The Vicious Cycle of Obesity and Low Back Pain: A Comprehensive Review
Abstract
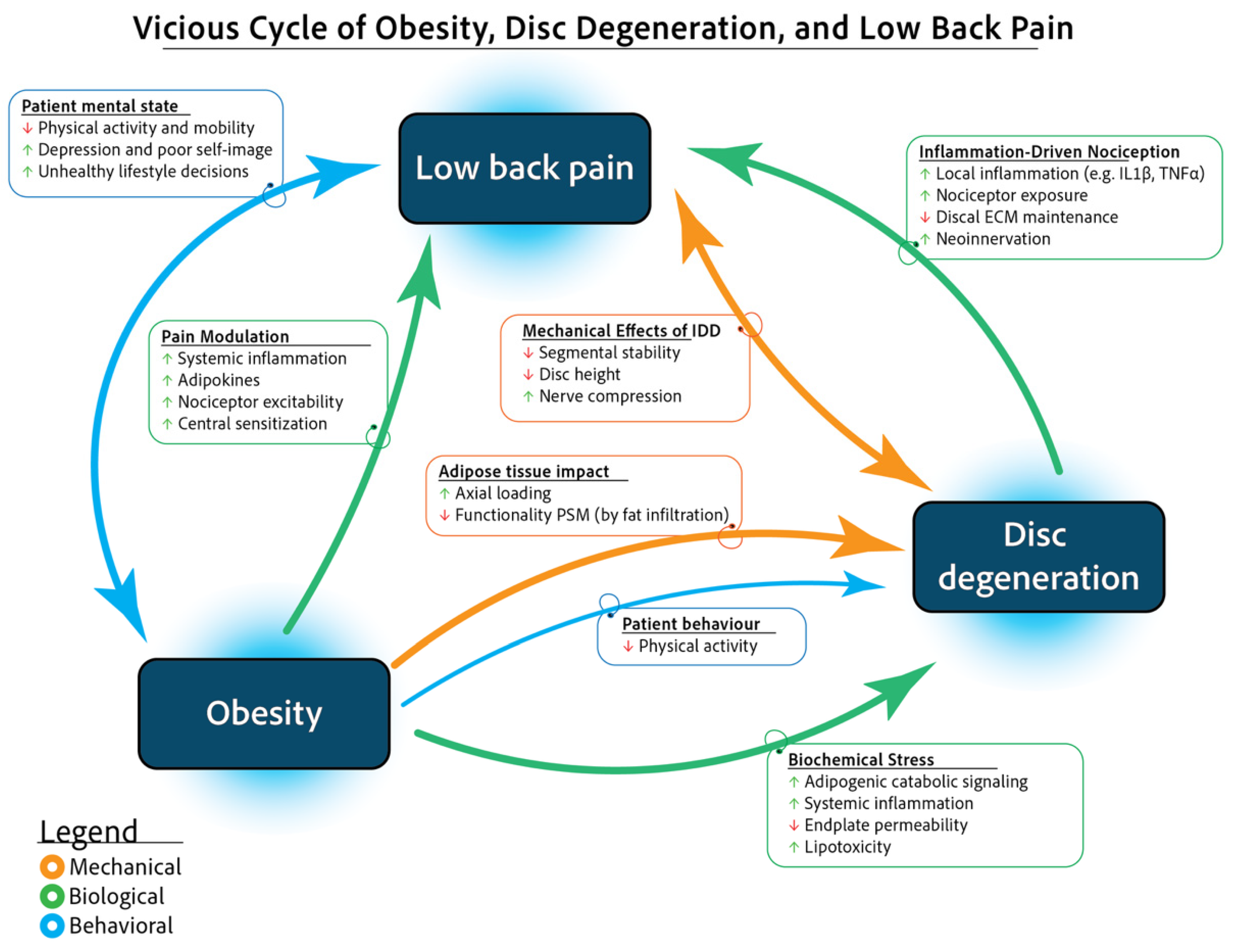
1. Introduction
2. Obesity
3. Low Back Pain
4. Spinal Changes Related to Obesity
4.1. Intervertebral Disc
4.2. Facet Joints
4.3. Paraspinal Muscles
4.4. Epidural Fat
4.5. Nervous System
5. The Biomechanical Impact of Obesity on the Spine
6. The Biochemical Impact of Obesity on the Spine
6.1. Lipid Dysregulation
6.2. Metabolic Alterations
6.3. The Adipokine Cascade
6.4. Gut Microbiome and Metabolic Inflammation
6.5. Hypoxia and Angiogenesis
7. Therapeutic Strategies Targeting the Obesity–LBP Connection
7.1. Addressing Obesity as a Pathway to Alleviate LBP
| Type | Author (Year) | Design | Intervention | N | Age (Years) Mean (±SD) | BMI (Baseline) Mean (±SD) | T2DM n (%) | LBP Rate (Baseline) n/N (%) | LBP Score (Baseline) Mean (±SD) | FU (Months) | BMI (Final FU) Mean (±SD) | LBP Rate (Final FU) n/N (%) | LBP Score (Final FU) Mean (±SD) | Imaging Findings | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgical | McGoey (1990) [226] | Prospective single-arm | Banding | 104 | 33.4 | unspecified | unspecified | 65/104 (62.5%) | - | 22 (average) | unspecified | 11/104 (10.6%) | - | NA | Two patients that lost weight and then regained it, had matching LBP relief and reoccurrence. |
| Melissas (2003) [227] | Prospective two-arm | Banding | 50 | 37.5 (±10.2) | 46.7 (±7.7) | unspecified | 29/50 (58.0%) | - | 24 | 33.6 (±5.6) | 10/50 (20.0%) | - | NA | - | |
| Melissas (2005) [228] | unspecified | Banding | 29 | 37.5 (±11.2) | 47.2 (±8.8) | unspecified | NA | 5.5 (±2.0) * | 24 | 32.9 (±6.3) | NA | 2.1 (±1.9) * | NA | - | |
| Khoueir (2009) [229] | Prospective single-arm | Bypass or sleeve | 38 | 48.4 (±10.1) | 52.3 (±12.6) | unspecified | NA | 5.2 (±3.4) | 12 | 38.3 (±9.7) | NA | 2.9 (±3.1) | NA | - | |
| Vincent (2012) [230] | Prospective two arm | Bypass or banding | 25 | 41 (±11) | 47 (±7) | unspecified | 6/25 (25.0%) | 5.2 | 3 | 39.1 | 15/25 (61.1%) | 2.4 | NA | Follow-up too short and lack of BMI change. | |
| Lidar (2012) [110] | Prospective single arm | Bypass, sleeve, or banding | 30 | 49 (±10.4) | 42.8 (±4.8) | unspecified | 26/30 (86.7%) | 5.7 (±3.1) | 12 | 29.7 (±3.4) | unspecified | 1.3 (±2.1) | Sign. increase L4/5-disc height (from 6.8 (±1) to 8.8 (±1) mm) | - | |
| Çakır (2015) [231] | Prospective single arm | Sleeve | 39 | 37.7 (±11.3) | 46.5 | unspecified | unspecified | 6.7 (±2.7) | 6 | 32.3 | unspecified | 1.97 (±2.2) | NA | Work does not focus specifically on back pain patients | |
| Taylor (2018) [232] | Prospective single arm | Abdominoplasty | 214 | 42.1 (±8.7) | 26.3 (±4.3) | unspecified | 195/214 (91.2%) | unspecified | 6 | unspecified | unspecified | unspecified | NA | Significant improvement in ODI disability scores related to LBP. | |
| Bhandari (2019) [233] | Prospective single arm | Bypass or Sleeve | 45 | 54.7 (±8.5) | 54.2 (±8.6) | 23 (51.1%) | 34/45 (75.5%) | 7.3 (±1.4) | 12 | unspecified | 25/45 (55.6%) | 2.3 (±1.4) | NA | - | |
| Soteropulos (2020) [234] | Retrospective | Abdominoplasty | 143 | 48 | unspecified | unspecified | 51/143 (35.7%) | NA | 120 | unspecified | unspecified | NA | NA | Improvement in ODI and SF36 scores for patients with LBP pre-operation. | |
| Patel (2023) [235] | Prospective single arm | Abdominoplasty | 30 | 48.4 (±14.3) | 27.8 (±4.2) | 3 (10%) | 21/30 (70%) | 3.95 | 6 | unspecified | unspecified | 0.53 | NA | - | |
| Lifestyle/programme | Roffey (2011) [236] | Prospective single arm | Diet, exercise, and counselling | 46 | 50.1 (±12.9) | 44.7 (±7.6) | unspecified | NA | 3.3 (±2.2) | 12 | 39.6 (±8.2) | NA | 2.6 (±2.5) | NA | - |
| Silişteanu (2015) [220] | Prospective two arm | Diet (+physio, NSAID, analgesics, etc.) | 90 | unclear | unclear | unspecified | unclear | unclear | unclear | unclear | unclear | unclear | NA | Significant correlation BMI loss and VAS improvement. | |
| Williams (2018) [237] | RCT | Diet and exercise | 79 | 56.0 (±13.3) | 32.4 (±3.5) | unspecified | NA | 6.7 (±1.8) | 6 | 32.7 (±4.3) | NA | 5.8 (±2.7) | NA | Diet was not effective in reducing BMI. | |
| Dunlevy (2018) [238] | Retrospective | Diet, exercise, and counselling | 476 | 45.1 (±12.0) | 50.8 (±8.1) | 66 (29.3%) | 281/476 (59.0%) | 5.9 (±3.8) | 6 | 47.6 (±8.0) | 236/476 (49.6%) | 4.5 (±3.7) | NA | - | |
| Safari (2020) [239] | RCT | Diet (+NSAIDs) | 48 | 39.7 (±10.7) | 28.4 (±3.5) | unspecified | NA | 2.2 (±0.5) | 2 | unspecified | NA | 1.0 (±1.0) | NA | Follow-up too short. Diet unable to result in relevant BMI loss. | |
| Ward (2024) [240] | Prospective single arm | Diet | 136 | 47.7 (±10.7) | 30.7 (±2.3) | Excluded | 43/136 (31.8%) | unclear | 3 | unclear | 18/136 (13.6%) | unclear | NA | - | |
| Pharmaco- logical | None identified | ||||||||||||||
7.2. Modulating Adipokine Signalling to Alleviate Obesity-Linked Discogenic Pain
7.3. Limitations and Considerations
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- GBD 2021 Other Musculoskeletal Disorders Collaborators. Global, regional, and national burden of other musculoskeletal disorders, 1990–2020, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e670–e682. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Low Back Pain Collaborators. Global, regional, and national burden of low back pain, 1990–2020, its attributable risk factors, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e316–e329. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, L.; Mazzuca, G.; Maguolo, A.; Russo, F.; Cannata, F.; Vadala, G.; Maffeis, C.; Papalia, R.; Denaro, V. The burden of low back pain in children and adolescents with overweight and obesity: From pathophysiology to prevention and treatment strategies. Ther. Adv. Musculoskelet. Dis. 2023, 15, 1759720X231188831. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fernandez, C.; Francisco, V.; Pino, J.; Mera, A.; Gonzalez-Gay, M.A.; Gomez, R.; Lago, F.; Gualillo, O. Molecular Relationships among Obesity, Inflammation and Intervertebral Disc Degeneration: Are Adipokines the Common Link? Int. J. Mol. Sci. 2019, 20, 2030. [Google Scholar] [CrossRef]
- Singh, N.K.; Singh, N.K.; Verma, R.; Diwan, A.D. Validation and Estimation of Obesity-Induced Intervertebral Disc Degeneration through Subject-Specific Finite Element Modelling of Functional Spinal Units. Bioengineering 2024, 11, 344. [Google Scholar] [CrossRef]
- Cannata, F.; Vadala, G.; Ambrosio, L.; Fallucca, S.; Napoli, N.; Papalia, R.; Pozzilli, P.; Denaro, V. Intervertebral disc degeneration: A focus on obesity and type 2 diabetes. Diabetes Metab. Res. Rev. 2019, 10, e3224. [Google Scholar] [CrossRef]
- Shiri, R.; Karppinen, J.; Leino-Arjas, P.; Solovieva, S.; Viikari-Juntura, E. The association between obesity and low back pain: A meta-analysis. Am. J. Epidemiol. 2010, 171, 135–154. [Google Scholar] [CrossRef]
- Lucha-Lopez, M.O.; Hidalgo-Garcia, C.; Monti-Ballano, S.; Marquez-Gonzalvo, S.; Ferrandez-Laliena, L.; Muller-Thyssen-Uriarte, J.; Lucha-Lopez, A.C. Body Mass Index and Its Influence on Chronic Low Back Pain in the Spanish Population: A Secondary Analysis from the European Health Survey (2020). Biomedicines 2023, 11, 2175. [Google Scholar] [CrossRef]
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 April 2020).
