1. Introduction
Cancer represents one of the leading causes of morbidity, mortality, and healthcare expenditure worldwide [
1]. According to the World Health Organization, cancer accounted for nearly 10 million deaths globally in 2020. Estimates from the OECD suggest that healthcare costs for oncological care in high-income countries are expected to exceed USD 500 billion annually by 2030 [
2]. In worldwide healthcare systems, the rising incidence of cancer driven by an aging population and increased life expectancy is placing unprecedented strain on sustainability and resource allocation [
3]. To meet this challenge, precision oncology is undergoing a profound transformation powered by the convergence of genomics and artificial intelligence (AI). High-throughput sequencing technologies, such as next-generation sequencing (NGS), have revolutionized our understanding of tumor biology by enabling the identification of both somatic and germline pathogenic variants [
4]. Meanwhile, AI, particularly through its subsets, machine learning (ML), which learns from data without explicit programming, and deep learning (DL), as a more advanced form utilizing artificial neural networks, is emerging as a critical partner in extracting actionable insights from complex, high-dimensional genomic datasets [
5]. This synergy is already reshaping cancer care. From guiding the development of targeted therapies and predicting therapeutic response to enhancing early detection through circulating tumor DNA (ctDNA) analysis, AI-powered genomics is opening new frontiers in diagnosis, prognosis, and individualized treatment. However, the integration of these tools into clinical practice also poses challenges related to data standardization, interpretability, and equitable access. In light of these opportunities and limitations, there is a growing need to critically examine how genomics and AI are being implemented across the cancer care continuum. The necessity of this review emerges not only from the rapid evolution of these fields but also the heterogeneity of current knowledge, which often overlooks the full scope and complexity of their combined application [
6,
7]. While existing reviews frequently focus on either genomics or AI in oncology, few offer a comprehensive synthesis that systematically analyzes the applications, benefits, and, crucially, the limitations of their synergy across all phases of cancer management, from risk assessment to resistance monitoring [
8,
9]. The primary contribution of this review is to provide an updated and integrated overview of the transformative impact of genomics and AI in oncology, highlighting not only their synergies, which lead to personalized therapeutic strategies and improved early detection, but also the significant challenges that must be addressed for their full and equitable clinical implementation. These challenges include data standardization, the interpretability of AI algorithms, overcoming biases in datasets, and ethical considerations related to patient privacy [
10].
To guide the reader through this complex landscape, the manuscript is structured around the main domains of application of genomics and AI in oncology. We first examine the contributions of genomic technologies to cancer risk assessment, diagnosis, treatment (including targeted therapies and immunotherapy), prognosis, and early detection. We then explore the role of epigenetics as both a biomarker and a therapeutic target. Finally, we focus on how AI is leveraged to interpret genomic data, personalize treatments, enable drug repurposing, and drive therapeutic innovation. A dedicated section discusses current and future challenges, including ethical, technical, and clinical considerations, offering a comprehensive view of the path forward.
2. Genomics in Cancer Diagnosis
Genomics has significantly contributed to cancer diagnosis by identifying specific genetic alterations that support clinical decision making. Both somatic pathogenic variants (sPVs), which arise in tumor cells, and germline pathogenic variants (gPVs), which are inherited, play a crucial role in cancer development and progression. sPVs occur in the DNA of individual cells and are not transmitted to offspring, typically resulting from environmental factors or errors in DNA replication. gPVs, on the other hand, are present in the DNA of every cell of an individual and can be inherited from one or both parents, predisposing individuals to various types of cancer [
11,
12]. PVs are identified using a combination of bioinformatics tools, functional assays, and clinical guidelines, such as those provided by the American College of Medical Genetics and Genomics (ACMG). Criteria include the type of mutation (e.g., nonsense, frameshift), its frequency in the general population, segregation with disease in families, computational predictions, and available experimental or clinical evidence linking the variant to disease [
13].
One of the most well-characterized genetic signatures in oncology includes gPVs in
BRCA1 and
BRCA2 genes, which confer susceptibility to breast and ovarian cancers, accounting for 5–10% of breast cancer cases and 5–25% of ovarian cases [
14]. PVs in
BRCA1 and
BRCA2 impair the repair of DNA damage, leading to an accumulation of mutations in cells, thereby increasing the risk of cancer. These gPVs are inherited in an autosomal dominant pattern, meaning an individual with a variant in either gene has a 50% chance of passing it on to their offspring. Additionally,
BRCA-gPVs not only affect breast and ovarian cancers but also increase the risk of other cancers, such as prostate and pancreatic cancer [
15]. Somatic genetic testing of
BRCA1 and
BRCA2 is currently employed in breast, ovarian, pancreatic, and prostatic cancers to guide the administration of PARP inhibitor therapy. BRCA1/2 are involved in the homologous recombination DNA repair pathway; their loss leads to homologous recombination deficiency. PARP inhibitors exploit this defect through a mechanism of synthetic lethality, as inhibition of PARP-mediated single-strand break repair leads to the accumulation of DNA damage and subsequent cell death in BRCA-deficient tumor cells [
16]. When a PV is identified through somatic testing, patients are often referred for genetic counseling to determine if they are germline mutation carriers. This distinction is crucial, as germline mutation carriers may benefit from advanced screening, risk-reduction strategies, and genetic testing for identifying family members at risk. Other hereditary breast-cancer-associated genes include high-penetrance genes (e.g.,
CDH1,
PALB2,
PTEN,
STK11,
TP53) and moderate-penetrance genes (e.g.,
ATM,
BARD1,
BRIP1,
CHEK2,
RAD51C,
RAD51D), where “penetrance” describes the proportion of individuals carrying a pathogenic variant who actually develop the associated disease [
17].
