The Impact of Dextrin-Activated Expanded Perlite and Vermiculite Particles on the Performance of Thermal Insulating Rapeseed Oil-Based Polyurethane Foam Composites
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of Dextrin Modified Fillers and Polyurethane Foam Composites
2.3. Testing Methods
3. Results and Discussion
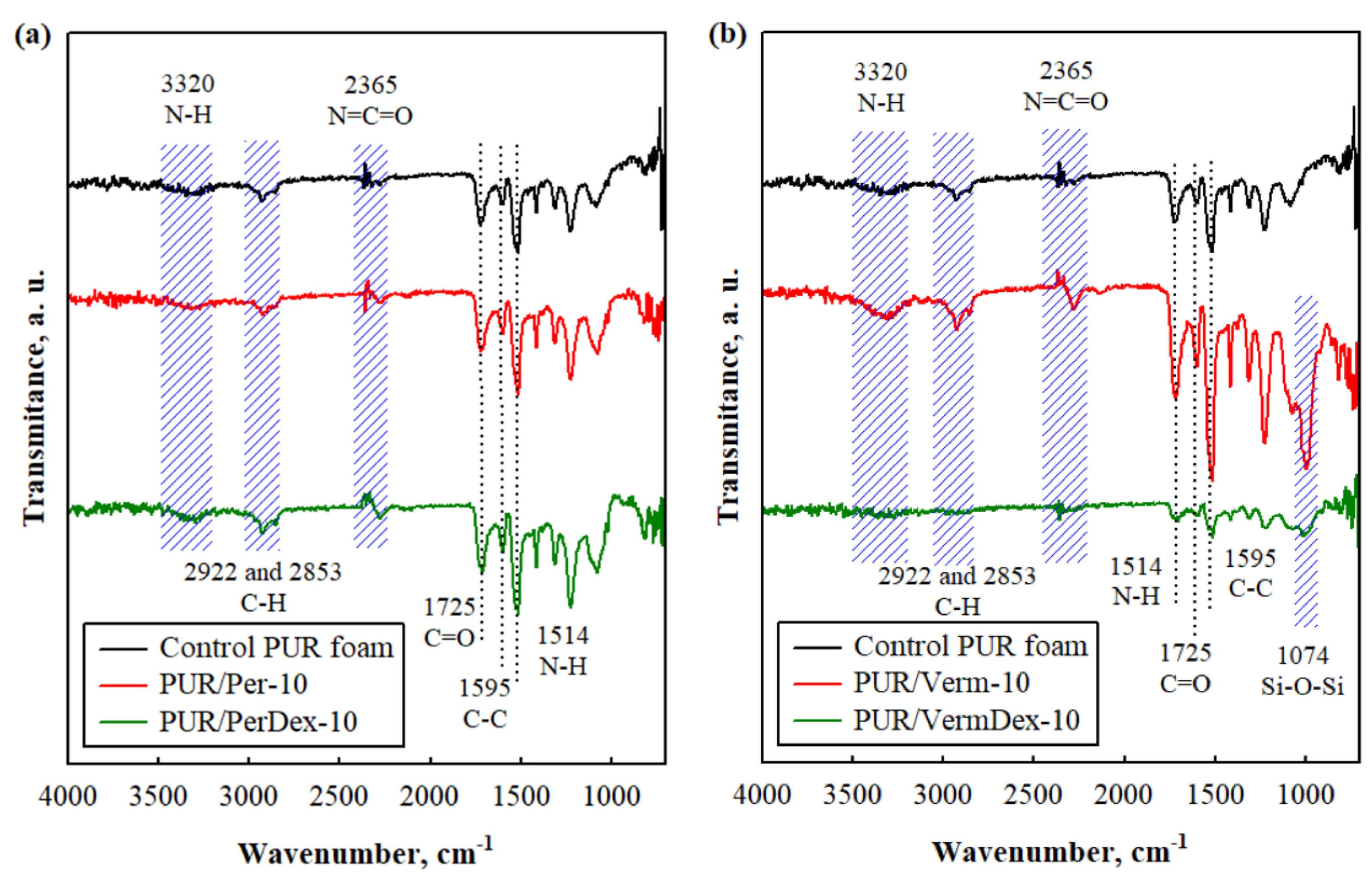
3.1. Characterization of Fillers
3.2. Reaction Kinetics of Premixes
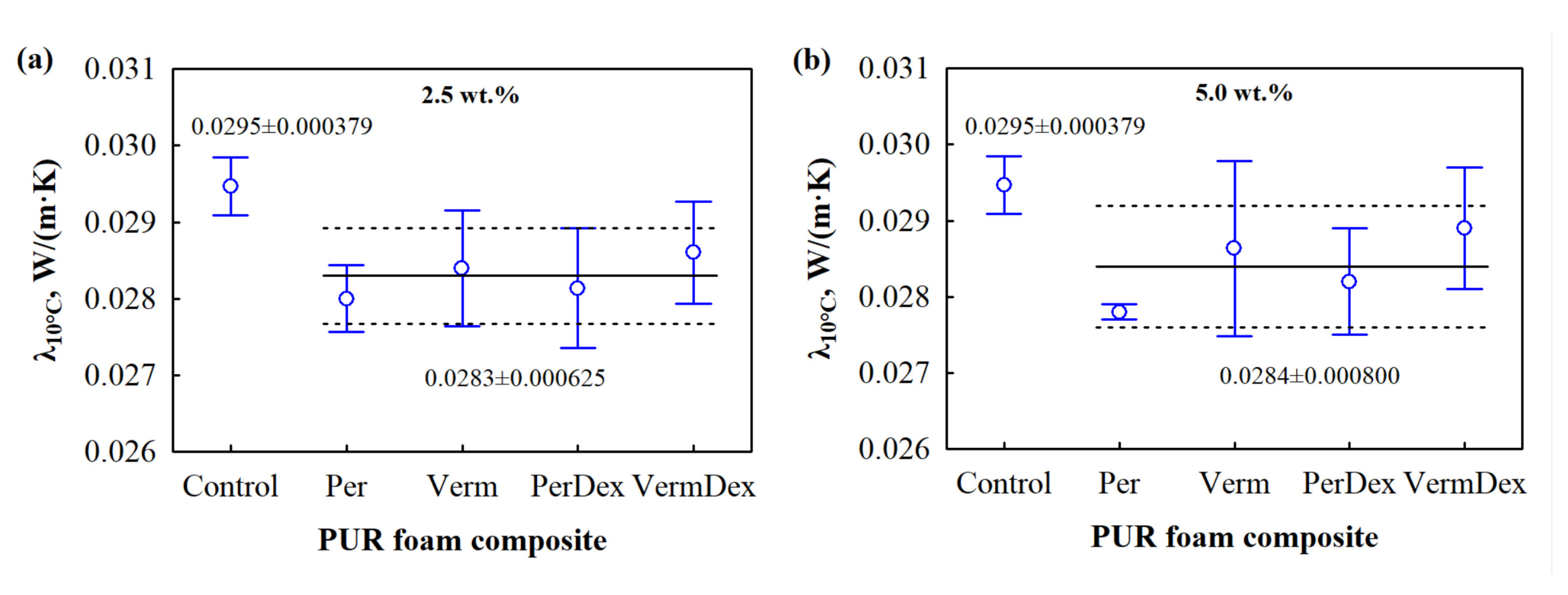
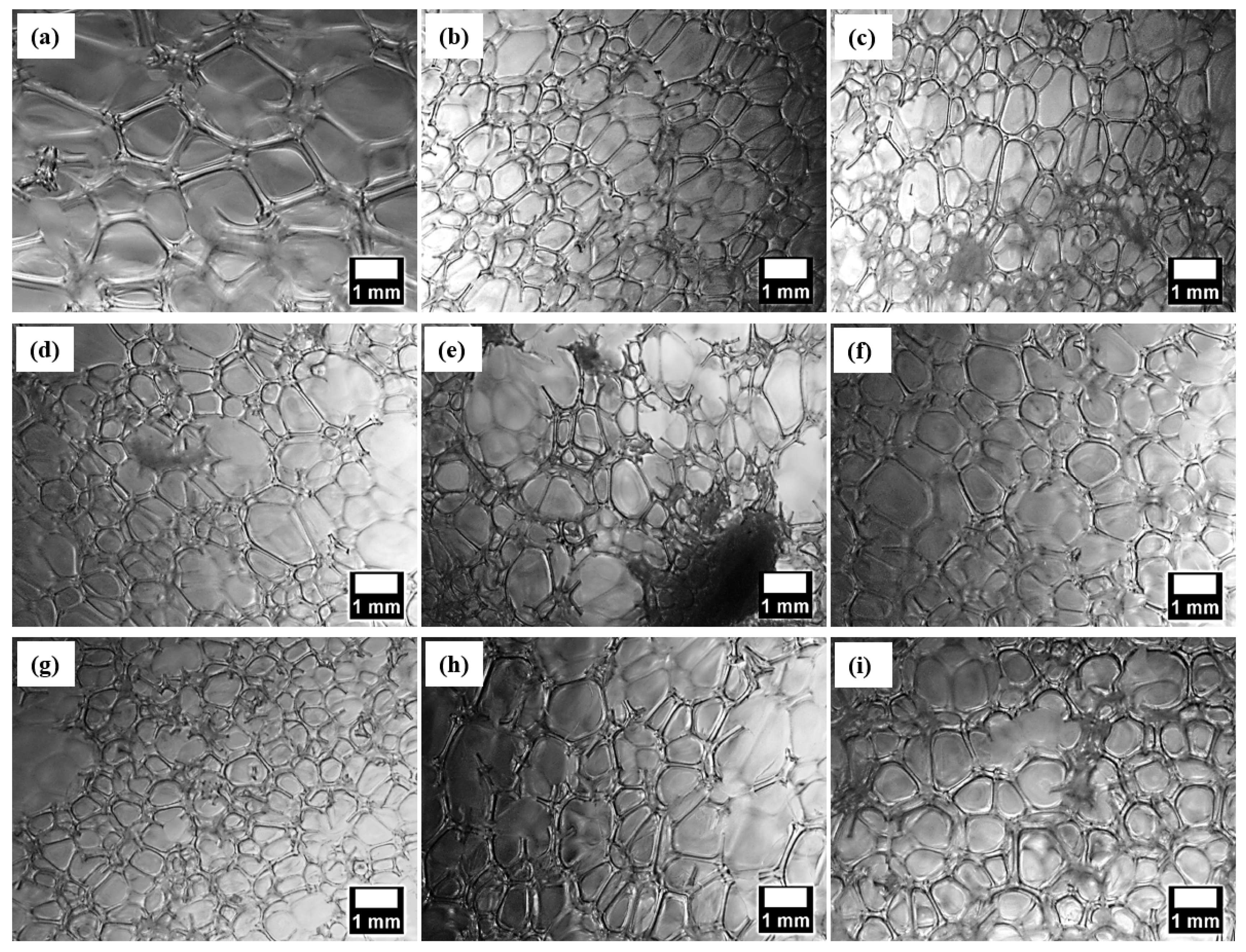
3.3. Microstructure and Thermal Conductivity