- Freisling, H.; Viallon, V.; Lennon, H.; Bagnardi, V.; Ricci, C.; Butterworth, A.S.; Sweeting, M.; Muller, D.; Romieu, I.; Bazelle, P.; et al. Lifestyle factors and risk of multimorbidity of cancer and cardiometabolic diseases: A multinational cohort study. BMC Med. 2020, 18, 5. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.R.F. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Singh-Manoux, A.; Fayosse, A.; Sabia, S.; Tabak, A.; Shipley, M.; Dugravot, A.; Kivimaki, M. Clinical, socioeconomic, and behavioural factors at age 50 years and risk of cardiometabolic multimorbidity and mortality: A cohort study. PLoS Med. 2018, 15, e1002571. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Global cost of overweight and obesity will hit $4.32tn a year by 2035, report warns. BMJ 2023, 380, 523. [Google Scholar] [CrossRef] [PubMed]
- Romero-Corral, A.; Somers, V.K.; Sierra-Johnson, J.; Thomas, R.J.; Collazo-Clavell, M.L.; Korinek, J.; Allison, T.G.; Batsis, J.A.; Sert-Kuniyoshi, F.H.; Lopez-Jimenez, F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int. J. Obes. 2008, 32, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Sweatt, K.; Garvey, W.T.; Martins, C. Strengths and Limitations of BMI in the Diagnosis of Obesity: What is the Path Forward? Curr. Obes. Rep. 2024, 13, 584–595. [Google Scholar] [CrossRef]
- Thaker, V.V. Genetic and Epigenetic Causes of Obesity. Adolesc. Med. State Art. Rev. 2017, 28, 379–405. [Google Scholar]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Heindel, J.J.; Howard, S.; Agay-Shay, K.; Arrebola, J.P.; Audouze, K.; Babin, P.J.; Barouki, R.; Bansal, A.; Blanc, E.; Cave, M.C.; et al. Obesity II: Establishing causal links between chemical exposures and obesity. Biochem. Pharmacol. 2022, 199, 115015. [Google Scholar] [CrossRef]
- Klimentidis, Y.C.; Beasley, T.M.; Lin, H.Y.; Murati, G.; Glass, G.E.; Guyton, M.; Newton, W.; Jorgensen, M.; Heymsfield, S.B.; Kemnitz, J.; et al. Canaries in the coal mine: A cross-species analysis of the plurality of obesity epidemics. Proc. Biol. Sci. 2011, 278, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.M.; Beserra, B.T.S.; Silva, N.G.; Lima, C.L.; Rocha, P.R.S.; Coelho, M.S.; Neves, F.A.R.; Amato, A.A. Exposure to endocrine-disrupting chemicals and anthropometric measures of obesity: A systematic review and meta-analysis. BMJ Open 2020, 10, e033509. [Google Scholar] [CrossRef]
- Apovian, C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22, s176–s185. [Google Scholar]
- Minana-Signes, V.; Monfort-Panego, M.; Bosh-Bivia, A.H.; Noll, M. Prevalence of Low Back Pain among Primary School Students from the City of Valencia (Spain). Healthcare 2021, 9, 270. [Google Scholar] [CrossRef] [PubMed]
- Gunzburg, R.; Balague, F.; Nordin, M.; Szpalski, M.; Duyck, D.; Bull, D.; Melot, C. Low back pain in a population of school children. Eur. Spine J. 1999, 8, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Louie, P.K.; Phillips, F.M.; An, H.S.; Samartzis, D. Low back pain in children: A rising concern. Eur. Spine J. 2019, 28, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Parreira, P.; Maher, C.G.; Steffens, D.; Hancock, M.J.; Ferreira, M.L. Risk factors for low back pain and sciatica: An umbrella review. Spine J. Off. J. North Am. Spine Soc. 2018, 18, 1715–1721. [Google Scholar] [CrossRef]
- Fujii, T.; Matsudaira, K. Prevalence of low back pain and factors associated with chronic disabling back pain in Japan. Eur. Spine J. 2013, 22, 432–438. [Google Scholar] [CrossRef]
- Bevan, S.; Quadrello, T.; McGee, R.; Mahdon, M.; Vavrovsky, A.; Barham, L. Fit for Work? Musculoskeletal Disorders in the European Workforce; The Work Foundation: London, UK, 2009. [Google Scholar]
- Diwan, A.D.; Melrose, J. Intervertebral disc degeneration and how it leads to low back pain. JOR Spine 2023, 6, e1231. [Google Scholar] [CrossRef]
- Nicol, V.; Verdaguer, C.; Daste, C.; Bisseriex, H.; Lapeyre, E.; Lefevre-Colau, M.M.; Rannou, F.; Roren, A.; Facione, J.; Nguyen, C. Chronic Low Back Pain: A Narrative Review of Recent International Guidelines for Diagnosis and Conservative Treatment. J. Clin. Med. 2023, 12, 1685. [Google Scholar] [CrossRef]
- Zhou, M.; Theologis, A.A.; O’Connell, G.D. Understanding the etiopathogenesis of lumbar intervertebral disc herniation: From clinical evidence to basic scientific research. JOR Spine 2023, 7, e1289. [Google Scholar] [CrossRef]
- Rustenburg, C.M.E.; Emanuel, K.S.; Peeters, M.; Lems, W.F.; Vergroesen, P.A.; Smit, T.H. Osteoarthritis and intervertebral disc degeneration: Quite different, quite similar. JOR Spine 2018, 1, e1033. [Google Scholar] [CrossRef]
- Schol, J.; Ambrosio, L.; Tamagawa, S.; Joyce, K.; Ruiz-Fernandez, C.; Nomura, A.; Sakai, D. Enzymatic chemonucleolysis for lumbar disc herniation-an assessment of historical and contemporary efficacy and safety: A systematic review and meta-analysis. Sci. Rep. 2024, 14, 12846. [Google Scholar] [CrossRef]
- Tamagawa, S.; Sakai, D.; Nojiri, H.; Nakamura, Y.; Warita, T.; Matsushita, E.; Schol, J.; Soma, H.; Ogasawara, S.; Munesada, D.; et al. SOD2 orchestrates redox homeostasis in intervertebral discs: A novel insight into oxidative stress-mediated degeneration and therapeutic potential. Redox Biology 2024, 71, 103091. [Google Scholar] [CrossRef] [PubMed]
- Stover, J.D.; Trone, M.A.R.; Weston, J.; Lewis, C.; Levis, H.; Philippi, M.; Zeidan, M.; Lawrence, B.; Bowles, R.D. Therapeutic TNF-alpha Delivery After CRISPR Receptor Modulation in the Intervertebral Disc. Biorxiv 2023, 32, 3955–3973. [Google Scholar] [CrossRef]
- Wang, S.; Sun, G.; Fan, P.; Huang, L.; Chen, Y.; Chen, C. Distinctive roles of tumor necrosis factor receptor type 1 and type 2 in a mouse disc degeneration model. J. Orthop. Transl. 2021, 31, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hu, B.; Liu, W.; Wang, P.; Lv, X.; Chen, S.; Shao, Z. The role of structure and function changes of sensory nervous system in intervertebral disc-related low back pain. Osteoarthr. Cartil. 2021, 29, 17–27. [Google Scholar] [CrossRef]
- Ma, J.; Stefanoska, D.; Stone, L.S.; Hildebrand, M.; van Donkelaar, C.C.; Zou, X.; Basoli, V.; Grad, S.; Alini, M.; Peroglio, M. Hypoxic stress enhances extension and branching of dorsal root ganglion neuronal outgrowth. JOR Spine 2020, 3, e1090. [Google Scholar] [CrossRef]
- Lama, P.; Le Maitre, C.L.; Harding, I.J.; Dolan, P.; Adams, M.A. Nerves and blood vessels in degenerated intervertebral discs are confined to physically disrupted tissue. J. Anat. 2018, 233, 86–97. [Google Scholar] [CrossRef]
- Song, C.; Cai, W.; Liu, F.; Cheng, K.; Guo, D.; Liu, Z. An in-depth analysis of the immunomodulatory mechanisms of intervertebral disc degeneration. JOR Spine 2022, 5, e1233. [Google Scholar] [CrossRef]
- Ye, F.; Lyu, F.J.; Wang, H.; Zheng, Z. The involvement of immune system in intervertebral disc herniation and degeneration. JOR Spine 2022, 5, e1196. [Google Scholar] [CrossRef]
- Heimann, M.K.; Thompson, K.; Gunsch, G.; Tang, S.N.; Klamer, B.; Corps, K.; Walter, B.A.; Moore, S.A.; Purmessur, D. Characterization and modulation of the pro-inflammatory effects of immune cells in the canine intervertebral disk. JOR Spine 2024, 7, e1333. [Google Scholar] [CrossRef]
- Sakai, D.; Schol, J.; Watanabe, M. Clinical Development of Regenerative Medicine Targeted for Intervertebral Disc Disease. Medicina 2022, 58, 267. [Google Scholar] [CrossRef]
- WHO Guidelines Approved by the Guidelines Review Committee. In WHO Guideline for Non-Surgical Management of Chronic Primary Low Back Pain in Adults in Primary and Community Care Settings; World Health Organization: Geneva, Switzerland, 2023.
