Developing a Superhydrophilic/Underwater Superoleophobic Plasma-Modified PVDF Microfiltration Membrane with Copolymer Hydrogels for Oily Water Separation
Abstract
1. Introduction
2. Experimental
2.1. Materials and Chemicals
2.2. Characterizations
2.3. Water Absorbency Measurement of the AM/NaA Hydrogel
2.4. Preparation of the AM-NaA/PVDF Membrane
2.5. Oily Water Preparation
2.6. Oily Water Separation
2.7. Analysis of the Residual Oil Concentration in the Permeate
3. Results and Discussion
3.1. Effect of Reaction Time on Grafting and Wettability
3.2. Surface Morphology
3.3. Structural Characterization of the Membranes by FT-IR Analysis
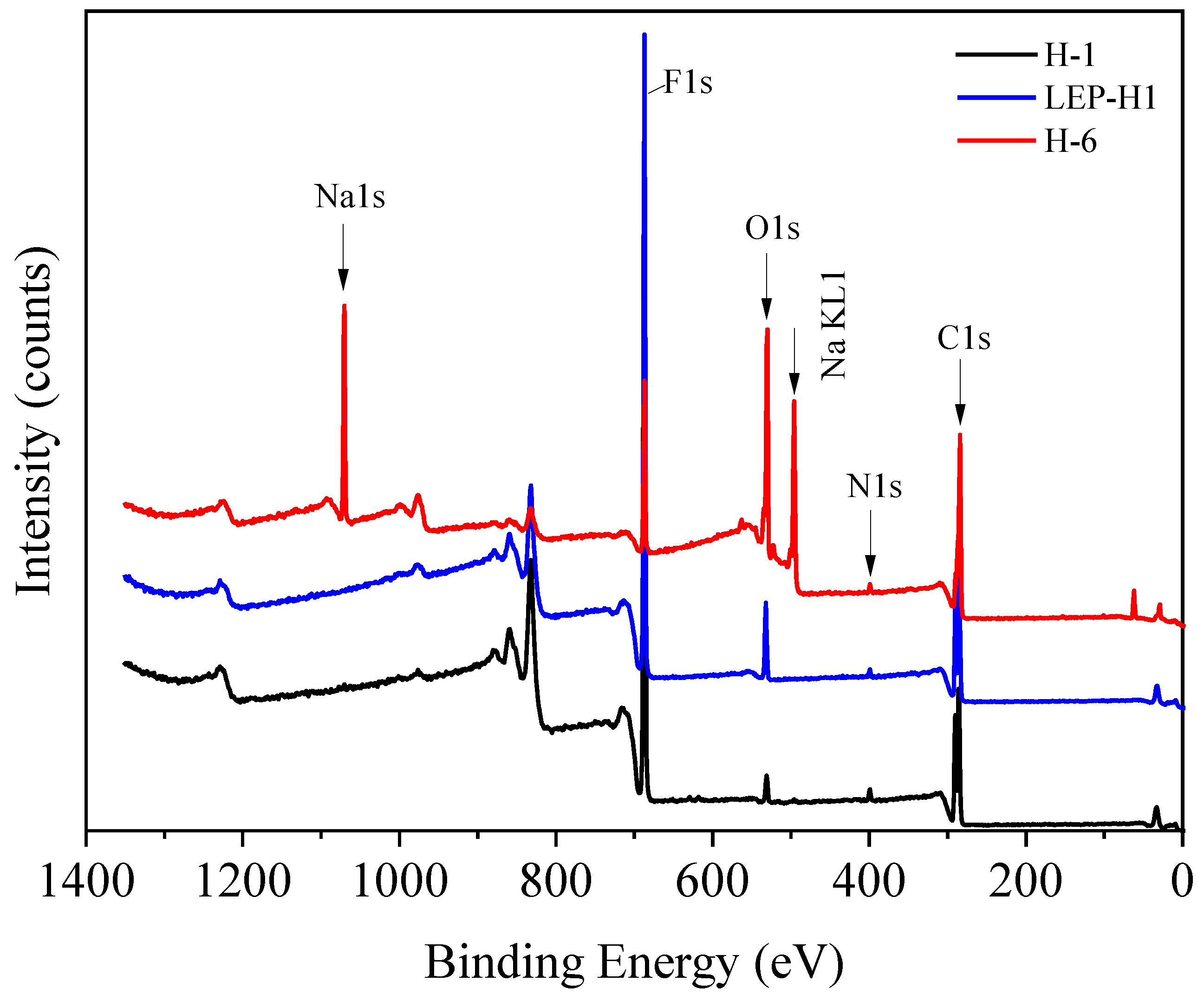
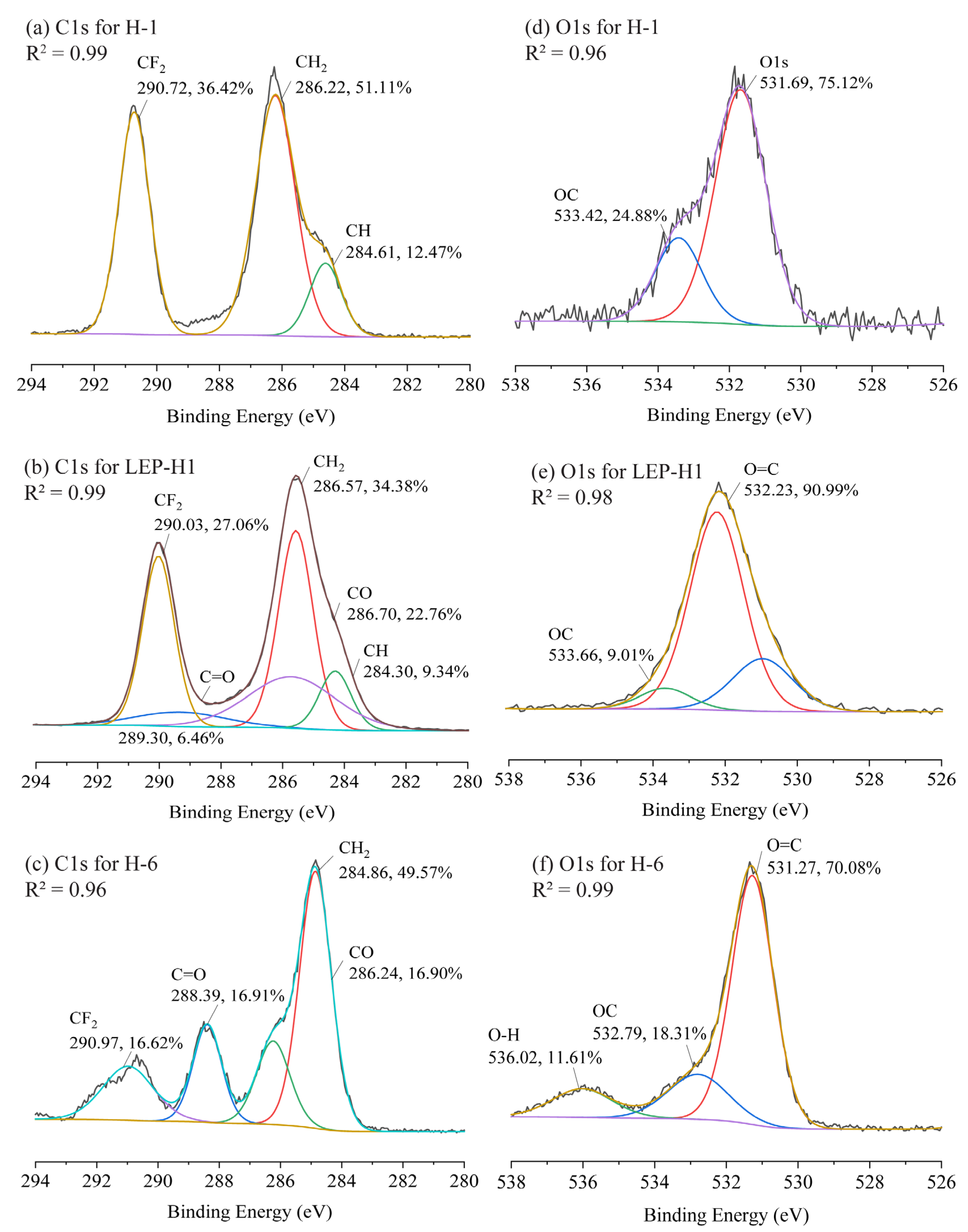
3.4. Structural Characterization of the Membranes by XPS Analysis
3.5. Wettability of the Membranes
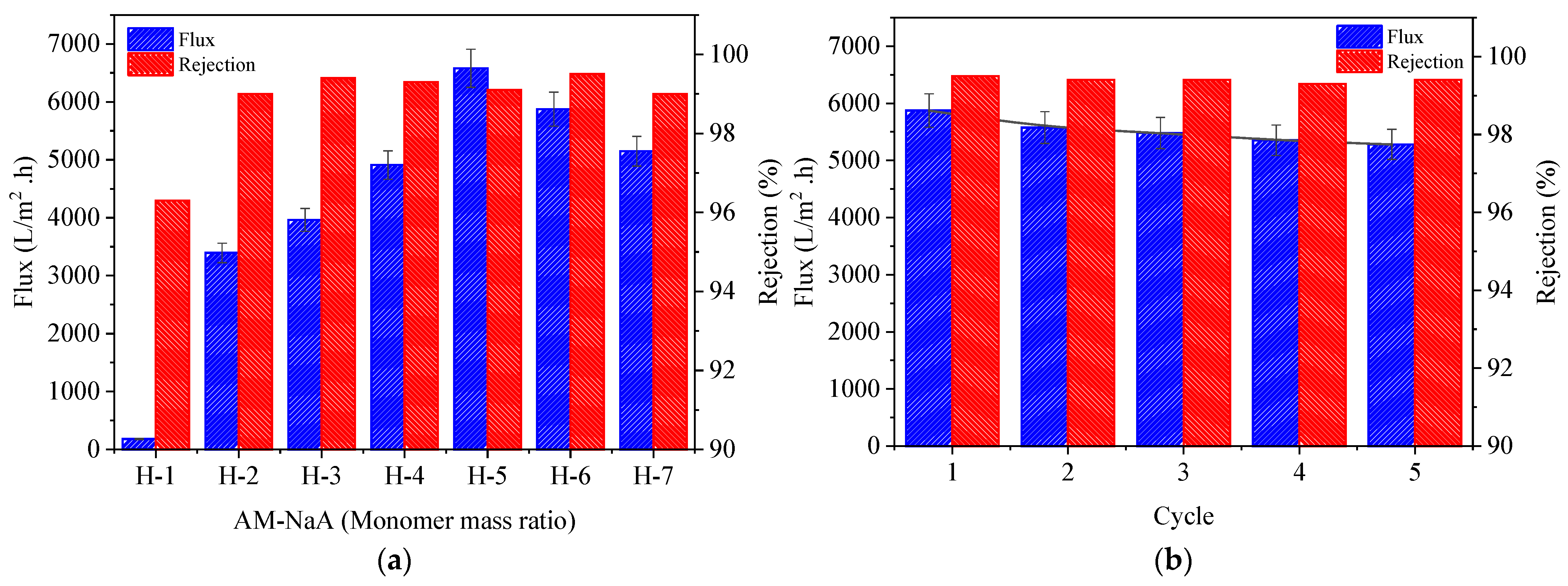
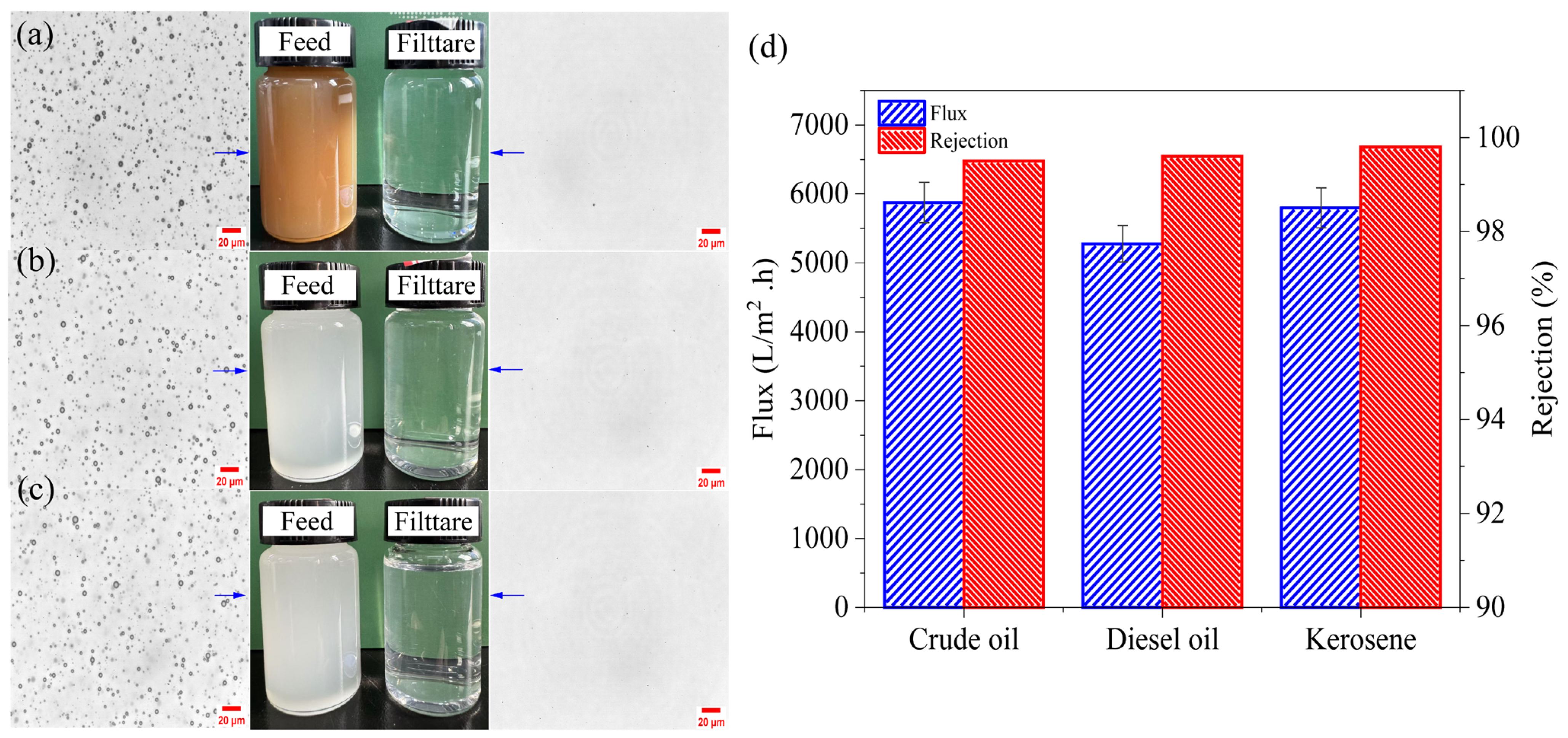
3.6. Oil-in-Water Emulsion Separation and Reusability Performance
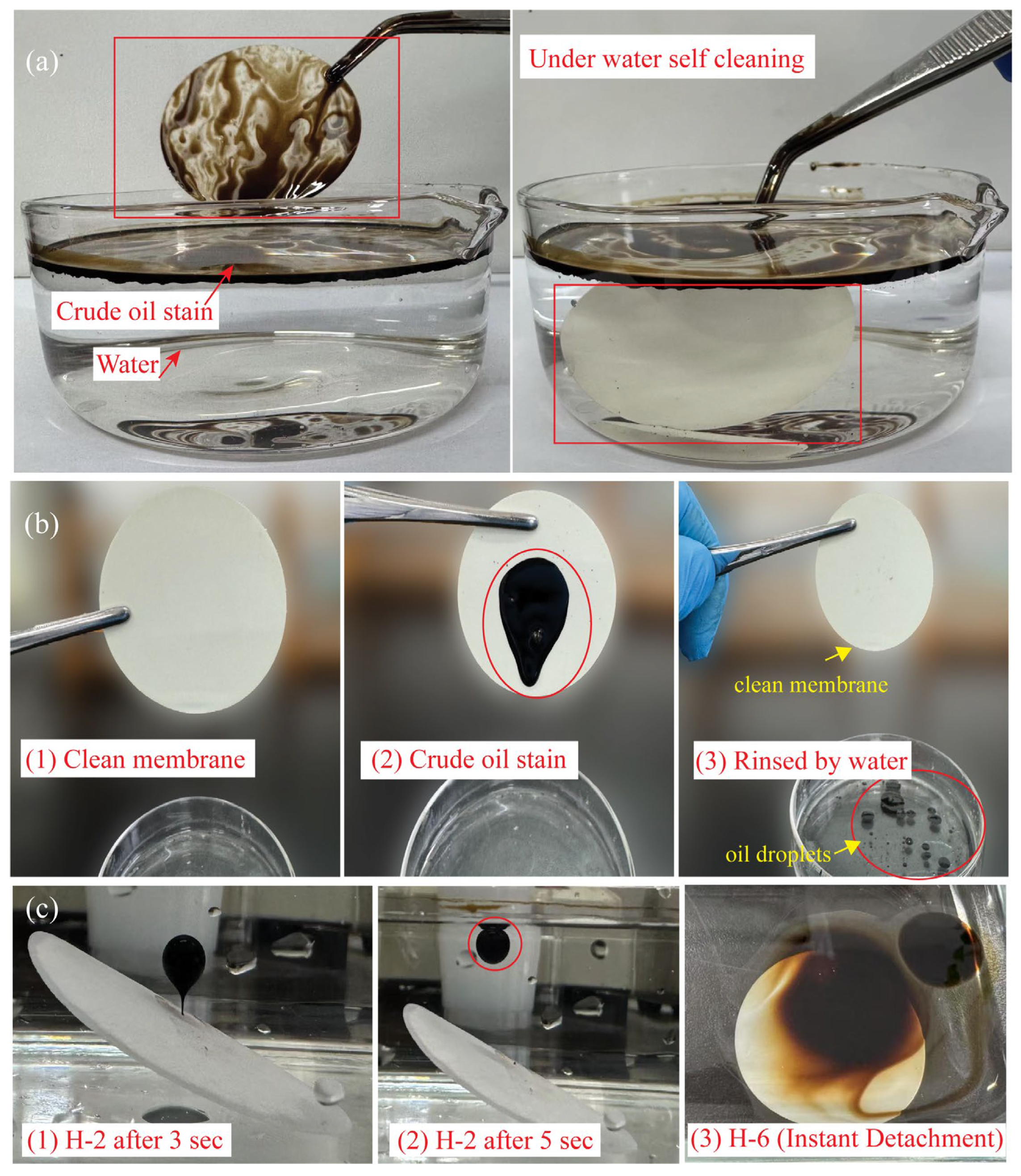
3.7. Chemical Stability and Self-Cleaning Function
4. Demulsification of Crude Oil-in-Water Emulsions for Enhanced Membrane Separation
5. Comparison Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajbongshi, A.; Gogoi, S.B. A review on oilfield produced water and its treatment technologies. Pet. Res. 2024, 9, 640–656. [Google Scholar] [CrossRef]
- Ghafoori, S.; Omar, M.; Koutahzadeh, N.; Zendehboudi, S.; Malhas, R.N.; Mohamed, M.; Al-Zubaidi, S.; Redha, K.; Baraki, F.; Mehrvar, M. New advancements, challenges, and future needs on treatment of oilfield produced water: A state-of-the-art review. Sep. Purif. Technol. 2022, 289, 120652. [Google Scholar] [CrossRef]
- Yadav, D.; Rangabhashiyam, S.; Verma, P.; Singh, P.; Devi, P.; Kumar, P.; Hussain, C.M.; Gaurav, G.K.; Kumar, K.S. Environmental and health impacts of contaminants of emerging concerns: Recent treatment challenges and approaches. Chemosphere 2021, 272, 129492. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Dai, M.; Wu, Y.; Peng, C. Emulsion system, demulsification and membrane technology in oil–water emulsion separation: A comprehensive review. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1254–1278. [Google Scholar] [CrossRef]
- Amakiri, K.T.; Ogolo, N.A.; Angelis-Dimakis, A.; Albert, O. Physicochemical assessment and treatment of produced water: A case study in Niger delta Nigeria. Pet. Res. 2023, 8, 87–95. [Google Scholar] [CrossRef]
- Zainal, B.S.; Ker, P.J.; Mohamed, H.; Ong, H.C.; Fattah, I.; Rahman, S.A.; Nghiem, L.D.; Mahlia, T.M.I. Recent advancement and assessment of green hydrogen production technologies. Renew. Sustain. Energy Rev. 2024, 189, 113941. [Google Scholar] [CrossRef]
- Lee, B.X.; Kjaerulf, F.; Turner, S.; Cohen, L.; Donnelly, P.D.; Muggah, R.; Davis, R.; Realini, A.; Kieselbach, B.; MacGregor, L.S.; et al. Transforming our world: Implementing the 2030 agenda through sustainable development goal indicators. J. Public Health Policy 2016, 37, 13–31. [Google Scholar] [CrossRef]
