Extraction, Detection, and Quantification Methods for Analyzing Glyphosate and AMPA in Foods: Challenges and Opportunities
Abstract
1. Introduction
2. Glyphosate and AMPA
3. Glyphosate and AMPA in Biological and Non-Biological Samples
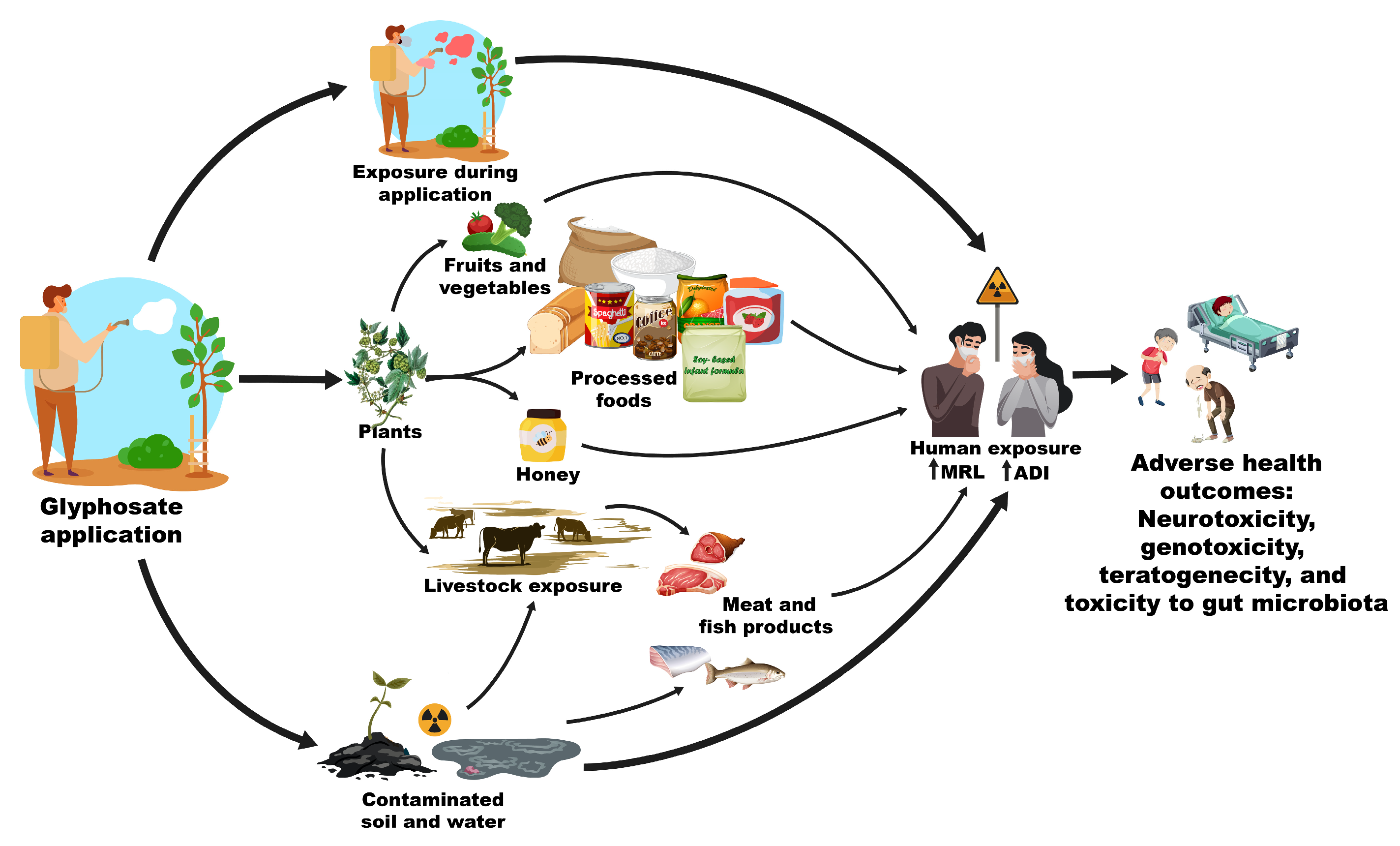
4. Glyphosate and AMPA in Foods
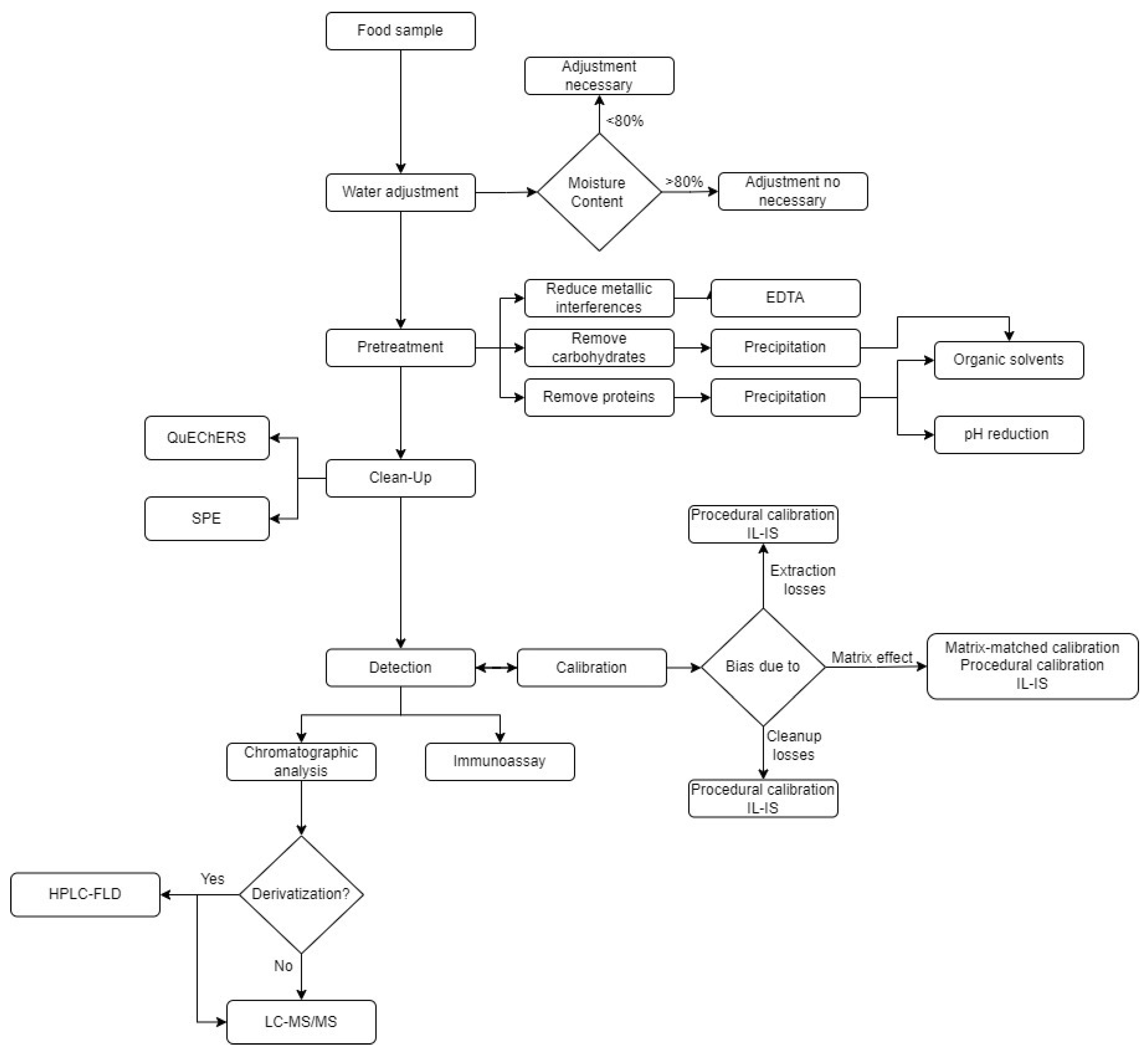
4.1. Sample Pretreatment
4.2. Sample Cleanup
4.3. Detection and Quantification
4.4. Analytical Method Validation
5. Challenges and Opportunities
- The high polarity of glyphosate and AMPA prevents their retention on reversed-phase chromatographic columns and limits the use of traditional extraction solvents, such as ethanol, methanol, and acetonitrile.
- Their chelating ability facilitates the formation of complexes with metallic elements commonly found in food matrices.
- The lack of chromophore groups on the GLYP structure hinders its detection using fluorescence and diode array detectors.
- The low concentrations of GLYP and AMPA present in foods necessitate the implementation of more sensitive and robust methodologies, with detection and quantification limits tailored to each food group and the established maximum residue limits.
