Comparative Measurement of Mercury Release Values from Amalgam Restorations with Different Surface Numbers: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Size Calculation
2.3. Application of Amalgam Restorations Following Cavity Preparation
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geurtsen, W.; Schoeler, U. A 4-Year Retrospective Clinical Study of Class I and Class II Composite Restorations. J. Dent. 1997, 25, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Bailer, J.; Rist, F.; Rudolf, A.; Staehle, H.J.; Eickholz, P.; Triebig, G.; Bader, M.; Pfeifer, U. Adverse Health Effects Related to Mercury Exposure from Dental Amalgam Fillings: Toxicological or Psychological Causes? Psychol. Med. 2001, 31, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Gay, D.; Cox, R.; Reinhardt, J. Chewing Releases Mercury from Fillings. Lancet 1979, 313, 985–986. [Google Scholar] [CrossRef]
- Rathore, M.; Singh, A.; Pant, V.A. The Dental Amalgam Toxicity Fear: A Myth or Actuality. Toxicol. Int. 2012, 19, 81. [Google Scholar]
- Newman, S.M. Amalgam Alternatives: What Can Compete? J. Am. Dent. Assoc. 1991, 122, 67–71. [Google Scholar] [CrossRef]
- SB, C. The Benefits and Risks of Dental Amalgam. J. Am. Dent. Assoc. 1994, 125, 381–388. [Google Scholar]
- Bates, M.N. Mercury Amalgam Dental Fillings: An Epidemiologic Assessment. Int. J. Hyg. Environ. Health 2006, 209, 309–316. [Google Scholar] [CrossRef]
- Eley, B.M. The Future of Dental Amalgam: A Review of the Literature. Part 6: Possible Harmful Effects of Mercury from Dental Amalgam. Br. Dent. J. 1997, 182, 455–459. [Google Scholar] [CrossRef]
- Makhija, S.K.; Gordan, V.V.; Gilbert, G.H.; Litaker, M.S.; Rindal, D.B.; Pihlstrom, D.J.; Qvist, V.; Dental Practice-Based Research Network Collaborative Group. Practitioner, Patient and Carious Lesion Characteristics Associated with Type of Restorative Material: Findings from The Dental Practice-Based Research Network. J. Am. Dent. Assoc. 2011, 142, 622–632. [Google Scholar] [CrossRef]
- Berdouses, E.; Vaidyanathan, T.K.; Dastane, A.; Weisel, C.; Houpt, M.; Shey, Z. Mercury Release from Dental Amalgams: An in Vitro Study under Controlled Chewing and Brushing in an Artificial Mouth. J. Dent. Res. 1995, 74, 1185–1193. [Google Scholar] [CrossRef]
- Björkman, L.; Sandborgh-Englund, G.; Ekstrand, J. Mercury in Saliva and Feces after Removal of Amalgam Fillings. Toxicol. Appl. Pharmacol. 1997, 144, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.M.; Warren, P.J. Mercury Vapour Released during the Removal of Old Amalgam Restorations. Br. Dent. J. 1985, 159, 231–232. [Google Scholar] [CrossRef] [PubMed]
- Haikel, Y.; Gasser, P.; Salek, P.; Voegel, J.C. Exposure to Mercury Vapor during Setting, Removing, and Polishing Amalgam Restorations. J. Biomed. Mater. Res. 1990, 24, 1551–1558. [Google Scholar] [CrossRef]
- Vimy, M.J.; Takahashi, Y.; Lorscheider, F.L. Maternal-Fetal Distribution of Mercury (203Hg) Released from Dental Amalgam Fillings. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1990, 258, R939–R945. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.W.; Charlton, D.G. The Release of Mercury from Amalgam Restorations and Its Health Effects: A Review. Oper. Dent. 2009, 34, 605–614. [Google Scholar] [CrossRef]
