1. Introduction
Tablets represent the most widespread and preferred oral dosage form due to their ease of administration, precise dosing, long-term stability, and cost-effectiveness [
1,
2,
3,
4]. Despite their convenience, the manufacture of high-quality tablets requires meeting rigorous pharmaceutical standards, including uniform active pharmaceutical ingredient (API) content, consistent dissolution behavior, appropriate mechanical strength, biocompatibility of excipients, and stability over the product’s shelf life [
5,
6,
7].
Among the most common technological approaches in tablet production are direct [
8,
9] compression and wet granulation followed by compression [
10,
11]. Direct compression simplifies the manufacturing process by eliminating the need for a granulation step. This reduction in processing stages translates to lower production costs, shorter process times, and the avoidance of water or organic solvents—making it an attractive strategy, particularly for heat- or moisture-sensitive APIs. However, this method imposes strict requirements on the physicochemical properties of the formulation blend, including adequate flowability and compressibility [
12].
In contrast, wet granulation is a more robust technique for improving powder flow, density, and content uniformity [
13]. It involves agglomerating fine powders with a binder solution to form granules, which are then dried and compressed. Although effective, wet granulation is resource-intensive and involves multiple unit operations that increase energy consumption and processing time [
14,
15].
In this study, sodium naproxen was chosen as the model API due to its known poor flow and compactibility characteristics [
16,
17]. It presents substantial formulation challenges in direct compression. These properties are representative of many widely used APIs with problematic physical characteristics, such as, ibuprofen [
18]. By contrast, actives such as lactose-based model drugs are known for their better processing behavior and can be formulated more easily using direct compression [
19]. Therefore, sodium naproxen serves as an appropriate benchmark for evaluating the potential of alternative processing strategies, such as vibratory ball mill mixing.
The formulation also included dolomite, a naturally occurring mineral composed of calcium and magnesium carbonates, used here as a low-cost and environmentally friendly filler. Owing to its abundance and non-toxic profile, dolomite is gaining attention as a sustainable excipient in solid dosage form development. Studies have demonstrated its potential as a filler in polymer composites, where it can enhance mechanical properties and thermal stability. For instance, research by Syed Bakar et al. highlighted the use of dolomite as a filler in recycled polypropylene matrices, noting improvements in tensile strength and modulus at certain loading levels [
20].
However, while dolomite offers many advantages [
21], it can present processing difficulties during compression, such as segregation and heterogeneity in die filling, especially when combined with other cohesive or electrostatically charged materials. These challenges often necessitate granulation prior to tableting to ensure homogeneity and tablet quality. Granulation processes, particularly wet granulation, are extensively used in the pharmaceutical industry to improve powder flowability and prevent segregation of constituents due to differences in particle size or density [
22]. Additionally, studies have shown that granulation narrows the particle size distribution of a tablet formulation’s bulk powder, eliminating segregation problems and ensuring superior compressibility in the tableting process [
23].
The formulation was complemented with polyvinylpyrrolidone (PVP) as a binder to facilitate granule formation, microcrystalline cellulose as a compressible filler, and magnesium stearate as a lubricant to reduce die wall friction during compression.
To address the limitations associated with conventional processing of such formulations, this study explores the use of a vibrating ball mill as an alternative to traditional granulation [
24,
25]. This approach offers several notable advantages, including a simplified manufacturing process with fewer unit operations, the elimination of solvent use, and improved cost-effectiveness. Furthermore, unlike many marketed naproxen sodium tablet formulations—which typically require the addition of glidants such as colloidal silicon dioxide (e.g., AEROSIL) [
26] to compensate for the poor flow properties of the active pharmaceutical ingredient—the proposed formulation achieves satisfactory flowability without the need for such additives. Although not a granulation technique per se, vibratory milling enables intense mechanical mixing and particle rearrangement, which may improve blend homogeneity and performance in direct compression [
27]. Its potential to simplify the manufacturing process while maintaining or even enhancing tablet quality is of particular industrial interest.
The objective of this study was to compare the performance of tablets produced by direct compression using a vibrating ball mill with those manufactured via pan granulation followed by compression. Special attention is given to the influence of raw material behavior, intermediate granule properties, and processing parameters on the final tablet characteristics, including drug content uniformity, mechanical strength, and overall manufacturability. The findings may support the development of more economical and sustainable production methods, especially for formulations involving naturally derived excipients such as dolomite.
2. Results
The physical properties of the raw materials used in this study were thoroughly evaluated, with results presented in
Table 1. Sodium naproxen and dolomite (calcium carbonate) exhibited the most problematic flow characteristics, reflected in their high angles of repose (47.2° and 46.1°, respectively) and large Hausner ratios (1.81 and 1.58). These values significantly exceed the acceptable thresholds for good powder flow and compressibility. In contrast, excipients such as polyvinylpyrrolidone (PVP) and cellulose demonstrated much lower compressibility indices (17.4% and 17.8%) and Hausner ratios close to 1.2, indicating favorable flow behavior.
These initial properties influenced the subsequent choice of formulation strategy. Poor-flowing materials typically require granulation to ensure uniform die filling and consistent compression.
Table 1 serves as a strong justification for exploring both direct compression and granulation routes in parallel, particularly considering the use of materials such as sodium naproxen and dolomite.
Table 2 summarizes the experimental design, detailing the processing parameters for each batch. The reference batch (REF) was produced using a conventional V-type blender, which is widely used in the pharmaceutical industry. However, tablets from this batch showed the weakest performance across multiple quality criteria, including mass uniformity and mechanical strength. This highlights a key limitation of standard blending for challenging powders.
In contrast, direct compression batches prepared using the vibratory ball mill (DT1–DT3) exhibited markedly improved outcomes. Increased mixing time resulted in better homogeneity, with DT4 (10 min) yielding tablets comparable to those obtained via indirect tableting. This result suggests that vibratory blending not only compensates for poor flow properties but also improves content uniformity without the need for granulation. The implications for industrial production are significant, as the vibratory approach reduces the number of process steps and eliminates solvent use.
Indirect tableting (IT1–IT3), involving pan granulation at varying pan inclination angles (10°, 30°, and 50°), demonstrated how intermediate granule properties affect final tablet quality. At a 30° inclination (IT2), granules exhibited optimal flow and compaction behavior, leading to tablets with the best balance of mechanical and uniformity parameters. The granules produced at 10° (IT3) were insufficiently developed, whereas excessive tilt at 50° (IT1) led to particle segregation and less consistent compaction.