- Compliance with international regulations for glyphosate and AMPA residue limits presents a significant analytical challenge.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sapkota, R.; Stenger, J.; Ostlie, M.; Flores, P. Towards Reducing Chemical Usage for Weed Control in Agriculture Using UAS Imagery Analysis and Computer Vision Techniques. Sci. Rep. 2023, 13, 6548. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.; Silva, L.; Duarte, S.; Pena, A.; Pereira, A. Glyphosate Use, Toxicity and Occurrence in Food. Foods 2021, 10, 2785. [Google Scholar] [CrossRef] [PubMed]
- Hudek, L.; Enez, A.; Bräu, L. Comparative Analyses of Glyphosate Alternative Weed Management Strategies on Plant Coverage, Soil and Soil Biota. Sustainability 2021, 13, 11454. [Google Scholar] [CrossRef]
- Gębura, K.; Wieczorek, P.P.; Poliwoda, A. Determination of Glyphosate and AMPA in Food Samples Using Membrane Extraction Technique for Analytes Preconcentration. Membranes 2022, 12, 20. [Google Scholar] [CrossRef]
- Myers, J.P.; Antoniou, M.N.; Blumberg, B.; Carroll, L.; Colborn, T.; Everett, L.G.; Hansen, M.; Landrigan, P.J.; Lanphear, B.P.; Mesnage, R.; et al. Concerns over Use of Glyphosate-Based Herbicides and Risks Associated with Exposures: A Consensus Statement. Environ. Health 2016, 15, 19. [Google Scholar] [CrossRef]
- Kolakowski, B.M.; Miller, L.; Murray, A.; Leclair, A.; Bietlot, H.; Van De Riet, J.M. Analysis of Glyphosate Residues in Foods from the Canadian Retail Markets between 2015 and 2017. J. Agric. Food Chem. 2020, 68, 5201–5211. [Google Scholar] [CrossRef]
- Caiati, C.; Pollice, P.; Favale, S.; Lepera, M.E. The Herbicide Glyphosate and Its Apparently Controversial Effect on Human Health: An Updated Clinical Perspective. Endocr. Metab. Immune Disord. Drug Targets 2019, 20, 489–505. [Google Scholar] [CrossRef]
- Maggi, F.; la Cecilia, D.; Tang, F.H.M.; McBratney, A. The Global Environmental Hazard of Glyphosate Use. Sci. Total Environ. 2020, 717, 137167. [Google Scholar] [CrossRef]
- Yu, X.; Sun, Y.; Lin, C.; Wang, P.; Shen, Z.; Zhao, Y. Development of Transgenic Maize Tolerant to Both Glyphosate and Glufosinate. Agronomy 2023, 13, 226. [Google Scholar] [CrossRef]
- Klingelhöfer, D.; Braun, M.; Brüggmann, D.; Groneberg, D.A. Glyphosate: How Do Ongoing Controversies, Market Characteristics, and Funding Influence the Global Research Landscape? Sci. Total Environ. 2021, 765, 144271. [Google Scholar] [CrossRef]
- van Bruggen, A.H.C.; Finckh, M.R.; He, M.; Ritsema, C.J.; Harkes, P.; Knuth, D.; Geissen, V. Indirect Effects of the Herbicide Glyphosate on Plant, Animal and Human Health Through Its Effects on Microbial Communities. Front. Environ. Sci. 2021, 9, 763917. [Google Scholar] [CrossRef]
- Costas-Ferreira, C.; Durán, R.; Faro, L.R.F. Toxic Effects of Glyphosate on the Nervous System: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 4605. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, J.V.; Court-Marques, D.; Tiramani, M.; Reich, H.; Pfeil, R.; Istace, F.; Crivellente, F. Glyphosate Toxicity and Carcinogenicity: A Review of the Scientific Basis of the European Union Assessment and Its Differences with IARC. Arch. Toxicol. 2017, 91, 2723–2743. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO Pesticide Residues in Food 2016. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/en/ (accessed on 19 December 2024).
- Davoren, M.J.; Schiestl, R.H. Glyphosate-Based Herbicides and Cancer Risk: A Post-IARC Decision Review of Potential Mechanisms, Policy and Avenues of Research. Carcinogenesis 2018, 39, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Kocadal, K.; Alkas, F.B.; Battal, D.; Saygi, S. A Review on Advances and Perspectives of Glyphosate Determination: Challenges and Opportunities. Arch. Environ. Prot. 2022, 48, 89–98. [Google Scholar]
- Ledoux, M.L.; Hettiarachchy, N.; Yu, X.; Howard, L.; Lee, S.O. Penetration of Glyphosate into the Food Supply and the Incidental Impact on the Honey Supply and Bees. Food Control 2020, 109, 106859. [Google Scholar] [CrossRef]
- Vicini, J.L.; Jensen, P.K.; Young, B.M.; Swarthout, J.T. Residues of Glyphosate in Food and Dietary Exposure. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5226–5257. [Google Scholar] [CrossRef]
- Peillex, C.; Pelletier, M. The Impact and Toxicity of Glyphosate and Glyphosate-Based Herbicides on Health and Immunity. J. Immunotoxicol. 2020, 17, 163–174. [Google Scholar] [CrossRef]
- Weisenburger, D.D. A Review and Update with Perspective of Evidence That the Herbicide Glyphosate (Roundup) Is a Cause of Non-Hodgkin Lymphoma. Clin. Lymphoma Myeloma Leuk. 2021, 21, 621–630. [Google Scholar] [CrossRef]
- Sang, Y.; Mejuto, J.C.; Xiao, J.; Simal-Gandara, J. Assessment of Glyphosate Impact on the Agrofood Ecosystem. Plants 2021, 10, 405. [Google Scholar] [CrossRef]
- Gill, J.P.K.; Sethi, N.; Mohan, A. Analysis of the Glyphosate Herbicide in Water, Soil and Food Using Derivatising Agents. Environ. Chem. Lett. 2017, 15, 85–100. [Google Scholar] [CrossRef]
- Rigobello-Masini, M.; Pereira, E.A.O.; Abate, G.; Masini, J.C. Solid-Phase Extraction of Glyphosate in the Analyses of Environmental, Plant, and Food Samples. Chromatographia 2019, 82, 1121–1138. [Google Scholar] [CrossRef]
- Marín, J.; Campillo, N.; Hernández-Córdoba, M.; Garrido, I.; Fenoll, J.; Viñas, P. Liquid-Liquid Microextraction of Glyphosate, Glufosinate and Aminomethylphosphonic Acid for the Analysis of Agricultural Samples by Liquid Chromatography. Anal. Methods 2020, 12, 2039–2045. [Google Scholar] [CrossRef]
- Lin, J.F.; Chang, F.C.; Sheen, J.F. Determination of Glyphosate, Aminomethylphosphonic Acid, and Glufosinate in River Water and Sediments Using Microwave-Assisted Rapid Derivatization and LC–MS/MS. Environ. Sci. Pollut. Res. 2022, 29, 46282–46292. [Google Scholar] [CrossRef]
- Anastassiades, M.; Kolberg, D.I.; Eichhorn, E.; Wachtler, A.-K.; Benkenstein, A.; Zechmann, S.; Mack, D.; Wildgrube, C.; Barth, A.; Sigalov, I.; et al. EU Reference Laboratory for Pesticides Requiring Single Residue Methods (EURL-SRM) Quick Method for the Analysis of Numerous Highly Polar Pesticides in Food Involving Extraction with Acidified Methanol and LC-MS/MS Measurement I. Food of Plant Origin (QuPPe-PO-Method), Version 12.3. 2021, pp. 1–73. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlSRM/EurlSrm_meth_QuPPe_PO_V12_3.pdf (accessed on 20 March 2025).
- Saurat, D.; Raffy, G.; Bonvallot, N.; Monfort, C.; Fardel, O.; Glorennec, P.; Chevrier, C.; Le Bot, B. Determination of Glyphosate and AMPA in Indoor Settled Dust by Hydrophilic Interaction Liquid Chromatography with Tandem Mass Spectrometry and Implications for Human Exposure. J. Hazard. Mater. 2023, 15, 130654. [Google Scholar] [CrossRef]
- Jansons, M.; Pugajeva, I.; Bartkevics, V.; Karkee, H.B. LC-MS/MS Characterisation and Determination of Dansyl Chloride Derivatised Glyphosate, Aminomethylphosphonic Acid (AMPA), and Glufosinate in Foods of Plant and Animal Origin. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2021, 1177, 122779. [Google Scholar] [CrossRef]
- Alferness, P.L.; Iwata, Y. Determination of Glyphosate and (Aminomethy1) Phosphonic Acid in Soil, Plant and Animal Matrices, and Water by Capillary Gas Chromatography with Mass-Selective Detection. J. Agric. Food Chem. 1994, 42, 2751–2759. [Google Scholar] [CrossRef]
- Gauglitz, G.; Wimmer, B.; Melzer, T.; Huhn, C. Glyphosate Analysis Using Sensors and Electromigration Separation Techniques as Alternatives to Gas or Liquid Chromatography. Anal. Bioanal. Chem. 2018, 410, 725–746. [Google Scholar] [CrossRef]
- de Castilhos Ghisi, N.; Zuanazzi, N.R.; Fabrin, T.M.C.; Oliveira, E.C. Glyphosate and Its Toxicology: A Scientometric Review. Sci. Total Environ. 2020, 733, 139359. [Google Scholar] [CrossRef]
- Zulet-González, A.; Barco-Antoñanzas, M.; Gil-Monreal, M.; Royuela, M.; Zabalza, A. Increased Glyphosate-Induced Gene Expression in the Shikimate Pathway Is Abolished in the Presence of Aromatic Amino Acids and Mimicked by Shikimate. Front. Plant Sci. 2020, 11, 459. [Google Scholar] [CrossRef]
- Fuchs, B.; Saikkonen, K.; Helander, M. Glyphosate-Modulated Biosynthesis Driving Plant Defense and Species Interactions. Trends Plant Sci. 2021, 26, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Carretta, L.; Cardinali, A.; Marotta, E.; Zanin, G.; Masin, R. A New Rapid Procedure for Simultaneous Determination of Glyphosate and AMPA in Water at Sub Μg/L Level. J. Chromatogr. A 2019, 1600, 65–72. [Google Scholar] [CrossRef] [PubMed]
- de Morais Valentim, J.M.B.; Coradi, C.; Viana, N.P.; Fagundes, T.R.; Micheletti, P.L.; Gaboardi, S.C.; Fadel, B.; Pizzatti, L.; Candiotto, L.Z.P.; Panis, C. Glyphosate as a Food Contaminant: Main Sources, Detection Levels, and Implications for Human and Public Health. Foods 2024, 13, 1697. [Google Scholar] [CrossRef] [PubMed]
- PubChem. Glyphosate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/3496 (accessed on 27 March 2025).