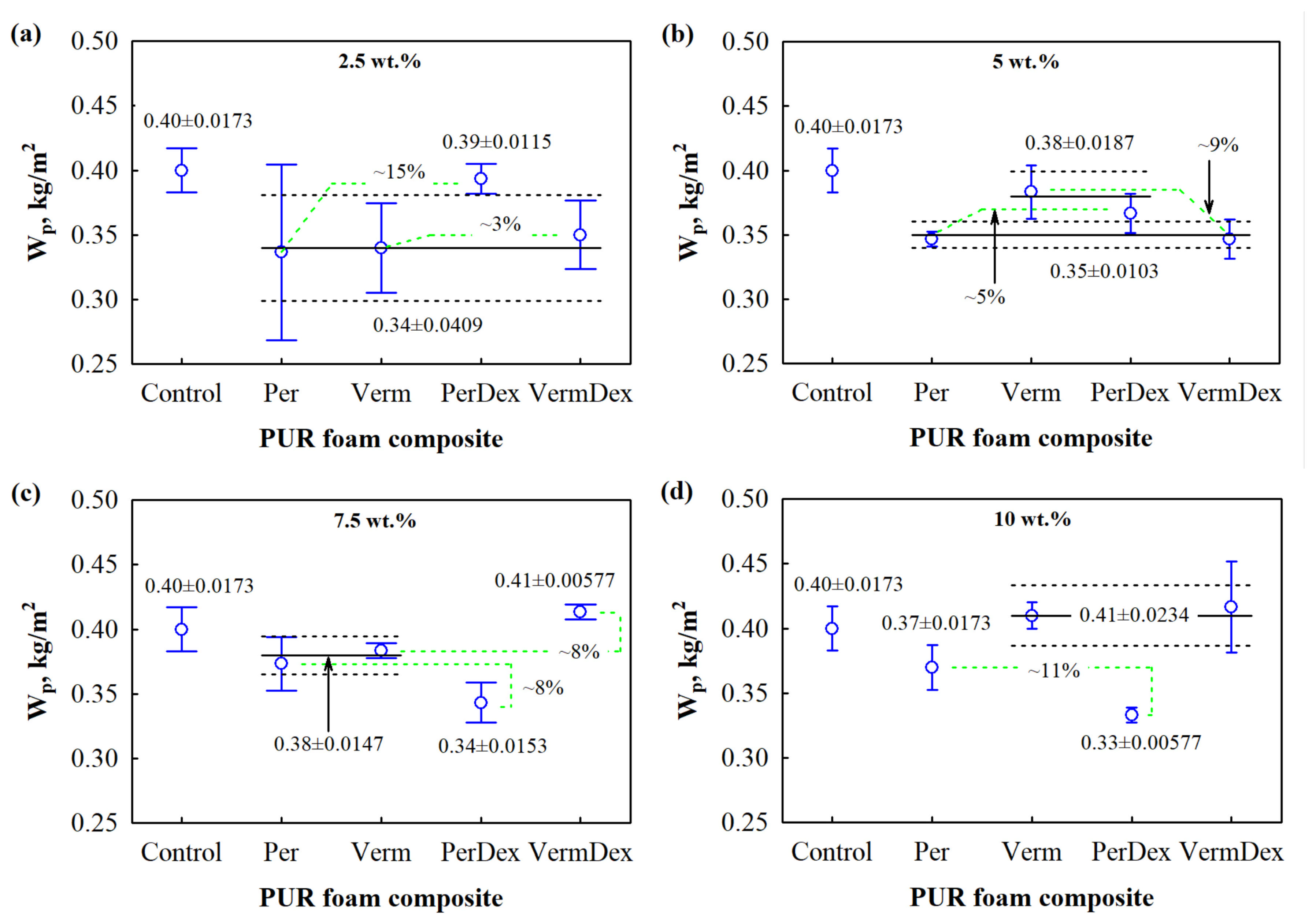
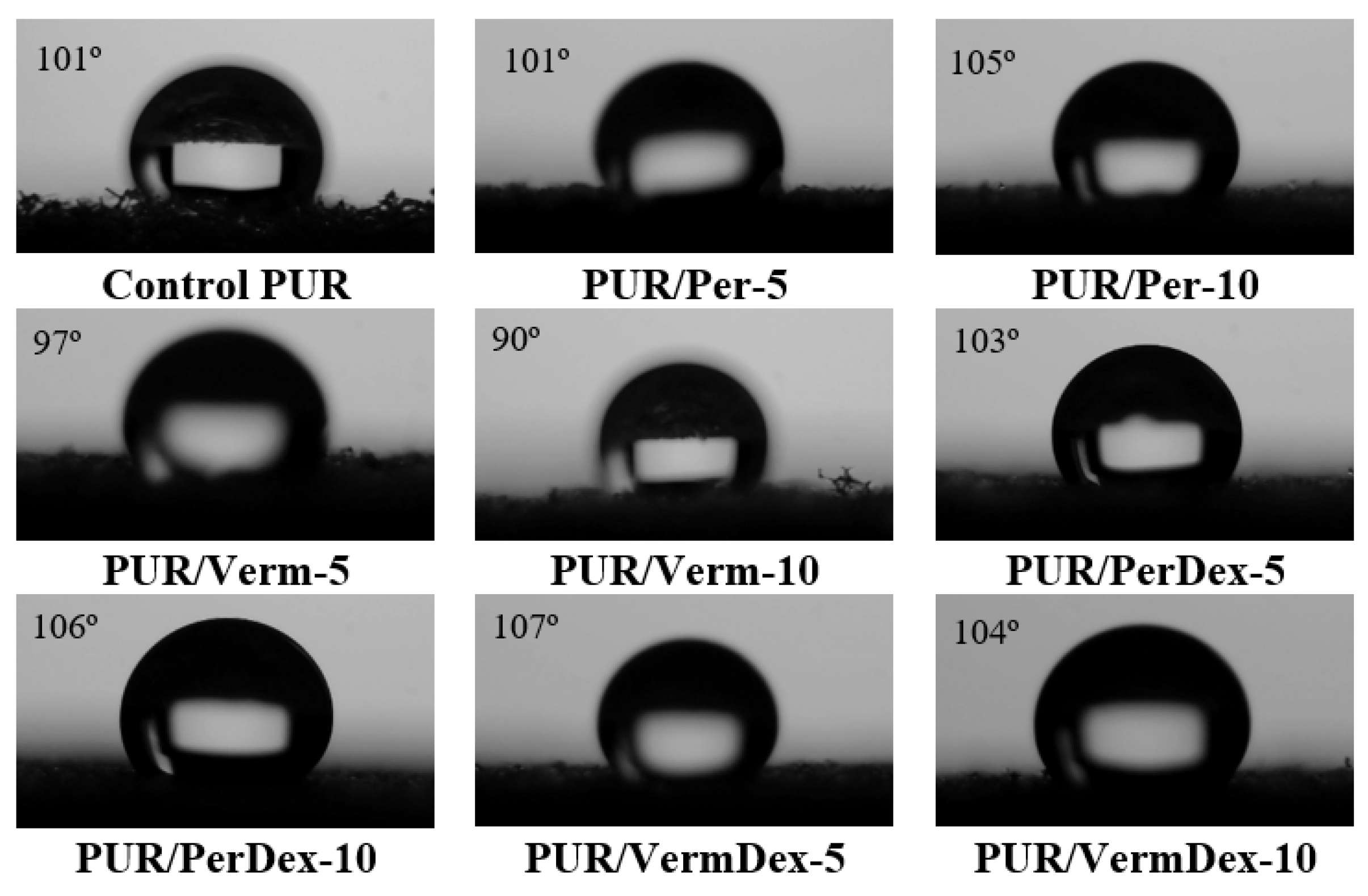
3.4. Water Resistance Performance
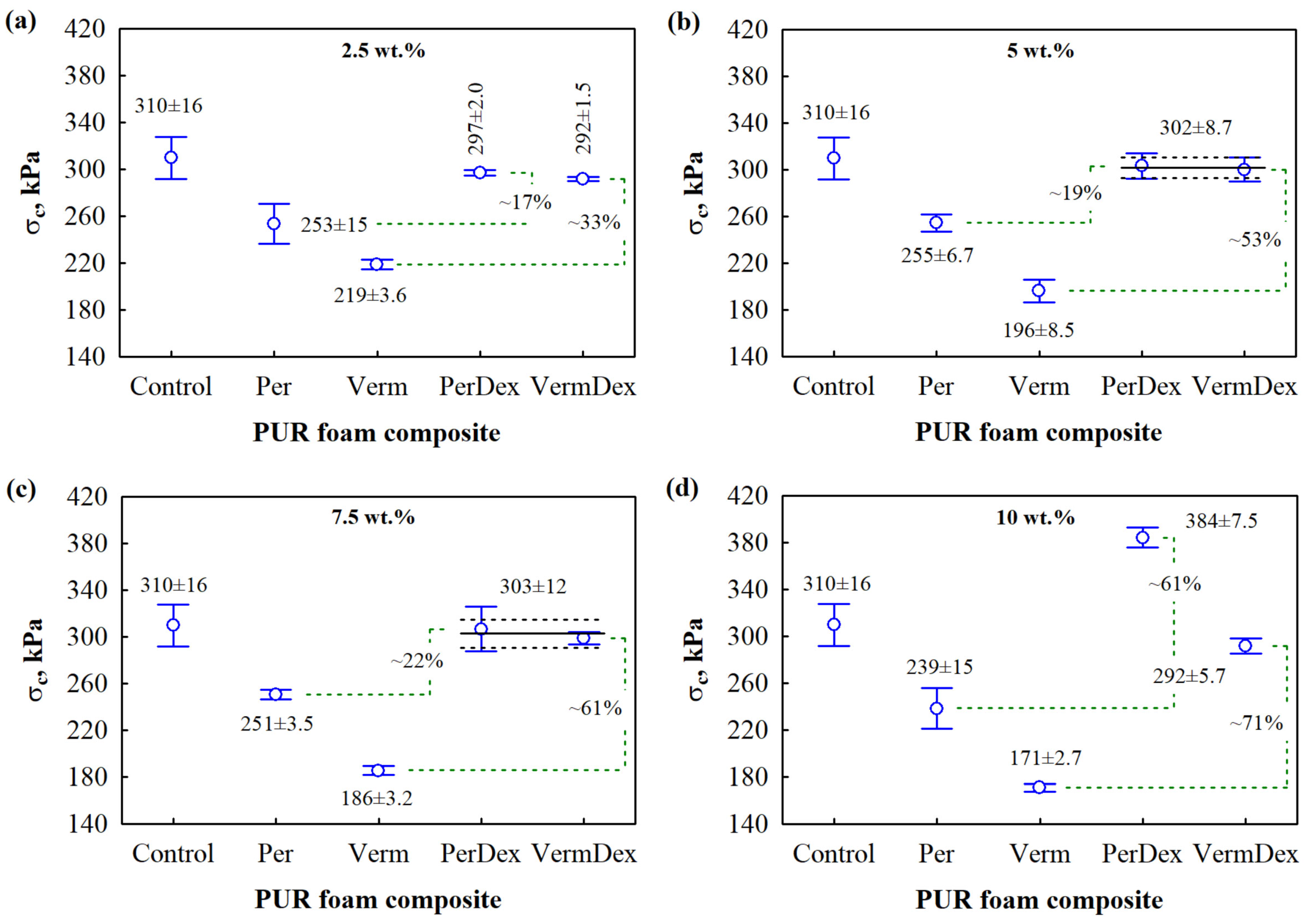
3.5. Mechanical Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PUR | Rigid polyurethane foam |
| Dex | Dextrin |
| Verm | Expanded vermiculite |
| Per | Expanded perlite |
| VermDex | Dextrin-activated expanded vermiculite |
| PerDex | Dextrin-activated expanded perlite |
References
- Cheng, C.; Zhu, Z.; Wang, M.; Li, J.; Sun, H.; Jiao, R.; Li, A. Enhanced flame retardancy and thermal insulation of flexible