- Clarkson, T.W.; Magos, L.; Cox, C.; Greenwood, M.R.; Amin-Zaki, L.; Majeed, M.A.; Al-Damluji, S.F. Tests of Efficacy of Antidotes for Removal of Methylmercury in Human Poisoning during the Iraq Outbreak. J. Pharmacol. Exp. Ther. 1981, 218, 74–83. [Google Scholar] [CrossRef]
- Murata, K.; Karita, K. Minamata Disease. In Overcoming Environmental Risks to Achieve Sustainable Development Goals: Lessons from the Japanese Experience; Springer: Singapore, 2022; pp. 9–19. [Google Scholar]
- Berry, T.G.; Summitt, J.B.; Chung, A.K.H.; Osborne, J.W. Amalgam at the New Millennium. J. Am. Dent. Assoc. 1998, 129, 1547–1556. [Google Scholar] [CrossRef]
- Lauterbach, M.; Martins, I.P.; Castro-Caldas, A.; Bernardo, M.; Luis, H.; Amaral, H.; Leitão, J.; Martin, M.D.; Townes, B.; Rosenbaum, G. Neurological Outcomes in Children with and without Amalgam-Related Mercury Exposure: Seven Years of Longitudinal Observations in a Randomized Trial. J. Am. Dent. Assoc. 2008, 139, 138–145. [Google Scholar] [CrossRef]
- ADA Council on Scientific Affairs. Dental Amalgam: Update on Safety Concerns. J. Am. Dent. Assoc. 1998, 129, 494–503. [Google Scholar] [CrossRef]
- Mercury, W.H.O.I. Environmental Health Criteria 118; World Health Organization: Geneva, Switzerland, 1991; Volume 107, Available online: https://apps.who.int/iris/bitstream/handle/10665/40626/IPCS_EHC_118.pdf (accessed on 5 June 2025).
- Fredin, B. Mercury Release from Dental Amalgam Fillings. Int. J. Risk Saf. Med. 1994, 4, 197–208. [Google Scholar] [CrossRef]
- Shenoy, A. Is It the End of the Road for Dental Amalgam? A Critical Review. J. Conserv. Dent. 2008, 11, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Osborne, J.W.; Swift Jr, E.J. Safety of Dental Amalgam. J. Esthet. Restor. Dent. 2004, 16, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.S.; Martin, M.D.; Leroux, B.G.; DeRouen, T.A.; Leitão, J.G.; Bernardo, M.F.; Luis, H.S.; Simmonds, P.L.; Kushleika, J.V.; Huang, Y. The Contribution of Dental Amalgam to Urinary Mercury Excretion in Children. Environ. Health Perspect. 2007, 115, 1527–1531. [Google Scholar] [CrossRef]
- TW, C. The Three Modern Faces of Mercury. Environ. Health Perspect. 2002, 110, 11–23. [Google Scholar]
- Eneström, S.; Hultman, P. Does Amalgam Affect the Immune System? A Controversial Issue (Part 2 of 2). Int. Arch. Allergy Immunol. 1995, 106, 192–203. [Google Scholar] [CrossRef]
- Fathi, M.; Mortazavi, V. A Review on Dental Amalgam Corrosion and Its Consequences. J. Res. Med. Sci. 2004, 1, 42–51. [Google Scholar]
- Skare, I.; Engqvist, A. Human Exposure to Mercury and Silver Released from Dental Amalgam Restorations. Arch. Environ. Health Int. J. 1994, 49, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W.; Magos, L.; Myers, G.J. The Toxicology of Mercury—Current Exposures and Clinical Manifestations. N. Engl. J. Med. 2003, 349, 1731–1737. [Google Scholar] [CrossRef]