- Bento, C.P.M.; Yang, X.; Gort, G.; Xue, S.; van Dam, R.; Zomer, P.; Mol, H.G.J.; Ritsema, C.J.; Geissen, V. Persistence of Glyphosate and Aminomethylphosphonic Acid in Loess Soil under Different Combinations of Temperature, Soil Moisture and Light/Darkness. Sci. Total Environ. 2016, 572, 301–311. [Google Scholar] [CrossRef]
- Gimsing, A.L.; Borggaard, O.K. Competitive Adsorption and Desorption of Glyphosate and Phosphate on Clay Silicates and Oxides. Clay Miner. 2002, 37, 509–515. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Glyphosate. Available online: https://comptox.epa.gov/dashboard/chemical/properties/DTXSID1024122 (accessed on 22 December 2024).
- Kanissery, R.; Gairhe, B.; Kadyampakeni, D.; Batuman, O.; Alferez, F. Glyphosate: Its Environmental Persistence and Impact on Crop Health and Nutrition. Plants 2019, 8, 499. [Google Scholar] [CrossRef]
- Schönbrunn, E.; Eschenburg, S.; Shuttleworth, W.A.; Schloss, J.V.; Amrhein, N.; Evans, J.N.S.; Kabsch, W. Interaction of the Herbicide Glyphosate with Its Target Enzyme 5-Enolpyruvylshikimate 3-Phosphate Synthase in Atomic Detail. Pnas 2001, 98, 1376–1380. [Google Scholar] [CrossRef]
- Galicia-Andrés, E.; Tunega, D.; Gerzabek, M.H.; Oostenbrink, C. On Glyphosate–Kaolinite Surface Interactions. A Molecular Dynamic Study. Eur. J. Soil. Sci. 2021, 72, 1231–1242. [Google Scholar] [CrossRef]
- Martin-Reina, J.; Dahiri, B.; Carbonero-Aguilar, P.; Soria-Dıaz, M.E.; González, A.G.; Bautista, J.; Moreno, I. Validation of a Simple Method for the Determination of Glyphosate and Aminomethylphosphonic Acid in Human Urine by UPLC-MS/MS. Microchem. J. 2021, 170, 106760. [Google Scholar] [CrossRef]
- Von Ehrenstein, O.S.; Ling, C.; Cui, X.; Cockburn, M.; Park, A.S.; Yu, F.; Wu, J.; Ritz, B. Prenatal and Infant Exposure to Ambient Pesticides and Autism Spectrum Disorder in Children: Population Based Case-Control Study. BMJ 2019, 364, 1962. [Google Scholar] [CrossRef]
- Zhao, J.; Pacenka, S.; Wu, J.; Richards, B.K.; Steenhuis, T.; Simpson, K.; Hay, A.G. Detection of Glyphosate Residues in Companion Animal Feeds. Environ. Pollut. 2018, 243, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Bonansea, R.I.; Filippi, I.; Wunderlin, D.A.; Marino, D.J.G.; Amé, M.V. The Fate of Glyphosate and AMPA in a Freshwater Endorheic Basin: An Ecotoxicological Risk Assessment. Toxics 2018, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Yusà, V.; Sanchís, Y.; Dualde, P.; Carbonell, E.; Coscollà, C. Quick Determination of Glyphosate and AMPA at Sub Μg/L in Drinking Water by Direct Injection into LC-MS/MS. Talanta Open 2021, 4, 100061. [Google Scholar] [CrossRef]
- Poiger, T.; Buerge, I.J.; Bächli, A.; Müller, M.D.; Balmer, M.E. Occurrence of the Herbicide Glyphosate and Its Metabolite AMPA in Surface Waters in Switzerland Determined with On-Line Solid Phase Extraction LC-MS/MS. Environ. Sci. Pollut. Res. 2017, 24, 1588–1596. [Google Scholar] [CrossRef]
- Wirth, M.A.; Schulz-Bull, D.E.; Kanwischer, M. The Challenge of Detecting the Herbicide Glyphosate and Its Metabolite AMPA in Seawater–Method Development and Application in the Baltic Sea. Chemosphere 2021, 262, 128327. [Google Scholar] [CrossRef]
- Alonso, B.; Griffero, L.; Bentos Pereira, H.; Pareja, L.; Pérez Parada, A. Determination of Glyphosate and AMPA in Freshwater and Soil from Agroecosystems by 9-Fluorenylmethoxycarbonyl Chloride Derivatization and Liquid Chromatography-Fluorescence Detection and Tandem Mass Spectrometry. MethodsX 2022, 9, 101730. [Google Scholar] [CrossRef]
- Camiccia, M.; Candiotto, L.Z.P.; Gaboardi, S.C.; Panis, C.; Kottiwitz, L.B.M. Determination of Glyphosate in Breast Milk of Lactating Women in a Rural Area from Paraná State, Brazil. Braz. J. Med. Biol. Res. 2022, 55, e12194. [Google Scholar] [CrossRef]
- Conrad, A.; Schröter-Kermani, C.; Hoppe, H.W.; Rüther, M.; Pieper, S.; Kolossa-Gehring, M. Glyphosate in German Adults—Time Trend (2001 to 2015) of Human Exposure to a Widely Used Herbicide. Int. J. Hyg. Environ. Health 2017, 220, 8–16. [Google Scholar] [CrossRef]
- Filippi, I.; Fernández, P.; Grimalt, J.O.; Butinof, M.; Amé, M.V.; Muñoz, S.E. Glyphosate and AMPA in Saliva and Other Traditional Human Matrices. New Findings for Less Invasive Biomonitoring to the Exposure to Pesticides. Environ. Adv. 2024, 15, 100474. [Google Scholar] [CrossRef]
- Zouaoui, K.; Dulaurent, S.; Gaulier, J.M.; Moesch, C.; Lachâtre, G. Determination of Glyphosate and AMPA in Blood and Urine from Humans: About 13 Cases of Acute Intoxication. Forensic Sci. Int. 2013, 226, e20–e25. [Google Scholar] [CrossRef]
- Ruiz, P.; Dualde, P.; Coscollà, C.; Fernández, S.F.; Carbonell, E.; Yusà, V. Biomonitoring of Glyphosate and AMPA in the Urine of Spanish Lactating Mothers. Sci. Total Environ. 2021, 801, 149688. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Smith, S.; Smith, G.; Wang, W.; Li, Y. Glyphosate Contamination in Grains and Foods: An Overview. Food Control 2019, 106, 106710. [Google Scholar] [CrossRef]
- CXG-90. Guidelines on Performance Criteria for Methods Ofanal-Ysis for the Determination of Pesticide Residues in Food and Feed. 2017. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXG%2B90-2017%252FCXG_090e.pdf (accessed on 19 December 2024).
- Presidential Decree DOF: 31/12/2020; Decree Establishing the Actions to be Taken by the Agencies and Entities Comprising the Federal Public Administration, within the Scope of Their Powers, to Gradually Replace the Use, Acquisition, Distribution, Promotion, and Importation of the Chemical Substance Glyphosate and the Agrochemicals Used in Our Country that Contain It as an Active ingredient, with sustainable and culturally appropriate alternatives that allow for the maintenance of production and are safe for human Health, the Country’s Biocultural Diversity, and the Environment. Presidency of the Mexican Republic. 2025. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5609365&fecha=31/12/2020#gsc.tab=0 (accessed on 5 June 2025).