- Sadek, S.A.; Al-Jubouri, S.M. Structure and performance of polyvinylchloride microfiltration membranes improved by green silicon oxide nanoparticles for oil-in-water emulsion separation. Mater. Today Sustain. 2023, 24, 100600. [Google Scholar] [CrossRef]
- Forero, J.E.; Ortíz, O.P.; Nariño, F.A.; Díaz, J.; Peña, H. Design and development of a high efficiency tank for crude oil dehydration (I). CT&F Ciencia Tecnología y Futuro 2008, 3, 185–199. [Google Scholar]
- Shi, P.; Gou, Y.; Li, J.; Zheng, Q.; Zhong, X.; Duan, M.; Pu, W. Recycling of crude oil from oily wastewater via a novel hydrogel coalescer. Fuel 2022, 313, 123040. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Liang, L.; Tu, F.; Li, Z.; Tang, X.; Dai, L.; Li, L. Research Progress on Membrane Separation Technology for Oily Wastewater Treatment. Toxics 2024, 12, 794. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Qi, B.; Guo, Z.; Wang, D.; Jiao, T. Recent developments in the application of membrane separation technology and its challenges in oil-water separation: A review. Chemosphere 2023, 327, 138528. [Google Scholar] [CrossRef] [PubMed]
- Manouchehri, M. A comprehensive review on state-of-the-art antifouling super (wetting and anti-wetting) membranes for oily wastewater treatment. Adv. Colloid Interface Sci. 2024, 323, 103073. [Google Scholar] [CrossRef] [PubMed]
- Sutrisna, P.D.; Kurnia, K.A.; Siagian, U.W.; Ismadji, S.; Wenten, I.G. Membrane fouling and fouling mitigation in oil–water separation: A review. J. Environ. Chem. Eng. 2022, 10, 107532. [Google Scholar] [CrossRef]
- Sadek, S.A.; Al-Jubouri, S.M. Highly efficient oil-in-water emulsion separation based on innovative stannic oxide/polyvinylchloride (SnO2/PVC) microfiltration membranes. J. Ind. Eng. Chem. 2024, 140, 577–588. [Google Scholar] [CrossRef]
- Mansi, A.; El-Marsafy, S.; Elhenawy, Y.; Bassyouni, M. Assessing the potential and limitations of membrane-based technologies for the treatment of oilfield produced water. Alex. Eng. J. 2023, 68, 787–815. [Google Scholar] [CrossRef]
- Adday, A.S.; Al-Jubouri, S.M. Developing a versatile visible-light-driven polyvinylidene fluoride/Ag2O@CRA photocatalytic membrane for efficient treatment of organic pollutants-contained wastewater. J. Water Process Eng. 2025. 73, 107713. [CrossRef]
- Sadek, S.A.; Al-Jubouri, S.M.; Al-Batty, S. Investigating the fouling models of the microfiltration mixed matrix membranes-based oxide nanoparticles applied for oil-in-water emulsion separation. Iraqi J. Chem. Pet. Eng. 2024, 25, 1–16. [Google Scholar] [CrossRef]
- Dargaville, T.R.; A George, G.; Hill, D.J.; Whittaker, A.K. High energy radiation grafting of fluoropolymers. Prog. Polym. Sci. 2003, 28, 1355–1376. [Google Scholar] [CrossRef]