- US Food and Drug Administration. White Paper: FDA Update/Review of Potential Adverse Health Risks Associated with Exposure to Mercury in Dental Amalgam; National Center for Toxicological Research: Jefferson, AR, USA, 2010. [Google Scholar]
- Sandborgh-Englund, G.; Elinder, C.-G.; Johanson, G.; Lind, B.; Skare, I.; Ekstrand, J. The Absorption, Blood Levels, and Excretion of Mercury after a Single Dose of Mercury Vapor in Humans. Toxicol. Appl. Pharmacol. 1998, 150, 146–153. [Google Scholar] [CrossRef]
- Kingman, A.; Albertini, T.; Brown, L.J. Mercury Concentrations in Urine and Whole Blood Associated with Amalgam Exposure in a US Military Population. J. Dent. Res. 1998, 77, 461–471. [Google Scholar] [CrossRef]
- Maserejian, N.N.; Trachtenberg, F.L.; Assmann, S.F.; Barregard, L. Dental Amalgam Exposure and Urinary Mercury Levels in Children: The New England Children’s Amalgam Trial. Environ. Health Perspect. 2008, 116, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.E.; Trachtenberg, F.L.; Barregard, L.; Bellinger, D.; McKinlay, S. Scalp Hair and Urine Mercury Content of Children in the Northeast United States: The New England Children’s Amalgam Trial. Environ. Res. 2008, 107, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Mackert, J.R.; Berglund, A. Mercury Exposure From Dental Amalgam Fillings: Absorbed Dose and the Potential for Adverse Health Effects. Crit. Rev. Oral Biol. Med. 1997, 8, 410–436. [Google Scholar] [CrossRef]
- Salomone, P.; Bueno, R.P.R.; Trinidade, R.F.; Nascimento, P.C.; Pozzobon, R.T. Assessment of the Release of Mercury from Silver Amalgam Alloys Exposed to Different 10% Carbamide Peroxide Bleaching Agents. Gen. Dent. 2013, 61, 33–35. [Google Scholar] [PubMed]
- Ekstrand, J.; Björkman, L.; Edlund, C.; Sandborgh-Englund, G. Toxicological Aspects on the Release and Systemic Uptake of Mercury from Dental Amalgam. Eur. J. Oral Sci. 1998, 106, 678–686. [Google Scholar] [CrossRef]
- Pizzichini, M.; Fonzi, M.; Sugherini, L.; Fonzi, L.; Comporti, M.; Gasparoni, A.; Pompella, A. Release of Mercury from Dental Amalgam and Its Influence on Salivary Antioxidant Activity. Bull. Group Int. Rech. Sci. Stomatol. Odontol. 2000, 42, 94–100. [Google Scholar] [CrossRef]
- Berglund, A. Estimation by a 24-Hour Study of the Daily Dose of Intra-Oral Mercury Vapor Inhaled after Release from Dental Amalgam. J. Dent. Res. 1990, 69, 1646–1651. [Google Scholar] [CrossRef]
- Sakaguchi, R.; Ferracane, J.; Powers, J. Testing of Dental Materials and Biomechanics. In Craig’s Restorative Dental Materials, 14th ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Campus, G.; Garcia-Godoy, F.; Gaspa, L.; Panzanelli, A.; Piu, P.C.; Micera, G.; Lugliè, P.; Sanna, G. Dependence of Kinetic Variables in the Short-Term Release of Hg2+, Cu2+ and Zn2+ Ions into Synthetic Saliva from an High-Copper Dental Amalgam. J. Mater. Sci. Mater. Med. 2007, 18, 1521–1527. [Google Scholar] [CrossRef]
- Beach, L.M. Evaluation of the Mercury Concentration Accessory for US EPA Methodology. Varian Instruments at Work. No. AA-108 1992, 1–5. Available online: https://www.perlan.com.pl/uploaded/AppBundleEntityProductApplication/fileKey/286/aa108.pdf (accessed on 5 June 2025).