- Thompson, T.S.; van den Heever, J.P.; Limanowka, R.E. Determination of Glyphosate, AMPA, and Glufosinate in Honey by Online Solid-Phase Extraction-Liquid Chromatography-Tandem Mass Spectrometry. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Zoller, O.; Rhyn, P.; Rupp, H.; Zarn, J.A.; Geiser, C. Glyphosate Residues in Swiss Market Foods: Monitoring and Risk Evaluation. Food Addit. Contam. Part B Surveill. 2018, 11, 83–91. [Google Scholar] [CrossRef]
- Karise, R.; Raimets, R.; Bartkevics, V.; Pugajeva, I.; Pihlik, P.; Keres, I.; Williams, I.H.; Viinalass, H.; Mänd, M. Are Pesticide Residues in Honey Related to Oilseed Rape Treatments? Chemosphere 2017, 188, 389–396. [Google Scholar] [CrossRef]
- Raimets, R.; Bontšutšnaja, A.; Bartkevics, V.; Pugajeva, I.; Kaart, T.; Puusepp, L.; Pihlik, P.; Keres, I.; Viinalass, H.; Mänd, M.; et al. Pesticide Residues in Beehive Matrices Are Dependent on Collection Time and Matrix Type but Independent of Proportion of Foraged Oilseed Rape and Agricultural Land in Foraging Territory. Chemosphere 2020, 238, 124555. [Google Scholar] [CrossRef]
- Berg, C.J.; Peter King, H.; Delenstarr, G.; Kumar, R.; Rubio, F.; Glaze, T. Glyphosate Residue Concentrations in Honey Attributed through Geospatial Analysis to Proximity of Large-Scale Agriculture and Transfer off-Site by Bees. PLoS ONE 2018, 13, e0198876. [Google Scholar] [CrossRef]
- de Souza, A.P.F.; Rodrigues, N.R.; Reyes, F.G.R. Glyphosate and Aminomethylphosphonic Acid (AMPA) Residues in Brazilian Honey. Food Addit. Contam. Part B Surveill. 2021, 14, 40–47. [Google Scholar] [CrossRef]
- Ruiz-Toledo, J.; Sánchez, D. Glyphosate Contamination: Implications for Honeybee Apis Mellifera and Consumers in Southeastern Mexico. Agro Product. 2024, 6, 33–45. [Google Scholar] [CrossRef]
- Savini, S.; Bandini, M.; Sannino, A. An Improved, Rapid, and Sensitive Ultra-High-Performance Liquid Chromatography-High-Resolution Orbitrap Mass Spectrometry Analysis for the Determination of Highly Polar Pesticides and Contaminants in Processed Fruits and Vegetables. J. Agric. Food Chem. 2019, 67, 2716–2722. [Google Scholar] [CrossRef]
- Lemos, J.; Sampedro, M.C.; de Ariño, A.; Ortiz, A.; Barrio, R.J. Risk Assessment of Exposure to Pesticides through Dietary Intake of Vegetables Typical of the Mediterranean Diet in the Basque Country. J. Food Comp. Anal. 2016, 49, 35–41. [Google Scholar] [CrossRef]
- Gotti, R.; Fiori, J.; Bosi, S.; Dinelli, G. Field-Amplified Sample Injection and Sweeping Micellar Electrokinetic Chromatography in Analysis of Glyphosate and Aminomethylphosphonic Acid in Wheat. J. Chromatogr. A 2019, 1601, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Berthion, J.M.; Colet, I.; Merlo, M.; Nougadère, A.; Hu, R. Validation and Application of Analytical Method for Glyphosate and Glufosinate in Foods by Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2018, 1549, 31–38. [Google Scholar] [CrossRef]
- Bou-Mitri, C.; Mekanna, A.N.; Dagher, S.; Moukarzel, S.; Farhat, A. Occurrence and Exposure to Glyphosate Present in Bread and Flour Products in Lebanon. Food Control 2022, 136, 108894. [Google Scholar] [CrossRef]
- Department of Agriculture, Water, and the Environmnet (DAWE). Wheat (Flour) Residue Testing Annual Datasets 2019–20. 2019. Available online: https://www.agriculture.gov.au/sites/default/files/documents/wheat-flour-residue-testing-datasets-2019-20.pdf (accessed on 5 January 2025).
- Cruz, J.M.; Murray, J.A. Determination of Glyphosate and AMPA in Oat Products for the Selection of Candidate Reference Materials. Food Chem. 2021, 342, 128213. [Google Scholar] [CrossRef]
- Viljoen, C.D.; Koortzen, B.J.; Sreenivasan Tantuan, S. Determining the Presence of Glyphosate and Glyphosate-Tolerant Events in Maize and Soybean Food Products in South Africa. Food Addit. Contam. Part B Surveill. 2021, 14, 91–97. [Google Scholar] [CrossRef]
- Wang, K.; Jiao, B.; Gao, H.; Pan, X.; Wu, X.; Xu, J.; Dong, F.; Zheng, Y. Residue and Dietary Risk Assessment of Glyphosate, Glufosinate-Ammonium, and Their Metabolites in Maize and Soybean. J. Food Comp. Anal. 2023, 120, 105298. [Google Scholar] [CrossRef]
- Stephenson, C.L.; Harris, C.A. An Assessment of Dietary Exposure to Glyphosate Using Refined Deterministic and Probabilistic Methods. Food Chem. Toxicol. 2016, 95, 28–41. [Google Scholar] [CrossRef]
- Wumbei, A.; Goeteyn, L.; Lopez, E.; Houbraken, M.; Spanoghe, P. Glyphosate in Yam from Ghana. Food Addit. Contam. Part B Surveill. 2019, 12, 231–235. [Google Scholar] [CrossRef]
- Carrasco Cabrera, L.; Di Piazza, G.; Dujardin, B.; Marchese, E.; Medina Pastor, P. The 2022 European Union Report on Pesticide Residues in Food. EFSA J. 2024, 22, 8753. [Google Scholar]
- Rodrigues, N.R.; de Souza, A.P.F. Occurrence of Glyphosate and AMPA Residues in Soy-Based Infant Formula Sold in Brazil. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Alarape, S.A.; Fagbohun, A.F.; Ipadeola, O.A.; Adeigbo, A.A.; Adesola, R.O.; Adeyemo, O.K. Assessment of Glyphosate and Its Metabolites’ Residue Concentrations in Cultured African Catfish Offered for Sale in Selected Markets in Ibadan, Oyo State, Nigeria. Front. Toxicol. 2023, 5, 1250137. [Google Scholar] [CrossRef] [PubMed]
- Jansons, M.; Pugajeva, I.; Bartkevičs, V. Occurrence of Glyphosate in Beer from the Latvian Market. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Hyötyläinen, T. Critical Evaluation of Sample Pretreatment Techniques. Anal. Bioanal. Chem. 2009, 394, 743–758. [Google Scholar] [CrossRef]
- Chamkasem, N.; Harmon, T. Direct Determination of Glyphosate, Glufosinate, and AMPA in Soybean and Corn by Liquid Chromatography/Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2016, 408, 4995–5004. [Google Scholar] [CrossRef]
- Santilio, A.; Pompili, C.; Giambenedetti, A. Determination of Glyphosate Residue in Maize and Rice Using a Fast and Easy Method Involving Liquid Chromatography–Mass Spectrometry (LC/MS/MS). J. Environ. Sci. Health B 2019, 54, 205–210. [Google Scholar] [CrossRef]