- Saleem, A.G.; Al-Jubouri, S.M. Separation performance of cationic and anionic dyes from water using polyvinylidene fluoride-based ultrafiltration membrane incorporating polyethylene glycol. Desalination Water Treat. 2024, 319, 100546. [Google Scholar] [CrossRef]
- Saleem, A.G.; Al-Jubouri, S.M.; Al-Batty, S.; Hakami, M.W. Determination of controlling fouling mechanism using the Hermia models and estimation of the manufacturing costs of the modified polyvinylidene fluoride-based ultrafiltration membranes. Iraqi J. Chem. Pet. Eng. 2025, 26, 67–76. [Google Scholar] [CrossRef]
- Xin, Y.; Qi, B.; Wu, X.; Yang, C.; Li, B. Different types of membrane materials for oil-water separation: Status and challenges. Colloid Interface Sci. Commun. 2024, 59, 100772. [Google Scholar] [CrossRef]
- Alrasheedi, N.F.; Abdulazeez, I.; Baig, N.; Salhi, B.; Asmaly, H.A.; Haladu, S.A.; Elsharif, A.M. Antifouling macrocyclic-engineered PVDF membrane for the low-pressure separation of surfactant-stabilized oily wastewater. J. Environ. Chem. Eng. 2024, 12, 112850. [Google Scholar] [CrossRef]
- Dallaev, R.; Pisarenko, T.; Sobola, D.; Orudzhev, F.; Ramazanov, S.; Trčka, T. Brief Review of PVDF Properties and Applications Potential. Polymers 2022, 14, 4793. [Google Scholar] [CrossRef]
- Bansal, S.; von Arnim, V.; Stegmaier, T.; Planck, H. Effect of fibrous filter properties on the oil-in-water-emulsion separation and filtration performance. J. Hazard Mater 2011, 190, 45–50. [Google Scholar] [CrossRef]
- Abbas, S.M.; Al-Jubouri, S.M. ZrO2 Nanoparticles Filler-Based Mixed Matrix Polyethersulfone/Cellulose Acetate Microfiltration Membrane for Oily Wastewater Separation. Appl. Sci. Eng. Prog. 2025, 18, 7599. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Yuan, Y.; Jiang, L.; Wu, H.; Dong, Y. Biomimetic hydrophilic modification of poly (vinylidene fluoride) membrane for efficient oil-in-water emulsions separation. Sep. Purif. Technol. 2024, 329, 125227. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, S.; Wang, N. Fabrication of modified PVDF membranes with PAA network polymer for highly efficient oil/water emulsion separation. Sep. Purif. Technol. 2025, 357, 130138. [Google Scholar] [CrossRef]
- Li, S.; Zhang, M.; Sun, J.; Sun, J.; Wang, Y. Preparation and characterization of superior hydrophilic PVDF/DA membranes by the self-polymerization approach of dopamine. Front. Chem. 2023, 11, 1162348. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, T.; Tong, L.; Gao, Y.; Zhang, H.; Zhang, Y.; Wang, Z.; Zhu, S. Non-free Fe dominated PMS activation for enhancing electro-Fenton efficiency in neutral wastewater. J. Electroanal. Chem. 2023, 928, 117062. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, B.; Fu, Z.; Li, J.; Yu, M.; Li, L.; Li, J. Poly (vinyl alcohol) modification of poly (vinylidene fluoride) microfiltration membranes for oil/water emulsion separation via an unconventional radiation method. J. Membr. Sci. 2021, 619, 118792. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, J.; Zhang, F.; Gao, S.; Wang, A.; Fang, W.; Jin, J. Zwitterionic Nanohydrogel Grafted PVDF Membranes with Comprehensive Antifouling Property and Superior Cycle Stability for Oil-in-Water Emulsion Separation. Adv. Funct. Mater. 2018, 28, 1804121. [Google Scholar] [CrossRef]