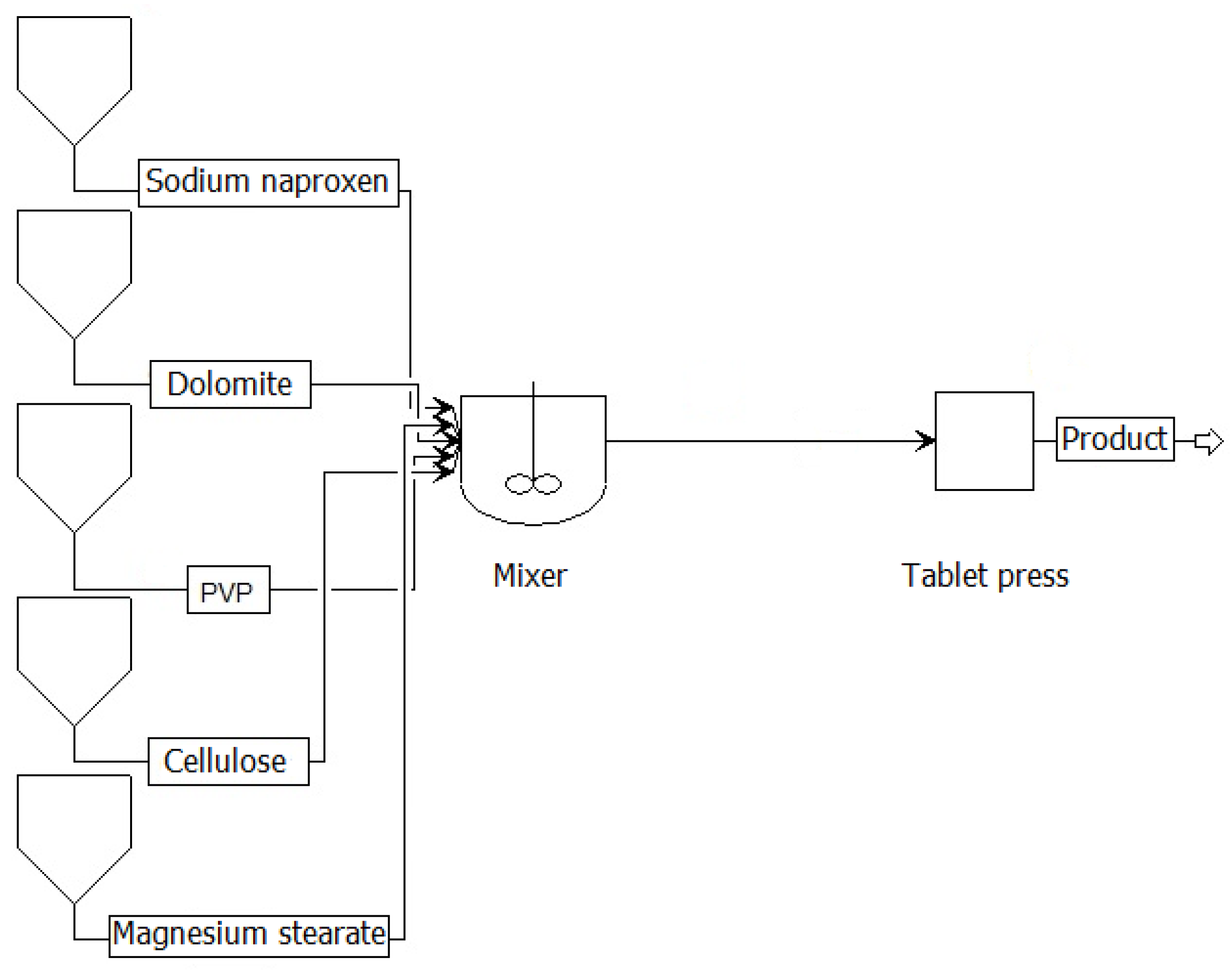
Figure 1 illustrates the process of direct tableting, in which all components of the formulation—sodium naproxen (API), dolomite and cellulose (fillers), polyvinylpyrrolidone (binder), and magnesium stearate (lubricant)—are dry-blended in a mixer. The homogeneous powder mixture is then fed directly into a tablet press for compression. This method eliminates the need for a granulation step, reducing the number of operations and processing time.
Figure 2 shows the process of indirect tableting, which incorporates a wet granulation stage. After initial mixing of the raw materials, a binder solution is added—typically water in combination with air atomization—to form granules in a granulator. The granulated material is then dried, sieved if needed, and fed into the tablet press. This approach improves powder flow and compressibility, especially beneficial when processing formulations containing cohesive or poorly compactable ingredients.
The particle size distribution of the individual raw materials used in the formulation is shown in
Figure 3. The curves reveal distinct differences in particle size profiles among the components. Magnesium stearate exhibits the narrowest distribution and the finest particles, with a dominant fraction below 50 µm. Sodium naproxen and cellulose display broader distributions, peaking around 100–200 µm, while dolomite is characterized by a wider spread, with a significant fraction of particles above 200 µm, reflecting its more heterogeneous structure. These differences in particle size and distribution are expected to influence both powder flowability and blend homogeneity during processing. The particle size distribution of polyvinylpyrrolidone (PVP) was not experimentally determined in this study due to its high solubility in a wide range of liquids, including those commonly used as dispersants in wet measurement systems. Laser diffraction analysis was conducted using a wet dispersion unit, which precluded accurate assessment of PVP particle size. According to the manufacturer’s specifications, the average particle size of the PVP used in the formulation is approximately 20 µm.
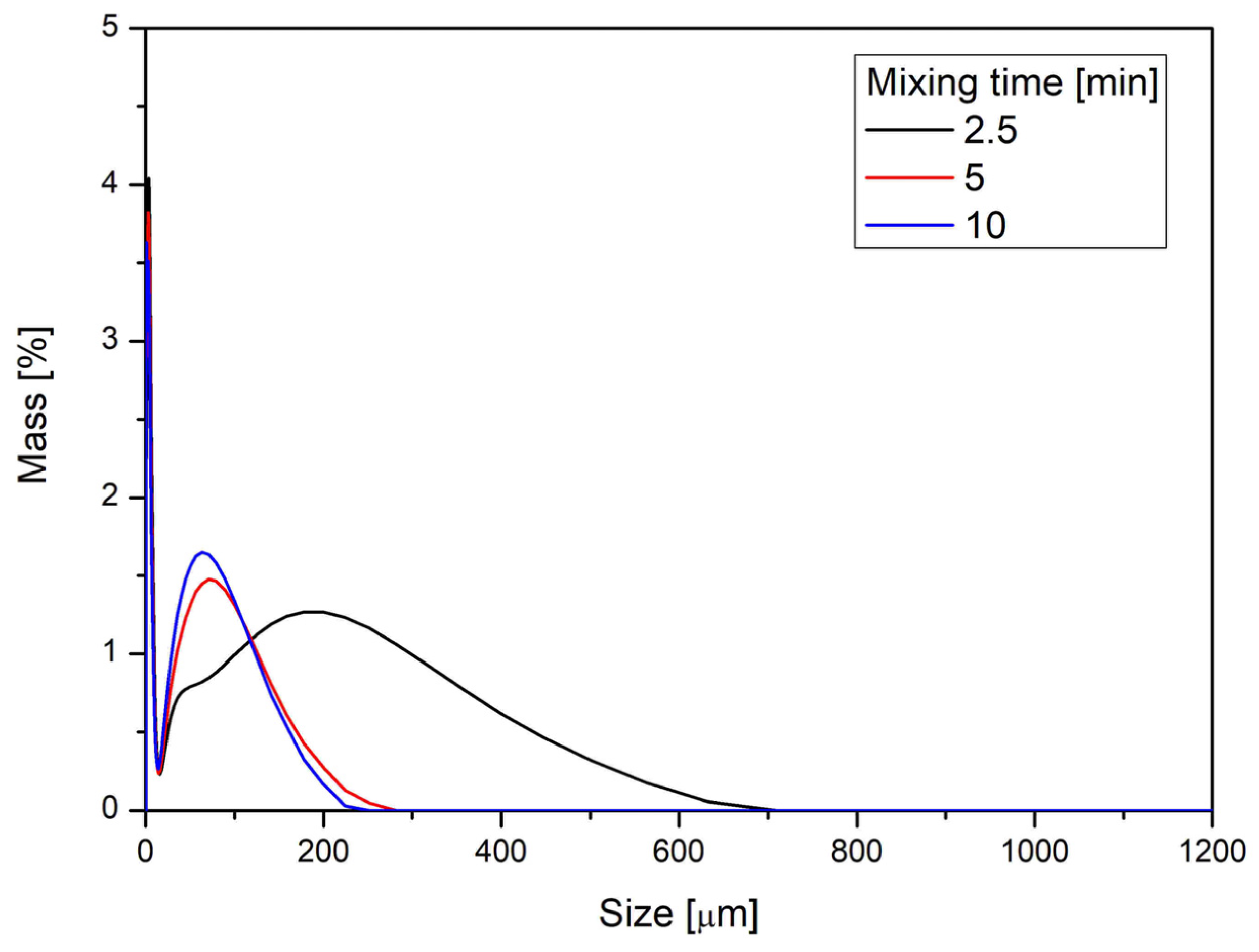
Figure 4 shows the particle size distribution of powder blends prepared by vibratory ball mill mixing at three different mixing times: 2.5, 5, and 10 min. As the mixing time increases, a noticeable shift of the distribution towards larger particle sizes is observed, along with peak broadening. This suggests a tendency for particle agglomeration or mechanical fusion during prolonged vibration. The most intensive mixing (10 min) produces a broader distribution with a reduced proportion of fine particles, indicating more cohesive interactions and possible granule formation effects. These trends can significantly affect downstream processability, particularly in direct compression.