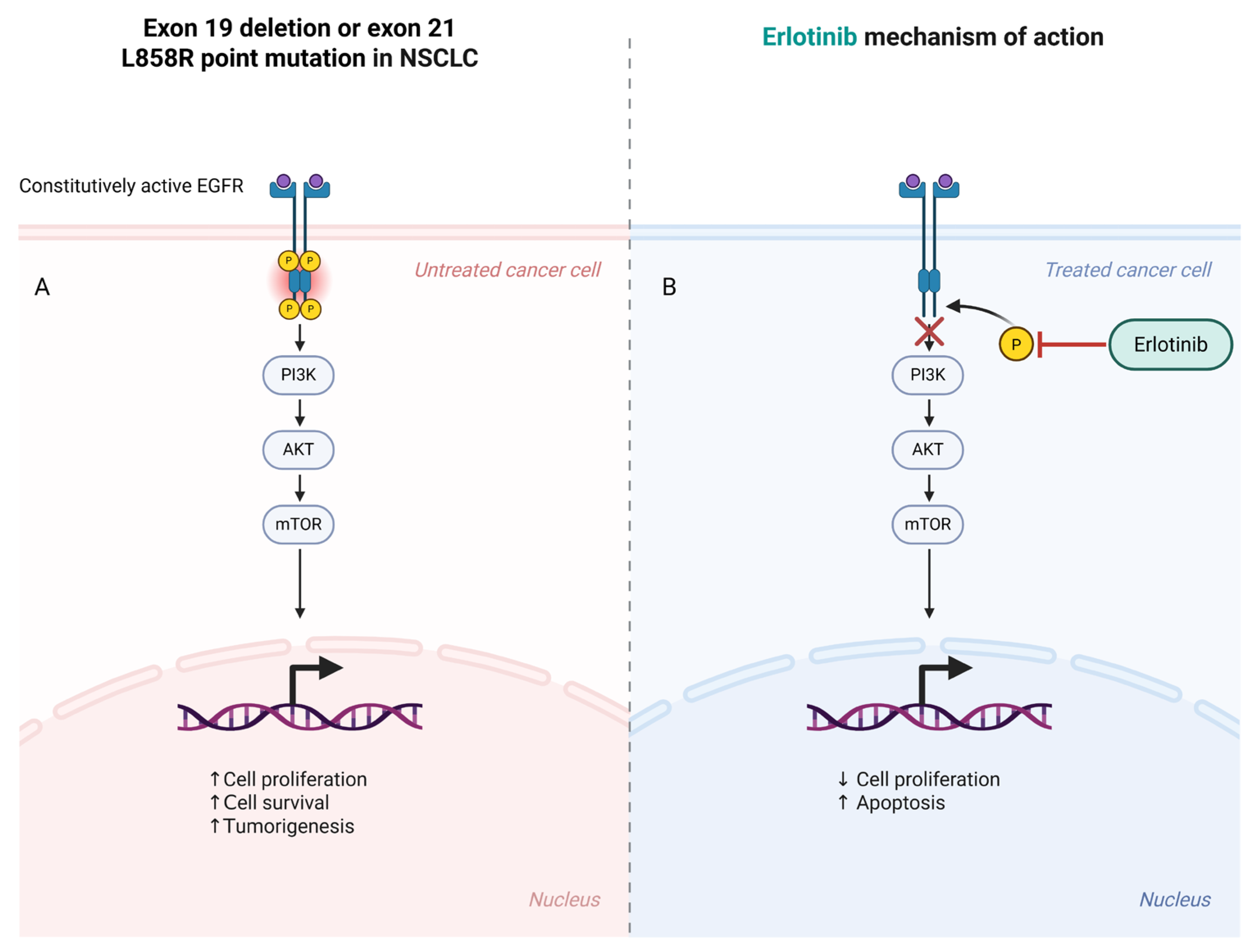
Similarly, PVs in
EGFR are somatic alterations commonly observed in non-small cell lung cancer (NSCLC). These variants typically occur in the epidermal growth factor receptor gene, which regulates cell growth and survival, and they cause the receptor to be constantly active, promoting tumor cell proliferation. Targeted therapies, such as tyrosine kinase inhibitors (TKIs), specifically inhibit this mutated receptor, offering a more personalized treatment approach for patients with NSCLC [
18].
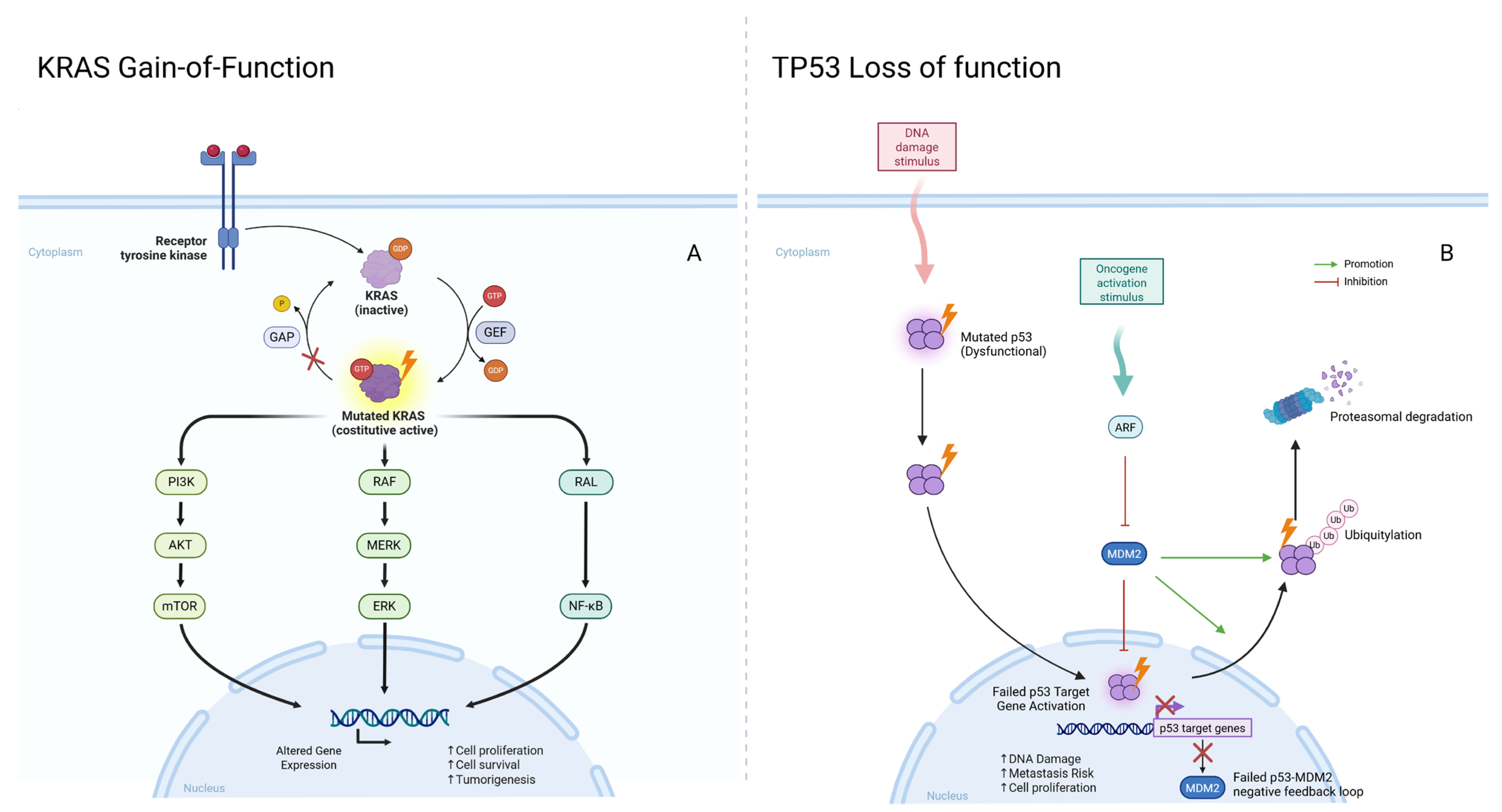
Other key genomic alterations include
KRAS variants in colorectal and pancreatic cancers and
TP53 variants, which are found in a wide range of malignancies and often indicate poor prognosis.
