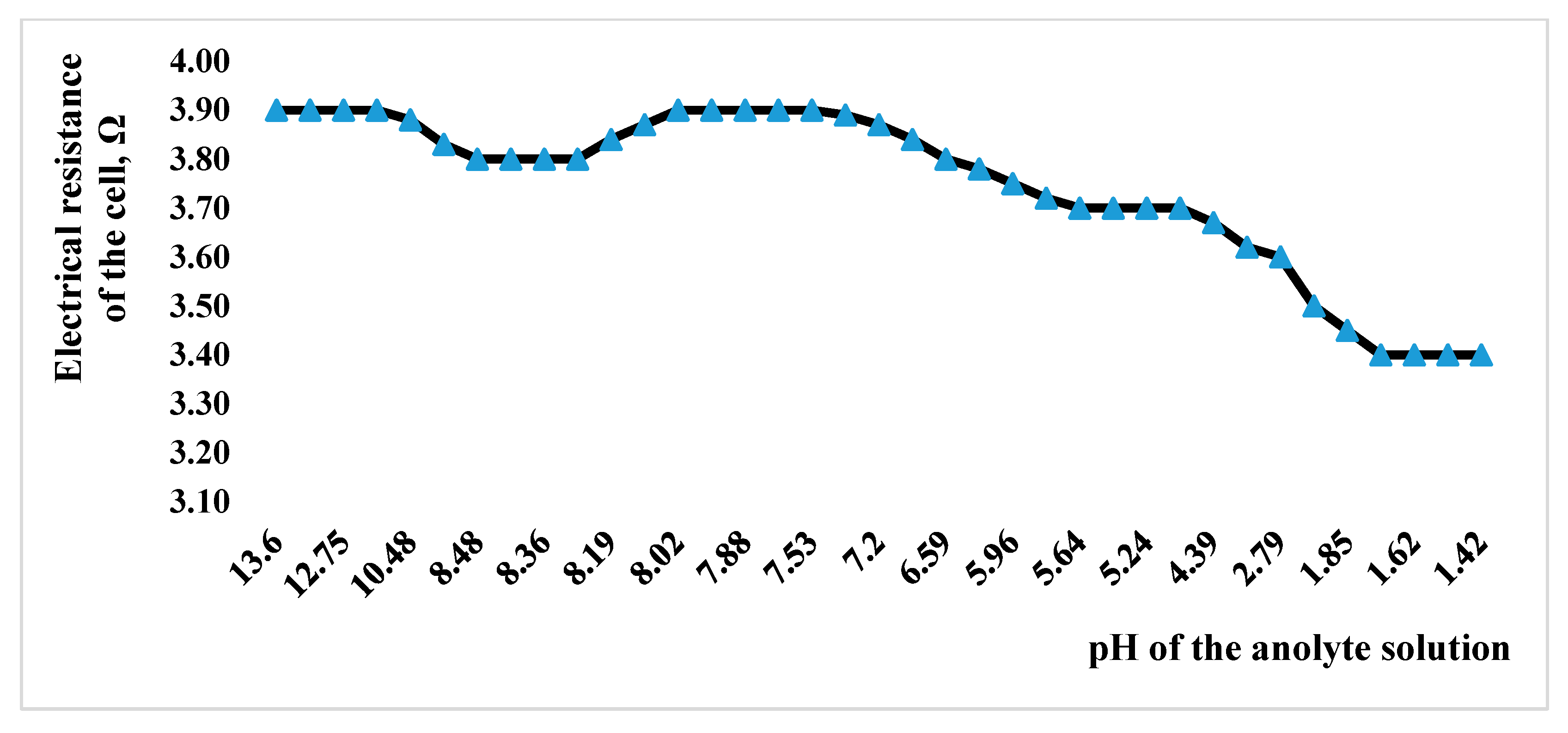
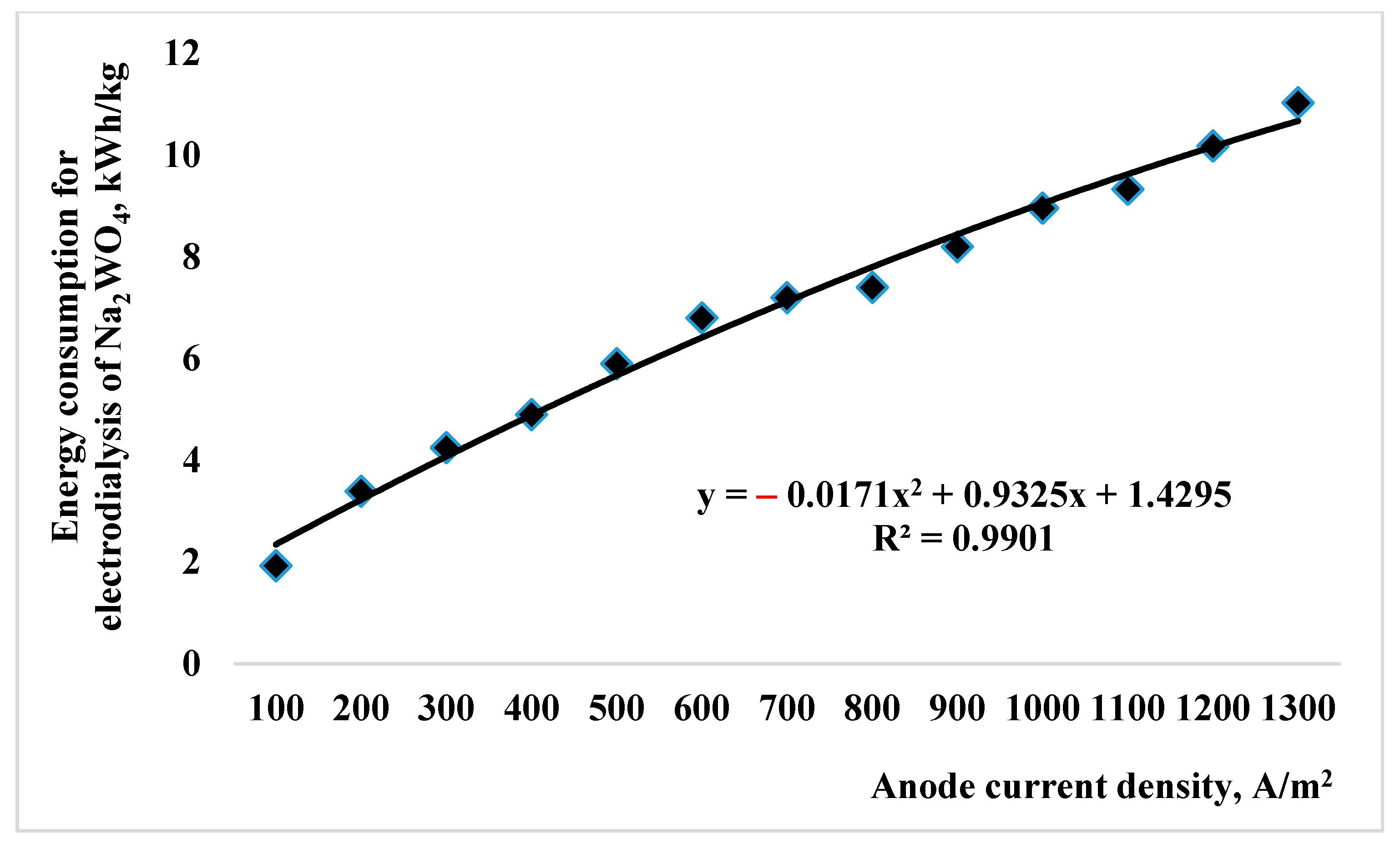

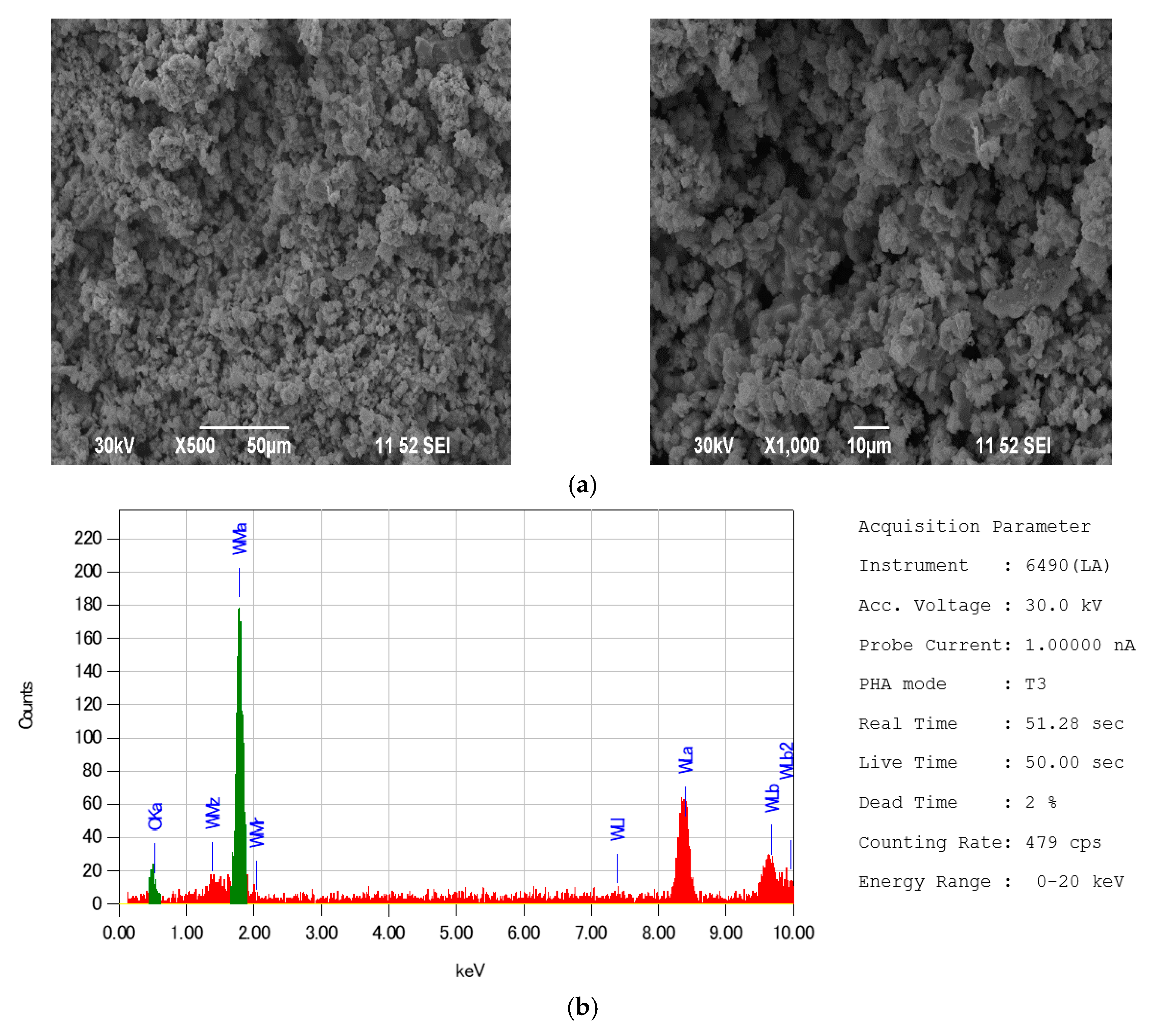
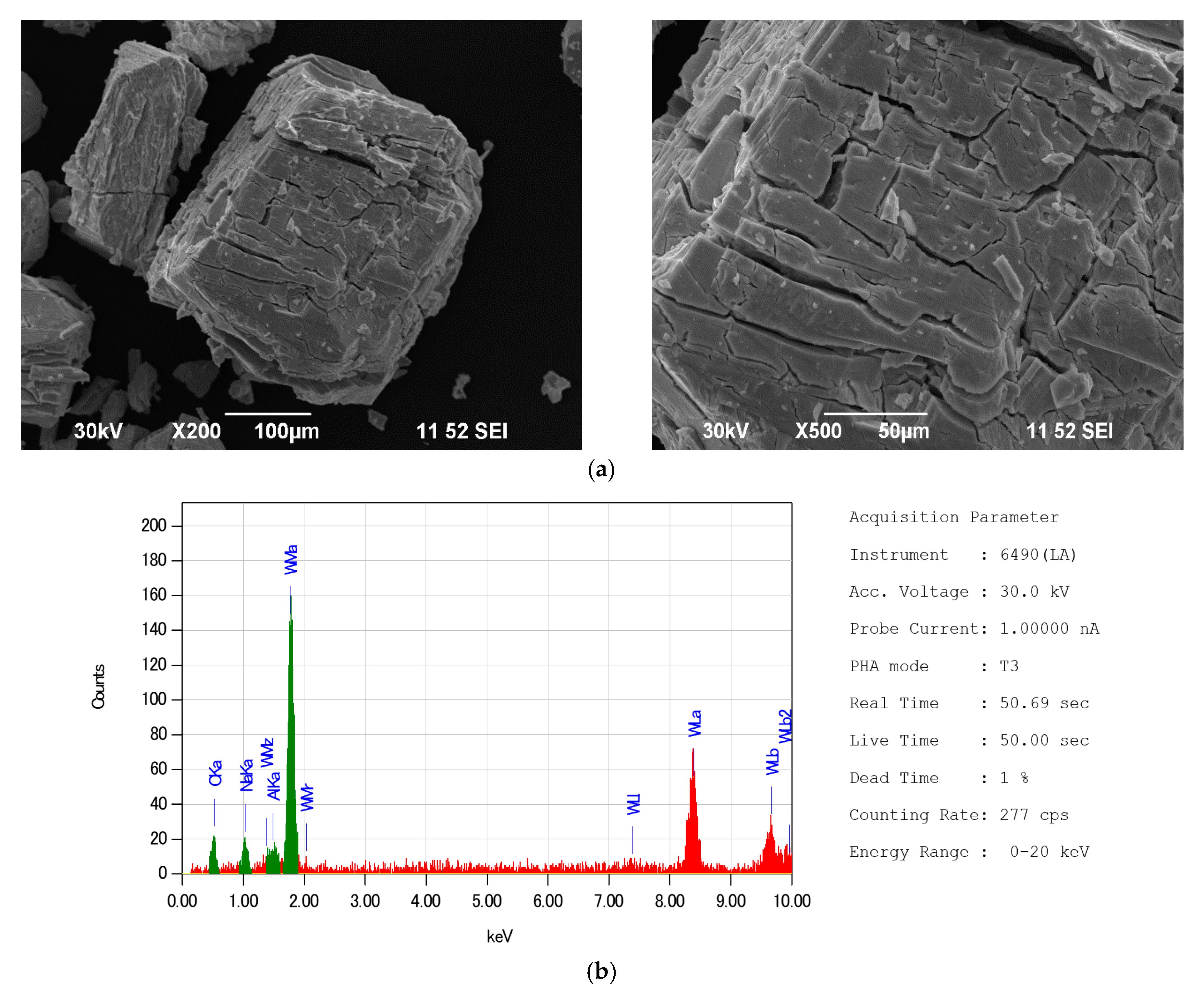
| Indicator Name | Mass % |
|---|---|
| Mass fraction of Na2WO4·2H2O, %, not less than | 99.0 |
| Mass fraction of water-insoluble substances, %, not more than | 0.01 |
| Mass fraction of nitrogen (N) from nitrates, nitrites, etc., %, not more than | 0.01 |
| Mass fraction of sulfates (SO4), %, not more than | 0.01 |
| Mass fraction of chlorides (Cl), %, not more than | 0.003 |
| Mass fraction of iron (Fe), %, not more than | 0.0005 |