- Haro, H.; Ebata, S.; Inoue, G.; Kaito, T.; Komori, H.; Ohba, T.; Sakai, D.; Sakai, T.; Seki, S.; Shiga, Y.; et al. Japanese Orthopaedic Association (JOA) clinical practice guidelines on the management of lumbar disc herniation, third edition–secondary publication. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2022, 27, 31–78. [Google Scholar] [CrossRef] [PubMed]
- Mannion, A.F.; Leivseth, G.; Brox, J.I.; Fritzell, P.; Hagg, O.; Fairbank, J.C. ISSLS Prize winner: Long-term follow-up suggests spinal fusion is associated with increased adjacent segment disc degeneration but without influence on clinical outcome: Results of a combined follow-up from 4 randomized controlled trials. Spine 2014, 39, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Mannion, A.F.; Brox, J.I.; Fairbank, J.C. Consensus at last! Long-term results of all randomized controlled trials show that fusion is no better than non-operative care in improving pain and disability in chronic low back pain. Spine J. Off. J. North Am. Spine Soc. 2016, 16, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Mannion, A.F.; Brox, J.I.; Fairbank, J.C. Comparison of spinal fusion and nonoperative treatment in patients with chronic low back pain: Long-term follow-up of three randomized controlled trials. Spine J. Off. J. North Am. Spine Soc. 2013, 13, 1438–1448. [Google Scholar] [CrossRef]
- Härtl, R.; Bonassar, L.; Bonassar, L.J. Biological Approaches to Spinal Disc Repair and Regeneration for Clinicians; Thieme Medical Publishers, Incorporated: New York, NY, USA, 2017. [Google Scholar]
- Rudnik-Jansen, I.; van Kruining Kodele, S.; Creemers, L.; Joosten, B. Biomolecular therapies for chronic discogenic low back pain: A narrative review. JOR Spine 2024, 7, e1345. [Google Scholar] [CrossRef]
- Schol, J.; Tamagawa, S.; Volleman, T.N.E.; Ishijima, M.; Sakai, D. A comprehensive review of cell transplantation and platelet-rich plasma therapy for the treatment of disc degeneration-related back and neck pain: A systematic evidence-based analysis. JOR Spine 2024, 7, e1348. [Google Scholar] [CrossRef]
- Schol, J.; Sakai, D.; Warita, T.; Nukaga, T.; Sako, K.; Wangler, S.; Tamagawa, S.; Zeiter, S.; Alini, M.; Grad, S. Homing of vertebral-delivered mesenchymal stromal cells for degenerative intervertebral discs repair–an in vivo proof-of-concept study. JOR Spine 2023, 6, e1228. [Google Scholar] [CrossRef]
- Schol, J.; Sakai, D. Comprehensive narrative review on the analysis of outcomes from cell transplantation clinical trials for discogenic low back pain. North Am. Spine Soc. J. 2023, 13, 100195. [Google Scholar] [CrossRef]
- Harmon, M.D.; Ramos, D.M.; Nithyadevi, D.; Bordett, R.; Rudraiah, S.; Nukavarapu, S.P.; Moss, I.L.; Kumbar, S.G. Growing a backbone–functional biomaterials and structures for intervertebral disc (IVD) repair and regeneration: Challenges, innovations, and future directions. Biomater. Sci. 2020, 8, 1216–1239. [Google Scholar] [CrossRef]
- Yamada, K.; Iwasaki, N.; Sudo, H. Biomaterials and Cell-Based Regenerative Therapies for Intervertebral Disc Degeneration with a Focus on Biological and Biomechanical Functional Repair: Targeting Treatments for Disc Herniation. Cells 2022, 11, 602. [Google Scholar] [CrossRef]
- Ogasawara, S.; Schol, J.; Sakai, D.; Warita, T.; Susumu, T.; Nakamura, Y.; Sako, K.; Tamagawa, S.; Matsushita, E.; Soma, H.; et al. Alginate vs. Hyaluronic Acid as Carriers for Nucleus Pulposus Cells: A Study on Regenerative Outcomes in Disc Degeneration. Cells 2024, 13, 1984. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, L.; Schol, J.; Ruiz-Fernandez, C.; Tamagawa, S.; Soma, H.; Tilotta, V.; Di Giacomo, G.; Cicione, C.; Nakayama, S.; Kamiya, K.; et al. ISSLS PRIZE in Basic Science 2024: Superiority of nucleus pulposus cell- versus mesenchymal stromal cell-derived extracellular vesicles in attenuating disc degeneration and alleviating pain. Eur. Spine J. 2024, 33, 1713–1727. [Google Scholar] [CrossRef]
- DiStefano, T.J.; Vaso, K.; Danias, G.; Chionuma, H.N.; Weiser, J.R.; Iatridis, J.C. Extracellular Vesicles as an Emerging Treatment Option for Intervertebral Disc Degeneration: Therapeutic Potential, Translational Pathways, and Regulatory Considerations. Adv. Healthc. Mater. 2022, 11, e2100596. [Google Scholar] [CrossRef]
- Ambrosio, L.; Schol, J.; Tilotta, V.; Di Giacomo, G.; Cicione, C.; Russo, F.; Sakai, D.; Vadalà, G.; Denaro, V. Extracellular vesicles for intervertebral disc regeneration. In Extracellular Vesicles for Therapeutic and Diagnostic Applications; Elsevier Science: Amsterdam, Netherlands, 2025; pp. 347–395. [Google Scholar]
- Ambrosio, L.; Petrucci, G.; Russo, F.; Cicione, C.; Papalia, R.; Vadala, G.; Denaro, V. Why clinical trials in disc regeneration strive to achieve completion: Insights from publication status and funding sources. JOR Spine 2024, 7, e1329. [Google Scholar] [CrossRef]
- Takahashi, T.; Donahue, R.P.; Nordberg, R.C.; Hu, J.C.; Currall, S.C.; Athanasiou, K.A. Commercialization of regenerative-medicine therapies. Nat. Rev. Bioeng. 2023, 1, 906–929. [Google Scholar] [CrossRef]
- Sakai, D.; Schol, J.; Foldager, C.B.; Sato, M.; Watanabe, M. Regenerative technologies to bed side: Evolving the regulatory framework. J. Orthop. Transl. 2017, 9, 1–7. [Google Scholar] [CrossRef]
- Binch, A.L.A.; Fitzgerald, J.C.; Growney, E.A.; Barry, F. Cell-based strategies for IVD repair: Clinical progress and translational obstacles. Nat. Rev. Rheumatol. 2021, 17, 158–175. [Google Scholar] [CrossRef]
- Sheng, B.; Feng, C.; Zhang, D.; Spitler, H.; Shi, L. Associations between Obesity and Spinal Diseases: A Medical Expenditure Panel Study Analysis. Int. J. Environ. Res. Public. Health 2017, 14, 183. [Google Scholar] [CrossRef]
- Zhou, J.; Mi, J.; Peng, Y.; Han, H.; Liu, Z. Causal Associations of Obesity with the Intervertebral Degeneration, Low Back Pain, and Sciatica: A Two-Sample Mendelian Randomization Study. Front. Endocrinol. 2021, 12, 740200. [Google Scholar] [CrossRef]
- Dario, A.B.; Ferreira, M.L.; Refshauge, K.M.; Lima, T.S.; Ordonana, J.R.; Ferreira, P.H. The relationship between obesity, low back pain, and lumbar disc degeneration when genetics and the environment are considered: A systematic review of twin studies. Spine J. Off. J. North Am. Spine Soc. 2015, 15, 1106–1117. [Google Scholar] [CrossRef]
- Segar, A.H.; Baroncini, A.; Urban, J.P.G.; Fairbank, J.; Judge, A.; McCall, I. Obesity increases the odds of intervertebral disc herniation and spinal stenosis; an MRI study of 1634 low back pain patients. Eur. Spine J. 2024, 33, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Kerr, G.J.; To, B.; White, I.; Millecamps, M.; Beier, F.; Grol, M.W.; Stone, L.S.; Seguin, C.A. Diet-induced obesity leads to behavioral indicators of pain preceding structural joint damage in wild-type mice. Arthritis Res. Ther. 2021, 23, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, J.; Huang, B.; Wang, J.; Shan, Z.; Liu, J.; Chen, Y.; Li, S.; Fan, S.; Zhao, F. Obesity Mediates Apoptosis and Extracellular Matrix Metabolic Imbalances via MAPK Pathway Activation in Intervertebral Disk Degeneration. Front. Physiol. 2019, 10, 1284. [Google Scholar] [CrossRef]
- Tseng, C.; Han, Y.; Lv, Z.; Song, Q.; Wang, K.; Shen, H.; Chen, Z. Glucose-stimulated PGC-1α couples with CBP and Runx2 to mediate intervertebral disc degeneration through transactivation of ADAMTS4/5 in diet-induced obesity mice. Bone 2023, 167, 116617. [Google Scholar] [CrossRef]
- Lintz, M.; Walk, R.E.; Tang, S.Y.; Bonassar, L.J. The degenerative impact of hyperglycemia on the structure and mechanics of developing murine intervertebral discs. JOR Spine 2022, 5, e1191. [Google Scholar] [CrossRef]
- Krishnamoorthy, D.; Hoy, R.C.; Natelson, D.M.; Torre, O.M.; Laudier, D.M.; Iatridis, J.C.; Illien-Jünger, S. Dietary advanced glycation end-product consumption leads to mechanical stiffening of murine intervertebral discs. Dis. Models Mech. 2018, 11, dmm036012. [Google Scholar] [CrossRef]
- Fournier, D.E.; Kiser, P.K.; Shoemaker, J.K.; Battie, M.C.; Seguin, C.A. Vascularization of the human intervertebral disc: A scoping review. JOR Spine 2020, 3, e1123. [Google Scholar] [CrossRef]
- Crump, K.B.; Alminnawi, A.; Bermudez-Lekerika, P.; Compte, R.; Gualdi, F.; McSweeney, T.; Munoz-Moya, E.; Nuesch, A.; Geris, L.; Dudli, S.; et al. Cartilaginous endplates: A comprehensive review on a neglected structure in intervertebral disc research. JOR Spine 2023, 6, e1294. [Google Scholar] [CrossRef]
- Han, Y.C.; Ma, B.; Guo, S.; Yang, M.; Li, L.J.; Wang, S.J.; Tan, J. Leptin regulates disc cartilage endplate degeneration and ossification through activation of the MAPK-ERK signalling pathway in vivo and in vitro. J. Cell. Mol. Med. 2018, 22, 2098–2109. [Google Scholar] [CrossRef]
- Videman, T.; Gibbons, L.E.; Kaprio, J.; Battie, M.C. Challenging the cumulative injury model: Positive effects of greater body mass on disc degeneration. Spine J. Off. J. North Am. Spine Soc. 2010, 10, 26–31. [Google Scholar] [CrossRef]
- Rade, M.; Maatta, J.H.; Freidin, M.B.; Airaksinen, O.; Karppinen, J.; Williams, F.M.K. Vertebral Endplate Defect as Initiating Factor in Intervertebral Disc Degeneration: Strong Association Between Endplate Defect and Disc Degeneration in the General Population. Spine 2018, 43, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Rudisill, S.S.; Hornung, A.L.; Kia, C.; Mallow, G.M.; Aboushaala, K.; Lim, P.; Martin, J.; Wong, A.Y.L.; Toro, S.; Kozaki, T.; et al. Obesity in children with low back pain: Implications with imaging phenotypes and opioid use. Spine J. 2023, 23, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.S.; Kjaer, P.; Korsholm, L.; Bendix, T.; Sorensen, J.S.; Manniche, C.; Leboeuf-Yde, C. Predictors of new vertebral endplate signal (Modic) changes in the general population. Eur. Spine J. 2009, 19, 129–135. [Google Scholar] [CrossRef]
- Han, C.; Kuang, M.-J.; Ma, J.-X.; Ma, X.-L. Prevalence of Modic changes in the lumbar vertebrae and their associations with workload, smoking and weight in northern China. Sci. Rep. 2017, 7, 46341. [Google Scholar] [CrossRef]
- Jentzsch, T.; Geiger, J.; Slankamenac, K.; Werner, C.M. Obesity measured by outer abdominal fat may cause facet joint arthritis at the lumbar spine. J. Back Musculoskelet. Rehabil. 2015, 28, 85–91. [Google Scholar] [CrossRef]