- Yan, L.; Yang, X.; Zhao, Y.; Wu, Y.; Moutloali, R.M.; Mamba, B.B.; Sorokin, P.; Shao, L. Bio-inspired mineral-hydrogel hybrid coating on hydrophobic PVDF membrane boosting oil/water emulsion separation. Sep. Purif. Technol. 2022, 285, 120383. [Google Scholar] [CrossRef]
- Fan, J.B.; Song, Y.; Wang, S.; Meng, J.; Yang, G.; Guo, X.; Feng, L.; Jiang, L. Directly Coating Hydrogel on Filter Paper for Effective Oil-Water Separation in Highly Acidic, Alkaline, and Salty Environment. Adv. Funct. Mater. 2015, 25, 5368–5375. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Ma, C.; Yin, H.; Gong, L.; Duh, Y.; Feng, R. Durable, cost-effective and superhydrophilic chitosan-alginate hydrogel-coated mesh for efficient oil/water separation. Carbohydr. Polym. 2019, 226, 115279. [Google Scholar] [CrossRef]
- Chen, J.; Yu, Q.; Wang, M.; Liu, D.; Dong, L.; Cui, Z.; He, B.; Li, J.; Yan, F. Superhydrophilic/underwater superoleophobic PVDF ultrafiltration membrane with pH-responsive self-cleaning performance for efficient oil-water separation. Sep. Purif. Technol. 2024, 330, 125420. [Google Scholar] [CrossRef]
- Gao, S.; Liu, P.; Jin, J. In-situ ionized construction of PVDF/sodium polyacrylate-grafted-PVDF blend ultrafiltration membrane with stable anti-oil-fouling ability for efficient oil-in-water emulsion separation. Front. Membr. Sci. Technol. 2024, 3, 1355773. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, L.; Li, H.; Xue, J.; Ma, C.; Yin, Y.; Qiao, X.; Sun, D.; Xue, Q. Multifunctional charged hydrogel nanofibrous membranes for metal ions contained emulsified oily wastewater purification. J. Membr. Sci. 2021, 621, 118950. [Google Scholar] [CrossRef]
- Pi, J.-K.; Yang, J.; Xu, Z.-K. One-pot mussel-inspiration and silication: A platform for constructing oil-repellent surfaces toward crude oil/water separation. J. Membr. Sci. 2020, 601, 117915. [Google Scholar] [CrossRef]
- Liu, H.; Xie, J.; Zhao, J.; Wang, R.; Qi, Y.; Lv, Z.; Yu, Y.; Sun, S. Construction of gradient SA-TiO2 hydrogel coated PVDF-g-IL fibre membranes with high hydrophilicity and self-cleaning for the efficient separation of oil-water emulsion and dye wastewater. J. Membr. Sci. 2024, 697, 122580. [Google Scholar] [CrossRef]
- Matsubayashi, T.; Tenjimbayashi, M.; Komine, M.; Manabe, K.; Shiratori, S. Bioinspired Hydrogel-Coated Mesh with Superhydrophilicity and Underwater Superoleophobicity for Efficient and Ultrafast Oil/Water Separation in Harsh Environments. Ind. Eng. Chem. Res. 2017, 56, 7080–7085. [Google Scholar] [CrossRef]