- Bates, M.N.; Fawcett, J.; Garrett, N.; Cutress, T.; Kjellstrom, T. Health Effects of Dental Amalgam Exposure: A Retrospective Cohort Study. Int. J. Epidemiol. 2004, 33, 894–902. [Google Scholar] [CrossRef]
- Syversen, T.; Kaur, P. The Toxicology of Mercury and Its Compounds. J. Trace Elem. Med. Biol. 2012, 26, 215–226. [Google Scholar] [CrossRef]
- Craig, R.G. Restorative Dental Materials; Mosby: Maryland Heights, MO, USA, 2006; ISBN 0801638666. [Google Scholar]
- Vimy, M.J.; Lorscheider, F.L. Serial Measurements of Intra-Oral Air Mercury: Estimation of Daily Dose from Dental Amalgam. J. Dent. Res. 1985, 64, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.E.; Weissberg, B.G.; Dennison, P.J. Mercury in Human Breath from Dental Amalgams. Bull. Environ. Contam. Toxicol. 1985, 34, 459–468. [Google Scholar] [CrossRef]
- Abraham, J.E.; Svare, C.W.; Frank, C.W. The Effect of Dental Amalgam Restorations on Blood Mercury Levels. J. Dent. Res. 1984, 63, 71–73. [Google Scholar] [CrossRef]
- Berglund, A.; Pohl, L.; Olsson, S.; Bergman, M. Determination of the Rate of Release of Intra-Oral Mercury Vapor from Amalgam. J. Dent. Res. 1988, 67, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Langworth, S.; Kölbeck, K.G.; Akesson, A. Mercury Exposure from Dental Fillings. II. Release and Absorption. Swed. Dent. J. 1988, 12, 71–72. [Google Scholar]
- Mackert, J.R., Jr. Factors Affecting Estimation of Dental Amalgam Mercury Exposure from Measurements of Mercury Vapor Levels in Intra-Oral and Expired Air. J. Dent. Res. 1987, 66, 1775–1780. [Google Scholar] [CrossRef] [PubMed]
- Halbach, S. Amalgam Tooth Fillings and Man’s Mercury Burden. Hum. Exp. Toxicol. 1994, 13, 496–501. [Google Scholar] [CrossRef]
- Clarkson, T.W. Principles of Risk Assessment. Adv. Dent. Res. 1992, 6, 22–27. [Google Scholar] [CrossRef]
- Guzzi, G.; Grandi, M.; Cattaneo, C.; Calza, S.; Minoia, C.; Ronchi, A.; Gatti, A.; Severi, G. Dental Amalgam and Mercury Levels in Autopsy Tissues: Food for Thought. Am. J. Forensic Med. Pathol. 2006, 27, 42–45. [Google Scholar] [CrossRef]
- Jokstad, A.; Thomassen, Y.; Bye, E.; Clench-Aas, J.; Aaseth, J. Dental Amalgam and Mercury. Pharmacol. Toxicol. 1992, 70, 308–313. [Google Scholar] [CrossRef]