polyurethane foam decorated with DMMP-loaded CuO hollow spheres. Colloids Surf. A Physicochem. Eng. Asp. 2025, 718, 136961. [Google Scholar] [CrossRef]
- Mendija, L.C.C.; Dingcong, R.G., Jr.; Alfeche, F.L.A.M.; Al-Moameri, H.H.; Dumancas, G.G.; Tan, N.P.B.; Malaluan, R.M.; Alguno, A.C.; Lubguban, A.A. Elucidating the Impact of Polyol Functional Moieties on Exothermic Poly(urethane-urea) Polymerization: A Thermo-Kinetic Simulation Approach. Sustainability 2024, 16, 4587. [Google Scholar] [CrossRef]
- Mouren, A.; Avérous, L. Sustainable cycloaliphatic polyurethanes: From synthesis to applications. Chem. Soc. Rev. 2023, 52, 277–317. [Google Scholar] [CrossRef]
- Malucelli, G. Flame retardant surface treatments for rigid polyurethane foams used in the building sector: Current state-of-the-art and perspectives. Constr. Build. Mater. 2025, 472, 140947. [Google Scholar] [CrossRef]
- Chen, M.; Yuan, Y.; Wang, W.; Xu, L. Recent Advances in Flame-Retardant Flexible Polyurethane Foams. Fire 2025, 8, 90. [Google Scholar] [CrossRef]
- Polyurethane Market. Available online: https://www.marketsandmarkets.com (accessed on 21 March 2025).