| Mass fraction of molybdenum (Mo), %, not more than | 0.002 |
| Mass fraction of arsenic (As), %, not more than | 0.0005 |
| Mass fraction of heavy metals (Pb), %, not more than | 0.001 |
| pH of a 5% solution of the reagent | 8–10 |
| Parameter | Unit | Value |
|---|---|---|
| External dimensions of the cell | mm | 100 × 180 × 8 |
| Internal dimensions of the cell | mm | 70 × 140 × 8 |
| Cathode—stainless steel | mm | 100 × 182 × 1 |
| Effective surface area of the cathode | cm2 | 98 |
| Surface area of the cation-exchange membrane | cm2 | 98 |
| Surface area of the platinized anode | cm2 | 50 |
| Distance from cathode to membrane | cm | 2.0 |
| Volume of the cathode compartment | mL | 98 |
| Distance from anode to membrane | cm | 2.0 |
| Volume of the anode compartment | mL | 98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dauletbakova, A.; Baimbetov, B.; Tazhiyev, Y.; Moldabayeva, G. Investigation of the Electrodialysis of Sodium Tungstate Solutions for the Production of Tungstic Acid. Appl. Sci. 2025, 15, 7033. https://doi.org/10.3390/app15137033
Dauletbakova A, Baimbetov B, Tazhiyev Y, Moldabayeva G. Investigation of the Electrodialysis of Sodium Tungstate Solutions for the Production of Tungstic Acid. Applied Sciences. 2025; 15(13):7033. https://doi.org/10.3390/app15137033
Chicago/Turabian StyleDauletbakova, Adelya, Bolotpay Baimbetov, Yeleussiz Tazhiyev, and Gulnara Moldabayeva. 2025. "Investigation of the Electrodialysis of Sodium Tungstate Solutions for the Production of Tungstic Acid" Applied Sciences 15, no. 13: 7033. https://doi.org/10.3390/app15137033
APA StyleDauletbakova, A., Baimbetov, B., Tazhiyev, Y., & Moldabayeva, G. (2025). Investigation of the Electrodialysis of Sodium Tungstate Solutions for the Production of Tungstic Acid. Applied Sciences, 15(13), 7033. https://doi.org/10.3390/app15137033