- Manchikanti, L.; Pampati, V.; Singh, V.; Beyer, C.; Damron, K.; Fellows, B. Evaluation of the role of facet joints in persistent low back pain in obesity: A controlled, prospective, comparative evaluation. Pain Physician 2001, 4, 266–272. [Google Scholar] [CrossRef]
- Searcy, S.; Mora, J.; Meroney, M.; Jafari, A.; Henson, C.; Jenkins, K.; Mallett, B.; Shibu, V.; Ogbemudia, B.; Vasilopoulos, T.; et al. Retrospective Review of the Efficacy of Lumbar Radiofrequency Ablation for Lumbar Facet Arthropathy: The Influence of Gender and Obesity. Pain Physician 2023, 26, E695–E701. [Google Scholar]
- Wang, Z.; Zhao, Z.; Han, S.; Hu, X.; Ye, L.; Li, Y.; Gao, J. Advances in research on fat infiltration and lumbar intervertebral disc degeneration. Front. Endocrinol. 2022, 13, 1067373. [Google Scholar] [CrossRef]
- Uçar, İ.; Karartı, C.; Cüce, İ.; Veziroğlu, E.; Özüdoğru, A.; Koçak, F.A.; Dadalı, Y. The relationship between muscle size, obesity, body fat ratio, pain and disability in individuals with and without nonspecific low back pain. Clin. Anat. 2021, 34, 1201–1207. [Google Scholar] [CrossRef]
- Özcan-Ekşi, E.E.; Turgut, V.U.; Küçüksüleymanoğlu, D.; Ekşi, M.Ş. Obesity could be associated with poor paraspinal muscle quality at upper lumbar levels and degenerated spine at lower lumbar levels: Is this a domino effect? J. Clin. Neurosci. 2021, 94, 120–127. [Google Scholar] [CrossRef]
- Walker, P.B.; Sark, C.; Brennan, G.; Smith, T.; Sherman, W.F.; Kaye, A.D. Spinal Epidural Lipomatosis: A Comprehensive Review. Orthop. Rev. 2021, 13, 25571. [Google Scholar] [CrossRef] [PubMed]
- Rigsby, R.K.; Barnes, S.; Sabaté, J.; Oyoyo, U.; Chowdhury, S.; Peters, E.M. Correlation of spinal epidural fat volume with body mass index: A longitudinal study. Clin. Imaging 2023, 98, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, D.; Urquhart, J.C.; Gurr, K.R.; Siddiqi, F.; Bailey, C.S. Obesity and spinal epidural lipomatosis in cauda equina syndrome. Spine J. 2018, 18, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yuan, H.; Hu, L.; Saad, M. Obesity is a risk factor for epidural lipomatosis: A meta-analysis. J. Orthop. Surg. 2021, 29, 23094990211027391. [Google Scholar] [CrossRef]
- Ishihara, S.; Fujita, N.; Azuma, K.; Michikawa, T.; Yagi, M.; Tsuji, T.; Takayama, M.; Matsumoto, H.; Nakamura, M.; Matsumoto, M.; et al. Spinal epidural lipomatosis is a previously unrecognized manifestation of metabolic syndrome. Spine J. 2019, 19, 493–500. [Google Scholar] [CrossRef]
- Cannata, F.; Vadala, G.; Ambrosio, L.; Napoli, N.; Papalia, R.; Denaro, V.; Pozzilli, P. Osteoarthritis and type 2 diabetes: From pathogenetic factors to therapeutic intervention. Diabetes Metab. Res. Rev. 2020, 36, e3254. [Google Scholar] [CrossRef]
- Yi, J.; Zhou, Q.; Huang, J.; Niu, S.; Ji, G.; Zheng, T. Lipid metabolism disorder promotes the development of intervertebral disc degeneration. Biomed. Pharmacother. 2023, 166, 115401. [Google Scholar] [CrossRef]
- Cai, Y.T.; Zhong, X.X.; Mo, L.; Huang, R.Z.; Lin, Q.; Liu, C.J.; Zhang, S.C. Evaluating the causal effect of atherosclerosis on the risk of intervertebral disc degeneration. JOR Spine 2024, 7, e1319. [Google Scholar] [CrossRef]
- McDonnell, E.E.; Buckley, C.T. Consolidating and re-evaluating the human disc nutrient microenvironment. JOR Spine 2022, 5, e1192. [Google Scholar] [CrossRef]
- Kodama, J.; Wilkinson, K.J.; Otsuru, S. Nutrient metabolism of the nucleus pulposus: A literature review. North Am. Spine Soc. J. 2023, 13, 100191. [Google Scholar] [CrossRef]
- Luo, X.H.; Guo, L.J.; Yuan, L.Q.; Xie, H.; Zhou, H.D.; Wu, X.P.; Liao, E.Y. Adiponectin stimulates human osteoblasts proliferation and differentiation via the MAPK signaling pathway. Exp. Cell Res. 2005, 309, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Kalichman, L.; Klindukhov, A.; Li, L.; Linov, L. Indices of Paraspinal Muscles Degeneration: Reliability and Association with Facet Joint Osteoarthritis: Feasibility Study. Clin. Spine Surg. 2016, 29, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Li, X.; Xu, Z.; Wang, L.; Cai, W.; Yang, S.; Liao, W.; Cheng, X. Age-related fatty infiltration of lumbar paraspinal muscles: A normative reference database study in 516 Chinese females. Quant. Imaging Med. Surg. 2020, 10, 1590–1601. [Google Scholar] [CrossRef]
- Steele, J.; Bruce-Low, S.; Smith, D. A review of the clinical value of isolated lumbar extension resistance training for chronic low back pain. PM R. J. Inj. Funct. Rehabil. 2015, 7, 169–187. [Google Scholar] [CrossRef]
- Fujita, N.; Hosogane, N.; Hikata, T.; Iwanami, A.; Watanabe, K.; Shiono, Y.; Okada, E.; Ishikawa, M.; Tsuji, T.; Shimoda, M.; et al. Potential Involvement of Obesity-Associated Chronic Inflammation in the Pathogenesis of Idiopathic Spinal Epidural Lipomatosis. Spine 2016, 41, E1402–E1407. [Google Scholar] [CrossRef]
- Plaza-Briceño, W.; Velásquez, V.B.; Silva-Olivares, F.; Ceballo, K.; Céspedes, R.; Jorquera, G.; Cruz, G.; Martínez-Pinto, J.; Bonansco, C.; Sotomayor-Zárate, R. Chronic Exposure to High Fat Diet Affects the Synaptic Transmission That Regulates the Dopamine Release in the Nucleus Accumbens of Adolescent Male Rats. Int. J. Mol. Sci. 2023, 24, 4703. [Google Scholar] [CrossRef]
- Dragano, N.R.; Haddad-Tovolli, R.; Velloso, L.A. Leptin, Neuroinflammation and Obesity. Front. Horm. Res. 2017, 48, 84–96. [Google Scholar] [CrossRef]
- Song, J.; Kim, O. Perspectives in Lipocalin-2: Emerging Biomarker for Medical Diagnosis and Prognosis for Alzheimer’s Disease. Clin. Nutr. Res. 2018, 7, 1. [Google Scholar] [CrossRef]
- Liang, Y.; Ma, Y.; Wang, J.; Nie, L.; Hou, X.; Wu, W.; Zhang, X.; Tian, Y. Leptin Contributes to Neuropathic Pain via Extrasynaptic NMDAR-nNOS Activation. Mol. Neurobiol. 2021, 58, 1185–1195. [Google Scholar] [CrossRef]
- Guo, M.; Huang, T.-Y.; Garza, J.C.; Chua, S.C.; Lu, X.-Y. Selective deletion of leptin receptors in adult hippocampus induces depression-related behaviours. Int. J. Neuropsychopharmacol. 2013, 16, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, X.; Wu, W. Association Between Overweight or Obesity and Lumbar Disk Diseases: A Meta-Analysis. J. Spinal Disord. Tech. 2015, 28, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Samartzis, D.; Karppinen, J.; Chan, D.; Luk, K.D.; Cheung, K.M. The association of lumbar intervertebral disc degeneration on magnetic resonance imaging with body mass index in overweight and obese adults: A population-based study. Arthritis Rheum. 2012, 64, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Ergin, A.; Ergin, E.; Atasever, A.; Çiyiltepe, H.; Fersahoğlu, M.M.; Esen Bulut, N.; Taşdelen, İ.; Güneş, Y.; Teke, E.; Yılmaz, C.; et al. Investigatıon of the effect of weight loss after laparoscopic sleeve gastrectomy on cobb angle, waist and back pain: A prospective study. Surg. Obes. Relat. Dis. 2023, 19, 1357–1365. [Google Scholar] [CrossRef]
- Lidar, Z.; Behrbalk, E.; Regev, G.J.; Salame, K.; Keynan, O.; Schweiger, C.; Appelbaum, L.; Levy, Y.; Keidar, A. Intervertebral disc height changes after weight reduction in morbidly obese patients and its effect on quality of life and radicular and low back pain. Spine 2012, 37, 1947–1952. [Google Scholar] [CrossRef]
- Ghezelbash, F.; Shirazi-Adl, A.; Plamondon, A.; Arjmand, N.; Parnianpour, M. Obesity and Obesity Shape Markedly Influence Spine Biomechanics: A Subject-Specific Risk Assessment Model. Ann. Biomed. Eng. 2017, 45, 2373–2382. [Google Scholar] [CrossRef]
- You, Q.; Jiang, Q.; Li, D.; Wang, T.; Wang, S.; Cao, S. Waist circumference, waist-hip ratio, body fat rate, total body fat mass and risk of low back pain: A systematic review and meta-analysis. Eur. Spine J. 2021, 31, 123–135. [Google Scholar] [CrossRef]
- Bayartai, M.-E.; Luomajoki, H.; Tringali, G.; De Micheli, R.; Abbruzzese, L.; Sartorio, A. Differences in spinal posture and mobility between adults with obesity and normal weight individuals. Sci. Rep. 2023, 13, 13409. [Google Scholar] [CrossRef]
- Valdovino, A.G.; Bastrom, T.P.; Reighard, F.G.; Cross, M.; Bartley, C.E.; Shah, S.A.; Yaszay, B.; Newton, P.O.; Upasani, V.V. Obesity Is Associated with Increased Thoracic Kyphosis in Adolescent Idiopathic Scoliosis Patients and Nonscoliotic Adolescents. Spine Deform. 2019, 7, 865–869. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, N.G.; Perez-Orribo, L.; Kalb, S.; Reyes, P.M.; Newcomb, A.G.; Hughes, J.; Theodore, N.; Crawford, N.R. The role of obesity in the biomechanics and radiological changes of the spine: An in vitro study. J. Neurosurg. Spine 2016, 24, 615–623. [Google Scholar] [CrossRef]
- Bahramian, M.; Arjmand, N.; El-Rich, M.; Parnianpour, M. Effect of obesity on spinal loads during load-reaching activities: A subject- and kinematics-specific musculoskeletal modeling approach. J. Biomech. 2023, 161, 111770. [Google Scholar] [CrossRef] [PubMed]
- Coppock, J.A.; Danyluk, S.T.; Englander, Z.A.; Spritzer, C.E.; Goode, A.P.; DeFrate, L.E. Increasing BMI increases lumbar intervertebral disc deformation following a treadmill walking stress test. J. Biomech. 2021, 121, 110392. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, R.; Shenoy, S.D.; Jaspal, S.S.; Nellikunja, S.; Fernandes, S. The differential effects of core stabilization exercise regime and conventional physiotherapy regime on postural control parameters during perturbation in patients with movement and control impairment chronic low back pain. BMC Sports Sci. Med. Rehabil. 2010, 2, 13. [Google Scholar] [CrossRef]
- Vanti, C.; Conti, C.; Faresin, F.; Ferrari, S.; Piccarreta, R. The Relationship Between Clinical Instability and Endurance Tests, Pain, and Disability in Nonspecific Low Back Pain. J. Manip. Physiol. Ther. 2016, 39, 359–368. [Google Scholar] [CrossRef]
- Unamuno, X.; Gomez-Ambrosi, J.; Rodriguez, A.; Becerril, S.; Fruhbeck, G.; Catalan, V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef]
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef]
- Poveda, L.; Hottiger, M.; Boos, N.; Wuertz, K. Peroxynitrite induces gene expression in intervertebral disc cells. Spine 2009, 34, 1127–1133. [Google Scholar] [CrossRef]
- Kauppila, L.I. Atherosclerosis and disc degeneration/low-back pain—A systematic review. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 661–670. [Google Scholar] [CrossRef]