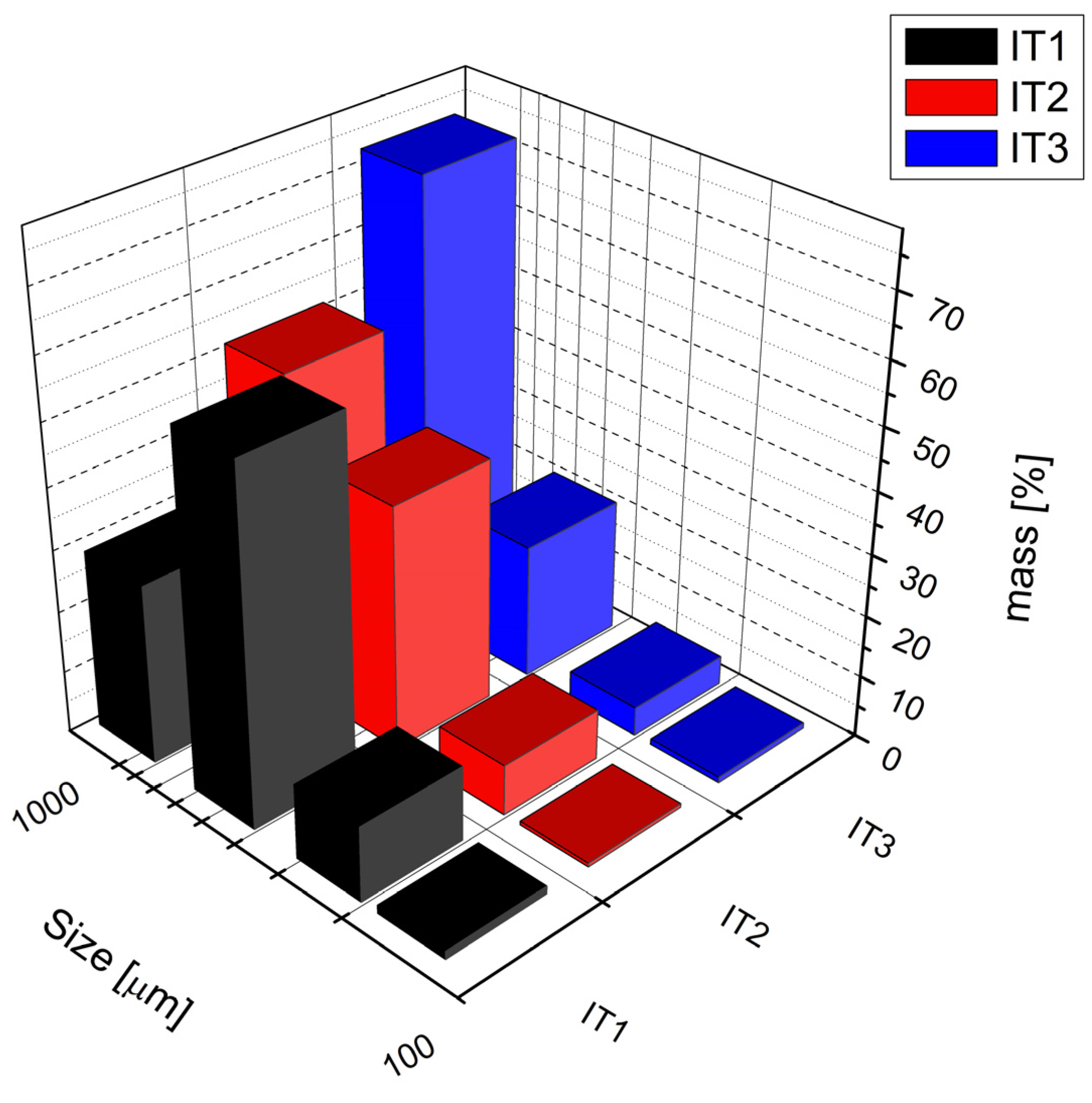
Among the granulated batches, granule size was found to correlate with the pan inclination angle used during the granulation process. Batch IT3, prepared at the highest angle of inclination (50°), produced the largest granules, followed by IT2 at 30°, and IT1 at the lowest angle (10°), which resulted in the smallest particle size (
Figure 5). The corresponding initial moisture content of the wet granules was 18.5% for IT1, 16.8% for IT2, and 14.8% for IT3. Each batch was dried to achieve a residual moisture content below 1%, a level shown to provide optimal conditions for subsequent tablet compression.
Flowability and packing parameters were assessed for the final compression blends of the REF and DT1–DT3 batches (
Table 3). All samples demonstrated cohesive flow behavior, with angles of repose above 44° and compressibility indices greater than 30%, indicating limited flow. However, the vibratory ball mill-treated blends showed slightly higher bulk and tapped densities than the reference, likely due to better packing efficiency achieved by redistribution of fine and coarse particles during mixing. This is consistent with observations for powders behaving according to Geldart Groups A and B.
Measurements were not conducted for the IT1–IT3 samples, as these were in the form of spherical granules produced by wet granulation. Due to their shape, granules are known to exhibit inherently good flowability, which is one of the primary purposes of the granulation process. This was indirectly confirmed by the low standard deviations observed in the physical characteristics of the resulting tablets.
The results obtained for the finished tablets are presented in
Figure 6,
Figure 7,
Figure 8,
Figure 9,
Figure 10,
Figure 11,
Figure 12 and
Figure 13. Given that batch-to-batch consistency is a critical parameter in industrial pharmaceutical processes, the graphs show not only the average values for each measured parameter, but also provide separate figures dedicated to standard deviations. These additional plots offer valuable insight into the reproducibility and reliability of each processing method. The magnitude of the standard deviations reflects the stability and uniformity of the formulation process across replicates within the same batch, allowing for a more comprehensive assessment of process robustness.