KRAS pathogenic variants drive uncontrolled cell proliferation in colorectal and pancreatic cancers, whereas
TP53 (called the “guardian of the genome”) pathogenic variants lead to the loss of a crucial tumor suppressor function, increasing metastasis risk [
19]. Both
KRAS and
TP53 pathogenic variants are typically somatic and correlate with aggressive cancer phenotypes and poor treatment response (
Figure 1).
Advancements in molecular diagnostic technologies have enabled rapid and precise identification of both somatic and germline pathogenic variants. Next-generation sequencing (NGS) allows for comprehensive genomic profiling of tumors, providing a detailed view of the genetic landscape of cancer. Whole-exome sequencing (WES) focuses on coding regions of the genome, thus identifying pathogenic variants that directly impact protein function. Whole-genome sequencing (WGS) analyzes the entire genome, including introns and non-coding regions, offering a comprehensive overview of potential genetic alterations. Although most clinically actionable variants lie within exons, WGS enables the detection of deep intronic variants that may affect splicing or regulatory functions. The interpretation of such intronic alterations, however, remains challenging and often requires integration with transcriptomic or functional studies to assess pathogenicity [
20]. However, due to the high costs and analytical complexity of WGS, WES still represents one of the most sustainable choices for many clinical applications. In addition, one of the most commonly implemented strategies in oncogenetics is the use of gene panels. These are custom-designed tools that analyze multiple genes simultaneously, allowing for efficient and targeted identification of variants involving different cancer-relevant genes. Gene panels are typically designed to focus on genes (such as
BRCA1,
BRCA2,
TP53,
EGFR, and
KRAS) mostly relevant to specific cancer types [
21]. By analyzing several genes at once, these panels provide deeper insight into the genetic mechanisms of cancer, allow clinicians to make more informed decisions about treatment strategies, and enable faster and more precise diagnoses. Parallel to these advancements, innovative approaches, such as the use of Feulgen–Thionin staining, a DNA stoichiometric stain, have also shown promise in improving prostate cancer diagnosis, highlighting how even established diagnostic techniques can be optimized for enhanced precision [
22].
Table 1 summarizes the most relevant genomic technologies currently applied in cancer diagnostics and risk assessment, highlighting their main applications, strengths, and limitations.
Contributions and Limitations of Genomics in Cancer Diagnosis
In summary, genomics has made substantial contributions to cancer diagnosis, enabling precise identification of genetic alterations crucial for clinical decision making. The analysis of somatic and germline variants, supported by technologies like NGS and gene panels, has allowed for more rapid and personalized diagnoses. However, significant limitations persist. The inherent complexity and high costs of WGS and, to some extent, WES, can restrict their widespread adoption [
23]. The interpretation of Variants of Uncertain Significance (VUS) remains a challenge, requiring specialized expertise and further research to ascertain their clinical relevance [
13]. Moreover, tumoral heterogeneity can complicate the detection of all relevant variants through a single tissue biopsy, potentially leading to incomplete diagnostic pictures [
24]. Addressing these limitations will be crucial for maximizing the full potential of genomics in oncological diagnostic practice and ensuring equitable access.
3. Genomics in Cancer Risk Assessment
Genomics plays a crucial role in cancer risk assessment, particularly for individuals with inherited predispositions to malignancies. Genetic testing enables the identification of germline variants associated with hereditary cancer syndromes, providing valuable information for early intervention and personalized risk management. Among the most well-known hereditary cancer syndromes are those linked to variants in genes, such as
BRCA1 and
BRCA2, which predispose individuals to breast, ovarian, and other cancers [
25]. This predominance is largely attributed to the hormone-sensitive nature and high proliferative rates of breast and ovarian tissues, which make them particularly vulnerable to the impaired DNA repair mechanisms caused by BRCA mutations [
26]. The identification of founder mutations in populations with shared ancestry, such as the Ashkenazi Jewish community, has facilitated targeted genetic screening and early detection efforts. Founder mutations in
BRCA1 (185delAG, 5382insC) and
BRCA2 (6174delT) are particularly well-characterized and have allowed for cost-effective screening programs in high-risk populations [
17]. These genetic variants significantly impact an individual’s lifetime cancer risk and have led to the development of preventive strategies, such as enhanced surveillance, chemoprevention, and risk-reducing surgeries.
Another notable hereditary cancer syndrome is Lynch syndrome (LS), an autosomal dominant condition caused by mutations in DNA mismatch repair (dMMR) genes, such as
MLH1,
MSH2,
MSH6, and
PMS2 [
27]. Mismatch repair (MMR) is a cellular mechanism responsible for identifying and correcting base–pair mismatches and small insertion/deletion loops that arise during DNA replication. Defects in MMR lead to microsatellite instability and an increased mutation rate, which contribute to cancer development [
28]. Lynch syndrome accounts for approximately 3% of unselected patients with colorectal or endometrial cancer and 10–15% of those with DNA mismatch repair-deficient tumors [
19]. In addition to colorectal and endometrial cancers, individuals with Lynch syndrome are at increased risk for other cancers, including those of the ovary, stomach, and small intestine [
27,
29]. Germline heterozygous variants in the mismatch repair genes predispose individuals to Lynch syndrome, and, in rare cases, genomic deletions at the 3′ end of EPCAM can lead to epigenetic silencing of MSH2, further contributing to genetic predisposition [
30]. Early detection through genetic testing allows for tailored surveillance programs and preventive measures to reduce cancer risk in individuals with Lynch syndrome, such as prophylactic surgeries [
31]. Other examples of hereditary cancer syndromes are hereditary retinoblastoma, caused by pathogenic variants in the
RB1 gene, which follows Knudson’s two-hit hypothesis [
32]. High-penetrance genes, such as
TP53, associated with Li–Fraumeni Syndrome, and
APC, responsible for Familial Adenomatous Polyposis, also require specific surveillance strategies [
33,
34]. A summary of key genes associated with hereditary cancer syndromes, their related malignancies, and clinical management approaches is provided in
Table 2.