- Gao, S.; Sun, J.; Liu, P.; Zhang, F.; Zhang, W.; Yuan, S.; Li, J.; Jin, J. A Robust Polyionized Hydrogel with an Unprecedented Underwater Anti-Crude-Oil-Adhesion Property. Adv. Mater. 2016, 28, 5307–5314. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, Y.; Yan, S.; Yin, X.; Chen, J. Development of alginate hydrogel modified multifunctional filtration membrane with robust anti-fouling property for efficient water purification. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123891. [Google Scholar] [CrossRef]
- Gao, S.; Zhu, Y.; Wang, J.; Zhang, F.; Li, J.; Jin, J. Layer-by-Layer Construction of Cu2+/Alginate Multilayer Modified Ultrafiltration Membrane with Bioinspired Superwetting Property for High-Efficient Crude-Oil-in-Water Emulsion Separation. Adv. Funct. Mater. 2018, 28, 1801944. [Google Scholar] [CrossRef]
- Xue, Z.; Wang, S.; Lin, L.; Chen, L.; Liu, M.; Feng, L.; Jiang, L. A Novel Superhydrophilic and Underwater Superoleophobic Hydrogel-Coated Mesh for Oil/Water Separation. Adv. Mater. 2011, 23, 4270–4273. [Google Scholar] [CrossRef]
- Liu, J.; Qu, S.; Suo, Z.; Yang, W. Functional hydrogel coatings. Natl. Sci. Rev. 2020, 8, 254. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, W.; Li, X.; Wang, Z. Surface engineering of filter membranes with hydrogels for oil-in-water emulsion separation. Sep. Purif. Technol. 2023, 304, 122340. [Google Scholar] [CrossRef]
- Perez, I.D.; dos Santos, F.B.; Miranda, N.T.; Vieira, M.G.A.; Fregolente, L.V. Polymer hydrogel for water removal from naphthenic insulating oil and marine diesel. Fuel 2022, 324, 124702. [Google Scholar] [CrossRef]
- He, S.; Zhang, F.; Cheng, S.; Wang, W. Synthesis of Sodium Acrylate and Acrylamide Copolymer/GO Hydrogels and Their Effective Adsorption for Pb2+ and Cd2+. ACS Sustain. Chem. Eng. 2016, 4, 3948–3959. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, S.; Kong, Q.; Li, R.; Zhou, C.; Li, Z.; Ren, X. Preparation of Poly (acrylic acid-co-acrylamide) Hydrogel-Modified PVDF Membrane and Its Separation and Antibacterial Properties. Macromol. Chem. Phys. 2023, 224, 2200436. [Google Scholar] [CrossRef]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.; Seyfoddin, A.; Fatihhi, S. Acrylic acid/acrylamide based hydrogels and its properties—A review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Lu, Q.; Li, N. Preparation of hydrophilic polyvinylidene fluoride/polyvinyl alcohol ultrafiltration membrane via polymer/non-solvent co-induced phase separation method towards enhance anti-fouling performance. J. Environ. Chem. Eng. 2021, 9, 106431. [Google Scholar] [CrossRef]
- Kim, E.-S.; Kim, Y.J.; Yu, Q.; Deng, B. Preparation and characterization of polyamide thin-film composite (TFC) membranes on plasma-modified polyvinylidene fluoride (PVDF). J. Membr. Sci. 2009, 344, 71–81. [Google Scholar] [CrossRef]