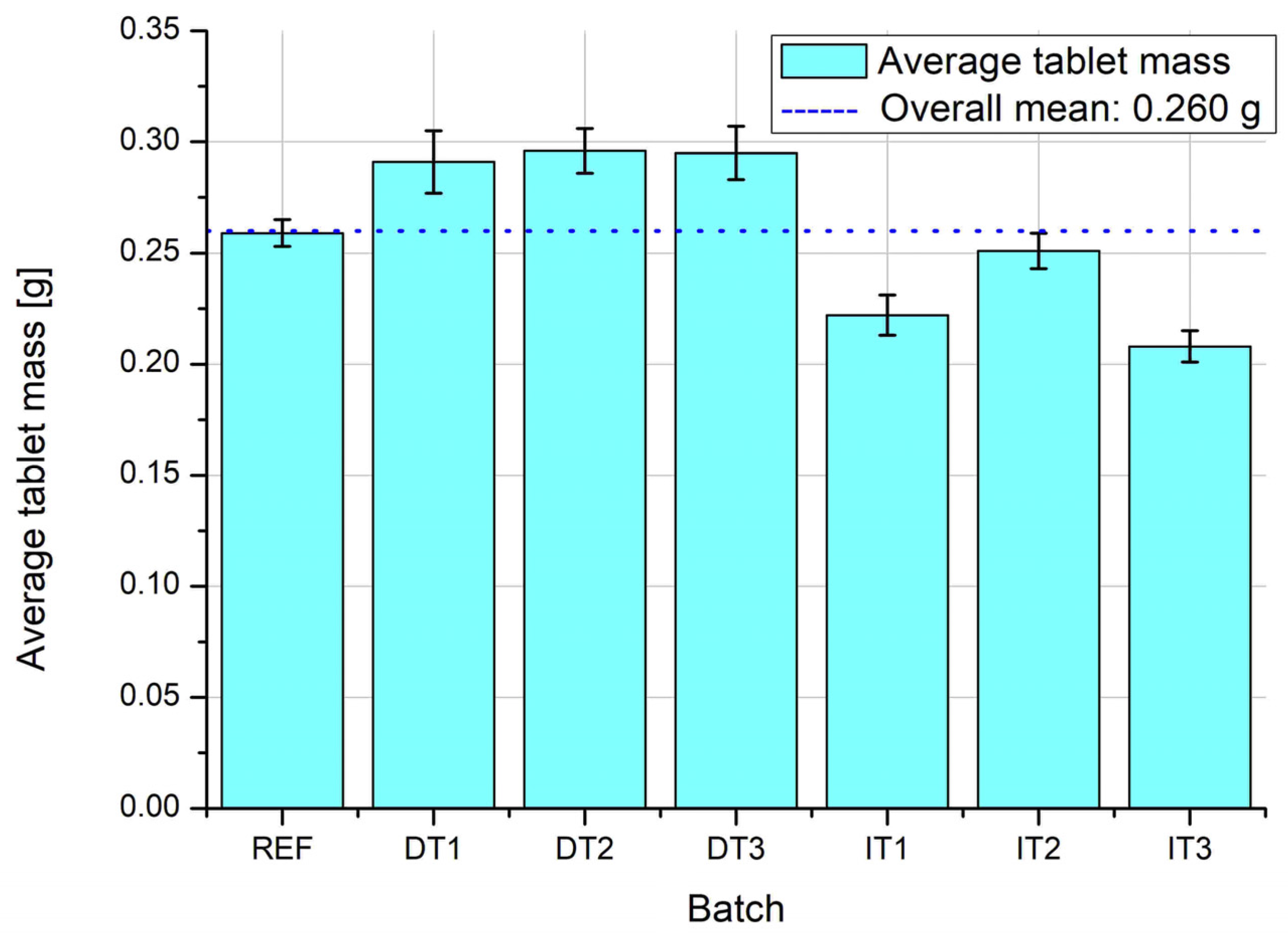
The average tablet mass across all experimental batches is depicted in
Figure 6. The dashed line represents the overall target mean of 0.260 g. Batches prepared by direct compression using the vibrating ball mill (DT1–DT3) show mass values very close to the target, with DT2 and DT3 slightly exceeding it. In contrast, tablets produced through indirect tableting (IT1–IT3), which includes pan granulation, show consistently lower average mass values, particularly in batches IT2 and IT3. The reference batch (REF), prepared using a conventional V-type mixer, is also slightly below the target value. These observations suggest that direct compression with vibratory mixing ensures more accurate die filling and mass control compared to the granulated series and the reference method.
Figure 7 displays the standard deviation of tablet mass for each batch, with a dashed line marking the overall mean SD value of 0.010 g. The REF batch demonstrates the lowest variability, possibly due to low average mass and limited powder cohesion leading to tightly packed tablets. Among the directly compressed tablets, DT2 and DT3 show higher mass variability, likely linked to slight fluctuations in flow behavior despite improved mixing. Notably, the granulated series (IT1–IT3) exhibits lower standard deviations than DT batches, indicating higher batch-to-batch reproducibility and better powder flow achieved through granulation. This highlights the trade-off between process simplicity (direct compression) and performance stability (wet granulation).
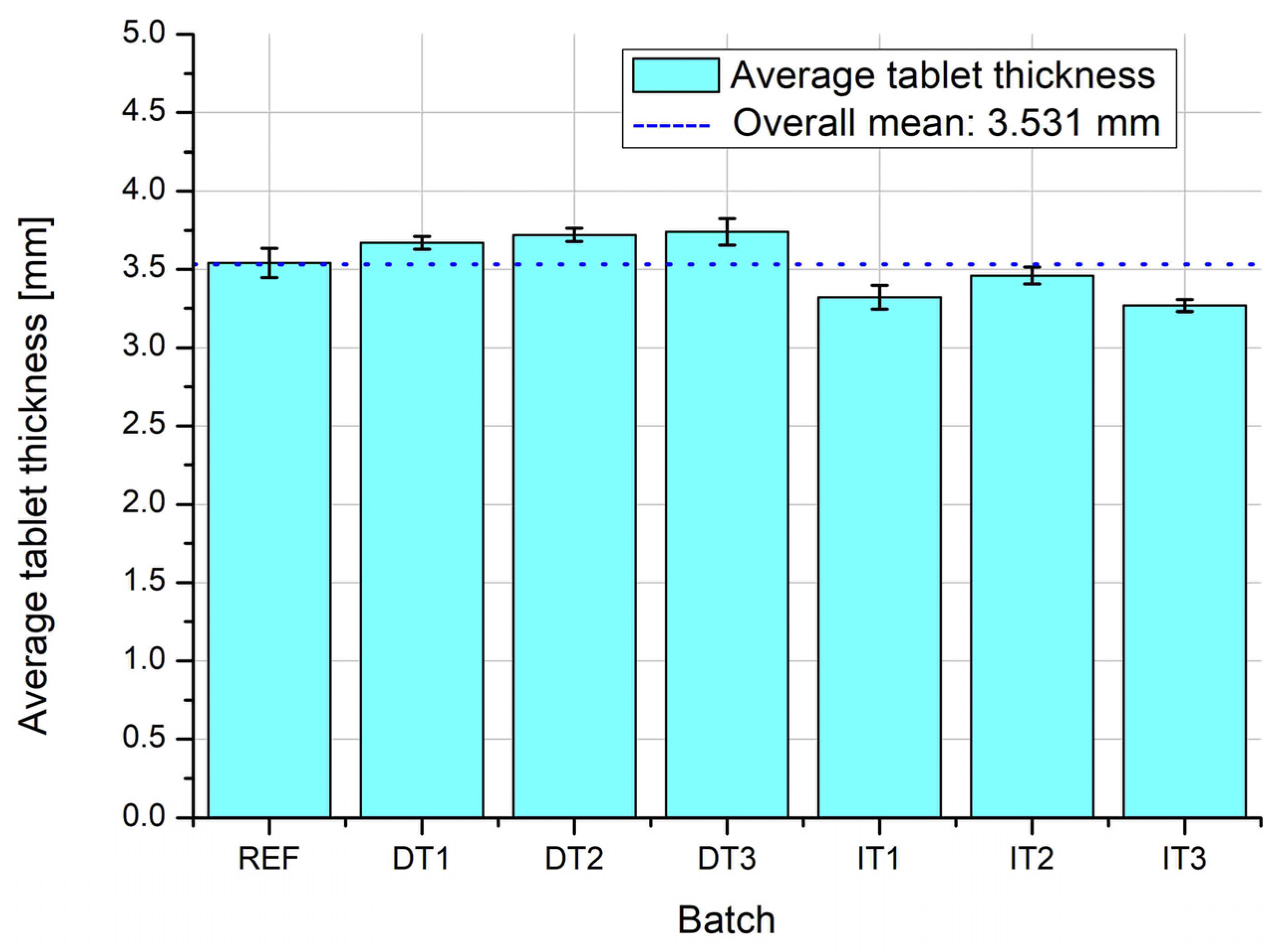
Figure 8 shows the average thickness of tablets across all production batches, with the overall mean indicated by the dashed line (3.531 mm). Tablets from the direct compression series (DT1–DT3) tend to be slightly thicker than the reference (REF) and granulated (IT1–IT3) batches, with DT2 and DT3 reaching the highest average values. These variations may result from differences in powder compressibility and die fill dynamics. Tablets from the indirect tableting process exhibit more uniform thicknesses and are closer to the overall mean.
Figure 9 presents the standard deviation of tablet thickness, providing insight into the consistency of tablet dimensions within each batch. The reference batch (REF) shows the highest variability, suggesting poor uniformity in die filling. The granulated batches (IT1–IT3) display notably lower standard deviations, indicating greater dimensional consistency due to improved flow and packing behavior of granules. Among the directly compressed batches, DT2 and DT3 demonstrate moderate variability, with DT1 achieving uniformity comparable to the IT series.