The advent of NGS has expanded the capacity to identify novel cancer susceptibility genes and rare variants with significant clinical impact. Germline testing for these variants has increasingly been integrated into oncology care, particularly in cases of early onset or multiple primary tumors. These insights have not only improved clinical outcomes but also support informed reproductive decisions, with preimplantation genetic diagnosis (PGD) now available for families with known hereditary cancer syndromes [
40].
With the continuous progression of genomic technologies and their integration into clinical practice, genetic counseling will remain a cornerstone of personalized cancer risk management. The ability to combine germline testing, somatic tumor profiling, and polygenic risk scores into a unified cancer risk assessment framework will allow oncologists and genetic counselors to provide more personalized recommendations, ultimately improving patient outcomes and reducing the impact of hereditary cancer.
Beyond single-gene variants, genomics has also fostered the use of polygenic risk scores (PRSs), which assess an individual’s risk of developing cancer based on the cumulative effect of multiple genetic variants. PRSs are calculated by aggregating the effects of numerous genetic variants across the genome, each weighted by their associated risk derived from large-scale genome-wide association studies (GWAS). This weighted sum reflects an individual’s genetic predisposition to a particular cancer [
41]. PRSs are particularly useful for evaluating cancers like breast, prostate, and lung cancer, where multiple genetic factors, along with lifestyle and environmental exposures, contribute to overall cancer risk. By integrating genetic information with non-genetic factors, a PRS offers a more comprehensive and personalized cancer risk assessment. This approach is further enhanced by the growing role of AI in genomic analysis [
4]. AI-driven tools can combine genetic data with environmental and lifestyle factors to create a more holistic understanding of cancer risk, enabling clinicians to make more precise, individualized recommendations for prevention and monitoring.
AI can be applied to implement screening programs, such as general population screening, targeted screening, and stratified screening [
42]. General population screening invites specific demographic groups to participate in screenings, targeted screening focuses on populations at risk due to genetic variants or pre-existing health conditions, and stratified screening customizes the frequency and type of testing based on the individualized risk level [
42]. The integration of genomic testing, including both inherited variants and PRSs, into clinical practice is greatly improving cancer risk assessments.
In addition to genetic testing, genetic counseling plays a crucial role in helping individuals understand their genetic susceptibility and make informed healthcare decisions [
43]. Genetic counselors assist patients in interpreting test results and discussing evidence-based options for cancer prevention, such as enhanced surveillance, chemoprevention, or preventive surgeries.
Recent advances in artificial intelligence have led to the exploration of chatbots as emerging tools to support genetic cancer risk assessment and counseling. Using Natural Language Processing (NLP), chatbots can simulate human conversation to help collect personal and family health history and preliminarily identify individuals at increased genetic risk for hereditary cancer syndromes. A recent systematic review and meta-analysis evaluated their implementation, reporting a completion rate of 36.7% (95% CI, 14.8–65.9%) for genetic risk assessments and 36.0% (95% CI, 27.3–45.7%) eligibility for genetic testing [
44]. Among eligible individuals, 47.4% proceeded with testing, and pathogenic variants were identified in 9.0% of cases. While these results indicate potential benefits in improving access and streamlining genetic services, further rigorous studies are necessary to compare chatbot-based models with traditional genetic counseling and assess their long-term clinical utility, cost-effectiveness, and impact on health equity.
5. Genomics in Prognosis and Early Detection
Genomics is revolutionizing the prognosis and early detection of cancer, offering more precise methods for assessing disease outcomes and detecting tumors in their early stages. Among the most important tools in cancer prognosis are genomic biomarkers, which help in characterizing the biological behavior of tumors and provide critical insights into the probability of recurrence or progression. Genomic biomarkers are able to predict how aggressive a cancer is and help guide decisions about the most appropriate treatment options. For example, Oncotype DX (Exact Sciences, Madison, WI, USA) and PAM50/Prosigna (NanoString Technologies, Seattle, WA, USA) are widely recognized tests that analyze the gene expression profiles of tumor samples to evaluate the risk of recurrence and the potential benefit of adjuvant therapies, particularly in breast cancer. Oncotype DX examines the activity of 21 genes involved in cancer progression, allowing clinicians to assign a recurrence score that guides treatment decisions, such as chemotherapy [
58]. PAM50, which is based on a 50-gene signature, classifies tumors into intrinsic subtypes and provides insight into the likely prognosis, helping clinicians tailor treatments to the individual’s tumor profile [
59]. These genomic tools enable a more personalized approach to cancer care by identifying patients who may benefit from aggressive treatment and sparing those who are less likely to benefit from unnecessary therapies.