- Polson, C.; Sarkar, P.; Incledon, B.; Raguvaran, V.; Grant, R.O. Ptimization of Protein Precipitation Based upon Effectiveness of Protein Removal and Ionization Effect in Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatograp. B 2003, 785, 263–275. [Google Scholar] [CrossRef]
- Dong, J.; Hu, Y.Q.; Su, X.L.; Yao, Y.X.; Zhou, Q.; Gao, M.Y. Low-Background Interference Detection of Glyphosate, Glufosinate, and AMPA in Foods Using UPLC-MS/MS without Derivatization. Anal. Bioanal. Chem. 2024, 416, 1561–1570. [Google Scholar] [CrossRef]
- Valle, A.L.; Mello, F.C.C.; Alves-Balvedi, R.P.; Rodrigues, L.P.; Goulart, L.R. Glyphosate Detection: Methods, Needs and Challenges. Environ. Chem. Lett. 2019, 17, 291–317. [Google Scholar] [CrossRef]
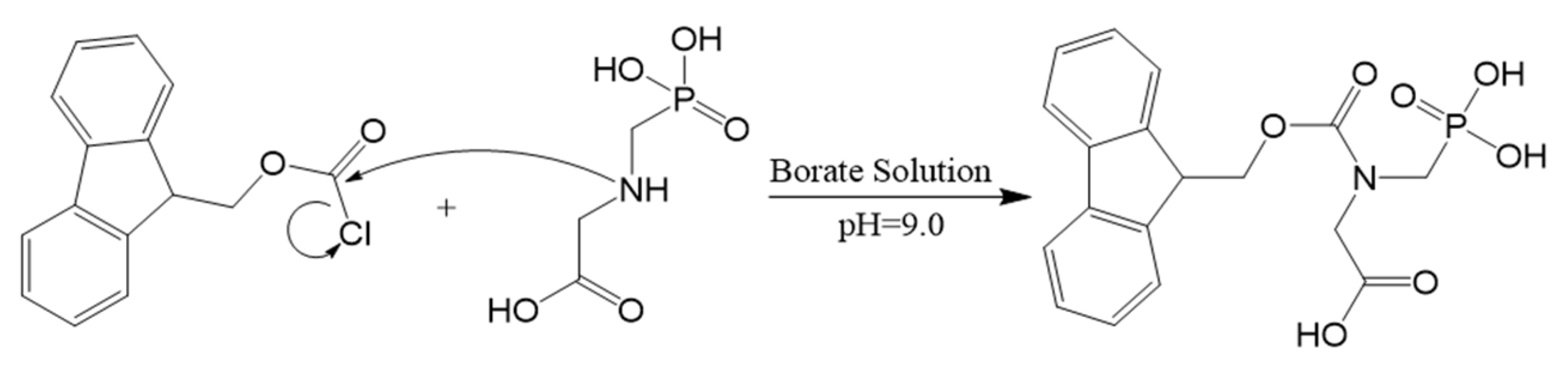
- Bressán, I.G.; Llesuy, S.F.; Rodriguez, C.; Ferloni, A.; Dawidowski, A.R.; Figar, S.B.; Giménez, M.I. Optimization and Validation of a Liquid Chromatography-Tandem Mass Spectrometry Method for the Determination of Glyphosate in Human Urine after Pre-Column Derivatization with 9-Fluorenylmethoxycarbonyl Chloride. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2021, 1171, 122616. [Google Scholar] [CrossRef]
- Campanale, C.; Triozzi, M.; Massarelli, C.; Uricchio, V.F. Development of a UHPLC-MS/MS Method to Enhance the Detection of Glyphosate, AMPA and Glufosinate at Sub-Microgram / L Levels in Water Samples. J. Chromatogr. A 2022, 1672, 463028. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.J.; He, K.; Blaney, L.; Hobbs, S.R. Advanced Liquid Chromatography with Tandem Mass Spectrometry Method for Quantifying Glyphosate, Glufosinate, and Aminomethylphosphonic Acid Using Pre-Column Derivatization. ACS ES T Water 2023, 3, 2407–2414. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.K.; Vetter, W.; Anastassiades, M. Improved Analysis of Glyphosate, Aminomethylphosphonic Acid, and Other Highly Polar Pesticides and Metabolites via the QuPPe Method by Employing Ethylenediaminetetraacetic Acid and IC-MS/MS. J. Agric. Food Chem. 2025, 73, 2645–2652. [Google Scholar] [CrossRef]
- Denžić Lugomer, M.; Bilandžić, N.; Pavliček, D.; Novosel, T. Direct Determination of Glyphosate and Its Metabolites in Foods of Animal Origin by Liquid Chromatography–Tandem Mass Spectrometry. Foods 2024, 13, 2451. [Google Scholar] [CrossRef]
- Ciasca, B.; Pecorelli, I.; Lepore, L.; Paoloni, A.; Catucci, L.; Pascale, M.; Lattanzio, V.M.T. Rapid and Reliable Detection of Glyphosate in Pome Fruits, Berries, Pulses and Cereals by Flow Injection–Mass Spectrometry. Food Chem. 2020, 310, 125813. [Google Scholar] [CrossRef]
- Chamkasem, N. Determination of Glyphosate, Maleic Hydrazide, Fosetyl Aluminum, and Ethephon in Grapes by Liquid Chromatography/Tandem Mass Spectrometry. J. Agric. Food Chem. 2017, 65, 7535–7541. [Google Scholar] [CrossRef]
- Hsu, C.C.; Whang, C.W. Microscale Solid Phase Extraction of Glyphosate and Aminomethylphosphonic Acid in Water and Guava Fruit Extract Using Alumina-Coated Iron Oxide Nanoparticles Followed by Capillary Electrophoresis and Electrochemiluminescence Detection. J. Chromatogr. A 2009, 1216, 8575–8580. [Google Scholar] [CrossRef]
- Chen, M.X.; Cao, Z.Y.; Jiang, Y.; Zhu, Z.W. Direct Determination of Glyphosate and Its Major Metabolite, Aminomethylphosphonic Acid, In Fruits and Vegetables by Mixed-Mode Hydrophilic Interaction/Weak Anion-Exchange Liquid Chromatography Coupled with Electrospray Tandem Mass Spectrometry. J. Chromatogr. A 2013, 1272, 90–99. [Google Scholar] [CrossRef]
- Varela-Martínez, D.A.; González-Sálamo, J.; González-Curbelo, M.Á.; Hernández-Borges, J. Quick, Easy, Cheap, Effective, Rugged, and Safe (QuECHERS) Extraction. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2019; pp. 399–437. [Google Scholar]
- Verdini, E.; Pecorelli, I. The current status of analytical methods applied to the determination of polar pesticides in food of animal origin: A brief review. Foods 2022, 11, 1527. [Google Scholar] [CrossRef]
- Chiesa, L.M.; Nobile, M.; Panseri, S.; Arioli, F. Detection of Glyphosate and Its Metabolites in Food of Animal Origin Based on Ion-Chromatography-High Resolution Mass Spectrometry (IC-HRMS). Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 592–600. [Google Scholar] [CrossRef]
- Granby, K.; Johannesen, S.; Vahl, M. Analysis of Glyphosate Residues in Cereals Using Liquid Chromatography-Mass Spectrometry (LC-MS/MS). Food Addit. Contam. 2003, 20, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Melton, L.M.; Taylor, M.J.; Flynn, E.E. The Utilisation of Ion Chromatography and Tandem Mass Spectrometry (IC-MS/MS) for the Multi-Residue Simultaneous Determination of Highly Polar Anionic Pesticides in Fruit and Vegetables. Food Chem. 2019, 298, 125028. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, M.; Jiménez-Medina, M.L.; Marín Saéz, J.; Moura, S.; Garrido Frenich, A.; Romero-González, R. Fast Analysis of Glufosinate, Glyphosate and Its Main Metabolite, Aminomethylphosphonic Acid, in Edible Oils, by Liquid Chromatographycoupled with Electrospray Tandem Mass Spectrometry. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.I.; El-Nouby, M.A.M.; Kimani, P.K.; Lim, L.W.; Rabea, E.I. A Review of the Modern Principles and Applications of Solid-Phase Extraction Techniques in Chromatographic Analysis. Anal. Sci. 2022, 38, 1457–1487. [Google Scholar] [CrossRef]
- González-Curbelo, M.Á.; Varela-Martínez, D.A.; Riaño-Herrera, D.A. Pesticide-residue analysis in soils by the QuEChERS method: A review. Molecules 2022, 27, 4323. [Google Scholar] [CrossRef]
- Pihlström, T.; Fernández-Alba, A.R.; Ferrer Amate, C.; Erecius Poulsen, M.; Lippold, R.; Carrasco Cabrera, L.; Pelosi, P.; Valverde, A.; Unterluggauer, H.; Mol, H.; et al. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Foood and Feed. SANTE/11312/021. Available online: https://www.eurl-pesticides.eu/docs/public/tmplt_article.asp?CntID=727 (accessed on 27 March 2025).