- Dakal, T.C.; Xiao, F.; Bhusal, C.K.; Sabapathy, P.C.; Segal, R.; Chen, J.; Bai, X. Lipids dysregulation in diseases: Core concepts, targets and treatment strategies. Lipids Health Dis. 2025, 24, 61. [Google Scholar] [CrossRef]
- Curic, G. Intervertebral Disc and Adipokine Leptin—Loves Me, Loves Me Not. Int. J. Mol. Sci. 2021, 22, 375. [Google Scholar] [CrossRef]
- Tu, S.; Dong, Y.; Li, C.; Jiang, M.; Duan, L.; Zhang, W.; Chen, X. Phosphatidylcholine Ameliorates Palmitic Acid-Induced Lipotoxicity by Facilitating Endoplasmic Reticulum and Mitochondria Contacts in Intervertebral Disc Degeneration. JOR Spine 2025, 8, e70062. [Google Scholar] [CrossRef] [PubMed]
- Francisco, V.; Ait Eldjoudi, D.; Gonzalez-Rodriguez, M.; Ruiz-Fernandez, C.; Cordero-Barreal, A.; Marques, P.; Sanz, M.J.; Real, J.T.; Lago, F.; Pino, J.; et al. Metabolomic signature and molecular profile of normal and degenerated human intervertebral disc cells. Spine J. Off. J. North Am. Spine Soc. 2023, 23, 1549–1562. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Jing, D.; Huang, X.; Yang, W.; Shao, Z. Drp1-mediated mitochondrial fission is involved in oxidized low-density lipoprotein-induced AF cella poptosis. J. Orthop. Res. 2021, 39, 1496–1504. [Google Scholar] [CrossRef]
- Yang, R.Z.; Xu, W.N.; Zheng, H.L.; Zheng, X.F.; Li, B.; Jiang, L.S.; Jiang, S.D. Involvement of oxidative stress-induced annulus fibrosus cell and nucleus pulposus cell ferroptosis in intervertebral disc degeneration pathogenesis. J. Cell. Physiol. 2021, 236, 2725–2739. [Google Scholar] [CrossRef]
- Lee, J.M.; Park, S.; Lee, D.; Ginting, R.P.; Lee, M.R.; Lee, M.W.; Han, J. Reduction in endoplasmic reticulum stress activates beige adipocytes differentiation and alleviates high fat diet-induced metabolic phenotypes. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166099. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, Q.; Ren, Q.; Luo, L.; Ji, G.; Zheng, T. Endoplasmic reticulum stress associates with the development of intervertebral disc degeneration. Front Endocrinol. 2022, 13, 1094394. [Google Scholar] [CrossRef]
- Cheng, X.; Ni, B.; Zhang, F.; Hu, Y.; Zhao, J. High Glucose-Induced Oxidative Stress Mediates Apoptosis and Extracellular Matrix Metabolic Imbalances Possibly via p38 MAPK Activation in Rat Nucleus Pulposus Cells. J. Diabetes Res. 2016, 2016, 3765173. [Google Scholar] [CrossRef]
- Richardson, S.M.; Knowles, R.; Tyler, J.; Mobasheri, A.; Hoyland, J.A. Expression of glucose transporters GLUT-1, GLUT-3, GLUT-9 and HIF-1alpha in normal and degenerate human intervertebral disc. Histochem. Cell Biol. 2008, 129, 503–511. [Google Scholar] [CrossRef]
- Yang, F.; Zhu, D.; Wang, Z.; Ma, Y.; Huang, L.; Kang, X.; Ma, B. Role of Advanced Glycation End Products in Intervertebral Disc Degeneration: Mechanism and Therapeutic Potential. Oxid. Med. Cell Longev. 2022, 2022, 7299005. [Google Scholar] [CrossRef]
- Vlassara, H.; Striker, G.E. AGE restriction in diabetes mellitus: A paradigm shift. Nat. Rev. Endocrinol. 2011, 7, 526–539. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, S.; Cho, H.; Kim, J.H.; Choi, H. Adipokine human Resistin promotes obesity-associated inflammatory intervertebral disc degeneration via pro-inflammatory cytokine cascade activation. Sci. Rep. 2022, 12, 8936. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A. The Role of Adipokines in Intervertebral Disc Degeneration. Med. Sci 2018, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Francisco, V.; Pino, J.; Gonzalez-Gay, M.A.; Lago, F.; Karppinen, J.; Tervonen, O.; Mobasheri, A.; Gualillo, O. A new immunometabolic perspective of intervertebral disc degeneration. Nat. Rev. Rheumatol. 2022, 18, 47–60. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Segar, A.H.; Fairbank, J.C.T.; Urban, J. Leptin and the intervertebral disc: A biochemical link exists between obesity, intervertebral disc degeneration and low back pain-an in vitro study in a bovine model. Eur. Spine J. 2019, 28, 214–223. [Google Scholar] [CrossRef]
- Yuan, B.; Huang, L.; Yan, M.; Zhang, S.; Zhang, Y.; Jin, B.; Ma, Y.; Luo, Z. Adiponectin Downregulates TNF-alpha Expression in Degenerated Intervertebral Discs. Spine 2018, 43, E381–E389. [Google Scholar] [CrossRef]
- Fan, C.; Wang, W.; Yu, Z.; Wang, J.; Xu, W.; Ji, Z.; He, W.; Hua, D.; Wang, W.; Yao, L.; et al. M1 macrophage-derived exosomes promote intervertebral disc degeneration by enhancing nucleus pulposus cell senescence through LCN2/NF-kappaB signaling axis. J. Nanobiotechnol. 2024, 22, 301. [Google Scholar] [CrossRef]
- Cui, H.; Du, X.; Liu, C.; Chen, S.; Cui, H.; Liu, H.; Wang, J.; Zheng, Z. Visfatin promotes intervertebral disc degeneration by inducing IL-6 expression through the ERK/JNK/p38 signalling pathways. Adipocyte 2021, 10, 201–215. [Google Scholar] [CrossRef]
- Tian, S.; Chen, X.; Wu, W.; Lin, H.; Qing, X.; Liu, S.; Wang, B.; Xiao, Y.; Shao, Z.; Peng, Y. Nucleus pulposus cells regulate macrophages in degenerated intervertebral discs via the integrated stress response-mediated CCL2/7-CCR2 signaling pathway. Exp. Mol. Med. 2024, 56, 408–421. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Yang, Y.; Qin, J.; Wang, R.; Wang, S.; Fu, W.; Niu, Q.; Wang, Y.; Li, C.; et al. Osteopontin deficiency promotes cartilaginous endplate degeneration by enhancing the NF-kappaB signaling to recruit macrophages and activate the NLRP3 inflammasome. Bone Res. 2024, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Cao, S.; Zhu, M.D.; Liu, J.Q.; Chen, J.J.; Gao, Y.J. Contribution of chemokine CCL2/CCR2 signaling in the dorsal root ganglion and spinal cord to the maintenance of neuropathic pain in a rat model of lumbar disc herniation. J. Pain 2014, 15, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Nakawaki, M.; Uchida, K.; Miyagi, M.; Inoue, G.; Kawakubo, A.; Kuroda, A.; Satoh, M.; Takaso, M. Sequential CCL2 Expression Profile After Disc Injury in Mice. J. Orthop. Res. 2020, 38, 895–901. [Google Scholar] [CrossRef]
- Gruber, H.E.; Hoelscher, G.L.; Ingram, J.A.; Bethea, S.; Cox, M.; Hanley, E.N., Jr. Proinflammatory cytokines modulate the chemokine CCL2 (MCP-1) in human annulus cells in vitro: CCL2 expression and production. Exp. Mol. Pathol. 2015, 98, 102–105. [Google Scholar] [CrossRef]
- Lee, S.; Jang, S.H.; Suzuki-Narita, M.; Gregoire, S.; Millecamps, M.; Stone, L.S. Voluntary running attenuates behavioural signs of low back pain: Dimorphic regulation of intervertebral disc inflammation in male and female SPARC-null mice. Osteoarthr. Cartil. 2022, 30, 110–123. [Google Scholar] [CrossRef]
- Aboushaala, K.; Chee, A.V.; Toro, S.J.; Vucicevic, R.; Yuh, C.; Dourdourekas, J.; Patel, I.K.; Espinoza-Orias, A.; Oh, C.; Al-Harthi, L.; et al. Discovery of circulating blood biomarkers in patients with and without Modic changes of the lumbar spine: A preliminary analysis. Eur. Spine J. 2024, 33, 1398–1406. [Google Scholar] [CrossRef]
- Krock, E.; Millecamps, M.; Anderson, K.M.; Srivastava, A.; Reihsen, T.E.; Hari, P.; Sun, Y.R.; Jang, S.H.; Wilcox, G.L.; Belani, K.G.; et al. Interleukin-8 as a therapeutic target for chronic low back pain: Upregulation in human cerebrospinal fluid and pre-clinical validation with chronic reparixin in the SPARC-null mouse model. EBioMedicine 2019, 43, 487–500. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Tian, Q.Y.; Liu, B.; Cuellar, J.; Richbourgh, B.; Jia, T.H.; Liu, C.J. Progranulin knockout accelerates intervertebral disc degeneration in aging mice. Sci. Rep. 2015, 5, 9102. [Google Scholar] [CrossRef]
- Wang, S.; Wei, J.; Fan, Y.; Ding, H.; Tian, H.; Zhou, X.; Cheng, L. Progranulin Is Positively Associated with Intervertebral Disc Degeneration by Interaction with IL-10 and IL-17 Through TNF Pathways. Inflammation 2018, 41, 1852–1863. [Google Scholar] [CrossRef]
- Ding, H.; Wei, J.; Zhao, Y.; Liu, Y.; Liu, L.; Cheng, L. Progranulin derived engineered protein Atsttrin suppresses TNF-α-mediated inflammation in intervertebral disc degenerative disease. Oncotarget 2017, 8, 109692. [Google Scholar] [CrossRef]
- Xu, K.; Zhang, Y.; Ilalov, K.; Carlson, C.S.; Feng, J.Q.; Di Cesare, P.E.; Liu, C.J. Cartilage oligomeric matrix protein associates with granulin-epithelin precursor (GEP) and potentiates GEP-stimulated chondrocyte proliferation. J. Biol. Chem. 2007, 282, 11347–11355. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Shao, Z.; Zhang, C.; Chen, L.; Mamun, A.A.; Zhao, N.; Cai, J.; Lou, Z.; Wang, X.; Chen, J. Chemerin facilitates intervertebral disc degeneration via TLR4 and CMKLR1 and activation of NF-kB signaling pathway. Aging 2020, 12, 11732–11753. [Google Scholar] [CrossRef] [PubMed]
- Takano, M.; Hirose, N.; Sumi, C.; Yanoshita, M.; Nishiyama, S.; Onishi, A.; Asakawa, Y.; Tanimoto, K. ANGPTL2 Promotes Inflammation via Integrin alpha5beta1 in Chondrocytes. Cartilage 2021, 13, 885S–897S. [Google Scholar] [CrossRef]
- Cui, L.; Wei, H.; Li, Z.M.; Dong, X.B.; Wang, P.Y. TGF-beta1 aggravates degenerative nucleus pulposus cells inflammation and fibrosis through the upregulation of angiopoietin-like protein 2 expression. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12025–12033. [Google Scholar] [CrossRef]
- Hoyland, J.A.; Le Maitre, C.; Freemont, A.J. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatol. 2008, 47, 809–814. [Google Scholar] [CrossRef]
- Vrselja, Z.; Curic, G. Vertebral marrow adipose tissue adipokines as a possible cause of intervertebral disc inflammation. Jt. Bone Spine 2018, 85, 143–146. [Google Scholar] [CrossRef]
- Yan, X.A.; Shen, E.; Cui, A.; Zhou, F.; Zhuang, Y. Assessing the causal relationship between CRP, IL-1alpha, IL-1beta, and IL-6 levels and intervertebral disc degeneration: A two-sample Mendelian randomization study. Sci. Rep. 2024, 14, 23716. [Google Scholar] [CrossRef]
- Weber, K.T.; Alipui, D.O.; Sison, C.P.; Bloom, O.; Quraishi, S.; Overby, M.C.; Levine, M.; Chahine, N.O. Serum levels of the proinflammatory cytokine interleukin-6 vary based on diagnoses in individuals with lumbar intervertebral disc diseases. Arthritis Res. Ther. 2016, 18, 3. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, L.; Wang, H.X.; Ye, L.; Shi, Y.Y.; Yang, W.Y.; Li, Y.N.; Li, Y. Interleukin-18 Inhibition Protects Against Intervertebral Disc Degeneration via the Inactivation of Caspase-3/9 Dependent Apoptotic Pathways. Immunol. Investig. 2022, 51, 1895–1907. [Google Scholar] [CrossRef]