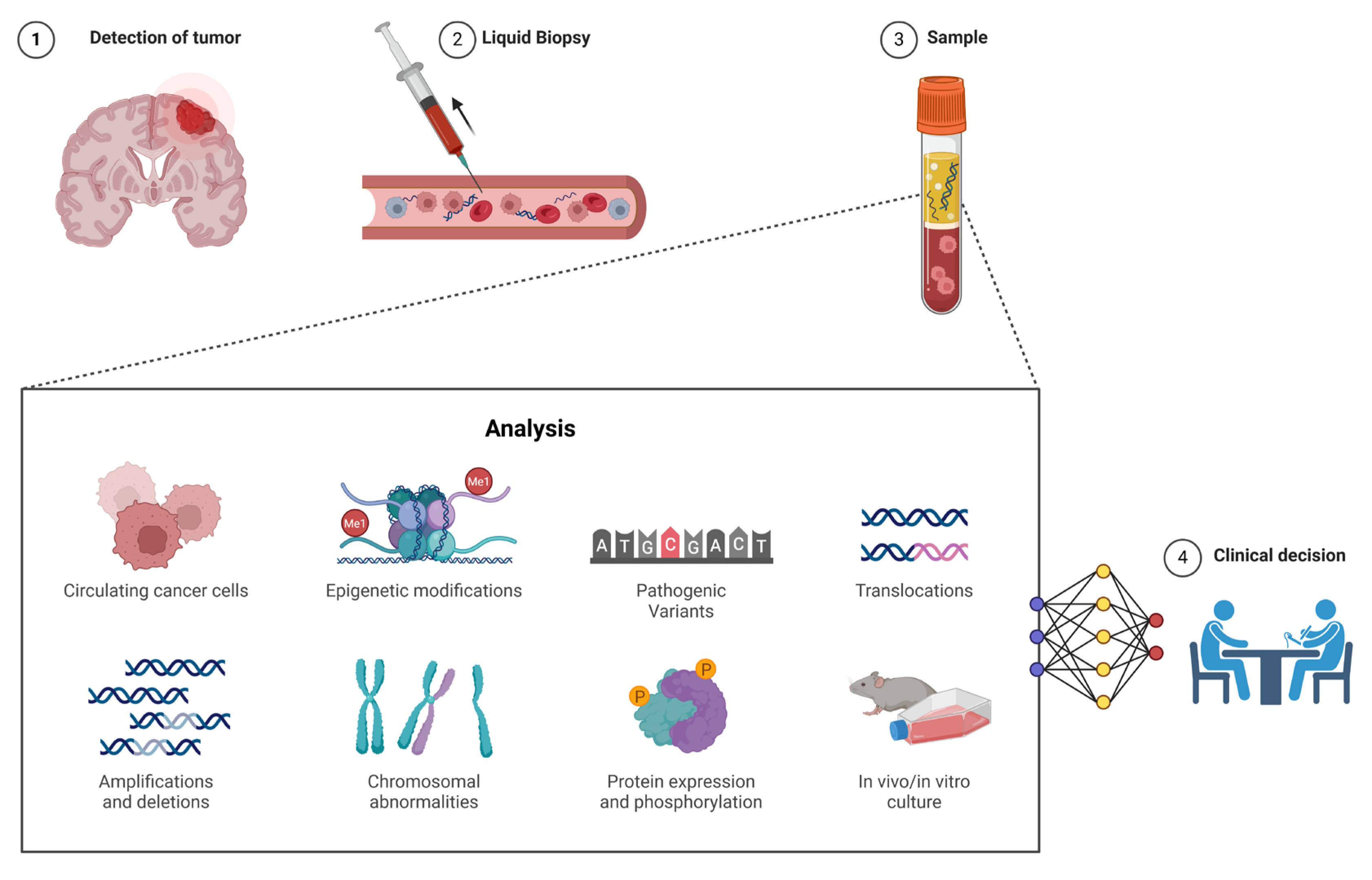
Another significant development in cancer monitoring and early detection is the use of ctDNA and cfDNA [
60]. Liquid biopsy refers to the analysis of genetic material, such as DNA fragments, circulating freely in body fluids like blood, as opposed to traditional biopsies, which require the extraction of solid tissue samples. cfDNA consists of small fragments of DNA released into the bloodstream from both normal and cancerous cells, whereas ctDNA is a subset of cfDNA that specifically originates from tumor cells and therefore reflects the tumor’s unique genetic alterations. This distinction is important because ctDNA provides a more precise snapshot of the tumor’s genomic profile [
61]. Liquid biopsy, by analyzing ctDNA, allows clinicians to detect pathogenic variants and copy number variations in the tumor genome without the need for invasive tissue biopsies. ctDNA analysis is increasingly used for early cancer detection, especially in cancers where traditional screening methods are limited, such as pancreatic, lung, and colorectal cancers [
61]. It is also valuable for monitoring disease progression and detecting minimal residual disease (MRD) after treatment, offering insights into the likelihood of relapse [
62]. While liquid biopsy provides a less invasive and more dynamic means to monitor cancer, its sensitivity and specificity can vary depending on tumor type, stage, and the amount of ctDNA shed into the bloodstream, and it may not yet fully replace traditional tissue biopsy, which remains the gold standard for initial diagnosis and detailed histopathological analysis. A comparative summary of the key features distinguishing liquid biopsy from conventional tissue biopsy is presented in
Table 4, highlighting the advantages of liquid biopsy in terms of minimal invasiveness, longitudinal monitoring, and the detection of MRD.
By combining genetic information with clinical factors, healthcare providers can categorize patients into different risk groups, allowing for more tailored monitoring strategies.
Continuous advancements in genomic technologies, including NGS and liquid biopsy platforms, are driving the integration of genomics into routine clinical practice. As the cost of genomic testing decreases and accuracy improves, genomics will become an even more integral part of cancer care, helping to detect cancer earlier, predict outcomes more accurately, and tailor treatments to each patient’s unique genetic profile.
Limitations in Genomics for Prognosis and Early Detection
In summary, genomics offers transformative contributions to cancer prognosis and early detection through the application of genomic biomarkers and liquid biopsy techniques. Genomic biomarkers like Oncotype DX and PAM50 provide crucial insights into tumor aggressiveness and recurrence risk, enabling personalized treatment intensification or de-escalation [
58,
59]. Liquid biopsies, specifically ctDNA and cfDNA analysis, allow for minimally invasive, real-time monitoring of genetic alterations, facilitating earlier cancer detection, tracking disease progression, and identifying MRD more effectively than traditional methods [
62]. These advancements collectively lead to more precise risk stratification and timely clinical interventions. However, several limitations challenge the widespread application of genomics in prognosis and early detection. While promising, the sensitivity and specificity of liquid biopsies for early cancer detection are not yet universally optimal across all cancer types, potentially leading to false positives or negatives [
61]. The clinical utility of MRD detection still requires further validation in large-scale prospective trials to establish definitive actionable thresholds and standardize testing protocols. Furthermore, the high cost of comprehensive genomic profiling and liquid biopsy platforms remains a barrier to equitable access, particularly in low-resource settings. The interpretation of complex genomic data for prognostic purposes requires specialized expertise, and integrating these insights seamlessly into routine clinical workflows can be challenging. Ongoing research is needed to refine these technologies, reduce costs, and develop clearer guidelines for their optimal implementation.
7. The Role of AI in Cancer Genomics
The integration of artificial intelligence in cancer genomics is revolutionizing the interpretation and application of genomic data in clinical practice. AI enables the processing of large and complex datasets, revealing critical insights that were previously difficult to identify [
73].
7.1. AI Approaches and Applications: Overview
AI includes various technologies designed to simulate human cognitive processes, allowing machines to perform tasks that typically require human intelligence. Fundamentally, AI involves various approaches, such as machine learning, deep learning, and natural language processing, each serving different functions and applications [
74].
ML is a subset of AI that allows systems to learn from data without being explicitly programmed [
74]. ML algorithms identify patterns and relationships in large datasets, making predictions or decisions based on new data. These algorithms can be divided into supervised learning, where the system is trained on labeled data, and unsupervised learning, where the system identifies patterns on its own. One example of ML in practice is clustering algorithms, which group data into categories based on similarities, a technique used in diverse fields, like marketing and finance.