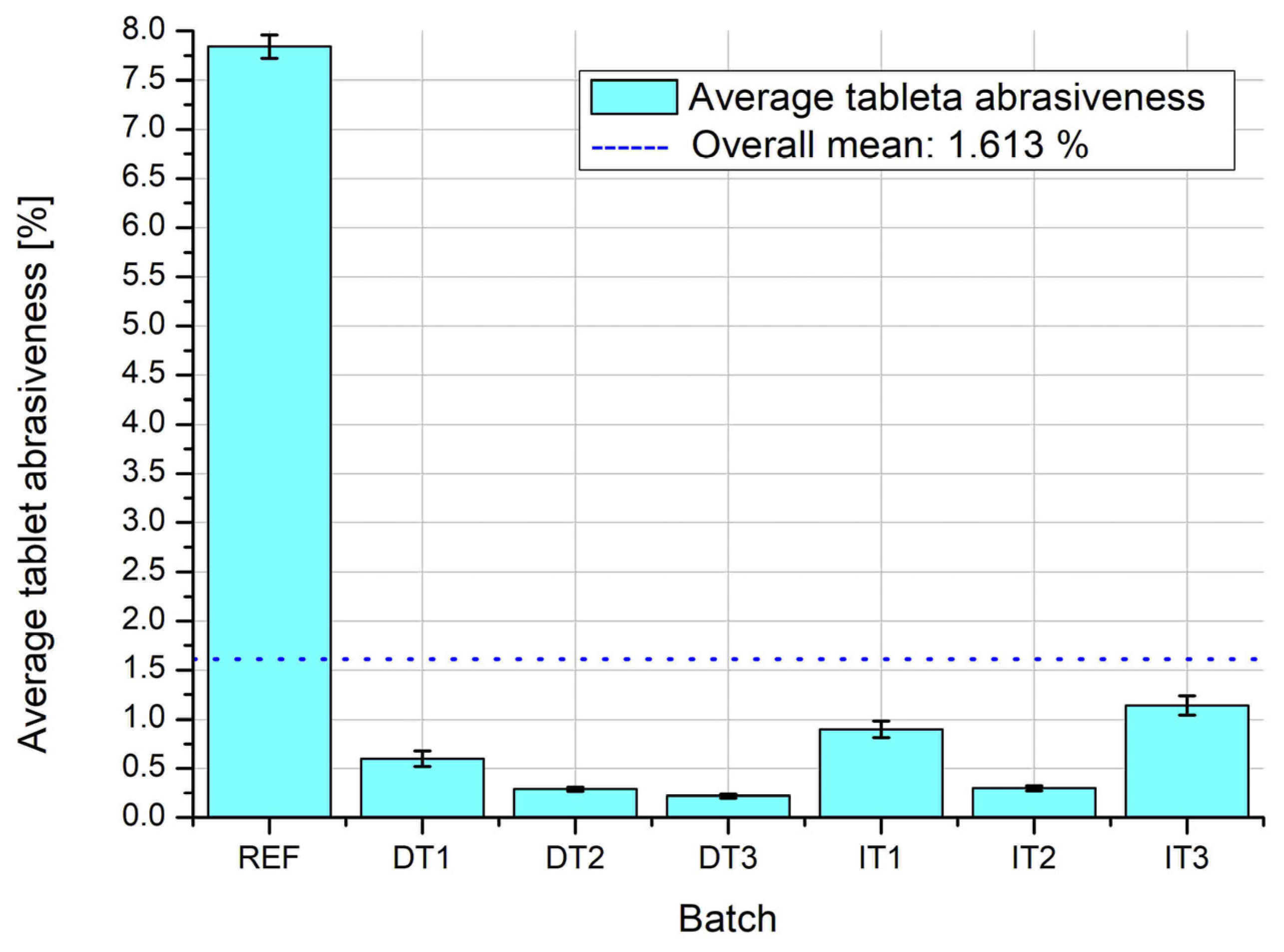
Figure 10 presents the average tablet abrasiveness, a critical quality parameter reflecting the mechanical resistance of tablets to surface damage during handling. The REF batch demonstrates excessively high abrasiveness (over 7%), far exceeding the acceptable threshold and the overall mean value of 1.613%. In contrast, all experimental batches (DT and IT series) show significantly reduced abrasiveness, with values well below 2%, indicating enhanced tablet integrity. This suggests that both direct compression (with vibratory mixing) and wet granulation considerably improve mechanical robustness.
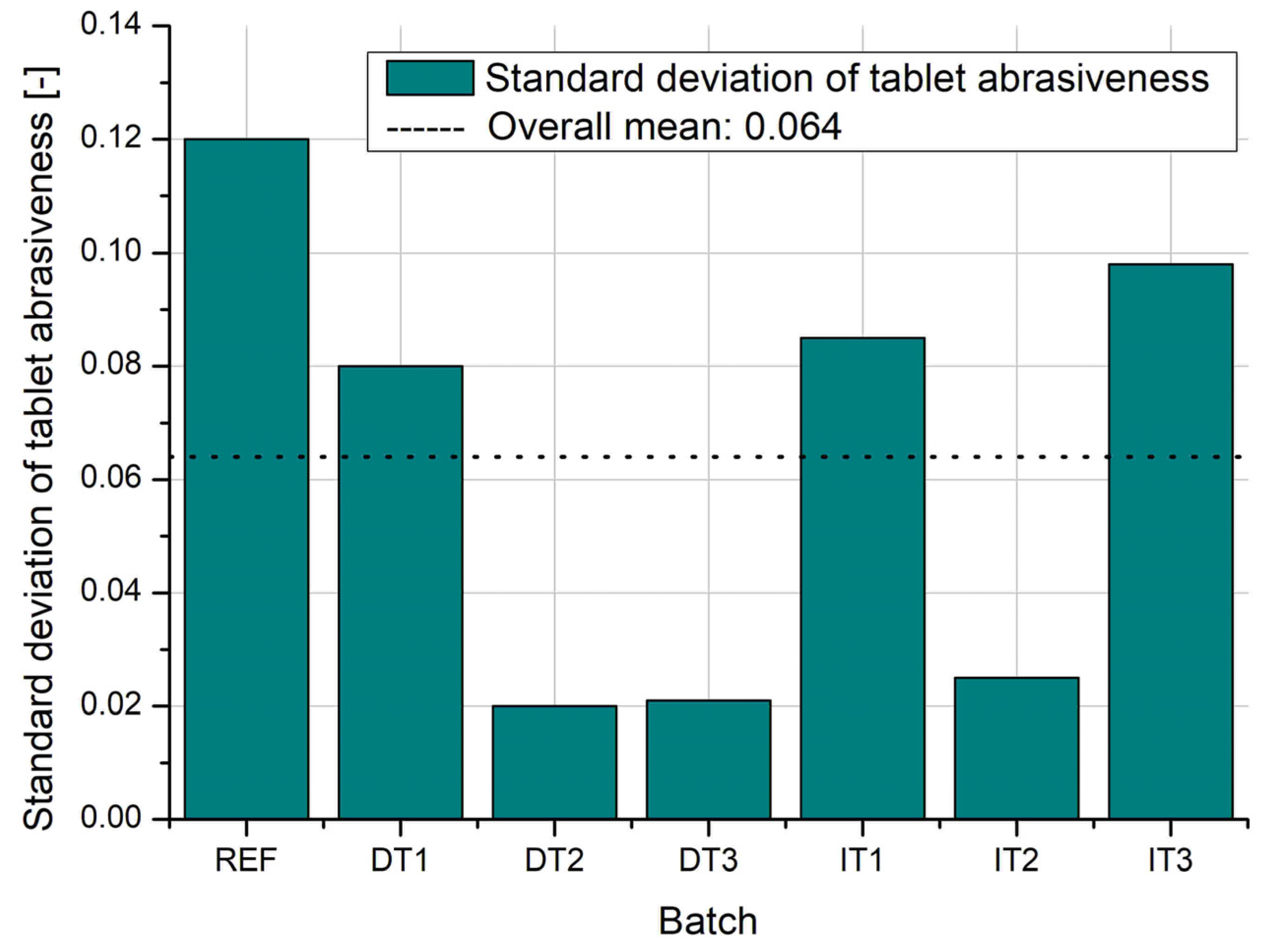
Figure 11 illustrates the standard deviation of tablet abrasiveness, further highlighting batch-to-batch variability. The REF batch again shows the highest inconsistency, followed by DT1. Batches DT2 and DT3 display low variability, suggesting stable processing conditions after extended mixing. Among the granulated batches, IT2 and IT3 show slightly higher variability compared to IT1 but remain within an acceptable range. Overall, granulation contributes to higher reproducibility in mechanical performance, while direct compression with appropriate mixing time (DT3) can yield similarly robust and consistent tablets.