- Ye, S.; Ju, B.; Wang, H.; Lee, K.B. Bone morphogenetic protein-2 provokes interleukin-18-induced human intervertebral disc degeneration. Bone Jt. Res. 2016, 5, 412–418. [Google Scholar] [CrossRef]
- Landy, E.; Carol, H.; Ring, A.; Canna, S. Biological and clinical roles of IL-18 in inflammatory diseases. Nat. Rev. Rheumatol. 2024, 20, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Li, J.K.; Nie, L.; Zhao, Y.P.; Zhang, Y.Q.; Wang, X.; Wang, S.S.; Liu, Y.; Zhao, H.; Cheng, L. IL-17 mediates inflammatory reactions via p38/c-Fos and JNK/c-Jun activation in an AP-1-dependent manner in human nucleus pulposus cells. J. Transl. Med. 2016, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Veras, M.A.; McCann, M.R.; Tenn, N.A.; Seguin, C.A. Transcriptional profiling of the murine intervertebral disc and age-associated changes in the nucleus pulposus. Connect. Tissue Res. 2020, 61, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Orlando, G.; Ferrante, C.; Chiavaroli, A.; Brunetti, L.; Leone, S. Adipokines: New Potential Therapeutic Target for Obesity and Metabolic, Rheumatic, and Cardiovascular Diseases. Front. Physiol. 2020, 11, 578966. [Google Scholar] [CrossRef]
- Natelson, D.M.; Lai, A.; Krishnamoorthy, D.; Hoy, R.C.; Iatridis, J.C.; Illien-Junger, S. Leptin signaling and the intervertebral disc: Sex dependent effects of leptin receptor deficiency and Western diet on the spine in a type 2 diabetes mouse model. PLoS ONE 2020, 15, e0227527. [Google Scholar] [CrossRef]
- Li, Z.; Liang, J.; Wu, W.K.; Yu, X.; Yu, J.; Weng, X.; Shen, J. Leptin activates RhoA/ROCK pathway to induce cytoskeleton remodeling in nucleus pulposus cells. Int. J. Mol. Sci. 2014, 15, 1176–1188. [Google Scholar] [CrossRef]
- Zhao, C.Q.; Liu, D.; Li, H.; Jiang, L.S.; Dai, L.Y. Expression of leptin and its functional receptor on disc cells: Contribution to cell proliferation. Spine 2008, 33, E858–E864. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Wang, Y.; Cao, F.; Chen, Z.; Hu, Z.; Yu, B.; Feng, H.; Ba, Z.; Liu, T.; et al. Intervertebral disc degeneration in mice with type II diabetes induced by leptin receptor deficiency. BMC Musculoskelet. Disord. 2020, 21, 77. [Google Scholar] [CrossRef]
- Ohnishi, H.; Zhang, Z.; Yurube, T.; Takeoka, Y.; Kanda, Y.; Tsujimoto, R.; Miyazaki, K.; Matsuo, T.; Ryu, M.; Kumagai, N.; et al. Anti-Inflammatory Effects of Adiponectin Receptor Agonist AdipoRon against Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2023, 24, 8566. [Google Scholar] [CrossRef]
- Terashima, Y.; Kakutani, K.; Yurube, T.; Takada, T.; Maeno, K.; Hirata, H.; Miyazaki, S.; Ito, M.; Kakiuchi, Y.; Takeoka, Y.; et al. Expression of adiponectin receptors in human and rat intervertebral disc cells and changes in receptor expression during disc degeneration using a rat tail temporary static compression model. J. Orthop. Surg. Res. 2016, 11, 147. [Google Scholar] [CrossRef]
- Khabour, O.F.; Abu-Rumeh, L.; Al-Jarrah, M.; Jamous, M.; Alhashimi, F. Association of adiponectin protein and ADIPOQ gene variants with lumbar disc degeneration. Exp. Ther. Med. 2014, 8, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Carrion, M.; Frommer, K.W.; Perez-Garcia, S.; Muller-Ladner, U.; Gomariz, R.P.; Neumann, E. The Adipokine Network in Rheumatic Joint Diseases. Int. J. Mol. Sci. 2019, 20, 4091. [Google Scholar] [CrossRef] [PubMed]
- Giardullo, L.; Corrado, A.; Maruotti, N.; Cici, D.; Mansueto, N.; Cantatore, F.P. Adipokine role in physiopathology of inflammatory and degenerative musculoskeletal diseases. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211015034. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, X.; Pan, H.; Yang, H.; Li, X.; Zhang, K.; Wang, H.; Zheng, Z.; Liu, H.; Wang, J. Resistin promotes CCL4 expression through toll-like receptor-4 and activation of the p38-MAPK and NF-kappaB signaling pathways: Implications for intervertebral disc degeneration. Osteoarthr. Cartil. 2017, 25, 341–350. [Google Scholar] [CrossRef]
- Liu, C.; Yang, H.; Gao, F.; Li, X.; An, Y.; Wang, J.; Jin, A. Resistin Promotes Intervertebral Disc Degeneration by Upregulation of ADAMTS-5 Through p38 MAPK Signaling Pathway. Spine 2016, 41, 1414–1420. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.; Qu, R.; Wang, W.; Chen, X.; Cheng, L.; Liu, Y.; Guo, L.; Zhao, Y.; Liu, C. Ghrelin protects against nucleus pulposus degeneration through inhibition of NF-kappaB signaling pathway and activation of Akt signaling pathway. Oncotarget 2017, 8, 91887–91901. [Google Scholar] [CrossRef]
- Cabral, V.L.F.; Wang, F.; Peng, X.; Gao, J.; Zhou, Z.; Sun, R.; Bao, J.; Wu, X. Omentin-1 promoted proliferation and ameliorated inflammation, apoptosis, and degeneration in human nucleus pulposus cells. Arch. Gerontol. Geriatr. 2022, 102, 104748. [Google Scholar] [CrossRef]
- Fujita, Y.; Yamashita, T. The Effects of Leptin on Glial Cells in Neurological Diseases. Front. Neurosci. 2019, 13, 828. [Google Scholar] [CrossRef]
- Kinfe, T.M.; Buchfelder, M.; Chaudhry, S.R.; Chakravarthy, K.V.; Deer, T.R.; Russo, M.; Georgius, P.; Hurlemann, R.; Rasheed, M.; Muhammad, S.; et al. Leptin and Associated Mediators of Immunometabolic Signaling: Novel Molecular Outcome Measures for Neurostimulation to Treat Chronic Pain. Int. J. Mol. Sci. 2019, 20, 4737. [Google Scholar] [CrossRef]
- Wuertz, K.; Haglund, L. Inflammatory mediators in intervertebral disk degeneration and discogenic pain. Glob. Spine J. 2013, 3, 175–184. [Google Scholar] [CrossRef]
- Shin, H.J.; Jin, Z.; An, H.S.; Park, G.; Lee, J.Y.; Lee, S.J.; Jang, H.M.; Jeong, E.A.; Kim, K.E.; Lee, J.; et al. Lipocalin-2 Deficiency Reduces Hepatic and Hippocampal Triggering Receptor Expressed on Myeloid Cells-2 Expressions in High-Fat Diet/Streptozotocin-Induced Diabetic Mice. Brain Sci. 2022, 12, 878. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.H.; Peng, Y.J.; Salter, D.M.; Lee, H.S. Nerve growth factor increases MMP9 activity in annulus fibrosus cells by upregulating lipocalin 2 expression. Eur. Spine J. 2015, 24, 1959–1968. [Google Scholar] [CrossRef] [PubMed]
- Boisson, M.; Borderie, D.; Henrotin, Y.; Teboul-Core, S.; Lefevre-Colau, M.M.; Rannou, F.; Nguyen, C. Serum biomarkers in people with chronic low back pain and Modic 1 changes: A case-control study. Sci. Rep. 2019, 9, 10005. [Google Scholar] [CrossRef]
- Khan, A.N.; Jacobsen, H.E.; Khan, J.; Filippi, C.G.; Levine, M.; Lehman, R.A., Jr.; Riew, K.D.; Lenke, L.G.; Chahine, N.O. Inflammatory biomarkers of low back pain and disc degeneration: A review. Ann. N. Y. Acad. Sci. 2017, 1410, 68–84. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Vasudevan, G.; Tangavel, C.; Ramachandran, K.; Nayagam, S.M.; Muthurajan, R.; Gopalakrishnan, C.; Anand, S.V.; Shetty, A.P.; Kanna, R.M. Does the gut microbiome influence disc health and disease? The interplay between dysbiosis, pathobionts, and disc inflammation: A pilot study. Spine J. Off. J. North Am. Spine Soc. 2024, 24, 1952–1963. [Google Scholar] [CrossRef]
- Aboushaala, K.; Chee, A.V.; Adnan, D.; Toro, S.J.; Singh, H.; Savoia, A.; Dhillon, E.S.; Yuh, C.; Dourdourekas, J.; Patel, I.K.; et al. Gut microbiome dysbiosis is associated with lumbar degenerative spondylolisthesis in symptomatic patients. JOR Spine 2024, 7, e70005. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Z.; Duan, J.; Liu, E.; Sun, F.; Yang, L.; Chen, L.; Yang, S. Unveiling the Gut-Disc Axis: How Microbiome Dysbiosis Accelerates Intervertebral Disc Degeneration. J. Inflamm. Res. 2024, 17, 8271–8280. [Google Scholar] [CrossRef]
- Gleason, B.; Chisari, E.; Parvizi, J. Osteoarthritis Can Also Start in the Gut: The Gut-Joint Axis. Indian. J. Orthop. 2022, 56, 1150–1155. [Google Scholar] [CrossRef]
- Boer, C.G.; Radjabzadeh, D.; Medina-Gomez, C.; Garmaeva, S.; Schiphof, D.; Arp, P.; Koet, T.; Kurilshikov, A.; Fu, J.; Ikram, M.A.; et al. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat. Commun. 2019, 10, 4881. [Google Scholar] [CrossRef]
- Hersoug, L.G.; Moller, P.; Loft, S. Role of microbiota-derived lipopolysaccharide in adipose tissue inflammation, adipocyte size and pyroptosis during obesity. Nutr. Res. Rev. 2018, 31, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Kobayashi, T.; Kakiuchi, T.; Esaki, M.; Tsukamoto, M.; Yoshihara, T.; Hirata, H.; Yabuki, S.; Mawatari, M. Gut-spine axis: A possible correlation between gut microbiota and spinal degenerative diseases. Front. Microbiol. 2023, 14, 1290858. [Google Scholar] [CrossRef] [PubMed]
- Lempesis, I.G.; van Meijel, R.L.J.; Manolopoulos, K.N.; Goossens, G.H. Oxygenation of adipose tissue: A human perspective. Acta Physiol. 2020, 228, e13298. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, J.W.; Osborne, O.; Oh, D.Y.; Sasik, R.; Schenk, S.; Chen, A.; Chung, H.; Murphy, A.; Watkins, S.M.; et al. Increased adipocyte O2 consumption triggers HIF-1alpha, causing inflammation and insulin resistance in obesity. Cell 2014, 157, 1339–1352. [Google Scholar] [CrossRef]
- Xiang, Q.; Wu, Z.; Zhao, Y.; Tian, S.; Lin, J.; Wang, L.; Jiang, S.; Sun, Z.; Li, W. Cellular and molecular mechanisms underlying obesity in degenerative spine and joint diseases. Bone Res. 2024, 12, 71. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Pan, D.; Xu, B.; Xing, X.; Zhou, H.; Zhang, B.; Zhou, S.; Ning, G.; Feng, S. The potential role and trend of HIF-1alpha in intervertebral disc degeneration: Friend or foe? (Review). Mol. Med. Rep. 2021, 23, 239. [Google Scholar] [CrossRef]
- Tran, C.M.; Shapiro, I.M.; Risbud, M.V. Molecular regulation of CCN2 in the intervertebral disc: Lessons learned from other connective tissues. Matrix Biol. J. Int. Soc. Matrix Biol. 2013, 32, 298–306. [Google Scholar] [CrossRef]
- Fainor, M.; Orozco, B.S.; Muir, V.G.; Mahindroo, S.; Gupta, S.; Mauck, R.L.; Burdick, J.A.; Smith, H.E.; Gullbrand, S.E. Mechanical crosstalk between the intervertebral disc, facet joints, and vertebral endplate following acute disc injury in a rabbit model. JOR Spine 2023, 6, e1287. [Google Scholar] [CrossRef]