- Rocío-Bautista, P.; Moreno-González, D.; Martínez-Piernas, A.B.; García-Reyes, J.F.; Molina-Díaz, A. Novel Liquid Chromatography/Mass Spectrometry-Based Approaches for the Determination of Glyphosate and Related Compounds: A Review. Trends Environ. Anal. Chem. 2022, 36, e00186. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, M.; Jia, M.; She, Y.; Wang, J.; Zheng, L.; Abd El-Aty, A.M. Development of a Specific and Sensitive Method for the Detection of Glyphosate Pesticide and Its Metabolite in Tea Using Dummy Molecularly Imprinted Solid-Phase Extraction Coupled with Liquid Chromatography-Tandem Quadrupole Mass Spectrometry. J. Chromatogr. A 2023, 1705, 464209. [Google Scholar] [CrossRef]
- Méndez-Barredo, L.H.; Monribot-Villanueva, J.L.; Bojórquez-Velázquez, E.; Elizalde-Contreras, J.M.; Guerrero-Analco, J.A.; Ruiz-May, E. Comparative Evaluation of Different Extraction Methods for Identification and Quantification of Glyphosate in Fortified Corn Flour. J. Mex. Chem. Soc. 2023, 67, 213–226. [Google Scholar] [CrossRef]
- Herrera López, S.; Scholten, J.; Kiedrowska, B.; de Kok, A. Method Validation and Application of a Selective Multiresidue Analysis of Highly Polar Pesticides in Food Matrices Using Hydrophilic Interaction Liquid Chromatography and Mass Spectrometry. J. Chromatogr. A 2019, 1594, 93–104. [Google Scholar] [CrossRef]
- López, S.H.; Dias, J.; de Kok, A. Analysis of Highly Polar Pesticides and Their Main Metabolites in Animal Origin Matrices by Hydrophilic Interaction Liquid Chromatography and Mass Spectrometry. Food Control. 2020, 115, 107289. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Qu, Q.; Wang, G.; Wang, C. Determination of Glyphosate and Aminomethylphosphonic Acid in Soybean Samples by High Performance Liquid Chromatography Using a Novel Fluorescent Labeling Reagent. Anal. Methods 2013, 5, 6465–6472. [Google Scholar] [CrossRef]
- Ding, J.; Jin, G.; Jin, G.; Shen, A.; Guo, Z.; Yu, B.; Jiao, Y.; Yan, J.; Liang, X. Determination of Underivatized Glyphosate Residues in Plant-Derived Food with Low Matrix Effect by Solid Phase Extraction-Liquid Chromatography-Tandem Mass Spectrometry. Food Anal. Methods 2016, 9, 2856–2863. [Google Scholar] [CrossRef]
- Ehling, S.; Reddy, T.M. Analysis of Glyphosate and Aminomethylphosphonic Acid in Nutritional Ingredients and Milk by Derivatization with Fluorenylmethyloxycarbonyl Chloride and Liquid Chromatography-Mass Spectrometry. J. Agric. Food Chem. 2015, 63, 10562–10568. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, H.; Imre, S.; Duca, R.C.; Farczádi, L. Methods and Strategies for Biomonitoring in Occupational Exposure to Plant Protection Products Containing Glyphosate. Int. J. Environ. Res. Public. Health 2023, 20, 3314. [Google Scholar] [CrossRef]
- Rampazzo, G.; Gazzotti, T.; Pagliuca, G.; Nobile, M.; Chiesa, L.; Carpino, S.; Panseri, S. Determination of Glyphosate, Glufosinate, and Metabolites in Honey Based on Different Detection Approaches Supporting Food Safety and Official Controls. LWT 2024, 200, 116159. [Google Scholar] [CrossRef]
- Jalali, Z.; Dhandapani, R.; Jack, R.; Tackett, B. Analysis of Underivatized Anionic and Cationic Pesticides in Reversed Phase and HILIC Modes Using a Single Mixed-mode HPLC Column. Phenomenex. Available online: https://www.phenomenex.com/-/jssmedia/phxjss/data/media/documents/an52500323-w.pdf?rev=d1916e7e72e24144a148eda0a0cf2374&srsltid=AfmBOorEu1G8Tyu-g1OArJsmHHW3E19Ebx3TD9FlzziSNxY5t5YS3pmu (accessed on 10 January 2025).
- Botero-Coy, A.M.; Ibáñez, M.; Sancho, J.V.; Hernández, F. Direct Liquid Chromatography-Tandem Mass Spectrometry Determination of Underivatized Glyphosate in Rice, Maize and Soybean. J. Chromatogr. A 2013, 1313, 157–165. [Google Scholar] [CrossRef]
- Stavra, E.; Petrou, P.S.; Koukouvinos, G.; Economou, A.; Goustouridis, D.; Misiakos, K.; Raptis, I.; Kakabakos, S.E. Fast, Sensitive and Selective Determination of Herbicide Glyphosate in Water Samples with a White Light Reflectance Spectroscopy Immunosensor. Talanta 2020, 214, 120854. [Google Scholar] [CrossRef]
- Olivo, V.E.; Tansini, A.; Carasek, F.; Cordenuzzi, D.; Fernandes, S.; Fiori, M.A.; Fragoso, A.; Magro, J.D. Rapid Method for Determination of Glyphosate in Groundwater Using High Performance Liquid Chromatography and Solid-Phase Extraction after Derivatization. Rev. Ambiente Agua 2014, 9, 445–458. [Google Scholar]
- Zhang, Y.; Dang, Y.; Lin, X.; An, K.; Li, J.; Zhang, M. Determination of Glyphosate and Glufosinate in Corn Using Multi-Walled Carbon Nanotubes Followed by Ultra High Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry. J. Chromatogr. A 2020, 1619, 460939. [Google Scholar] [CrossRef]
- Szternfeld, P.; Malysheva, S.V.; Hanot, V.; Joly, L. A Robust Transferable Method for the Determination of Glyphosate Residue in Liver After Derivatization by Ultra-High Pressure Liquid Chromatography–Tandem Mass Spectrometry. Food Anal. Methods 2016, 9, 1173–1179. [Google Scholar] [CrossRef]
- Goscinny, S.; Unterluggauer, H.; Aldrian, J.; Hanot, V.; Masselter, S. Determination of Glyphosate and Its Metabolite AMPA (Aminomethylphosphonic Acid) in Cereals After Derivatization by Isotope Dilution and UPLC-MS/MS”. Food Anal. Methods 2012, 5, 1177–1185. [Google Scholar] [CrossRef]
- Selvi, A.A.; Sreenivasa, M.A.; Manonmani, H.K. Enzyme-Linked Immunoassay for the Detection of Glyphosate in Food Samples Using Avian Antibodies. Food Agric. Immunol. 2011, 22, 217–228. [Google Scholar] [CrossRef]
- Bettazzi, F.; Natale, A.R.; Torres, E.; Palchetti, I. Glyphosate Determination by Coupling an Immuno-Magnetic Assay with Electrochemical Sensors. Sensors 2018, 18, 2965. [Google Scholar] [CrossRef]
- Côco, A.S.; Campos, F.V.; Díaz, C.A.R.; Guimarães, M.C.C.; Prado, A.R.; de Oliveira, J.P. Localized Surface Plasmon Resonance-Based Nanosensor for Rapid Detection of Glyphosate in Food Samples. Biosensors 2023, 13, 512. [Google Scholar] [CrossRef]
- Torul, H.; Boyaci, I.H.; Tamer, U. Attomole Detection of Glyphosate by Surface-Enhanced Raman Spectroscopy Using Gold Nanorods. J. Pharm. Sci. 2010, 35, 179–184. [Google Scholar]
- Guo, J.; Zhang, Y.; Luo, Y.; Shen, F.; Sun, C. Efficient Fluorescence Resonance Energy Transfer between Oppositely Charged CdTe Quantum Dots and Gold Nanoparticles for Turn-on Fluorescence Detection of Glyphosate. Talanta 2014, 125, 385–392. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Zhang, Q.; Xu, G.; Dai, H.; Lin, Y. Binding-Induced Internal-Displacement of Signal-on Photoelectrochemical Response: A Glyphosate Detection Platform Based on Graphitic Carbon Nitride. Sens. Actuators B Chem. 2016, 224, 798–804. [Google Scholar] [CrossRef]
- Taverniers, I.; De Loose, M.; Van Bockstaele, E. Trends in Quality in the Analytical Laboratory. II. Analytical Method. Validation and Quality Assurance. TrAC-Trends Anal. Chem. 2004, 23, 535–552. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar Dubey, J.; Katna, S.; Shandil, D.; Singh Brar, G.; Singh, S. Validation of Analytical Methods Used for Pesticide Residue Detection in Fruits and Vegetables. Food Anal. Methods 2021, 14, 1019–1926. [Google Scholar] [CrossRef]
- Mashuni, M.; Ritonga, H.; Jahiding, M.; Rubak, B.; Hamid, F.H. Highly Sensitive Detection of Carbaryl Pesticides Using Potentiometric Biosensor with Nanocomposite Ag/r-Graphene Oxide/Chitosan Immobilized Acetylcholinesterase Enzyme. Chemosensors 2022, 10, 138. [Google Scholar] [CrossRef]
- CCAYAC-P-058. Control Analítico y Ampliación de Cobertura. 2011. Available online: https://www.gob.mx/cofepris/acciones-y-programas/comision-de-control-analitico-y-ampliacion-de-cobertura (accessed on 20 December 2024).