- Zhang, Y.; Li, P.; Sun, S. Biopolyol synthesis via ring-opening of epoxidized soybean oil with glycerol utilizing a novel sulfonic-functionalized ionic liquid as the catalyst at low temperature. Ind. Crops Prod. 2024, 222, 119546. [Google Scholar] [CrossRef]
- Li, G.-Z.; Wang, Q.; Zhu, C.; Zhang, S.; Wang, F.; Tao, L.; Jiang, Y.; Zhang, Q.; Wang, W.; Han, R. Synthesis and Performance of Epoxy-Terminated Hyperbranched Polymers Based on Epoxidized Soybean Oil. Molecules 2025, 30, 583. [Google Scholar] [CrossRef]
- Gurgel, D.; Bresolin, D.; Sayer, C.; Filho, L.C.; de Araújo, P.H.H. Flexible polyurethane foams produced from industrial residues and castor oil. Ind. Crops Prod. 2021, 164, 113377. [Google Scholar] [CrossRef]
- Ciastowicz, Ż.; Pamuła, R.; Bobak, Ł.; Białowiec, A. Characterization of Vegetable Oils for Direct Use in Polyurethane-Based Adhesives: Physicochemical and Compatibility Assessment. Materials 2025, 18, 918. [Google Scholar] [CrossRef]
- Mekarat, S.; Suwan, A.; Chaisit, T.; Thongseenuch, S.; Saetung, A.; Saetung, N. Synthesis of palm oil-based bio-polyol by thiol-ene reaction: Preliminary study of its potential as cationic waterborne polyurethane for coating application. Prog. Org. Coat. 2024, 194, 108586. [Google Scholar] [CrossRef]
- Palaczek, K.; Kurańska, M.; Auguścik-Królikowska, M.; Prociak, A.; Ryszkowska, J. Open-cell polyurethane foams of very low density modified with various palm oil-based bio-polyols in accordance with cleaner production. J. Clean. Prod. 2021, 290, 125875. [Google Scholar] [CrossRef]
- Abolins, A.; Eihe, D.; Pomilovskis, R.; Fridrihsone, A.; Kirpluks, M. Rapeseed oil as feedstock for the polymeric materials via Michael addition reaction. Ind. Crops Prod. 2023, 204, 117367. [Google Scholar] [CrossRef]
- Aydoğmuş, E.; Kamişli, F. New commercial polyurethane synthesized with biopolyol obtained from canola oil: Optimization, characterization, and thermophysical properties. J. Mol. Struct. 2022, 1256, 132495. [Google Scholar] [CrossRef]
- Czifrák, K.; Lakatos, C.; Cserháti, C.; Vecsei, G.; Zsuga, M.; Kéki, S. Bio-Based Polyurethane Networks Containing Sunflower Oil Based Polyols. Int. J. Mol. Sci. 2024, 25, 7300. [Google Scholar] [CrossRef]
- Suthar, V.; Asare, M.A.; de Souza, F.M.; Gupta, R.K. Effect of Graphene Oxide and Reduced Graphene Oxide on the Properties of Sunflower Oil-Based Polyurethane Films. Polymers 2022, 14, 4974. [Google Scholar] [CrossRef]
- Polaczek, K.; Kurańska, M.; Prociak, A. Open-cell bio-polyurethane foams based on bio-polyols from used cooking oil. J. Clean. Prod. 2022, 359, 132107. [Google Scholar] [CrossRef]
- Olivito, F.; Jagdale, P.; Oza, G. Direct Production of Furfural from Fructose Catalyzed by Iron(III) Sulfate Using a Simple Distillation Apparatus. ACS Sustain. Chem. Eng. 2023, 11, 17595–17599. [Google Scholar] [CrossRef]
- Jang, J.Y.; Byun, Y.; You, S.; Kim, S.; Lee, D.-M.; Kim, S.-W.; Son, S.U. Polyurethanes Synthesized Using Biomass-derived Furan Diols as Sustainable Triboelectric Materials. Chem. Commun. 2024, 60, 9741–9744. [Google Scholar] [CrossRef]
- Chang, H.; Gilcher, E.B.; Huber, G.W.; Dumesic, J.A. Synthesis of performance-advantaged polyurethanes and polyesters from biomass-derived monomers by aldol-condensation of 5-hydroxymethyl furfural and hydrogenation. Green Chem. 2021, 23, 4355–4364. [Google Scholar] [CrossRef]
- Torresi, S.; Calvo-Correas, T.; Basasoro, S.; Guaresti, O.; Alonso-Varona, A.; Gabilondo, N.; Eceiza, A. Furan-containing biobased polyurethane nanofibers: A new versatile and green support clickable via Diels-Alder reaction. React. Funct. Polym. 2022, 178, 105353. [Google Scholar] [CrossRef]
- Skoczinski, P.; Cangahuala, M.K.E.; Maniar, D.; Albach, R.W.; Bittner, N.; Loos, K. Biocatalytic Synthesis of Furan-Based Oligomer Diols with Enhanced End-Group Fidelity. ACS Sustain. Chem. Eng. 2020, 8, 1068–1086. [Google Scholar] [CrossRef]
- Kurańska, M.; Pinto, J.A.; Salach, K.; Barreiro, M.F.; Prociak, A. Synthesis of thermal insulating polyurethane foams from lignin and rapeseed based polyols: A comparative study. Ind. Crops Prod. 2020, 143, 111882. [Google Scholar] [CrossRef]
- Tan, S.; Abraham, T.; Ference, D.; MacOsko, C.W. Rigid polyurethane foams from a soybean oil-based polyol. Polymer 2011, 52, 2840–2846. [Google Scholar] [CrossRef]
- Kurańska, M.; Marcovich, N.E.; Prociak, A.; Malewska, E.; Kulpa, K. Open cell semi-rigid polyurethane foams synthesized using palmoil-based bio-polyol. Ind. Crops Prod. 2017, 102, 88–96. [Google Scholar]
- Valipour, F.; Dehghan, S.F.; Hajizadeh, R. The effect of nano- and microfillers on thermal properties of Polyurethane foam. Int. J. Environ. Sci. Technol. 2022, 19, 541–552. [Google Scholar] [CrossRef]