- Shalash, W.; Ahrens, S.R.; Bardonova, L.A.; Byvaltsev, V.A.; Giers, M.B. Patient-specific apparent diffusion maps used to model nutrient availability in degenerated intervertebral discs. JOR Spine 2021, 4, e1179. [Google Scholar] [CrossRef]
- Arpinar, V.E.; Rand, S.D.; Klein, A.P.; Maiman, D.J.; Muftuler, L.T. Changes in perfusion and diffusion in the endplate regions of degenerating intervertebral discs: A DCE-MRI study. Eur. Spine J. 2015, 24, 2458–2467. [Google Scholar] [CrossRef]
- Ryan, D.H.; Yockey, S.R. Weight Loss and Improvement in Comorbidity: Differences at 5%, 10%, 15%, and Over. Curr. Obes. Rep. 2017, 6, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Haase, C.L.; Lopes, S.; Olsen, A.H.; Satylganova, A.; Schnecke, V.; McEwan, P. Weight loss and risk reduction of obesity-related outcomes in 0.5 million people: Evidence from a UK primary care database. Int. J. Obes. 2021, 45, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Steffens, D.; Maher, C.G.; Pereira, L.S.; Stevens, M.L.; Oliveira, V.C.; Chapple, M.; Teixeira-Salmela, L.F.; Hancock, M.J. Prevention of Low Back Pain: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2016, 176, 199–208. [Google Scholar] [CrossRef]
- de Campos, T.F.; Maher, C.G.; Fuller, J.T.; Steffens, D.; Attwell, S.; Hancock, M.J. Prevention strategies to reduce future impact of low back pain: A systematic review and meta-analysis. Br. J. Sports Med. 2021, 55, 468–476. [Google Scholar] [CrossRef]
- Jenkins, H.J.; Correa, L.; Brown, B.T.; Ferreira, G.E.; Nim, C.; Aspinall, S.L.; Wareham, D.; Choi, J.; Maher, C.G.; Hancock, M.J. Long-term effectiveness of non-surgical interventions for chronic low back pain: A systematic review and meta-analysis. Lancet Rheumatol. 2025, in press. [Google Scholar] [CrossRef]
- Vadalà, G.; Di Giacomo, G.; Ambrosio, L.; Cicione, C.; Tilotta, V.; Russo, F.; Papalia, R.; Denaro, V. The Effect of Irisin on Human Nucleus Pulposus Cells: New Insights into the Biological Crosstalk between the Muscle and Intervertebral Disc. Spine 2023, 48, 468–475, publish ahead of print. [Google Scholar] [CrossRef]
- Wang, P.; Lu, X.; Wen, M.; Li, X.; Gao, Q.; Qin, R. Association between muscle strength and low back pain among middle-aged and older adults: A cross-sectional study. BMC Public. Health 2025, 25, 1869. [Google Scholar] [CrossRef]
- Friend, D.M.; Devarakonda, K.; O’Neal, T.J.; Skirzewski, M.; Papazoglou, I.; Kaplan, A.R.; Liow, J.S.; Guo, J.; Rane, S.G.; Rubinstein, M.; et al. Basal Ganglia Dysfunction Contributes to Physical Inactivity in Obesity. Cell Metab. 2017, 25, 312–321. [Google Scholar] [CrossRef]
- Curran, F.; Davis, M.E.; Murphy, K.; Tersigni, N.; King, A.; Ngo, N.; O’Donoghue, G. Correlates of physical activity and sedentary behavior in adults living with overweight and obesity: A systematic review. Obes. Rev. 2023, 24, e13615. [Google Scholar] [CrossRef]
- Liu, E.; Chang, S.H. Self-esteem and weight status of young adults: Findings from a pilot study. J. Educ. Health Promot. 2022, 11, 263. [Google Scholar] [CrossRef]
- Osei-Assibey, G.; Kyrou, I.; Kumar, S.; Saravanan, P.; Matyka, K.A. Self-Reported Psychosocial Health in Obese Patients before and after Weight Loss. J. Obes. 2010, 2010, 372463. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lazaridou, A.; Paschali, M.; Zgierska, A.E.; Garland, E.L.; Edwards, R.R. Exploring the Relationship Between Endogenous Pain Modulation, Pain Intensity, and Depression in Patients Using Opioids for Chronic Low Back Pain. Clin. J. Pain 2022, 38, 595–600. [Google Scholar] [CrossRef]
- Codella, R.; Chirico, A. Physical Inactivity and Depression: The Gloomy Dual with Rising Costs in a Large-Scale Emergency. Int. J. Environ. Res. Public. Health 2023, 20, 1603. [Google Scholar] [CrossRef]
- Chen, L.H.; Weber, K.; Mehrabkhani, S.; Baskaran, S.; Abbass, T.; Macedo, L.G. The effectiveness of weight loss programs for low back pain: A systematic review. BMC Musculoskelet. Disord. 2022, 23, 488. [Google Scholar] [CrossRef]
- Silisteanu, S.; Covasa, M. Reduction of body weight through nutrition intervention reduces chronic low back pain. In Proceedings of the 2015 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 19 November 2015; IEEE: New York, NY, USA; pp. 1–3. [Google Scholar]
- Robson, E.; Kamper, S.J.; Lee, H.; Palazzi, K.; O’Brien, K.M.; Williams, A.; Hodder, R.K.; Williams, C.M. Compliance with telephone-based lifestyle weight loss programs improves low back pain but not knee pain outcomes: Complier average causal effects analyses of 2 randomised trials. Pain 2022, 163, e862–e868. [Google Scholar] [CrossRef]
- Moiz, A.; Levett, J.Y.; Filion, K.B.; Peri, K.; Reynier, P.; Eisenberg, M.J. Long-Term Efficacy and Safety of Once-Weekly Semaglutide for Weight Loss in Patients Without Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Cardiol. 2024, 222, 121–130. [Google Scholar] [CrossRef]
- Bliddal, H.; Bays, H.; Czernichow, S.; Hemmingsson, J.U.; Hjelmesæth, J.; Morville, T.H.; Koroleva, A.; Neergaard, J.S.; Sánchez, P.V.; Wharton, S.; et al. Once-Weekly Semaglutide in Persons with Obesity and Knee Osteoarthritis. N. Engl. J. Med. 2024, 391, 1573–1583. [Google Scholar] [CrossRef]
- He, Y.; Xu, B.; Zhang, M.; Chen, D.; Wu, S.; Gao, J.; Liu, Y.; Zhang, Z.; Kuang, J.; Fang, Q. Advances in GLP-1 receptor agonists for pain treatment and their future potential. J. Headache Pain 2025, 26, 46. [Google Scholar] [CrossRef]
- Shumnalieva, R.; Kotov, G.; Monov, S. Obesity-Related Knee Osteoarthritis—Current Concepts. Life 2023, 13, 1650. [Google Scholar] [CrossRef]
- McGoey, B.V.; Deitel, M.; Saplys, R.J.; Kliman, M.E. Effect of weight loss on musculoskeletal pain in the morbidly obese. J. Bone Jt. Surg. Br. Vol. 1990, 72, 322–323. [Google Scholar] [CrossRef]
- Melissas, J.; Volakakis, E.; Hadjipavlou, A. Low-back pain in morbidly obese patients and the effect of weight loss following surgery. Obes. Surg. 2003, 13, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Melissas, J.; Kontakis, G.; Volakakis, E.; Tsepetis, T.; Alegakis, A.; Hadjipavlou, A. The effect of surgical weight reduction on functional status in morbidly obese patients with low back pain. Obes. Surg. 2005, 15, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Khoueir, P.; Black, M.H.; Crookes, P.F.; Kaufman, H.S.; Katkhouda, N.; Wang, M.Y. Prospective assessment of axial back pain symptoms before and after bariatric weight reduction surgery. Spine J. Off. J. North Am. Spine Soc. 2009, 9, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Vincent, H.K.; Ben-David, K.; Conrad, B.P.; Lamb, K.M.; Seay, A.N.; Vincent, K.R. Rapid changes in gait, musculoskeletal pain, and quality of life after bariatric surgery. Surg. Obes. Relat. Dis. 2012, 8, 346–354. [Google Scholar] [CrossRef]
- Cakir, T.; Oruc, M.T.; Aslaner, A.; Duygun, F.; Yardimci, E.C.; Mayir, B.; Bulbuller, N. The effects of laparoscopic sleeve gastrectomy on head, neck, shoulder, low back and knee pain of female patients. Int. J. Clin. Exp. Med. 2015, 8, 2668–2673. [Google Scholar]
- Taylor, D.A.; Merten, S.L.; Sandercoe, G.D.; Gahankari, D.; Ingram, S.B.; Moncrieff, N.J.; Ho, K.; Sellars, G.D.; Magnusson, M.R. Abdominoplasty Improves Low Back Pain and Urinary Incontinence. Plast. Reconstr. Surg. 2018, 141, 637–645. [Google Scholar] [CrossRef]
- Bhandari, M.; Mathur, W.; Kosta, S.; Salvi, P.; Fobi, M. Assessment of functional ability of nonambulatory patients with obesity: After and before bariatric surgery. Surg. Obes. Relat. Dis. 2019, 15, 2087–2093. [Google Scholar] [CrossRef]
- Soteropulos, C.E.; Edinger, K.M.; Leibl, K.E.; Siebert, J.W. Improvement in Back Pain Following Abdominoplasty: Results of a 10-Year, Single-Surgeon Series. Aesthet. Surg. J. 2020, 40, 1309–1315. [Google Scholar] [CrossRef]
- Patel, S.D.; Joo, A.; Xu, J.; Palic, A.; Wood, J.J.; Sirls, E.R.; Tomczyk, E.G.; Rothkopf, D.M. Back Pain According to Roland-Morris Low Back Pain Scale After Abdominoplasty with Plication: A Prospective Case Series. Ann. Plast. Surg. 2023, 90, S704–S706. [Google Scholar] [CrossRef]
- Roffey, D.M.; Ashdown, L.C.; Dornan, H.D.; Creech, M.J.; Dagenais, S.; Dent, R.M.; Wai, E.K. Pilot evaluation of a multidisciplinary, medically supervised, nonsurgical weight loss program on the severity of low back pain in obese adults. Spine J. Off. J. North Am. Spine Soc. 2011, 11, 197–204. [Google Scholar] [CrossRef]
- Williams, A.; Wiggers, J.; O’Brien, K.M.; Wolfenden, L.; Yoong, S.L.; Hodder, R.K.; Lee, H.; Robson, E.K.; McAuley, J.H.; Haskins, R.; et al. Effectiveness of a healthy lifestyle intervention for chronic low back pain: A randomised controlled trial. Pain 2018, 159, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Dunlevy, C.; MacLellan, G.A.; O’Malley, E.; Blake, C.; Breen, C.; Gaynor, K.; Wallace, N.; Yoder, R.; Casey, D.; Mehegan, J.; et al. Does changing weight change pain? Retrospective data analysis from a national multidisciplinary weight management service. Eur. J. Pain 2019, 23, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Safari, M.B.; Nozad, A.; Ghaffari, F.; Ghavamzadeh, S.; Alijaniha, F.; Naseri, M. Efficacy of a Short-Term Low-Calorie Diet in Overweight and Obese Patients with Chronic Sciatica: A Randomized Controlled Trial. J. Altern. Complement. Med. 2020, 26, 508–514. [Google Scholar] [CrossRef]
- Ward, S.J.; Coates, A.M.; Carter, S.; Baldock, K.L.; Berryman, C.; Stanton, T.R.; Yandell, C.; Buckley, J.D.; Tan, S.Y.; Rogers, G.B.; et al. Effects of weight loss through dietary intervention on pain characteristics, functional mobility, and inflammation in adults with elevated adiposity. Front. Nutr. 2024, 11, 1274356. [Google Scholar] [CrossRef]
- Mohamed Mohamed, W.J.; Joseph, L.; Canby, G.; Paungmali, A.; Sitilertpisan, P.; Pirunsan, U. Are patient expectations associated with treatment outcomes in individuals with chronic low back pain? A systematic review of randomised controlled trials. Int. J. Clin. Pract. 2020, 74, e13680. [Google Scholar] [CrossRef]
- Eklund, A.; De Carvalho, D.; Page, I.; Wong, A.; Johansson, M.S.; Pohlman, K.A.; Hartvigsen, J.; Swain, M. Expectations influence treatment outcomes in patients with low back pain. A secondary analysis of data from a randomized clinical trial. Eur. J. Pain 2019, 23, 1378–1389. [Google Scholar] [CrossRef]