- Drexler, H.; Schaller, K.-H. The Mercury Concentration in Breast Milk Resulting from Amalgam Fillings and Dietary Habits. Environ. Res. 1998, 77, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Ganss, C.; Gottwald, B.; Traenckner, I.; Kupfer, J.; Eis, D.; Mönch, J.; Gieler, U.; Klimek, J. Relation between Mercury Concentrations in Saliva, Blood, and Urine in Subjects with Amalgam Restorations. Clin. Oral Investig. 2000, 4, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Marek, M. Dissolution of Mercury from Dental Amalgam at Different PH Values. J. Dent. Res. 1997, 76, 1308–1315. [Google Scholar] [CrossRef]
- Holland, R.I. Release of Mercury Vapor from Corroding Amalgam in Vitro. Dent. Mater. 1993, 9, 99–103. [Google Scholar] [CrossRef]
- Brune, D.; Gjerdet, N.; Paulsen, G. Gastrointestinal and in Vitro Release of Copper, Cadmium, Indium, Mercury and Zinc from Conventional and Copper-rich Amalgams. Eur. J. Oral Sci. 1983, 91, 66–71. [Google Scholar] [CrossRef]
- Okabe, T.; Ferracane, J.; Cooper, C.; Matsumoto, H.; Wagner, M. Dissolution of Mercury from Amalgam into Saline Solution. J. Dent. Res. 1987, 66, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Fritz, U.; Zellmann, M. Mercury Release from Amalgam Specimens. Dtsch. Zahnarztl. Z. 1991, 46, 553–554. [Google Scholar]
- Marek, M. The Release of Mercury from Dental Amalgam: The Mechanism and in Vitro Testing. J. Dent. Res. 1990, 69, 1167–1174. [Google Scholar] [CrossRef]
- Brune, D.; Evje, D.M. Man’s Mercury Loading from a Dental Amalgam. Sci. Total Environ. 1985, 44, 51–63. [Google Scholar] [CrossRef]
- Davies, R.A.; Ardalan, S.; Mu, W.-H.; Tian, K.; Farsaikiya, F.; Darvell, B.W.; Chass, G.A. Geometric, Electronic and Elastic Properties of Dental Silver Amalgam γ-(Ag3Sn), Γ1-(Ag2Hg3), Γ2-(Sn8Hg) Phases, Comparison of Experiment and Theory. Intermetallics 2010, 18, 756–760. [Google Scholar] [CrossRef]
- Lorscheider, F.L.; Vimy, M.J.; Summers, A.O. Mercury Exposure from “Silver” Tooth Fillings: Emerging Evidence Questions a Traditional Dental Paradigm. FASEB J. 1995, 9, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Kj, A. Phillips’ Science of Dental Materials; WB Saunders: St Louis, MO, USA, 2003; Volume 596. [Google Scholar]
- Brambilla, E.; García-Godoy, F.; Strohmenger, L. Principles of Diagnosis and Treatment of High-Caries-Risk Subjects. Dent. Clin. N. Am. 2000, 44, 507–540. [Google Scholar] [CrossRef] [PubMed]