- Wang, H.; Li, T.-T.; Ren, H.; Peng, H.; Huang, S.-Y.; Lin, Q.; Lin, J.-H.; Lou, C.-W. Expanded Vermiculite-Filled Polyurethane Foam-Core Bionic Composites: Preparation and Thermal, Compression, and Dynamic Cushion Properties. Polymers 2019, 11, 1028. [Google Scholar] [CrossRef]
- Ai, M.; Cao, L.; Zhao, X.; Xiang, Z.; Guo, X. Preparation and Characterization of Polyurethane Rigid Foam/Expanded Perlite Thermal Insulation Composites. Adv. Mater. Res. 2010, 96, 141–144. [Google Scholar] [CrossRef]
- Barczewski, M.; Kurańska, M.; Sałasińska, K.; Aniśko, J.; Szulc, J.; Szafraniak-Wiza, I.; Prociak, A.; Polaczek, K.; Uram, K.; Surmacz, K.; et al. Comprehensive Analysis of the Influence of Expanded Vermiculite on the Foaming Process and Selected Properties of Composite Rigid Polyurethane Foams. Polymers 2022, 14, 4967. [Google Scholar] [CrossRef]
- Cetin, M.S.; Toprakci, O.; Taskin, O.S.; Aksu, A.; Toprakci, H.A.K. Expanded vermiculite-filled flexible polymer composites. J. Elastomers Plast. 2022, 54, 145–168. [Google Scholar] [CrossRef]
- Jaworska, B.; Stańczak, D.; Łukowski, P. The Influence of Waste Perlite Powder on Selected Mechanical Properties of Polymer–Cement Composites. Buildings 2024, 14, 181. [Google Scholar] [CrossRef]
- Szadkowski, B.; Marzec, A.; Rybiński, P.; Żukowski, W.; Zaborski, M. Characterization of Ethylene–propylene Composites Filled with Perlite and Vermiculite Minerals: Mechanical, Barrier, and Flammability Properties. Materials 2020, 13, 585. [Google Scholar] [CrossRef]
- Erdem, R. Uses of Vermiculite and Perlite in FireRetardant Textile Applications. Fash. Technol. 2023, 8, 000691. [Google Scholar]
- Lanzón, M.; Castellón, F.J.; Ayala, M. Effect of the expanded perlite dose on the fire performance of gypsum plasters. Constr. Build. Mater. 2022, 346, 128494. [Google Scholar] [CrossRef]
- Joseph, E.J.; Akshayraj, V.R.; Panneerselvam, K. Surface modification of tungsten fillers for application in polymer matrix composites. Mater. Today Proc. 2021, 45, 7930–7933. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, Y.; Zheng, S.; Kang, W. Progress in Application of Silane Coupling Agent for Clay Modification to Flame Retardant Polymer. Molecules 2024, 29, 4143. [Google Scholar] [CrossRef]
- Li, H.; Song, S.-I.; Jang, K.-S. The Role of Surface Treatment and Coupling Agents for Adhesion between Stainless Steel (SUS) and Polyamide (PA) of Heterojunction Bilayer Composites. Polymers 2024, 16, 896. [Google Scholar] [CrossRef]
- Miedzińska, K.; Członka, S.; Strąkowska, A.; Strzelec, K. Vermiculite Filler Modified with Casein, Chitosan, and Potato Protein as a Flame Retardant for Polyurethane Foams. Int. J. Mol. Sci. 2021, 22, 10825. [Google Scholar] [CrossRef]
- Członka, S.; Kairytė, A.; Miedzińska, K.; Strąkowska, A. Casein/Apricot Filler in the Production of Flame-Retardant Polyurethane Composites. Materials 2021, 14, 3620. [Google Scholar] [CrossRef]
- Wang, W.; Wang, D.; Xia, B.; Li, T.; Wang, Y.; Zhang, X.; Bai, H.; Chen, M.; Dong, W. Rigid polyurethane foams based on dextrin and glycerol. Ind. Crops Prod. 2022, 177, 114479. [Google Scholar] [CrossRef]
- Ivdre, A.; Abolins, A.; Sevastyanova, I.; Kirpluks, M.; Cabulis, U.; Merijs-Meri, R. Rigid Polyurethane Foams with Various Isocyanate Indices Based on Polyols from Rapeseed Oil and Waste PET. Polymers 2020, 12, 738. [Google Scholar] [CrossRef]
- EN 14315-1; Thermal Insulating Products for Buildings—In-Situ Formed Sprayed Rigid Polyurethane (PUR) and Polyisocyanurate (PIR) Foam Products—Part 1: Specification for the Rigid Foam Spray System before Installation. European Standardization Committee: Brussels, Belgium, 2013.
- ISO 4590; Rigid Cellular Plastics—Determination of the Volume Percentage of Open Cells and of Closed Cells. International Standardization Organization: Geneva, Switzerland, 2016.
- EN 12667; Thermal Performance of Building Materials and Products—Determination of Thermal Resistance by Means of Guarded Hot Plate and Heat Flow Meter Methods—Products of High and Medium Thermal Resistance. European Standardization Committee: Brussels, Belgium, 2001.
- ISO 29767; Thermal Insulating Products for Building Applications—Determination of Short-Term Water Absorption by Partial Immersion. International Standardization Organization: Geneva, Switzerland, 2019.
- EN 1607; Thermal Insulating Products for Building Applications—Determination of Tensile Strength Perpendicular to Faces. European Standardization Committee: Brussels, Belgium, 2013.
- ISO 29469; Thermal Insulating Products for Building Applications—Determination of Compression Behaviour. International Standardization Organization: Geneva, Switzerland, 2022.