- Würfel, M.; Blüher, M.; Stumvoll, M.; Ebert, T.; Kovacs, P.; Tönjes, A.; Breitfeld, J. Adipokines as Clinically Relevant Therapeutic Targets in Obesity. Biomedicines 2023, 11, 1427. [Google Scholar] [CrossRef]
- Kenawy, H.M.; Nunez, M.I.; Morales, X.; Lisiewski, L.E.; Burt, K.G.; Kim, M.K.M.; Campos, L.; Kiridly, N.; Hung, C.T.; Chahine, N.O. Sex differences in the biomechanical and biochemical responses of caudal rat intervertebral discs to injury. JOR Spine 2023, 6, e1299. [Google Scholar] [CrossRef]
- Bonnheim, N.B.; Lazar, A.A.; Kumar, A.; Akkaya, Z.; Zhou, J.; Guo, X.; O’Neill, C.; Link, T.M.; Lotz, J.C.; Krug, R.; et al. ISSLS Prize in Bioengineering Science 2023: Age- and sex-related differences in lumbar intervertebral disc degeneration between patients with chronic low back pain and asymptomatic controls. Eur. Spine J. 2023, 32, 1517–1524. [Google Scholar] [CrossRef]
- Mosley, G.E.; Wang, M.; Nasser, P.; Lai, A.; Charen, D.A.; Zhang, B.; Iatridis, J.C. Males and females exhibit distinct relationships between intervertebral disc degeneration and pain in a rat model. Sci. Rep. 2020, 10, 15120. [Google Scholar] [CrossRef]
- Mosley, G.E.; Hoy, R.C.; Nasser, P.; Kaseta, T.; Lai, A.; Evashwick-Rogler, T.W.; Lee, M.; Iatridis, J.C. Sex Differences in Rat Intervertebral Disc Structure and Function Following Annular Puncture Injury. Spine 2019, 44, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Song, X.X.; Yu, Y.J.; Li, X.F.; Liu, Z.D.; Yu, B.W.; Guo, Z. Estrogen receptor expression in lumbar intervertebral disc of the elderly: Gender- and degeneration degree-related variations. Jt. Bone Spine 2014, 81, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Otani, Y.; Schol, J.; Sakai, D.; Nakamura, Y.; Sako, K.; Warita, T.; Tamagawa, S.; Ambrosio, L.; Munesada, D.; Ogasawara, S.; et al. Assessment of Tie2-Rejuvenated Nucleus Pulposus Cell Transplants from Young and Old Patient Sources Demonstrates That Age Still Matters. Int. J. Mol. Sci. 2024, 25, 8335. [Google Scholar] [CrossRef] [PubMed]
- Vo, N.V.; Piva, S.R.; Patterson, C.G.; McKernan, G.P.; Zhou, L.; Bell, K.M.; Anderst, W.; Greco, C.M.; Schneider, M.J.; Delitto, A.; et al. Toward the Identification of Distinct Phenotypes: Research Protocol for the Low Back Pain Biological, Biomechanical, and Behavioral (LB3P) Cohort Study and the BACPAC Mechanistic Research Center at the University of Pittsburgh. Pain Med. 2023, 24, S36–S47. [Google Scholar] [CrossRef]

| Tissue | Structural Changes | Functional Changes | Ref |
|---|---|---|---|
| NP | Loss of proteoglycans Decreased water content AGE accumulation Fibrotic changes | Reduced ability to resist compression Loss of mechanical shock absorption Loss in spinal height and stability | [33,67,68,69] |
| AF | Disruption of lamellar structure AGE accumulation Fibrotic changes Microtears | Decreased tensile strength and increased stiffness Loss of structural integrity and containment of NP | [70,71] |
| CEP | Thinning and calcification Impaired permeability Microfractures | Reduced nutrient and waste exchange Loss of NP environment containment Development of focal defects | [64,72,73,74,75,76,77,78,79] |
| Facet joints | Osteoarthritic changes Joint effusion | Reduced spinal stability Compression of neighbouring tissues | [80,81,82] |
| PSMs | Increased fatty infiltration Reduced muscle fibre size Muscle atrophy | Reduced spinal stability Decreased endurance and strength | [83,84,85] |
| Epidural fat | Excessive fat accumulation Compression of adjacent nervous structures | Increased risk of spinal stenosis and cauda equina syndrome Compression-related pain | [86,87,88,89,90] |
| Adipokine | Type | Function | Effect on IDD | Refs. |
|---|---|---|---|---|
| CCL2 | Chemokine | Regulates immune cell migration. Involved in neuroinflammation. | Increases macrophage infiltration, enhances NF-κB activation, and contributes to IDD. | [145,146,147,148,149] |
| CXCL5 | Chemokine | Pro-inflammatory mediator and attractant for granulocytes. | Elevated in LBP and IDD models and Modic-change patients. | [150,151,152] |
| Progranulin | Cytokine | Autocrine growth factor promoting chondrocyte differentiation and proliferation. Implicated in inflammation, host defence, wound healing, and rheumatic diseases. | Increases MMP-13, ADAMTS-5, and iNOS in AF and CEP cells. Activates NF-κB, Wnt–β-catenin signalling. It is increased in IDD and positively related to its clinical symptoms, playing a protecting role by inducing IL-10, reducing IL-17. | [153,154,155,156] |
| Lipocalin-2 | Cytokine | Pro-inflammatory adipokine regulating hematopoietic apoptosis, immune responses, and metabolic homeostasis. Acts as a sensor of mechanical load and inflammation. | Increases MMP9 activity via nerve growth factor. Promotes IDD through LCN2/NF-κB axis. | [138,143] |
| Chemerin | Cytokine | Links innate and adaptive immunity. Interacts with CMKLR1 to regulate inflammation. | Activates NF-κB via CMKLR1 and TLR4. Increases inflammatory mediators leading to ECM degradation. Chemerin and CMKLR1 increase with IDD, especially in obese individuals. | [157] |
| ANGPTL2 | Cytokine | Signalling pathway mediator in chronic low-grade inflammatory diseases, including obesity, diabetes mellitus, and cardiovascular diseases. Potential target of TGF-β, contributing to capillary formation. | Its upregulation aggravates inflammation and fibrosis in NP cells. Induced by TGF-β1 overexpression triggered by IL-1β. Promotes IDD by increasing IL-6, TNF-α, collagen I, and collagen III expression. | [158,159] |
| TNF-α | Cytokine | Multifunctional cytokine involved in autoimmune diseases and inflammatory responses. Strongly promotes interleukins and MMP production. | Promotes inflammation, induces IL-1, IL-17. Increases ECM degrading metalloproteinases leading to IDD. | [160] |
| Interleukins | Cytokine | Interleukins are a diverse family of cytokines regulating immune responses. IL-1 and IL-6 drive inflammation and share functional overlap with TLR signalling. IL-17 and IL-18 amplify autoimmune and inflammatory pathways, while IL-10 acts as a key anti-inflammatory mediator, limiting immune cell activation. | IL-1 triggers inflammatory cascades that drive IDD progression. IL-6 promotes IDD through ERK/JNK/p38 signalling and is elevated in IDD patients. IL-18 accelerates ECM degradation by upregulating MMP13 and downregulating type II collagen and SOX6 but may also exert protective effects by inhibiting caspase-3/9-dependent apoptosis. IL-17 is increased in lumbar disc herniation, enhancing inflammation and immune activation. IL-10, found at higher levels in IDD patients, regulates immune responses with potential anti-inflammatory effects. | [144,152,154,161,162,163,164,165,166,167] |
| SFRP5 | Cytokine | Anti-inflammatory adipokine. Blocks WNT5A signalling, regulating inflammation, proliferation, and differentiation. | Upregulated in NP growth. Plays a role in laminin and BMP receptor binding pathways. | [168,169] |
| Leptin | Hormone | Regulates appetite and energy balance via LepR and modulates innate immunity. Stimulates pro-inflammatory cytokine release and influences insulin secretion, lipid metabolism, thermogenesis, angiogenesis, and bone/cartilage homeostasis. | Expression in NP and AF cells. Increases cell proliferation, proteolytic enzyme synthesis, NO production. Reduces aggrecan levels, induces differentiation, regulates CEP degeneration, and inhibits apoptosis of NP cells. | [74,125,139,141,170,171,172,173] |
| Adiponectin | Hormone | Anti-inflammatory properties, promoting fatty acid oxidation and glucose uptake. Implicated in metabolic syndrome, cardiovascular diseases, osteoarthritis, and rheumatoid arthritis. | Increases TNF secretion in AF cells, reduces TNF in NP cells, expression reduced in degenerated NP cells. Exert anti-inflammatory effects in diseased disc tissues. Conflicting evidence regarding AdipoR1, AdipoR2 expression in NP cells. | [138,174,175,176] |
| Visfatin | Hormone | Expressed in visceral fat and involved in energy metabolism and inflammation. It promotes inflammatory cytokine production and modulates MAPK and NF-κB signalling pathways. Its expression is upregulated under hypoxic conditions, linking it to metabolic stress and inflammatory responses. | Highly concentrated in NP cells with severe IDD. Expression increased by IL-1β. Regulates autophagy. Promotes IL-6 production, mediates IL-1β-induced NP cell degeneration and apoptosis. | [127,144,177,178] |
| Resistin | Hormone | Links obesity and diabetes by promoting insulin resistance. Modulates angiogenesis and inflammatory environments in joints. | Highly expressed in degenerative disc cells. Increases ADAMTS-5 and CCL4 in NP cells. Promotes IDD via p38 MAPK. Enhances inflammatory responses and MMP expression. | [136,179,180] |
| Ghrelin | Hormone | Involved in growth hormone secretion, appetite regulation, glucose metabolism, bone/cartilage metabolism. | Counteracts IL-1-induced catabolic effects. Reduces IL-1β-induced ADAMTS-5, MMP-13, TNF-α. Induces ECM production via GHSR activation. | [181] |
| Omentin-1 | Hormone | Anti-inflammatory adipokine involved in immunity and metabolic processes. Its expression level is inversely correlated with the progression of various diseases, including diabetes, obesity, and osteoarthritis. | Activates PI3K/Akt and SIRT1 pathways. Protects NP cells from inflammation and apoptosis induced by IL-1β. | [169,182] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Fernandez, C.; Schol, J.; Ambrosio, L.; Sakai, D. The Vicious Cycle of Obesity and Low Back Pain: A Comprehensive Review. Appl. Sci. 2025, 15, 6660. https://doi.org/10.3390/app15126660
Ruiz-Fernandez C, Schol J, Ambrosio L, Sakai D. The Vicious Cycle of Obesity and Low Back Pain: A Comprehensive Review. Applied Sciences. 2025; 15(12):6660. https://doi.org/10.3390/app15126660
Chicago/Turabian StyleRuiz-Fernandez, Clara, Jordy Schol, Luca Ambrosio, and Daisuke Sakai. 2025. "The Vicious Cycle of Obesity and Low Back Pain: A Comprehensive Review" Applied Sciences 15, no. 12: 6660. https://doi.org/10.3390/app15126660
APA StyleRuiz-Fernandez, C., Schol, J., Ambrosio, L., & Sakai, D. (2025). The Vicious Cycle of Obesity and Low Back Pain: A Comprehensive Review. Applied Sciences, 15(12), 6660. https://doi.org/10.3390/app15126660