DL, a more advanced form of ML, uses artificial neural networks with many layers of processing (hence the term “deep”) to analyze complex data [
74]. DL excels in tasks like image and speech recognition, where it can process vast amounts of unstructured data, such as images or audio files. Convolutional neural networks (CNNs) are one of the most common types of DL models, used for tasks like facial recognition, while Recurrent Neural Networks (RNNs) are used for sequential data analysis, such as language translation or speech-to-text systems.
NLP is another essential area of AI that focuses on the interaction between computers and human language [
75]. NLP techniques enable machines to understand, interpret, and generate human language, allowing for applications like chatbots, feeling analysis, and automatic translation. NLP models can also assist in extracting useful information from large bodies of text, as seen in applications ranging from legal document analysis to customer service.
Together, these AI approaches form a powerful toolkit that is transforming industries from healthcare to finance, allowing for automation, enhanced decision making, and improved efficiency in managing complex tasks. In oncology, for example, these technologies are being applied to interpret genomic data, predict disease progression, and optimize treatment plans, but they have broad applications across various domains, including robotics, autonomous vehicles, and personalized recommendations in e-commerce.
7.2. Enhancing Genomic Data Interpretation
One of the primary applications of AI in cancer genomics is enhancing the interpretation of sequencing data. AI-powered models analyze WES, WGS, and targeted gene panels to distinguish PV from polymorphisms, enabling targeted therapies. Deep learning models, such as DeepVariant (Google, Mountain View, CA, USA) and DeepSEA (developed by researchers at Stanford University and Princeton University), enhance the accuracy of genomic analyses by improving variant calling and regulatory element identification [
76]. DeepVariant, uses convolutional neural networks (CNNs) to transform raw sequencing reads into high-accuracy variant calls, reducing false positives in genomic data interpretation. DeepSEA, on the other hand, predicts the functional impact of non-coding variants by analyzing DNA sequence patterns and chromatin accessibility, aiding in the identification of regulatory elements that influence gene expression in cancer [
77]. AlphaFold, a leading deep learning model, enhances protein structure prediction by analyzing amino acid distances and peptide bond angles [
78].
Beyond known pathogenic variants, AI algorithms stand out for detecting rare genetic variants and novel alterations that may influence cancer progression or treatment resistance.
7.3. Advancing Personalized Medicine
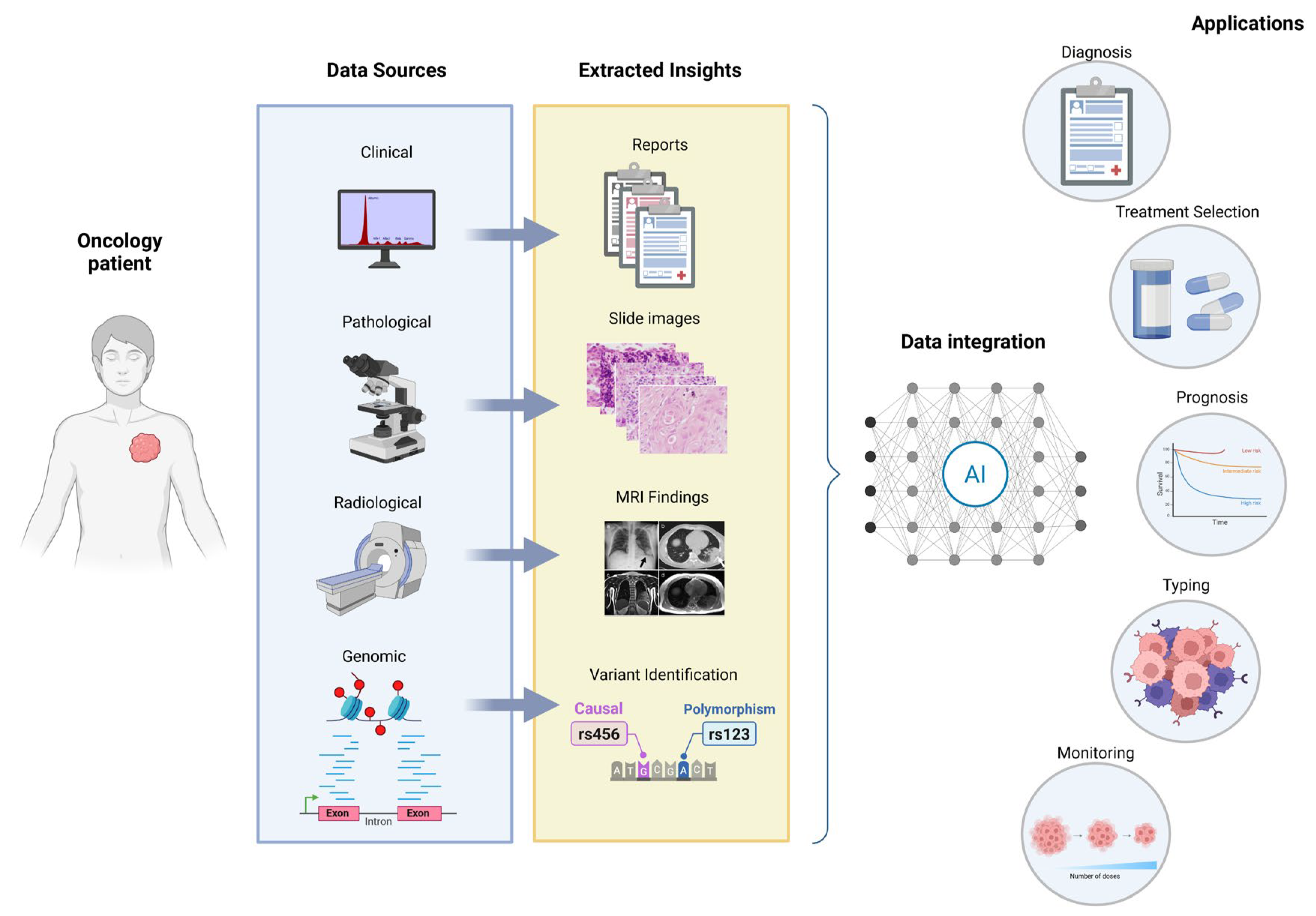
One of the most promising applications of AI in cancer genomics is its role in advancing personalized medicine. AI stands out as a powerful tool for merging genomic data (from tumor sequencing), clinical data (such as patient health history and treatment responses), and imaging data (such as CT scans or MRIs) to provide a holistic view of the patient’s cancer [
79]. The integration of these diverse data types is crucial because it offers a deeper overview of the disease, including not only genetic variants but also how those variants physically manifest (via imaging) and how they interact with a patient’s overall health and previous treatments. By combining these datasets, AI models can help oncologists developing personalized therapy plans that are specifically tailored to the patient’s genetic makeup, cancer type, stage, and other unique characteristics [
80].