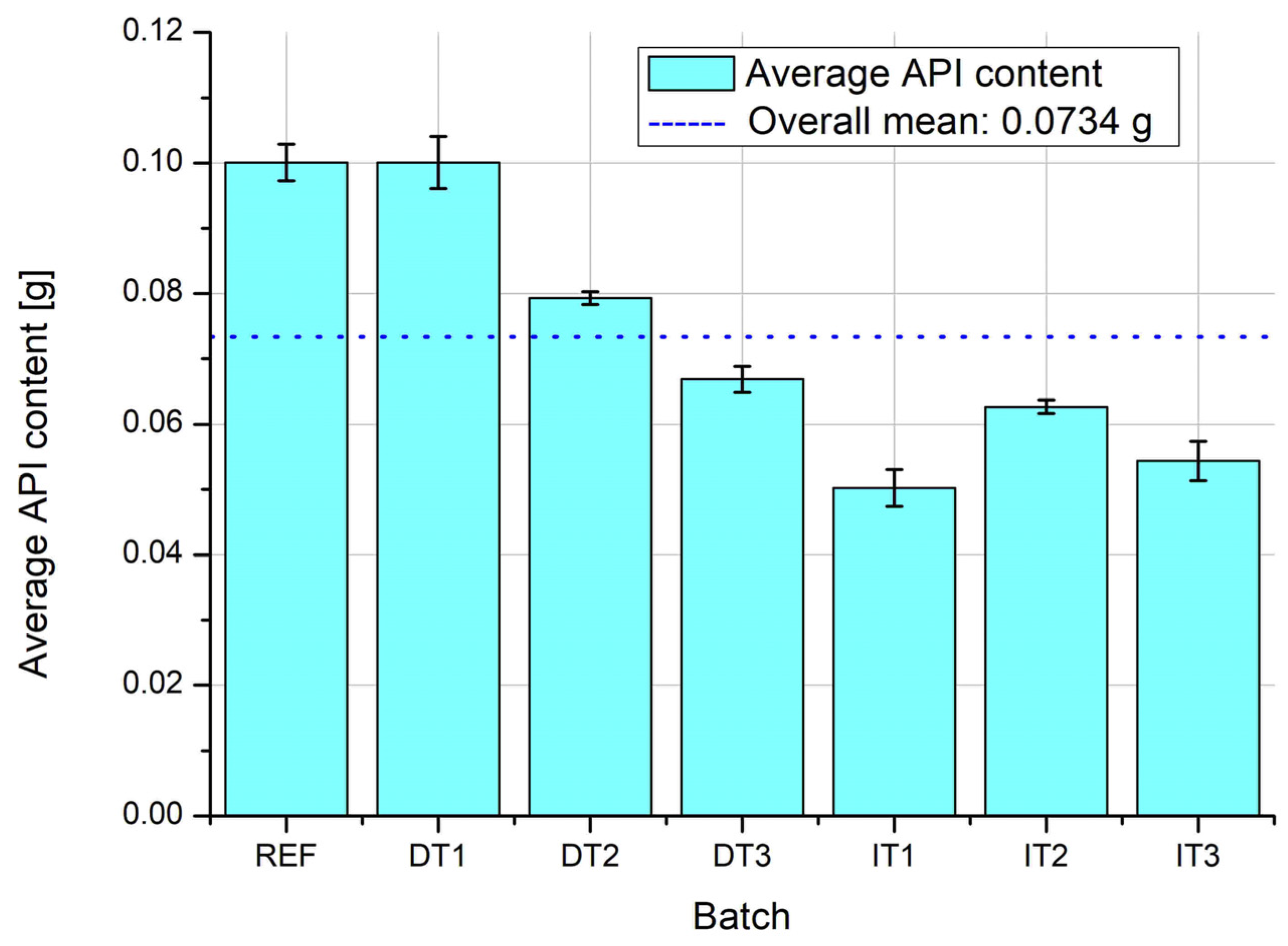
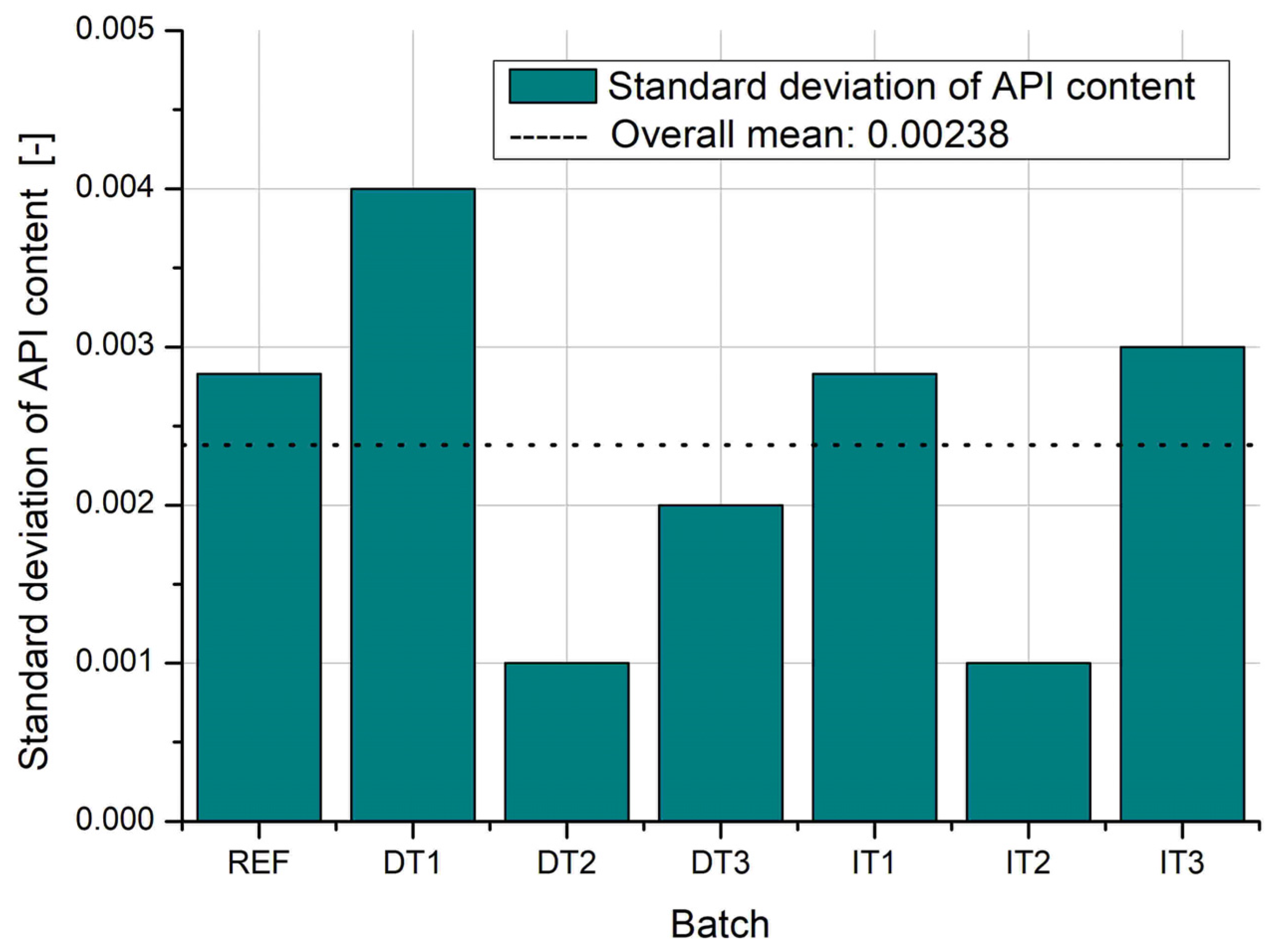
The results of API content analysis reveal distinct differences in both average values and variability across the tested batches (
Figure 12 and
Figure 13). The reference batch (REF) and DT1 (direct compression with shortest mixing time) exhibit the highest average API content, but also the greatest standard deviations, indicating poor homogeneity and significant variation between individual tablets. This is likely due to insufficient mixing and uneven distribution of the active substance in the bulk powder, especially in the absence of granulation or adequate blending time.