- Marek, M. The Effect of Tin on the Corrosion Behavior of the Ag-Hg Phase of Dental Amalgam and Dissolution of Mercury. J. Dent. Res. 1990, 69, 1786–1790. [Google Scholar] [CrossRef]
- Mueller, H.J.; Greener, E.H.; Crimmins, D.S. The Electrochemical Properties of Dental Amalgam. J. Biomed. Mater. Res. 1968, 2, 95–119. [Google Scholar] [CrossRef]
- Fovet, Y.; Gal, J.-Y. Tin Passivation by Copper in Dental Amalgams. Comptes Rendus L’académie Des Sci.—Ser. IIC—Chem. 2000, 3, 379–385. [Google Scholar] [CrossRef]
- Okabe, T.; Elvebak, B.; Carrasco, L.; Ferracane, J.L.; Keanini, R.G.; Nakajima, H. Mercury Release from Dental Amalgams into Continuously Replenished Liquids. Dent. Mater. 2003, 19, 38–45. [Google Scholar] [CrossRef]
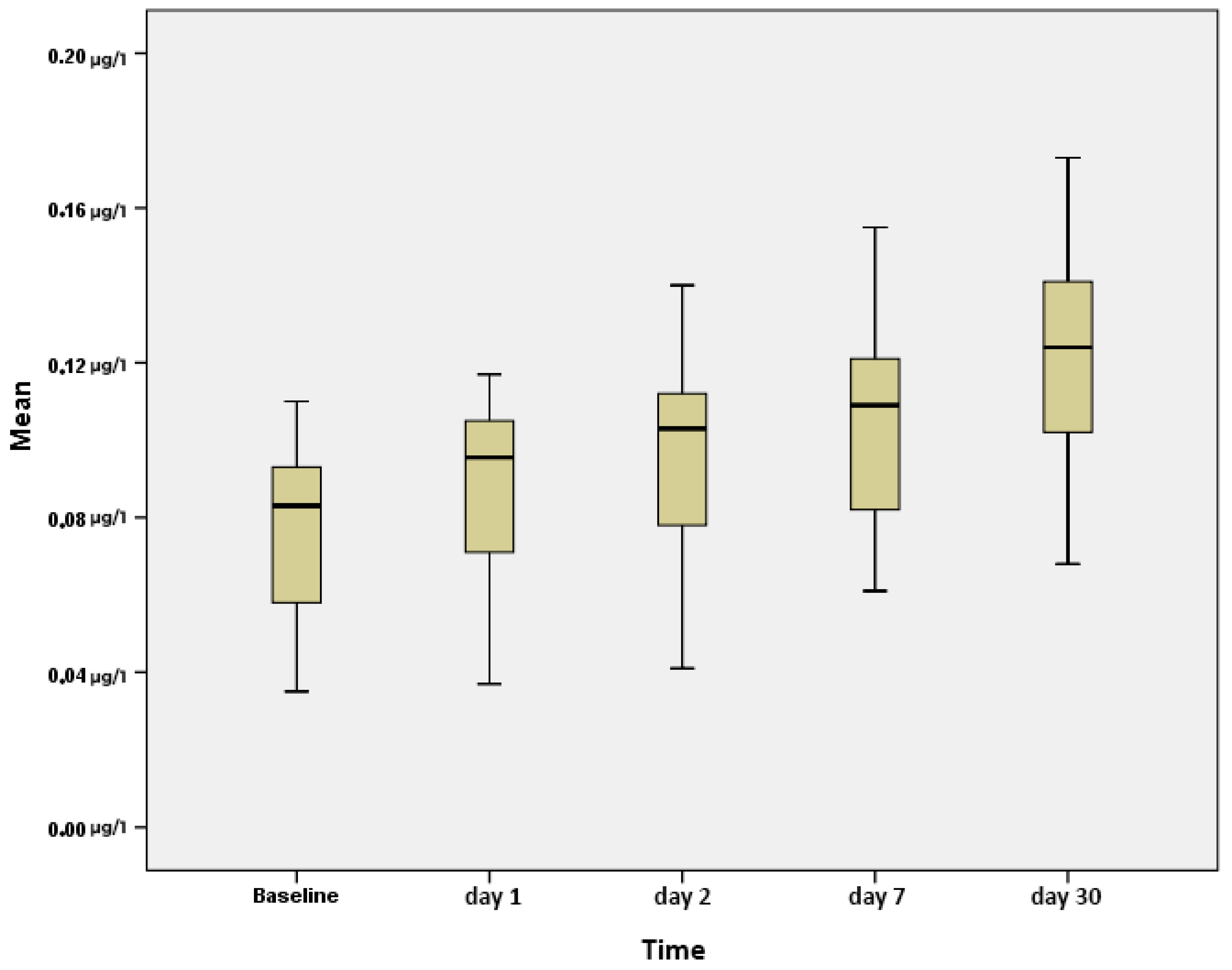

| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | a p | |
|---|---|---|---|---|---|---|---|
| Mean ± sd | Mean ± sd | Mean ± sd | Mean ± sd | Mean ± sd | Mean ± sd | ||
| Baseline | 0.043 ± 0.009 | 0.060 ± 0.004 | 0.083 ± 0.005 | 0.086 ± 0.006 | 0.094 ± 0.005 | 0.098 ± 0.006 | <0.001 ** |
| 1st day | 0.047 ± 0.011 | 0.072 ± 0.003 | 0.089 ± 0.007 | 0.097 ± 0.006 | 0.106 ± 0.003 | 0.113 ± 0.004 | <0.001 ** |
| 2nd day | 0.053 ± 0.013 | 0.080 ± 0.006 | 0.095 ± 0.007 | 0.105 ± 0.005 | 0.113 ± 0.004 | 0.125 ± 0.009 | <0.001 ** |
| 7th day | 0.069 ± 0.006 | 0.084 ± 0.005 | 0.103 ± 0.006 | 0.115 ± 0.007 | 0.120 ± 0.004 | 0.131 ± 0.012 | <0.001 ** |
| 30th day | 0.079 ± 0.006 | 0.103 ± 0.008 | 0.117 ± 0.004 | 0.129 ± 0.003 | 0.139 ± 0.006 | 0.157 ± 0.009 | <0.001 ** |
| b p | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** | |
| Post hoc | Mean ± sd | Mean ± sd | Mean ± sd | Mean ± sd | Mean ± sd | Mean ± sd | a p |
| Baseline–1st day | 0.004 ± 0.003 | 0.012 ± 0.003 ** | 0.007 ± 0.005 | 0.011 ± 0.006 * | 0.012 ± 0.003 ** | 0.016 ± 0.006 ** | <0.001 ** |
| Baseline–2nd day | 0.011 ± 0.006 * | 0.020 ± 0.005 ** | 0.012 ± 0.006 * | 0.019 ± 0.006 ** | 0.019 ± 0.005 ** | 0.027 ± 0.010 ** | 0.001 ** |
| Baseline–7th day | 0.026 ± 0.005 ** | 0.024 ± 0.004 ** | 0.021 ± 0.006 ** | 0.029 ± 0.009 ** | 0.026 ± 0.006 ** | 0.033 ± 0.013 ** | 0.094 |
| Baseline–30th day | 0.037 ± 0.007 ** | 0.043 ± 0.012 ** | 0.035 ± 0.002 ** | 0.043 ± 0.007 ** | 0.045 ± 0.007 ** | 0.059 ± 0.012 ** | <0.001 ** |