For example, AI can suggest the most appropriate targeted therapies based on the patient’s specific genomic alterations, such as the presence of EGFR variants in non-small cell lung cancer or BRCA1/2 variants in breast cancer, both of which can significantly affect treatment options [
81,
82]. AI can also identify the probability of treatment success or failure based on the patient’s genetic profile and historical data from similar cases. In addition, AI can help with optimizing treatment regimens by analyzing imaging data to track tumor progression and detect early signs of drug resistance. For instance, radiomics, an emerging field that applies machine learning to medical imaging, can extract quantitative features from imaging scans that reflect tumor characteristics [
83].
Another critical aspect of personalized medicine is the development of predictive models that estimate how a patient’s tumor will respond to specific drugs or treatment regimens. These models can be used to identify patients who may benefit from targeted therapies, immunotherapy, or chemotherapy, and they can help avoid unnecessary complications by predicting adverse reactions to drugs [
84].
DL algorithms are particularly valuable for creating these predictive models. They can analyze historical patient data to identify patterns and trends that would be difficult for clinicians to detect manually [
85] (
Figure 3).
Furthermore, predictive models can help with optimizing therapy over time by continuously learning from new data. As a patient progresses through treatment, new genomic or clinical data can be incorporated into the model, allowing it to adjust therapy plans in real time. For example, if a patient’s tumor develops resistance to a particular drug, AI can predict this resistance early and suggest alternative treatments that might be more effective [
86,
87]. Through AI-driven predictive modeling, personalized cancer therapy is becoming more precise and timely and tailored to each patient’s needs.
7.4. Drug Repurposing: A Cost-Effective Strategy Enhanced by Artificial Intelligence
The process of developing a new anti-cancer drug from the beginning is extremely time-consuming and expensive, often spanning 10–15 years and costing over USD 1 billion from initial discovery to clinical approval [
88]. Drug repurposing offers an excellent solution to this problem by identifying new therapeutic uses for existing drugs, bypassing early-stage development obstacles. In oncology, drug repurposing can significantly accelerate the availability of treatments because known drugs have established safety profiles, allowing researchers to move directly into Phase II or III trials for efficacy in cancer. Historically, drugs like thalidomide (now used in multiple myeloma) and metformin (investigated in breast and ovarian cancer) have had successful repurposing applications [
89].
Many other non-oncological drugs, such as antivirals, antiparasitics, or metabolic agents, have been investigated for anti-cancer properties, potentially expanding the arsenal of cancer therapies without the need to design a molecule from the ground up. This strategy can substantially reduce development costs and timelines, and it leverages the wealth of clinical data already available for approved medications.
AI has emerged as a key promoter of drug repurposing by rapidly processing and interpreting vast biomedical datasets. ML and DL algorithms can integrate transcriptomics, proteomics, and chemical structure data to predict novel drug–target interactions. For instance, models like AtomNet and Pafnucy use 3D convolutional neural networks to enhance molecular docking predictions, revealing binding affinities of existing drugs for new cancer targets [
90]. These systems outperform traditional methods by learning directly from real drug–target interaction data, achieving higher accuracy in predicting binding affinities (with AtomNet reporting AUC values around 0.85–0.90 compared to below 0.7 for classical docking) and reducing false positives in virtual screening [
91]. Pafnucy similarly improves binding affinity prediction accuracy, showing significant reductions in error metrics compared to conventional scoring functions, thus enabling more reliable and efficient drug discovery pipelines [
92].
Additionally, transcriptomic signature matching has been widely adopted. This involves identifying drugs that reverse disease-specific gene expression patterns. This approach compares gene expression profiles induced by disease states with those induced by drugs, identifying compounds capable of functionally reversing oncogenic transcriptional programs. Tools like CMap and algorithms like DrInsight or DeCoST have uncovered compounds with the potential to modulate key cancer-related signatures, particularly in hard-to-treat subtypes like triple-negative breast cancer [
93]. Notably, drugs like thioridazine and lovastatin have shown efficacy in targeting cancer stem cell traits or estrogen receptor activity through these in silico techniques.
Table 6 summarizes additional examples of repurposed drugs with established or emerging oncological applications, along with their mechanisms of action.
Beyond computational approaches, high-throughput screenings, organoid models, and phenotypic analyses (e.g., filopodia inhibition assays) offer complementary experimental strategies for identifying repurposing candidates. For example, tumoroid models allow for preclinical validation of repurposed drugs within personalized tumor microenvironments [
99].
Overall, drug repurposing, especially when coupled with AI, offers a powerful, cost-effective, and time-saving opportunity for expanding oncological treatment options. As part of the AI–genomics integration framework in oncology, drug repurposing exemplifies how computational power can uncover unexpected therapeutic opportunities from known pharmacological agents. Its integration into precision oncology could dramatically accelerate access to innovative therapies for patients while minimizing the attrition rates that typically plague de novo drug development.
7.5. Therapeutic Innovation in Oncology
As precision medicine evolves, new strategies are emerging to improve cancer therapy outcomes. Two promising and complementary approaches are the repurposing of existing drugs for new oncology uses and the development of entirely novel therapeutics with the help of advanced AI techniques. These innovations aim to address current gaps in treatment, reduce the time and cost of drug development, and ultimately improve patient survival and quality of life.
7.5.1. AI in Target Discovery and Design
AI is reshaping cancer drug discovery by introducing efficiencies in every stage of the development pipeline. Integrating AI techniques, ML, DL, and evolutionary algorithms into biomedical research enables scientists to analyze massive multi-omic datasets, predict molecular interactions, and design optimized drug candidates with high precision [
100]. In the early stages of development, AI plays a key role in identifying novel therapeutic targets. Deep learning models can scan thousands of genomic and transcriptomic datasets to uncover critical mutations or dysregulated pathways that drive tumorigenesis. These insights reveal targetable nodes in cancer signaling networks that may otherwise remain undetected [
100]. Notably, tools like AlphaFold have revolutionized structural biology by predicting the 3D conformation of cancer-related proteins. These high-accuracy structural models provide a foundation for rational drug design, allowing researchers to tailor molecules that bind precisely to oncogenic targets [
101].