In contrast, DT2 and IT2 demonstrate the lowest standard deviations, suggesting high uniformity of drug distribution within the batch. For DT2, this result highlights the effectiveness of vibratory ball mill mixing with optimized process duration (5 min) in achieving homogenous blends. Similarly, IT2 benefits from the improved granule formation at the optimal pan inclination angle (30°), enhancing blend uniformity and die filling consistency.
Notably, the indirect tableting batches IT1, IT2, and IT3 exhibit very similar average API content, which confirms the reliability and reproducibility of the granulation-based process. This consistency is particularly valuable in industrial settings, where regulatory compliance requires tight control over dosage uniformity.
These findings emphasize the importance of both mixing intensity and granulation conditions in achieving uniform API distribution, and further support the vibratory ball mill as a promising alternative to conventional granulation under optimized parameters.
3. Discussion
The results of this study demonstrate how both the choice of manufacturing method and process parameters significantly affect the quality, reproducibility, and performance of tablets containing sodium naproxen as the active pharmaceutical ingredient (API), dolomite and cellulose as fillers, polyvinylpyrrolidone (PVP) as a binder, and magnesium stearate as a lubricant.
The reference batch (REF), produced using a conventional V-type tumble mixer, consistently exhibited the weakest performance across multiple quality attributes. Although the average API content in REF tablets was relatively high, it was accompanied by the largest standard deviation, indicating poor content uniformity. Such variability is consistent with prior findings showing that conventional low-energy blending techniques can lead to content segregation in multi-component formulations, particularly when the particle size or density of excipients and API differ significantly [
28]. The high values of tablet abrasiveness and its variability further emphasize the limitations of this standard blending method, particularly when processing formulations with low natural cohesiveness or a tendency toward demixing [
29].
In contrast, the direct compression approach using a vibrating ball mill showed promising results, especially at extended mixing times. The DT2 and DT3 batches demonstrated improvements in API content uniformity, tablet mass consistency, and reduced abrasiveness compared to both REF and DT1. Notably, DT2 achieved the lowest standard deviation in API content, suggesting that a 5-min vibratory mixing duration may be sufficient to produce a homogeneous blend for direct compression. Vibratory mixing has been shown in other studies to promote more uniform particle distribution due to the intense mechanical energy and rapid particle agitation, which enhances homogeneity and can mitigate segregation risks [
30]. Tablet mass and thickness in the DT series were slightly higher than the overall mean, potentially reflecting better die fill due to improved powder rearrangement under vibratory energy [
31].
The indirect tableting process, which involved wet pan granulation, led to robust performance across all parameters. Batches IT1–IT3 showed consistently low values of tablet abrasiveness and dimensional variability, with IT2 exhibiting optimal performance, including the lowest API content standard deviation and high reproducibility in tablet properties. These results validate the granulation process as a highly effective method for stabilizing powder flow and improving compressibility, especially for multi-component formulations. The performance advantages of wet granulation are well documented, particularly in achieving enhanced uniformity and mechanical strength in tablets composed of poorly flowing or highly cohesive materials [
32]. The similar outcomes observed among IT1, IT2, and IT3 further underscore the robustness of the granulation step, even with variation in pan inclination angles.
Importantly, the findings indicate that vibratory ball mill mixing may serve as a viable and simpler alternative to wet granulation, particularly in formulations where direct compression is desired but hindered by challenging powder properties. When properly optimized, as seen in the DT2 batch, this method can yield tablets with quality attributes comparable to those obtained by conventional granulation—yet with reduced processing steps, lower equipment requirements, and no need for solvents or drying. This aligns with current trends in pharmaceutical manufacturing that prioritize continuous, solvent-free, and environmentally sustainable processes [
31].
In the context of industrial pharmaceutical manufacturing, where process efficiency, reproducibility, and cost-effectiveness are paramount, these results are highly relevant. They suggest that vibratory mixing may represent a scalable, energy-efficient, and environmentally favorable strategy for developing direct compression formulations, particularly those involving sustainable excipients such as dolomite. Recent studies have highlighted dolomite’s potential as a biocompatible and mineral-rich excipient with acceptable compaction behavior, making it suitable for use in eco-conscious pharmaceutical formulations [
30].
The performance of the tablets can be directly linked to the functional contributions of the individual formulation components. Dolomite, a brittle mineral filler, enhanced mechanical strength but required balancing with plastic excipients to ensure adequate compressibility. Cellulose not only provided compressibility but also supported disintegration through its porous structure. The presence of PVP as a binder contributed to improved granule cohesion and tablet integrity, particularly in wet granulation batches. Magnesium stearate, used as a lubricant, ensured smooth tableting but may have reduced wettability to some extent. Overall, the high solubility of sodium naproxen played a key role in enabling simplified composition without the need for a dedicated disintegrant.
4. Materials and Methods
4.1. Materials
The formulation was composed of the following excipients and active ingredient, each sourced from reputable manufacturers: sodium naproxen—sodium (2S)-2-(6-methoxynaphthalen-2-yl)propanoate p.a. (Divi’s Laboratories, Hyderabad, India), dolomite p.a. (Semav, Skały, Poland), cellulose p.a. (FMC BioPolymer, St. Louis, MO, USA), polyvinylpyrrolidone (PVP) p.a. and magnesium stearate p.a. (both from Sigma-Aldrich, St. Louis, MO, USA). These components were incorporated into the formulation mixture in the following mass percentage ratios: 20% sodium naproxen (serving as the active pharmaceutical ingredient, API), 65% dolomite and 7% cellulose (acting as fillers), 5% polyvinylpyrrolidone (used as a binder), and 3% magnesium stearate (functioning as a lubricant). Although the formulation does not include a dedicated disintegrant, cellulose was incorporated at 7% and serves as both a filler and a wicking-type disintegrant. Due to its porous structure and high capillarity, MCC facilitates water uptake and promotes disintegration. In combination with the high aqueous solubility of sodium naproxen, this composition was expected to provide adequate disintegration performance without the need for additional disintegrating agents.
4.2. Method of Preparing Mixtures for Tabletting
The powder blends intended for wet granulation (indirect tableting) as well as the reference mixture (REF) were prepared using a V-type tumble blender with a total volume of 750 cm3, manufactured by CDK (Gliwice, Poland). The blending process was conducted for 15 min at a rotational speed of 20 rpm, with the blender filled to 40% of its total capacity. This approach is commonly used in the pharmaceutical industry to ensure efficient and homogeneous mixing of powder components.
In contrast, the powder blends used for direct tableting were prepared with a vibrating ball mill (Model 8000M, SPEX SamplePrep LLC, Metuchen, NJ, USA). The device was equipped with a stainless steel mixing chamber with a volume of 50 cm3. Mixing was carried out using two stainless steel balls, each with a diameter of 10 mm. The process time varied from 2.5 to 10 min depending on the batch, and the chamber was filled to 30% of its volume to ensure efficient energy transfer and uniform blending. The use of a vibrating ball mill, despite not being part of standard granulation technology, proved effective in achieving powder homogeneity sufficient for direct compression.