- Pan, H.; Wang, G.; Pan, J.; Ye, G.; Sun, K.; Zhang, J.; Wang, J. Cold plasma-induced surface modification of heat-polymerized acrylic resin and prevention of early adherence of Candida albicans. Dent Mater J 2015, 34, 529–536. [Google Scholar] [CrossRef]
- Akashi, N.; Kuroda, S.-I. Protein immobilization onto poly (vinylidene fluoride) microporous membranes activated by the atmospheric pressure low temperature plasma. Polymer 2014, 55, 2780–2791. [Google Scholar] [CrossRef]
- Espiritu, R.; Mamlouk, M.; Scott, K. Study on the effect of the degree of grafting on the performance of polyethylene-based anion exchange membrane for fuel cell application. Int. J. Hydrogen Energy 2016, 41, 1120–1133. [Google Scholar] [CrossRef]
- Raju, M.K.; Raju, M.P. Synthesis of novel superabsorbing copolymers for agricultural and horticultural applications. Polym. Int. 2001, 50, 946–951. [Google Scholar] [CrossRef]
- Mandal, J.; Zhang, K.; Spencer, N.D. Oxygen inhibition of free-radical polymerization is the dominant mechanism behind the “mold effect” on hydrogels. Soft Matter 2021, 17, 6394–6403. [Google Scholar]
- Cho, E.; Kim, C.; Park, J.-Y.; Hwang, C.H.; Kim, J.H.; Kim, Y.A.; Endo, M.; Chang, D.R.; Kook, J.-K. Surface Modification of Electrospun Polyvinylidene Fluoride Nanofiber Membrane by Plasma Treatment for Protein Detection. J. Nanosci. Nanotechnol. 2013, 13, 674–677. [Google Scholar] [CrossRef]
- Yang, C.; Li, X.-M.; Gilron, J.; Kong, D.-F.; Yin, Y.; Oren, Y.; Linder, C.; He, T. CF4 plasma-modified superhydrophobic PVDF membranes for direct contact membrane distillation. J. Membr. Sci. 2014, 456, 155–161. [Google Scholar]
- Kormunda, M.; Ryšánek, P.; Hájková, P.; Štěpanovská, E.; Čapková, P.; Pavlík, J. Effect of low energy plasma treatment on surface chemistry and phase composition of electrospun polyvinylidene fluoride membrane. Surf. Interfaces 2021, 22, 100900. [Google Scholar] [CrossRef]
- Yang, X.; Wang, R.; Shi, L.; Fane, A.G.; Debowski, M. Performance improvement of PVDF hollow fiber-based membrane distillation process. J. Membr. Sci. 2011, 369, 437–447. [Google Scholar] [CrossRef]
- Riahinezhad, M.; Kazemi, N.; McManus, N.; Penlidis, A. Effect of ionic strength on the reactivity ratios of acrylamide/acrylic acid (sodium acrylate) copolymerization. J. Appl. Polym. Sci. 2014, 131, 40949. [Google Scholar] [CrossRef]
- Hashmi, S.; Nadeem, S.; Awan, Z.; Rehman, A.U.; Ghani, A.A. Synthesis, Applications and Swelling Properties of Poly (Sodium Acrylate-Coacrylamide) Based Superabsorbent Hydrogels. J. Chem. Soc. Pak. 2019, 41, 668–678. [Google Scholar]
- Hantsche, H. High resolution XPS of organic polymers, the scienta ESCA300 database. By G. Beamson and D. Briggs, Wiley, Chichester 1992, 295 pp., hardcover, £ 65.00, ISBN 0-471-93592-1. Adv. Mater. 1993, 5, 778. [Google Scholar] [CrossRef]