- Tiryaki, O. Validation of QuEChERS Method for the Determination of Some Pesticide Residues in Two Apple Varieties. J. Environ. Sci. Health B 2016, 51, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Agarski, M.; Bursić, V.; Vuković, G. Method validation for the determination of glyphosate and aminomethylphosphonic acid in water by LC-MS/MS. J. Agron. Technol. Eng. Manag. 2023, 6, 902–909. [Google Scholar] [CrossRef]
- Nomura, H.; Hamada, R.; Saito, I.; Nakane, K.; Sawa, R.; Ukai, M.; Shibata, E.; Sato, M.; Kamijima, M.; Ueyama, J. Optimization and validation of a highly sensitive method for determining glyphosate in human urine by solid-phase extraction and liquid chromatography with tandem mass spectrometry: A methodological study. Environ. Health Prev. Med. 2020, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.K.; Wujcik, C.E.; McGuire, M.K.; McGuire, M.A. Validation of reliable and selective methods for direct determination of glyphosate and aminomethylphosphonic acid in milk and urine using LC-MS/MS. J. Environ. Sci. Health Part B 2016, 51, 254–259. [Google Scholar] [CrossRef]
- Leyva-Morales, J.B.; Cabrera, R.; Bastidas-Bastidas, P.D.J.; Valenzuela-Quintanar, A.I.; Pérez-Camarillo, J.P.; González-Mendoza, V.M.; Perea-Domínguez, X.P.; Márquez-Pacheco, H.; Amiliano-Cisneros, J.M.; Badilla-Medina, C.N.; et al. Validation and application of liquid chromatography coupled with tandem mass spectrometry method for the analysis of glyphosate, aminomethylphosphonic acid (AMPA), and glufosinate in soil. Agriculture 2023, 13, 1131. [Google Scholar] [CrossRef]
- Li, Z.M.; Kannan, K. Analysis of Glyphosate, Aminomethylphosphonic Acid (AMPA), and Glufosinate in Human Urine Using Liquid Chromatography-Tandem Mass. Int. J. Environ. Res. Public Health 2022, 19, 1–14. [Google Scholar] [CrossRef]
- Kenny, L.; Sams, C.; Jones, K.; Polledri, E.; Mercadante, R.; Fustinoni, S.; Göen, T.; Hartwig, A.; Commission, M.A.K. Glyphosate–Determination of glyphosate and AMPA in urine by LC-MS/MS. MAK Collect. Occup. Health Saf. 2025, 10, 1. [Google Scholar]
- Masiá, A.; Suarez-Varela, M.M.; Llopis-Gonzalez, A.; Picó, Y. Determination of pesticides and veterinary drug residues in food by liquid chromatography-mass spectrometry: A review. Anal. Chim. Acta 2016, 936, 40–61. [Google Scholar] [CrossRef]
- Feldsine, P.; Abeyta, C.; Andrews, W.H. AOAC International Methods Committee Guidelines for Validation of Qualitative and Quantitative Food Microbiological Official Methods of Analysis. J. AOAC Int. 2002, 85, 1187–1200. [Google Scholar] [CrossRef]
- International Council of Harmonisation. Validation of Analytical Procedures Q2 (R2); European Pharmaceutical Review: Geneva, Switzerland, 2022; Available online: https://database.ich.org/sites/default/files/ICH_Q2-R2_Document_Step2_Guideline_2022_0324.pdf (accessed on 11 January 2025).
- ISO 17025 (2005); General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization: Vernier, Switzerland, 2005.
| Parameter | Property Value | |
|---|---|---|
| Glyphosate | Aminomethylphosphonic Acid | |
| Chemical structure | 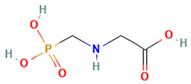 | 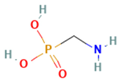 |
| Family | Organophosphorus compounds | Organophosphorus compounds |
| Function | Herbicide | Metabolite from glyphosate |
| IUPAC name | N-phosphonomethyl-glycine | Aminomethylphosphonic acid |
| CAS number | 1071-83-6 | 1066-51-9 |
| Molecular formula | C3H8NO5P | CH6NO3P |
| Molecular weight | 169.07 g/mol | 111.04 g/mol |
| Solubility | >353 g L−1 at 20 °CNot soluble in acetone, ethanol or xylene | 272.3 to 710.7 g L−1 at 20 °CNot soluble in acetone, ethanol or xylene |
| Melting point (°C) | 189 | 220 |
| Boiling point (°C) | Not defined | Not defined |
| Temperature of decomposition (°C) | 215 | 290 |
| Dissociation constant | pKa1 = 2.0; pKa2 = 2.6; pKa3 = 5.6; pKa4 = 10.6 | pKa1 = 1.6; pKa2 = 5.8 |
| pH | 2.5 | 3.0 |
| Density | 1.7 g/cm3 | 1.51 g/cm3 |
| Octanol–water coeff. (Kow) | −4.6 to −1.6 | −2.6 to −2.14 |
| Vapor pressure | 1.94 × 10−7 mmHg at 45 °C | 2.1 × 10−6 mmHg at 45 °C |
| Freundlich adsorption coefficient (Kads) (L Kg−1) | 0.6 to 303 | 5 to 500 |
| Degradation half-life in soil (T1/2) (days) | 7–60 | 26–44 |
| Sample | Glyphosate (µg/L) | AMPA (µg/L) | Ref. |
|---|---|---|---|
| Freshwater | 17.5–125 | 17.5–125 | [46] |
| Drinking water | <0.03–0.225 | <0.03–0.184 | [47] |
| Tap water | 170–2900 | 10–80 | [48] |
| Seawater | 0.00012–0.0022 | NR | [49] |
| Soil | 50–825 | 238–1182 | [50] |
| Animal feed | * 7.8−2140 | ND | [45] |
| Human milk | 0.11–3.32 | NR | [51] |
| Human urine | 0.2–5 | 0.2–5 | [52] |
| Human saliva | 0.00038–0.0028 | 6 × 10−5–0.00031 | [53] |
| Human blood cells | 600–7,480,000 | 100–60,000 | [54] |
| Human urine | 1600–400,000 | 500–1,000,000 |
| Country | Food Product | Number of Samples | Detection Frequency (%) GLYP/AMPA | Glyphosate Range (µg/kg) | AMPA Range (µg/kg) | Ref. |
|---|---|---|---|---|---|---|
| Canada | Honey | 200 | 98.5 | 1–49.8 | NR | [59] |
| Switzerland | Honey | 16 | 93.8 | ˂1–15.9 | NR | [60] |
| Estonia | Honey | 33 | 12.1 | 9–62 | NR | [61] |
| Estonia | Honey | 140 | 18 | 70 | NR | [62] |
| USA | Honey | 85 | 28.2 | 15–342 | NR | [63] |
| Brazil | Honey | 40 | 100 | 40 | 40 | [64] |
| Mexico | Pollen | 120 | 100 | 3.71–7.29 | NR | [65] |
| Switzerland | Fruit juice | 11 | 100/18 | 1.6–1.9 | 0.2–0.6 | [60] |
| Canada | Juice concentrates | 42 | NI | 4.2–38 | NR | [6] |
| USA | Frozen vegetables, fruit juice, baby fruit puree | 83 | 15–18 | 3–10 | NR | [66] |
| Spain | Vegetables | 221 | NI | NI | NR | [67] |
| Switzerland | Breakfast cereal | 10 | 80/30 | ˂1–291 | 2.5–10 | [60] |
| Switzerland | Wheat snacks | 11 | 36.4 | ˂1–421 | NR | [60] |
| Switzerland | Bread | 10 | 70 | ˂1–45.8 | NR | [60] |
| Switzerland | Wheat flower | 28 | 28.6 | ˂1–133 | NR | [60] |
| Switzerland | Pseudo cereals | 3 | ND | ˂1 | NR | [60] |
| Italy | Wheat seed | 1 | 100 | 243,000 | NR | [68] |
| France | Breakfast cereal | 2 | 100 | 6–34 | NR | [69] |
| Lebanon | Bread and wheat flour | 164 | 80 and 100 | 14–52 | NR | [70] |
| Australia | Wheat flour | 26 | 100 | ˂10 | NR | [71] |
| USA | Oat products | 310 | 100 | 0.04–1.1 | NR | [72] |
| South Africa | Soy milk | 8 | 100 | 32–142 | NR | [73] |
| South Africa | Texturized soy protein | 7 | 100 | 195–2257 | NR | [73] |
| Canada | Pasta | 221 | 5–1400 | NR | [59] | |
| China | Fresh maize and soybean | 234 | 11–18 | 40–290 | NR | [74] |
| South Africa | Maize pasta | 3 | 100 | 47–62 | NR | [73] |
| South Africa | Maize rice | 3 | 100 | 28–65 | NR | [73] |
| Switzerland | Potato and vegetables | 10 | 30/0 | ˂1–7.7 | NR | [60] |
| Europe and UK | Grains, rice, flour, bread, cereal based for infants | 2136 | 19.9 | 10–267 | NR | [75] |
| Switzerland | Pulses | 41 | 51.2/24 | ˂1–2948 | 3.1–25 | [60] |
| Switzerland | Meat and Fish | 13 | 23.1 | ˂1–4.9 | NR | [60] |
| Ghana | Yam | 68 | 20.5 | ˂120 | NR | [76] |
| Several European counties | Processed foods | 110,829 | 3.7 | Above MRL values | NR | [77] |
| Brazil | Soy-based infant formula | 105 | NI | 30–1080 | 20–170 | [78] |