| 1st–2nd day | 0.007 ± 0.006 | 0.008 ± 0.006 | 0.006 ± 0.002 ** | 0.007 ± 0.002 ** | 0.007 ± 0.003 ** | 0.011 ± 0.006 * | 0.345 |
| 1st–7th day | 0.022 ± 0.007 ** | 0.012 ± 0.004 ** | 0.014 ± 0.004 ** | 0.018 ± 0.005 ** | 0.015 ± 0.005 ** | 0.018 ± 0.010 * | 0.051 |
| 1st–30th day | 0.032 ± 0.009 ** | 0.031 ± 0.009 ** | 0.028 ± 0.005 ** | 0.032 ± 0.005 ** | 0.033 ± 0.006 ** | 0.044 ± 0.007 ** | 0.004 ** |
| 2nd–7th day | 0.016 ± 0.009 * | 0.004 ± 0.002 | 0.008 ± 0.002 ** | 0.010 ± 0.004 ** | 0.008 ± 0.003 * | 0.006 ± 0.004 | 0.001 ** |
| 2nd–30th day | 0.026 ± 0.011 ** | 0.023 ± 0.012 * | 0.022 ± 0.006 ** | 0.024 ± 0.003 ** | 0.026 ± 0.004 ** | 0.032 ± 0.006 ** | 0.182 |
| 7th–30th day | 0.010 ± 0.006 | 0.019 ± 0.010 * | 0.014 ± 0.005 ** | 0.014 ± 0.004 ** | 0.018 ± 0.003 ** | 0.026 ± 0.006 ** | 0.001 ** |
| Tests for Within-Group Effects | ||
|---|---|---|
| F | p | |
| Time | 546.254 | <0.001 ** |
| Time × Group | 4.174 | <0.001 ** |
| Tests for Between-Group Effects | ||
| F | p | |
| Group | 140.326 | <0.001 ** |
| Baseline | 1st Day | 2nd Day | 7th Day | 30th Day | |
|---|---|---|---|---|---|
| G1—G2 | <0.001 ** | <0.001 ** | <0.001 ** | 0.007 ** | <0.001 ** |
| G1—G3 | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** |
| G1—G4 | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** |
| G1—G5 | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** |
| G1—G6 | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** |
| G2—G3 | <0.001 ** | <0.001 ** | 0.017 * | <0.001 ** | 0.002 ** |
| G2—G4 | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** |
| G2—G5 | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** |
| G2—G6 | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** |
| G3—G4 | 0.999 | 0.325 | 0.415 | 0.058 | 0.021 * |
| G3—G5 | 0.021 * | <0.001 ** | 0.003 ** | 0.001 ** | <0.001 ** |
| G3—G6 | 0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** |
| G4—G5 | 0.310 | 0.267 | 0.999 | 0.999 | 0.113 |
| G4—G6 | 0.019 * | 0.001 ** | 0.001 ** | 0.003 ** | <0.001 ** |
| G5—G6 | 0.999 | 0.479 | 0.122 | 0.137 | <0.001 ** |
| B-1 | B-2 | B-7 | B-30 | 1-2 | 1-7 | 1-30 | 2-7 | 2-30 | 7-30 | |
|---|---|---|---|---|---|---|---|---|---|---|
| G1—G2 | 0.032 * | 0.128 | 0.999 | 0.999 | 0.999 | 0.051 | 0.999 | 0.001 ** | 0.999 | 0.138 |