- Deng, Y.; Peng, C.; Dai, M.; Lin, D.; Ali, I.; Alhewairini, S.S.; Zheng, X.; Chen, G.; Li, J.; Naz, I. Recent development of super-wettable materials and their applications in oil-water separation. J. Clean. Prod. 2020, 266, 121624. [Google Scholar] [CrossRef]
- Israelachvili, J.N. (Ed.) 17—Adhesion and Wetting Phenomena. In Intermolecular and Surface Forces, 3rd ed.; Academic Press: San Diego, CA, USA, 2011; pp. 415–467. [Google Scholar]
- Packham, D.E. Surface energy, surface topography and adhesion. Int. J. Adhes. Adhes. 2003, 23, 437–448. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Virga, E.; Field, R.W.; Biesheuvel, P.; de Vos, W.M. Theory of oil fouling for microfiltration and ultrafiltration membranes in produced water treatment. J. Colloid Interface Sci. 2022, 621, 431–439. [Google Scholar] [CrossRef]
- Al-Jubouri, S. Oily water treatment using ceramic memberane. J. Eng. 2011, 17, 252–264. [Google Scholar]
- Abuhantash, F.; Abuhasheesh, Y.H.; Hegab, H.M.; Aljundi, I.H.; Al Marzooqi, F.; Hasan, S.W. Hydrophilic, oleophilic and switchable Janus mixed matrix membranes for oily wastewater treatment: A review. J. Water Process Eng. 2023, 56, 104310. [Google Scholar] [CrossRef]
- Tong, K.; Zhang, Y.; Chu, P.K. Evaluation of calcium chloride for synergistic demulsification of super heavy oil wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2013, 419, 46–52. [Google Scholar] [CrossRef]
- Hiemenz, P.C.; Rajagopalan, R. Principles of Colloid and Surface Chemistry, Revised and Expanded; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Ray, J.P.; Engelhardt, F.R.; Engelhardt, F.R. Produced Water: Technological, environmental issues and solutions. In Proceedings of the 1992 International Produced Water Symposium, San Diego, CA, USA, 4–7 February 1992; Springer: New York, NY, USA, 1993. [Google Scholar]
- Zhang, M.; Wang, M.; Chen, J.; Dong, L.; Tian, Y.; Cui, Z.; Li, J.; He, B.; Yan, F. Demulsifier-Inspired Superhydrophilic/Underwater Superoleophobic Membrane Modified with Polyoxypropylene Polyoxyethylene Block Polymer for Enhanced Oil/Water Separation Properties. Molecules 2023, 28, 1282. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Yang, Y.; Zhu, L.; Zeng, Z.; Liu, S.; Li, Y.; Liang, Z. Poly (vinylidene fluoride) membranes with underwater superoleophobicity for highly efficient separation of oil-in-water emulsions in resisting fouling. Sep. Purif. Technol. 2022, 285, 120298. [Google Scholar] [CrossRef]
- Yang, H.-C.; Xie, Y.; Chan, H.; Narayanan, B.; Chen, L.; Waldman, R.Z.; Sankaranarayanan, S.K.R.S.; Elam, J.W.; Darling, S.B. Crude-Oil-Repellent Membranes by Atomic Layer Deposition: Oxide Interface Engineering. ACS Nano 2018, 12, 8678–8685. [Google Scholar] [CrossRef]
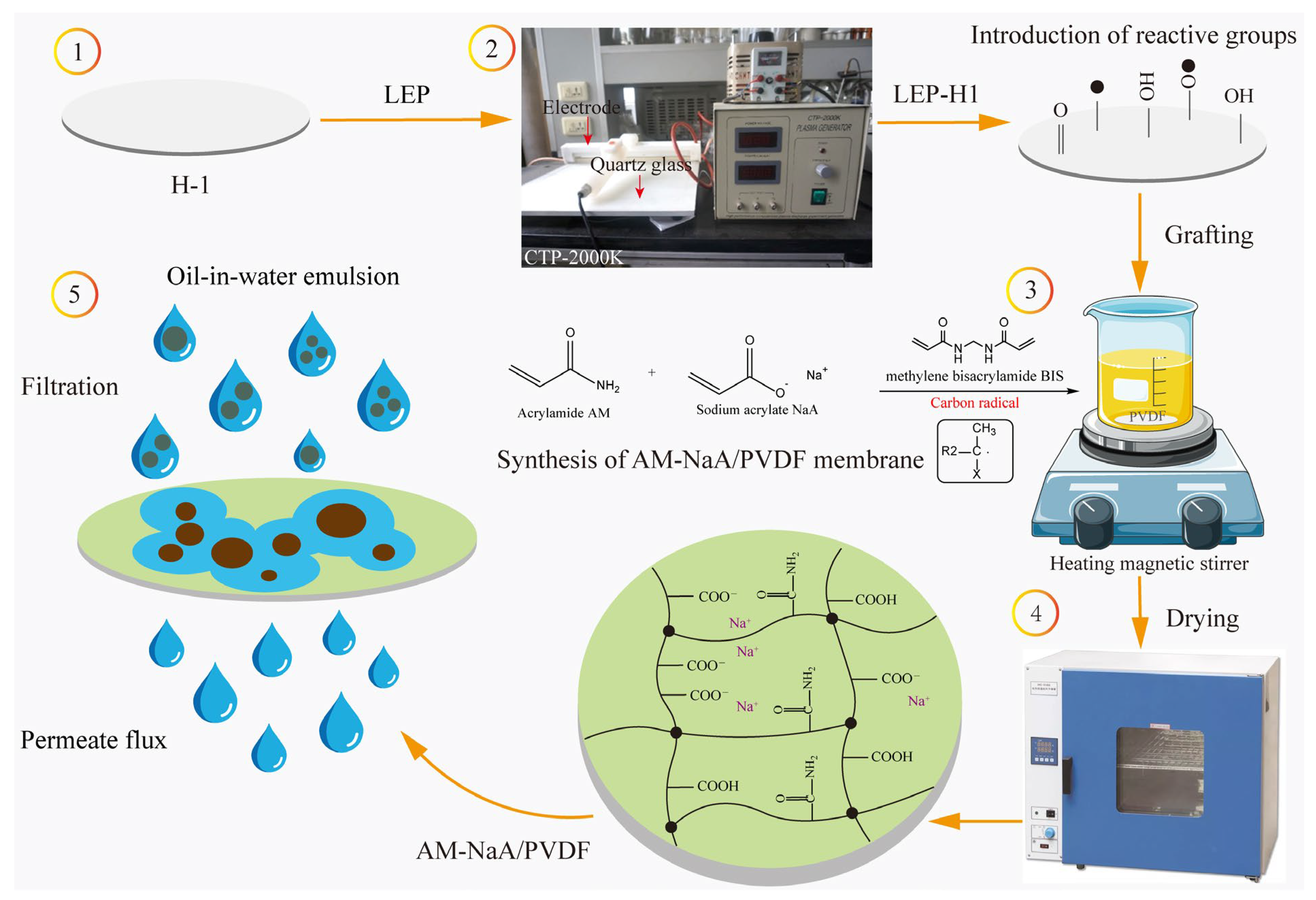
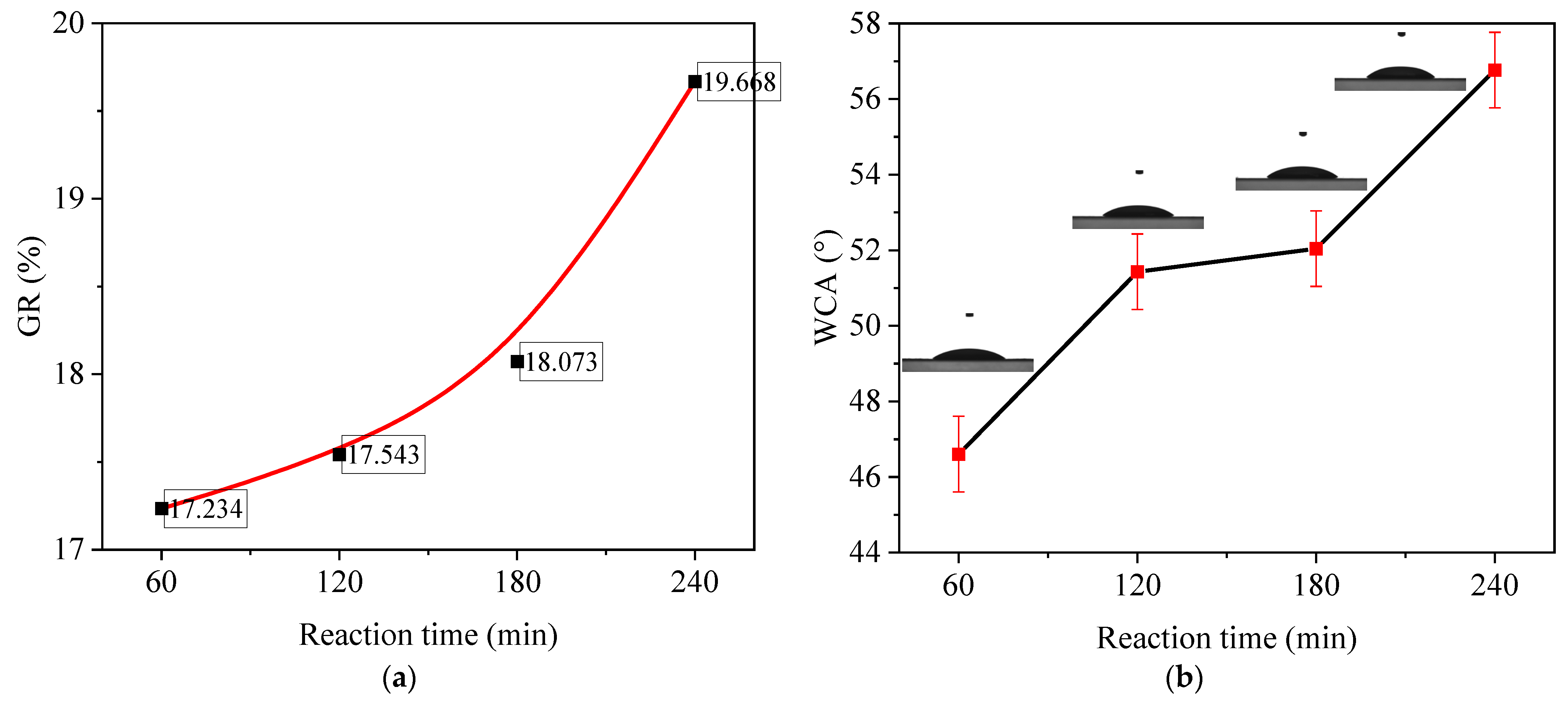


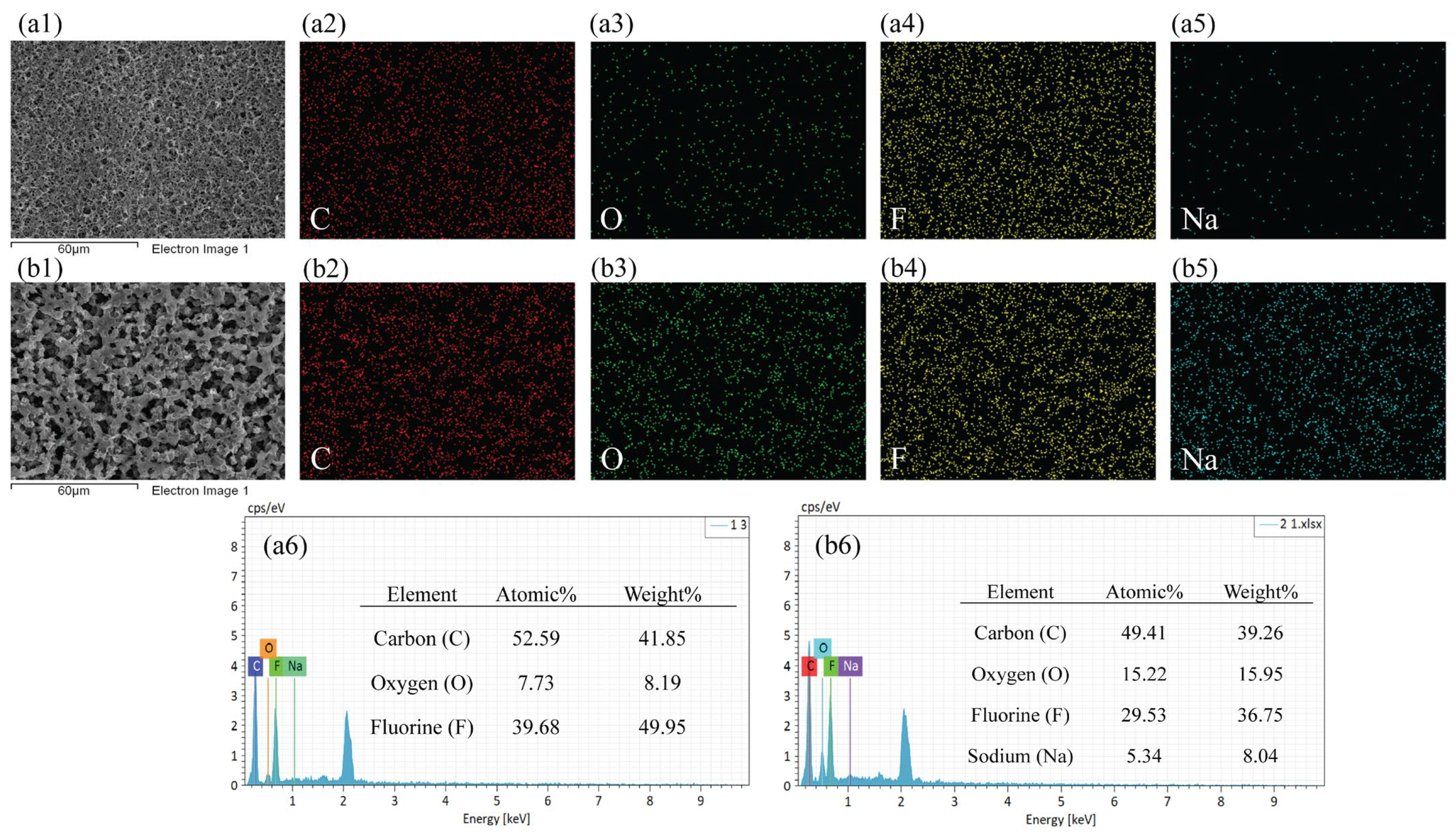
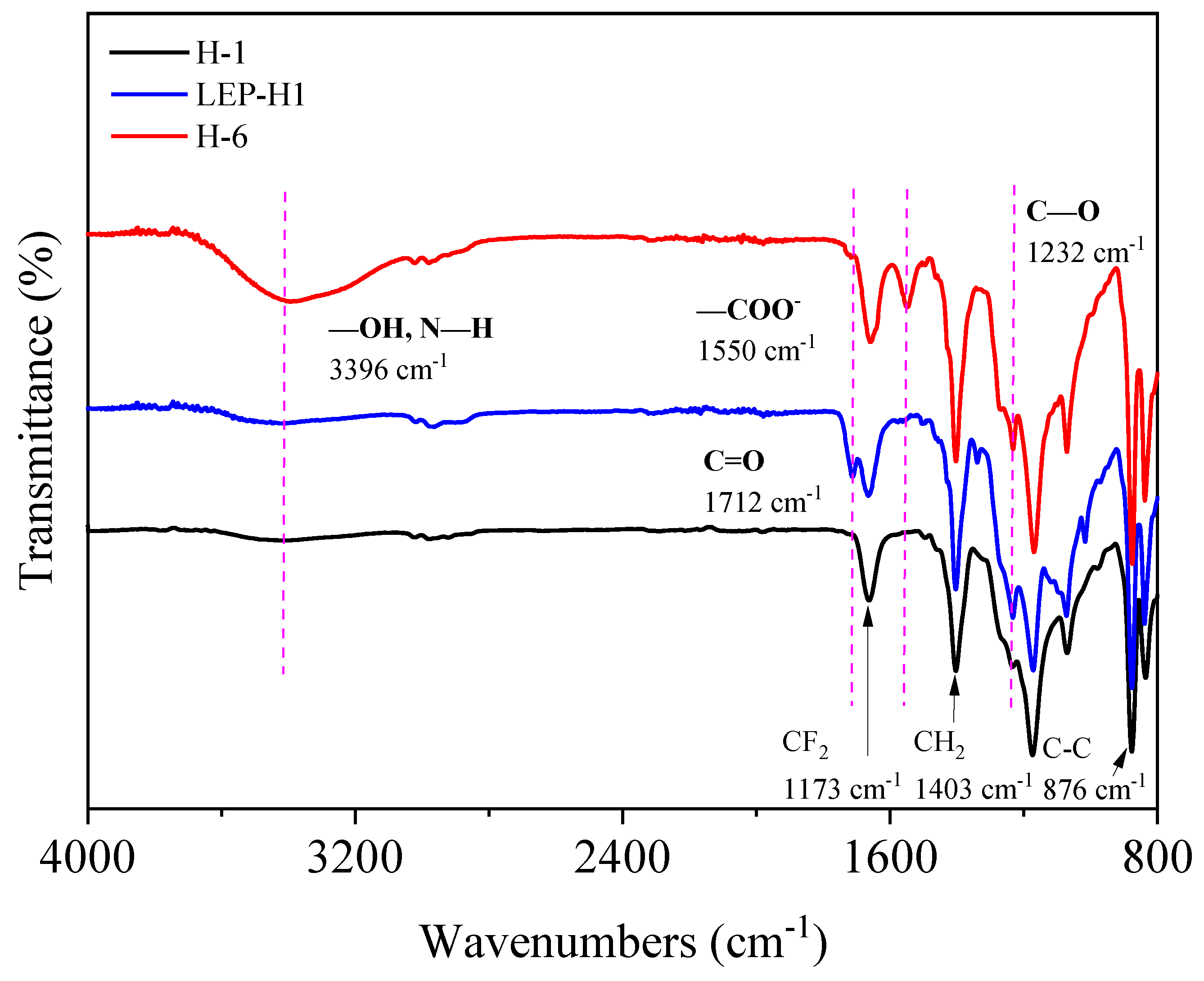