- Sakr, H.; Ammar, A.; Zaki, H.; Salama, M.A.; Ali, M. Impact of ball milling on physicochemical, structural, and functional properties of Moringa oleifera L. leaf powders. J. Food Meas. Charact. 2023, 18, 320–330. [Google Scholar] [CrossRef]
- Saufi, H.; El Alouani, M.; Alehyen, S.; El Achouri, M.; Aride, J.; Taibi, M. Photocatalytic Degradation of Methylene Blue from Aqueous Medium onto Perlite-Based Geopolymer. Int. J. Chem. Eng. 2020, 2020, 9498349. [Google Scholar] [CrossRef]
- Lee, W.K.W.; van Deventer, J.S.J. Use of Infrared Spectroscopy to Study Geopolymerization of Heterogeneous Amorphous Aluminosilicates. Langmuir 2003, 19, 8726–8734. [Google Scholar] [CrossRef]
- Tran, L.; Wu, P.; Zhu, Y.; Yang, L.; Zhu, N. Highly enhanced adsorption for the removal of Hg(II) from aqueous solution by Mercaptoethylamine/Mercaptopropyltrimethoxysilane functionalized vermiculites. J. Colloid Interface Sci. 2015, 445, 348–356. [Google Scholar] [CrossRef]
- Duman, O.; Tunç, S.; Polat, T.G. Determination of adsorptive properties of expanded vermiculite for the removal of C.I. Basic Red 9 from aqueous solution: Kinetic, isotherm and thermodynamic studies. Appl. Clay Sci. 2015, 109, 22–32. [Google Scholar] [CrossRef]
- Salih, S.S.; Kadhom, M.; Shihab, M.A.; Ghosh, T.K. Competitive Adsorption of Pb(II) and Phenol Onto Modified Chitosan/Vermiculite Adsorbents. J. Polym. Environ. 2022, 30, 4238–4251. [Google Scholar] [CrossRef]
- Mamiński, M.Ł.; Więcław-Midor, A.; Parzuchowski, P.G. The Effect of Silica-Filler on Polyurethane Adhesives Based on Renewable Resource for Wood Bonding. Polymers 2020, 12, 2177. [Google Scholar] [CrossRef]
- Sari, Y.; Utama, A.H.; Irawan, A.; Andiana, P.; Faradila, R.; Manab, A.; Radiati, L.E. Optimizing Dextrin Levels for Enhanced Physicochemical Properties and Microstructure of Sumbawa Mare’s Milk Powder. Adv. Anim. Vet. Sci. 2025, 13, 431–439. [Google Scholar] [CrossRef]
- Arai, Y.; Tomai, T.; Seong, G.; Ito, R.; Yoko, A.; Adschiri, T. Unified formulation of particle-size-dependent viscosity for Newtonian dispersions of micro- and nanoparticles. J. Mol. Liq. 2024, 411, 125659. [Google Scholar] [CrossRef]
- Dorokhov, A.S.; Goncharova, Y.A.; Sviridov, A.S.; Tuzhilin, S.P.; Alekhina, R.A. Measurement of the Dynamic Viscosity of Filled Composites on the Basis of Thermosetting Cast Polyurethane. Theor. Found. Chem. Eng. 2023, 57, 1116–1119. [Google Scholar] [CrossRef]
- Gama, N.V.; Amaral, C.; Silva, T.; Vicente, R.; Coutinho, J.A.P.; Barros-Timmons, A.; Ferreira, A. Thermal Energy Storage and Mechanical Performance of Crude Glycerol Polyurethane Composite Foams Containing Phase Change Materials and Expandable Graphite. Materials 2018, 11, 1896. [Google Scholar] [CrossRef]
- Lee, D.I.; Ha, Y.H.; Jeon, H.; Kim, S.H. Preparation and Properties of Polyurethane Composite Foams with Silica-Based Fillers. Appl. Sci. 2022, 12, 7418. [Google Scholar] [CrossRef]
- Soloveva, O.V.; Solovev, S.A.; Vankov, Y.V.; Shakurova, R.Z. Experimental Studies of the Effective Thermal Conductivity of Polyurethane Foams with Different Morphologies. Processes 2022, 10, 2257. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Raja, F.D. Experimental characterisation and evaluation of perlite as a sorbent for heavy metal ions in single and quaternary solutions. J. Water Process Eng. 2014, 4, 179–184. [Google Scholar] [CrossRef]
- Marcos, C. Effect of Water Immersion on Raw and Expanded Ugandan Vermiculite. Minerals 2022, 12, 23. [Google Scholar] [CrossRef]
- Assis Neto, P.C.D.; Sales, L.P.B.; Oliveira, P.K.S.; Silva, I.C.D.; Barros, I.M.D.S.; Nóbrega, A.F.D.; Carneiro, A.M.P. Expanded Vermiculite: A Short Review about Its Production, Characteristics, and Effects on the Properties of Lightweight Mortars. Buildings 2023, 13, 823. [Google Scholar] [CrossRef]
- Xia, Z.; Wang, Z. Rigid polyurethane/expanded vermiculite/melamine phenylphosphate composite foams with good flame retardant and mechanical properties. e-Polymers 2019, 19, 563–573. [Google Scholar] [CrossRef]
- Członka, S.; Bertino, M.F.; Strzelec, K.; Strąkowska, A.; Masłowski, M. Rigid polyurethane foams reinforced with solid waste generated in leather industry. Polym. Test. 2018, 69, 225–237. [Google Scholar] [CrossRef]
- de Souza, F.M.; Choi, J.; Bhoyate, S.; Kahol, P.K.; Gupta, R.K. Expendable Graphite as an Efficient Flame-Retardant for Novel Partial Bio-Based Rigid Polyurethane Foams. J. Carbon Res. 2020, 6, 27. [Google Scholar] [CrossRef]