| Nigeria | Fish | 75 | 100 | Below ADI and MRL values | NR | [79] |
| Food Sample | GLYP/AMPA | Glyphosate Recovery (%) | AMPA Recovery (%) | Extraction Method | Sorbent | Retention Mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| Apple | GLYP/AMPA | 103.1–115.6 | 84.2–105.6 | SPE | PRiME HLB | Reversed phase | [85] |
| Cucumber | 97.4–112.5 | 90.5–101.4 | |||||
| Potato | 99.3–102.7 | 95.9–103.4 | |||||
| Celery | 90.2–110.5 | 89.1–99.0 | |||||
| Grape | 91.3–107.5 | 98.1–114.4 | |||||
| Soybean | 106.1–111.4 | 91.4–94.0 | |||||
| Tea | 97.0–102.3 | 92.0–108.0 | |||||
| Kiwi | 87.3–105.6 | 96.0–97.5 | |||||
| Pumpkin | 87.0–101.3 | 99.7–104.5 | |||||
| Orange | 104.4–109.2 | 88.6–100.6 | |||||
| Lettuce | 88.6–104.7 | 91.7–103.1 | |||||
| Rice | 96.0–97.2 | 91.5–103.5 | |||||
| Wheat | 101.5–105.5 | 91.1–98.9 | |||||
| Soybean Corn | GLYP/AMPA | 96–98 | 96–113 | SPE | Oasis HLB | Reversed phase | [82] |
| Oat | GLYP/AMPA | 102 | NR | SPE | Oasis HLB | Reversed phase | [72] |
| Beer | GLYP | 87.0–123.0 | SPE | Strata-XA | Anionic exchange | [80] | |
| Egg | GLYP/AMPA | 94.12–139.3 | 96.83–106.8 | SPE | Plexa PCX | Cationic exchange | [91] |
| Sesame | GLYP/AMPA | 87 | 87 | dSPE | C18 | Reversed phase | [90] |
| Lentils | 57 | 60 | |||||
| Wheat | 60 | 74 | |||||
| Cocoa Bean | 50 | 47 | |||||
| Infant Food | 71 | 73 | |||||
| Milk | 93 | 93 |
| Food Sample | GLYP/AMPA | Analytical Methodology | LOD | LOQ | Recovery (%) | RSD% | R2 | Ref. |
|---|---|---|---|---|---|---|---|---|
| Tea leaves | GLYP/AMPA | LC-MS/MS | 0.0028 and 0.046 µg/mL | 0.0093 and 0.046 μg/mL | 98.69–106.26/79.95–83.84 | 0.91–1.18/6.4–7.45 | 0.999/0.991 | [106] |
| Commercial breakfast cereals | GLYP/AMPA | LC-MS/MS | 1–5 ng/g | 5 and 40 g/g | 92–111 | ˂8 | 0.9989/0.9987 | [72] |
| Baby formula, bovine liver and kidney | GLYP/AMPA | LC-ESI- MS/MS | NI | 10–25 µg/Kg | 104 | 5–25/11–38 | [28] | |
| Commercial corn flour | GLYP | UPLC-MS/MS | 0.1 and 0.2 µM | 0.2 µM 1.0 µM | 58.48–109 | NI | 0.9976/0.9980 | [107] |
| Beer | GLYP/AMPA | CE-TOF-MS | <5 μg/L/3.3 and 30.6 μg/L | NI | 94.3–110.7/80.2–100.4. | ~8.1%. | [30] | |
| Grapes, orange, lettuce, oat and soya beans. | GLYP/AMPA | LC-ESI-QTRAP-MS | NI | 0.02–0.05 mg/kg | 83–118/93–120 | 5–30/ 3–19 | [108] | |
| Pome fruits, berries, pulses and cereals | GLYP | FI-MS/MS and LC-MS/MS | NI | 0.5–2 mg/kg | 78–111 | ˂20% | [92] | |
| Cow milk, liver (bovine), kidney (bovine) and meat/egg chicken. | GLYP/AMPA | LC-ESI-TQ-MS | NI | 0.01–0.02 mg/kg | 70–120 | ≤20% | [109] | |
| Maize and rice | GLYP | LC-MS/MS | 0.002 and 0.004 mg/kg | 0.01 mg/kg | 70–105 | <20% | 0.9982 | [83] |
| Edible oils | GLYP/AMPA | LC-MS/MS | NI | 10 μg/kg 5 μg/kg | 81.4–119.4 | <20% | 0.996 | [101] |
| Honey, fish (bass) and bovine muscle | GLYP/AMPA | IC-HRMS | NI | 4.30–9.26 ng/g | 75–100/75–96 | 7–13/2–12 | [98] | |
| Soybean | GLYP/AMPA | HPLC | 0.002 mg/kg 0.001 mg/kg | NI | 85.4–94.1/87.3–95.2 | 3.1–4.7/3–4.4 | 0.999 | [110] |
| Food Sample | GLYP/AMPA | Analytical Methodology | Column | Derivatization | Results | Ref. |
|---|---|---|---|---|---|---|
| Soy-based products | GLYP/AMPA | LC-MS | ACQUITY UPLC BEH C18 1.8 μm, 2.1 × 100 mm, column | FMOC | LOQ was 50 and 5 µg/kg | [112] |
| Corn | GLYP | UHPLC-MS/MS | HSS T3 (1.8 μm, 2.1 mm × 100 mm) from Waters | FMOC-Cl | LOQ was 0.005 mg/kg | [119] |
| Baby formula, bovine liver and kidney | GLYP/AMPA | LC-ESI-MS/MS | Luna column (150 × 2 mm) with bonded 3 μm C18 | FMOC-Cl | LOQ: 10–25 μg/kg for both | [80] |
| Liver of animal origin | GLYP | UPLC-MS/MS | ACQUITYTM UPLC BEH C18 (1.7 μm, 2.1 × 100 mm) | FMOC-Cl | LOQ and LOD: 0.025 mg/kg | [120] |
| Oat and rye wheat | GLYP/AMPA | LC-MS/MS | ACQUITY™ BEH C18 column (1.7 μm; 2.1 × 100 mm) | FMOC-Cl | LOQ: 10 ng/mL | [121] |
| Honey | GLYP/AMPA | HPLC-FLD | Aminex-A9 potassium exchange column (100 mm × 4.6 mm, 5 μm) | OPA-MERC | LOQ: 10–25 μg/g for both | [64] |
| Soybean | GLYP/AMPA | HPLC | ODS column (150, 4.6 mm I.D., 5 mm) | DPCS-Cl | LOQ: 0.002 mg/kg for GLYP and 0.001 mg/kg for AMPA | [110] |
| Analytical Method | Matrix Effect Handling | Uncertainty Estimation | Guideline Referenced | Validation Summary | Ref. |
|---|---|---|---|---|---|
| LC-MS/MS | Controlled with standards | Estimated measurement uncertainty provided | SANTE/11312/2021 | Fully validated for GLYP and AMPA in water; high accuracy and reproducibility. | [133] |
| LC-MS/MS | Matrix effects assessed; internal standards used | Not specified | Not specified | Fast and reliable for GLYP and AMPA; suitable for biomonitoring. | [134] |
| LC-MS/MS | Matrix effects evaluated; internal standards used | Not specified | Not specified | Acceptable accuracy and precision good for GLYP/AMPA. | [135] |
| LC-MS/MS | Matrix effects evaluated; internal standards used | Not specified | Not specified | Validated for GLYP/AMPA in soil; applicable to field samples. | [136] |
| LC-MS/MS | Matrix effects evaluated; internal standards used | Not specified | Not specified | Robust method for large-scale GLYP/AMPA. | [137] |
| LC-MS/MS | Matrix effects evaluated; internal standards used | Not specified | Not specified | Suitable for GLYP/AMPA exposure assessment in workers. | [138] |
| LC-HRMS | Controlled with standards | Not elaborated | Not specified | Sensitive for GLYP in water; limited validation data. | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Cruz, A.D.; Anaya-Esparza, L.M.; Valenzuela-Chavira, I.; Martínez-Esquivias, F.; Ruvalcaba-Gómez, J.M.; Silva-Jara, J.M.; Velázquez-Carriles, C.A.; Balderas-León, I.; Arteaga-Garibay, R.I.; Villagrán, Z. Extraction, Detection, and Quantification Methods for Analyzing Glyphosate and AMPA in Foods: Challenges and Opportunities. Appl. Sci. 2025, 15, 6979. https://doi.org/10.3390/app15136979
González-Cruz AD, Anaya-Esparza LM, Valenzuela-Chavira I, Martínez-Esquivias F, Ruvalcaba-Gómez JM, Silva-Jara JM, Velázquez-Carriles CA, Balderas-León I, Arteaga-Garibay RI, Villagrán Z. Extraction, Detection, and Quantification Methods for Analyzing Glyphosate and AMPA in Foods: Challenges and Opportunities. Applied Sciences. 2025; 15(13):6979. https://doi.org/10.3390/app15136979
Chicago/Turabian StyleGonzález-Cruz, Andony David, Luis Miguel Anaya-Esparza, Ignacio Valenzuela-Chavira, Fernando Martínez-Esquivias, José Martín Ruvalcaba-Gómez, Jorge Manuel Silva-Jara, Carlos Arnulfo Velázquez-Carriles, Iván Balderas-León, Ramón I. Arteaga-Garibay, and Zuamí Villagrán. 2025. "Extraction, Detection, and Quantification Methods for Analyzing Glyphosate and AMPA in Foods: Challenges and Opportunities" Applied Sciences 15, no. 13: 6979. https://doi.org/10.3390/app15136979
APA StyleGonzález-Cruz, A. D., Anaya-Esparza, L. M., Valenzuela-Chavira, I., Martínez-Esquivias, F., Ruvalcaba-Gómez, J. M., Silva-Jara, J. M., Velázquez-Carriles, C. A., Balderas-León, I., Arteaga-Garibay, R. I., & Villagrán, Z. (2025). Extraction, Detection, and Quantification Methods for Analyzing Glyphosate and AMPA in Foods: Challenges and Opportunities. Applied Sciences, 15(13), 6979. https://doi.org/10.3390/app15136979