| Membrane Code | H-1 | H-2 | H-3 | H-4 | H-5 | H-6 | H-7 |
|---|---|---|---|---|---|---|---|
| AM/NaA (monomer mass ratio) | 0:0 | 5:0 | 1:1 | 4:1 | 3:2 | 2:3 | 0:5 |
| Membrane | C1s | O1s | O/C | ||
|---|---|---|---|---|---|
| Binding Energy (eV) | Atomic (%) | Binding Energy (eV) | Atomic (%) | ||
| PVDF | 286.23 | 52.03 | 531.74 | 4.03 | 0.077 |
| LEP/PVDF | 285.54 | 86.8 | 532.15 | 10.57 | 0.122 |
| AM-NaA/PVDF | 284.89 | 60.54 | 531.29 | 25.87 | 0.427 |
| Salt | Concentration (g/100 mL) | Ionic Strength (Ic) (mol/m3) | Double-Layer Thickness (k−1) (m) |
| CaCl2 | 0.1 | 27.03 | |
| 0.5 | 135.15 | ||
| 1.0 | 270.3 | ||
| MgCl2·6H2O | 0.1 | 14.76 | |
| 0.5 | 73.8 | ||
| 1.0 | 147.6 | ||
| NaCl | 0.1 | 17.1 | |
| 0.5 | 85.6 | ||
| 1.0 | 171 |
| Porous Materials | Substrate Type | Fabrication Method | Modification Conditions (h/°C) | Inlet Oil Content (mg/L) | Oil Removal (%) | Flux L/m2·h | Ref. |
|---|---|---|---|---|---|---|---|
| DMPA@SMA | PVDF | Grafting and co-blending | 9/60 | 1034.6 | 99.2 | 420 | [36] |
| PAAS@PVDF | PVDF | Grafting and blending | 48/85 | 1:99 oil/water | 99.97 | 350 | [37] |
| SA-TiO hydrogel | PVDF-g-IL | Surface coating | 8/60 | 1:99 oil/water | 99 | 1658 | [40] |
| F127@SMA | PVDF | Grafting and co-blending | 9/70 | 1000 | 99.1 | 272.4 | [76] |
| PVDF/PHEMA, TA/AEPPS | non-woven fabric | Vapor-induced phase co-deposited | –/85 | 1:99 oil/water | 99 | 1056 | [77] |
| Oxides (ZnO, Al2O3, TiO2 and SnO2) | PVDF | Atomic layer deposition (ALD) | 0.0125/100 | 2845 | 97.11 | 800 | [78] |
| Alginate hydrogel | Filter paper | Surface coating | 8/– | 1:20 oil/water | 99.9 | ~4000 | [43] |
| AM-NaA copolymer hydrogel | PVDF | Surface coating | 1/40 | 1000 | 99.5 | 5874 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayder, H.A.; Shi, P.; Al-Jubouri, S.M. Developing a Superhydrophilic/Underwater Superoleophobic Plasma-Modified PVDF Microfiltration Membrane with Copolymer Hydrogels for Oily Water Separation. Appl. Sci. 2025, 15, 6654. https://doi.org/10.3390/app15126654
Hayder HA, Shi P, Al-Jubouri SM. Developing a Superhydrophilic/Underwater Superoleophobic Plasma-Modified PVDF Microfiltration Membrane with Copolymer Hydrogels for Oily Water Separation. Applied Sciences. 2025; 15(12):6654. https://doi.org/10.3390/app15126654
Chicago/Turabian StyleHayder, Hasan Ali, Peng Shi, and Sama M. Al-Jubouri. 2025. "Developing a Superhydrophilic/Underwater Superoleophobic Plasma-Modified PVDF Microfiltration Membrane with Copolymer Hydrogels for Oily Water Separation" Applied Sciences 15, no. 12: 6654. https://doi.org/10.3390/app15126654
APA StyleHayder, H. A., Shi, P., & Al-Jubouri, S. M. (2025). Developing a Superhydrophilic/Underwater Superoleophobic Plasma-Modified PVDF Microfiltration Membrane with Copolymer Hydrogels for Oily Water Separation. Applied Sciences, 15(12), 6654. https://doi.org/10.3390/app15126654