- Hejna, A.; Olszewski, A.; Zedler, Ł.; Kosmela, P.; Formela, K. The Impact of Ground Tire Rubber Oxidation with H2O2 and KMnO4 on the Structure and Performance of Flexible Polyurethane/Ground Tire Rubber Composite Foams. Materials 2021, 14, 499. [Google Scholar] [CrossRef]






| Component | Amount of Component, pbw | ||||
|---|---|---|---|---|---|
| Control PUR | PUR-Verm | PUR-Per | PUR-VermDex | PUR-PerDex | |
| Component A: | |||||
| BioPolyol RD | 60 | 60 | 60 | 60 | 60 |
| ROKOPOL RF-152 V | 60 | 60 | 60 | 60 | 60 |
| Water | 3 | 3 | 3 | 3 | 3 |
| Polycat 9 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 |
| ST-52 | 3.6 | 3.6 | 3.6 | 3.6 | 3.6 |
| Filler, wt.% | 0 | 2.5; 5; 7.5; 10 | 2.5; 5; 7.5; 10 | 2.5; 5; 7.5; 10 | 2.5; 5; 7.5; 10 |
| Component B: | |||||
| Isocyanate | Isocyanate index for all compositions—125 | ||||
| Premix | Parameter | |||
|---|---|---|---|---|
| Cream Time, s | Gel Time, s | Tack-Free Time, s | Dynamic Viscosity, mPa·s | |
| Control PUR | 20 ± 2 | 76 ± 2 | 102 ± 4 | 521 ± 10 |
| PUR/Per-2.5 | 29 ± 2 | 114 ± 4 | 156 ± 3 | 1456 ± 15 |
| PUR/Per-5 | 22 ± 2 | 110 ± 2 | 159 ± 2 | 3875 ± 20 |
| PUR/Per-7.5 | 19 ± 3 | 104 ± 2 | 162 ± 3 | 28,699 ± 25 |
| PUR/Per-10 | 16 ± 2 | 96 ± 3 | 164 ± 3 | 50,987 ± 18 |
| PUR/Verm-2.5 | 28 ± 1 | 107 ± 2 | 140 ± 2 | 2350 ± 14 |
| PUR/Verm-5 | 24 ± 2 | 100 ± 3 | 165 ± 2 | 7172 ± 10 |
| PUR/Verm-7.5 | 21 ± 2 | 94 ± 3 | 197 ± 3 | 34,569 ± 15 |
| PUR/Verm-10 | 18 ± 2 | 140 ± 3 | 237 ± 3 | 59,230 ± 19 |
| PUR/PerDex-2.5 | 31 ± 3 | 112 ± 2 | 160 ± 3 | 988 ± 21 |
| PUR/PerDex-5 | 26 ± 2 | 110 ± 1 | 174 ± 2 | 1798 ± 18 |
| PUR/PerDex-7.5 | 20 ± 3 | 110 ± 2 | 181 ± 3 | 2230 ± 16 |
| PUR/PerDex-10 | 16 ± 2 | 109 ± 2 | 189 ± 4 | 3042 ± 19 |
| PUR/VermDex-2.5 | 30 ± 2 | 99 ± 2 | 179 ± 3 | 652 ± 5 |
| PUR/VermDex-5 | 28 ± 3 | 98 ± 3 | 184 ± 2 | 1153 ± 15 |
| PUR/VermDex-7.5 | 23 ± 2 | 95 ± 2 | 189 ± 4 | 1690 ± 12 |
| PUR/VermDex-10 | 20 ± 3 | 94 ± 2 | 195 ± 2 | 2742 ± 20 |
| PUR Foam Composite | Parameter | |||
|---|---|---|---|---|
| Apparent Density, kg/m3 | Closed Cell Content, vol.% | Cell Size, mm | Wall Thickness, μm | |
| Control PUR | 43.7 ± 4 | 94.4 ± 0.52 | 1.32 ± 0.12 | 177 ± 5 |
| PUR/Per-2.5 | 44.5 ± 3 | 94.9 ± 0.63 | 0.812 ± 0.11 | 100 ± 4 |
| PUR/Per-5 | 47.4 ± 5 | 95.4 ± 0.55 | 0.618 ± 0.12 | 88.6 ± 6 |
| PUR/Per-7.5 | 47.6 ± 2 | 97.3 ± 0.42 | 0.725 ± 0.10 | 74.5 ± 6 |
| PUR/Per-10 | 47.4 ± 3 | 94.6 ± 0.44 | 0.837 ± 0.16 | 65.5 ± 2 |
| PUR/Verm-2.5 | 45.7 ± 5 | 94.0 ± 0.22 | 0.882 ± 0.14 | 76.7 ± 6 |
| PUR/Verm-5 | 44.9 ± 4 | 91.4 ± 0.26 | 0.703 ± 0.19 | 71.6 ± 4 |
| PUR/Verm-7.5 | 46.8 ± 3 | 91.6 ± 0.38 | 0.754 ± 0.13 | 65.3 ± 3 |
| PUR/Verm-10 | 52.3 ± 2 | 91.2 ± 0.44 | 0.772 ± 0.12 | 62.7 ± 5 |
| PUR/PerDex-2.5 | 49.4 ± 4 | 97.6 ± 0.62 | 1.12 ± 0.22 | 138 ± 6 |
| PUR/PerDex-5 | 52.3 ± 5 | 97.3 ± 0.51 | 0.780 ± 0.14 | 155 ± 4 |
| PUR/PerDex-7.5 | 56.4 ± 5 | 97.9 ± 0.38 | 0.623 ± 0.16 | 168 ± 3 |
| PUR/PerDex-10 | 63.7 ± 4 | 97.0 ± 0.49 | 0.592 ± 0.12 | 185 ± 6 |
| PUR/VermDex-2.5 | 48.0 ± 3 | 96.1 ± 0.29 | 1.01 ± 0.23 | 113 ± 4 |
| PUR/VermDex-5 | 51.0 ± 2 | 98.0 ± 0.49 | 0.795 ± 0.14 | 115 ± 3 |
| PUR/VermDex-7.5 | 56.4 ± 3 | 94.4 ± 0.64 | 0.832 ± 0.16 | 119 ± 4 |
| PUR/VermDex-10 | 59.8 ± 2 | 94.2 ± 0.58 | 0.865 ± 0.17 | 119 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kairytė, A.; Levina, A. The Impact of Dextrin-Activated Expanded Perlite and Vermiculite Particles on the Performance of Thermal Insulating Rapeseed Oil-Based Polyurethane Foam Composites. Appl. Sci. 2025, 15, 6604. https://doi.org/10.3390/app15126604
Kairytė A, Levina A. The Impact of Dextrin-Activated Expanded Perlite and Vermiculite Particles on the Performance of Thermal Insulating Rapeseed Oil-Based Polyurethane Foam Composites. Applied Sciences. 2025; 15(12):6604. https://doi.org/10.3390/app15126604
Chicago/Turabian StyleKairytė, Agnė, and Aliona Levina. 2025. "The Impact of Dextrin-Activated Expanded Perlite and Vermiculite Particles on the Performance of Thermal Insulating Rapeseed Oil-Based Polyurethane Foam Composites" Applied Sciences 15, no. 12: 6604. https://doi.org/10.3390/app15126604
APA StyleKairytė, A., & Levina, A. (2025). The Impact of Dextrin-Activated Expanded Perlite and Vermiculite Particles on the Performance of Thermal Insulating Rapeseed Oil-Based Polyurethane Foam Composites. Applied Sciences, 15(12), 6604. https://doi.org/10.3390/app15126604