4.3. Method of Preparing Granules
Wet granulation was performed using a pilot-scale pan granulator, engineered for continuous processing. The granulation vessel featured a 400 mm internal diameter and 100 mm depth, providing adequate space for uniform particle movement and binder application. Powdered raw materials were initially loaded into a feed tank and continuously transferred into the rotating pan via a vibratory conveyor system, ensuring stable flow and preventing blockages.
A binder solution was applied through a top-spray nozzle, with liquid delivery managed by an external pump and atomization supported by a compressed air system. The pan’s rotary motion was driven by an electric motor (Model AR304, ERWEKA GmbH, Langen, Germany), and key process parameters—rotational speed, pan inclination angle, and material feed rates—were optimized based on preliminary trials aimed at obtaining granules with the best compaction and flow properties for tablet manufacturing.
During the experiments, the pan inclination angle was varied between 10° and 50° to assess its influence on granule formation and uniformity. All other settings were held constant to maintain experimental consistency: rotational speed was fixed at 40 rpm, powder feed rate at 200 g/min, and binder solution flow rate at 30 mL/min. These values were identified during the preliminary phase as optimal for producing granules with desirable mechanical and rheological characteristics.
To maintain process stability and reproducibility, the apparatus included a vibrating feed hopper to ensure uninterrupted powder flow and interchangeable binder reservoirs for flexibility in formulation. All process parameters—binder delivery, powder input, and airflow—were regulated using a centralized control unit (Model CE255, G.U.N.T. Gerätebau GmbH, Norderstedt, Germany). The entire setup, including structural components, was constructed from stainless steel, complying with pharmaceutical manufacturing standards.
4.4. Method of Preparing Tablets
In order to compare the quality of tablets obtained via direct compression and wet granulation followed by compression, the same compaction parameters were applied to all formulation series to ensure consistency and comparability of the results. Tablets were prepared using a manual single-punch tablet press (model TDP 0, LFA Machines Oxford LTD, Oxfordshire, UK).
Compression was performed using a 6 mm-diameter die with a spherical shape, featuring chamfered edges and a break-line marker on the upper punch to allow for tablet splitting. Each tablet was compressed using a pressing force of 3.5 kN, a value selected to ensure sufficient tablet cohesion and mechanical stability without compromising the integrity of thermosensitive components. This standardized approach allowed for a direct evaluation of the influence of the powder preparation method—either direct compression from a powder blend or compression of granules obtained by wet granulation—on the resulting tablet properties.
4.5. Methods for Measuring the Properties of Powder Materials
The flow and packing properties of the powdered raw materials were evaluated using a dedicated powder characterization device that complies with current pharmacopoeial standards. All measurements were performed with the Powder Tester PT-S (Hosokawa Micron B.V., Doetinchem, The Netherlands). The evaluated parameters included angle of repose, angle of fall, angle of difference, aerated bulk density, and packed bulk density. The angle of repose indicates the flowability of the powder based on the natural slope formed when the material is poured. The angle of fall is measured after the powder collapses from its initial repose state, while the angle of difference, calculated as the difference between the angles of repose and fall, serves as an indicator of powder cohesiveness. Aerated bulk density refers to the density of the loosely poured powder including interparticle voids, whereas packed bulk density is measured after the powder has been tapped or mechanically compacted in a fixed volume. The bulk density was determined by measuring the mass of a known volume of powder, taking into account the space between particles. Based on the obtained values of aerated and packed bulk densities, two derived parameters were calculated. The compressibility was calculated using the formula: Compressibility Index (%) = ((ρ_packed − ρ_aerated)/ρ_packed) × 100. The Hausner ratio was calculated using the formula: Hausner Ratio = ρ_packed/ρ_aerated, where ρ_aerated is the aerated bulk density and ρ_packed is the packed bulk density. These indices are commonly used in pharmaceutical preformulation to assess powder flowability and cohesiveness. The results provided critical insight into the handling behavior of the raw materials during both direct compression and wet granulation processes.
Particle size distribution of the raw materials was determined using laser diffraction with a wet dispersion technique. Measurements were conducted employing a Mastersizer 2000 Hydro MU (Malvern Instruments, Malvern, Worcestershire, UK), which allows for particle size analysis within the range of 0.01 to 2000 µm by means of dynamic light scattering. All measurements were performed in compliance with the ISO 13320-1:1999 standard [
33]. The instrument generates output data as particle size distributions (PSDs), which are mass-normalized for each analyzed sample.
4.6. Methods of Measuring the Properties of Granules
To determine the particle size distribution of the granules, sieve analysis was carried out using a laboratory sieve shaker equipped with a set of sieves with nominal mesh sizes of 2 mm, 1 mm, 0.5 mm, 250 µm, 150 µm, and 75 µm, all supplied by EKO-LAB-11-200 (Jasie, Poland).
The moisture content of the granules was measured using a RADWAG MA.R laboratory moisture analyzer (Radom, Poland). The method provided a measurement precision with an estimated error of approximately ±0.25%.
4.7. Methods for Measuring the Properties of Tablets
The tablets were subjected to comprehensive quality assessment through the evaluation of critical quality attributes, including hardness, mass uniformity, compressibility, and active pharmaceutical ingredient (API) content. Tablet mass was accurately determined using an Ohaus Pioneer PX224 analytical balance (Greifensee, Switzerland). Tablet thickness was measured using a precision caliper with an accuracy of 0.01 mm, with measurements conducted on 20 tablets from each batch. All measurements were performed in quadruplicate to ensure reproducibility.
Abrasiveness was evaluated utilizing a GUNT CE 245 drum mill (Barsbüttel, Germany). Approximately 50 g of tablets from each batch were introduced into a 1.15 dm3 drum chamber, which was subsequently mounted on rotating shafts operating at 200 revolutions per minute (rpm) for a duration of 5 min. Upon completion of the test, the contents of the drum were transferred onto a 500 µm mesh sieve and sieved. The mass of the material retained on the sieve was recorded. Abrasiveness was expressed as the percentage ratio of the powder mass retained on the sieve to the initial tablet mass. Each test was performed in quadruplicate to ensure statistical validity.
The quantification of the active pharmaceutical ingredient (API) was performed using a laboratory refractometer RL-1 (PZO Warsaw, Poland). One tablet was placed into each of ten 50 mL round-bottom flasks, followed by continuous agitation for 24 h to facilitate dissolution. Subsequently, the resulting mixture was filtered through a syringe filter with a pore size of 2 µm, and the refractive index of the filtrate was measured. The concentration of sodium naproxen in individual tablets was determined based on a previously established calibration curve.
5. Conclusions
This study demonstrated that both the manufacturing method and process parameters have a significant impact on the quality and reproducibility of tablets formulated with sodium naproxen, dolomite, cellulose, polyvinylpyrrolidone (PVP), and magnesium stearate. The reference formulation, prepared in a conventional V-type mixer, showed the greatest variability and poorest performance across several quality criteria. In contrast, tablets produced by direct compression using a vibrating ball mill exhibited improved uniformity and mechanical properties, particularly when mixed for longer durations. Wet granulation using a pan granulator consistently yielded tablets with excellent dimensional stability, low abrasiveness, and highly reproducible API content, with optimal results observed for the intermediate inclination angle (30°). Notably, vibratory ball mill mixing proved capable of producing tablets with comparable quality to those obtained via granulation, while requiring fewer processing steps and no solvents.
The observed tablet properties were closely linked to the functional roles of the formulation components. The use of dolomite enhanced tablet strength, while microcrystalline cellulose contributed to both compressibility and internal porosity. Polyvinylpyrrolidone improved granule cohesion in wet granulation, and magnesium stearate ensured efficient compression. Together with the high solubility of sodium naproxen, these components enabled the development of a simplified formulation with robust performance across different manufacturing methods.
This finding highlights the potential of this method as a cost-effective and scalable alternative for formulations containing challenging or poorly flowing materials, especially in applications aiming for greener and simpler manufacturing processes.