| G1—G3 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.288 | 0.999 | 0.109 | 0.999 | 0.999 |
| G1—G4 | 0.097 | 0.426 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.781 | 0.999 | 0.999 |
| G1—G5 | 0.061 | 0.426 | 0.999 | 0.999 | 0.999 | 0.391 | 0.999 | 0.052 | 0.999 | 0.288 |
| G1—G6 | 0.001 ** | 0.001 ** | 0.999 | <0.001 ** | 0.999 | 0.999 | 0.060 | 0.013 * | 0.999 | <0.001 ** |
| G2—G3 | 0.357 | 0.387 | 0.999 | 0.933 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 |
| G2—G4 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.190 | 0.999 | 0.999 |
| G2—G5 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 |
| G2—G6 | 0.999 | 0.961 | 0.559 | 0.017 * | 0.999 | 0.999 | 0.026 * | 0.999 | 0.387 | 0.693 |
| G3—G4 | 0.891 | 0.999 | 0.807 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 |
| G3—G5 | 0.609 | 0.999 | 0.999 | 0.541 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 |
| G3—G6 | 0.009 ** | 0.002 ** | 0.078 | <0.001 ** | 0.554 | 0.999 | 0.003 ** | 0.999 | 0.256 | 0.012 * |
| G4—G5 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 |
| G4—G6 | 0.999 | 0.319 | 0.999 | 0.013 * | 0.999 | 0.999 | 0.040 * | 0.999 | 0.782 | 0.011 * |
| G5—G6 | 0.999 | 0.319 | 0.999 | 0.034 * | 0.999 | 0.999 | 0.089 | 0.999 | 0.999 | 0.352 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazicioglu, O.; Ucuncu, M.K.; Aydin, S. Comparative Measurement of Mercury Release Values from Amalgam Restorations with Different Surface Numbers: An In Vitro Study. Appl. Sci. 2025, 15, 6646. https://doi.org/10.3390/app15126646
Yazicioglu O, Ucuncu MK, Aydin S. Comparative Measurement of Mercury Release Values from Amalgam Restorations with Different Surface Numbers: An In Vitro Study. Applied Sciences. 2025; 15(12):6646. https://doi.org/10.3390/app15126646
Chicago/Turabian StyleYazicioglu, Oktay, Musa Kazim Ucuncu, and Serdar Aydin. 2025. "Comparative Measurement of Mercury Release Values from Amalgam Restorations with Different Surface Numbers: An In Vitro Study" Applied Sciences 15, no. 12: 6646. https://doi.org/10.3390/app15126646
APA StyleYazicioglu, O., Ucuncu, M. K., & Aydin, S. (2025). Comparative Measurement of Mercury Release Values from Amalgam Restorations with Different Surface Numbers: An In Vitro Study. Applied Sciences, 15(12), 6646. https://doi.org/10.3390/app15126646