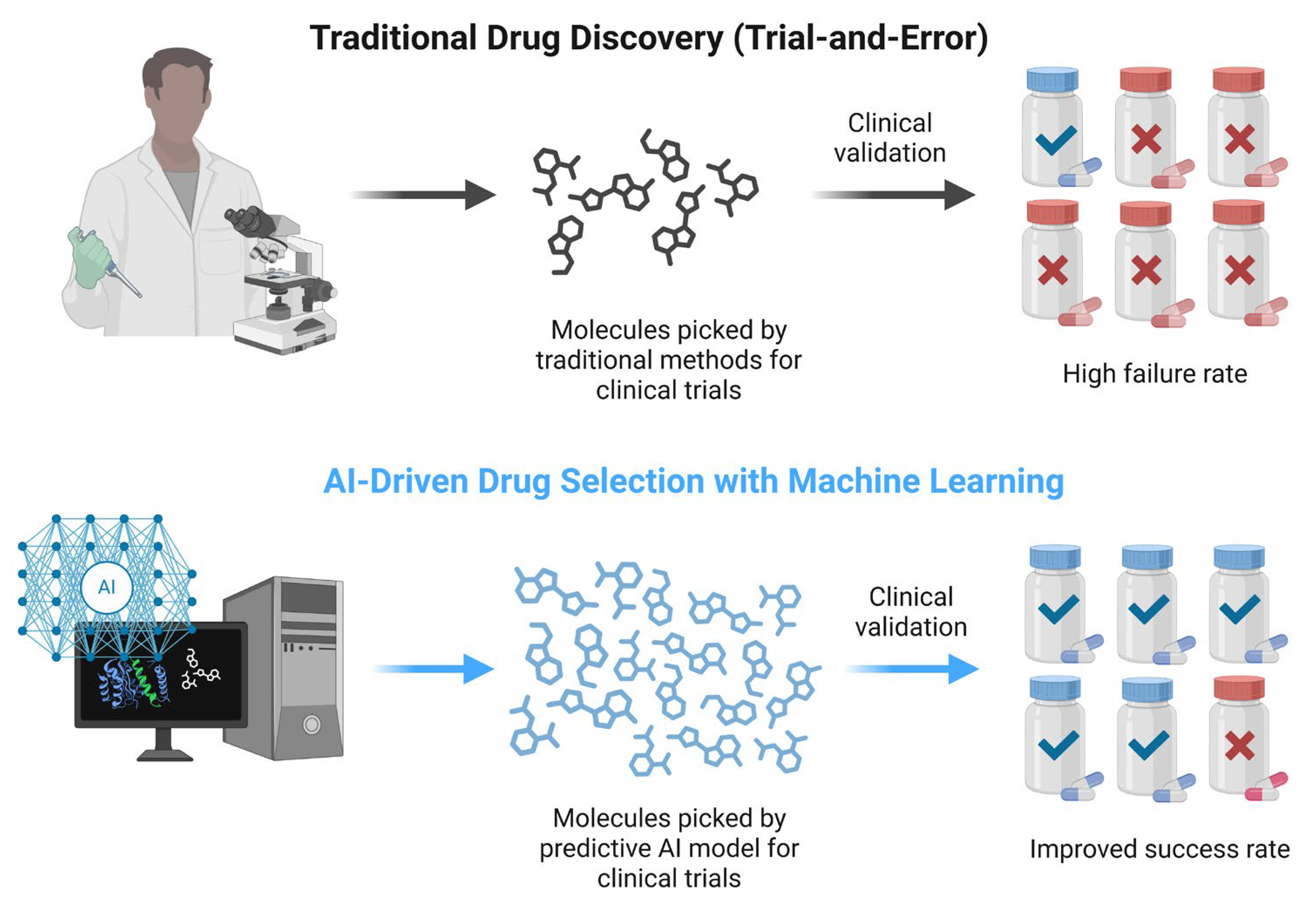
7.5.2. AI in Molecular Generation and Preclinical Optimization
Beyond target identification, AI revolutionizes the design of new anti-cancer molecules. Techniques like virtual screening powered by AI can rapidly evaluate millions of chemical compounds to identify those most likely to bind a given cancer target with high affinity. This computational screening dramatically filters the pool of potential drugs, focusing experimental efforts on the most promising candidates. In parallel, generative AI models are now used to create novel chemical entities de novo based on desired pharmacological profiles. These models propose structures that are optimized for properties like improved selectivity, reduced toxicity, or resistance evasion, which are essential for the design of next-generation cancer therapeutics [
100].
Figure 4 illustrates how AI tools, such as CNNs, can support early drug discovery stages. While these methods have shown improved predictive accuracy in preclinical models, their impact on overall clinical success rates remains under investigation.
AI is also proving crucial in downstream phases of drug development. Predictive models trained on pharmacological data from past compounds can anticipate the ADMET profile (absorption, distribution, metabolism, excretion, toxicity) of new molecules, identifying potential safety issues before clinical testing. This allows chemists to iteratively refine compounds, increasing their likelihood of success in trials [
100]. Additionally, AI can suggest optimal drug combinations by analyzing how different agents interact within cellular networks, pointing to multi-drug regimens that might be more effective than single agents. This is particularly relevant in cancer, where combinatorial treatments are often needed to prevent or overcome resistance.
7.5.3. Real-World Impact and Future Outlook
This AI-driven approach is not merely theoretical. Several compounds generated or optimized through AI are now advancing through preclinical pipelines and entering clinical trials. These include not only small molecule inhibitors but also biologics and cell therapies whose design has benefited from computational modeling.
In summary, AI is redefining the future of cancer therapeutics from molecular design to safety profiling and combination strategy development. Its integration into oncology drug discovery is poised to deliver more effective, targeted, and personalized treatments with greater speed and efficiency than ever before.
7.6. Early Detection and Monitoring
AI is playing an increasingly pivotal role in early cancer detection and the continuous monitoring of tumor evolution. With advancements in liquid biopsy technologies, particularly the analysis of ctDNA, AI is significantly improving the ability to detect cancer at its earliest, most treatable stages and to track the progression of the disease throughout treatment. These developments are helping to establish more dynamic, real-time approaches to cancer care, allowing for timely interventions and better patient prognosis.
AI is particularly effective in analyzing the complex datasets generated by liquid biopsies. By applying ML algorithms to large amounts of ctDNA data, AI systems can identify subtle genetic alterations associated with the presence of tumors, allowing for earlier detection of cancer. AI models can process variations in ctDNA levels and sequence data to detect even small quantities of tumor-derived DNA, improving the sensitivity of early detection [
85].
For example, in cancers like lung cancer, breast cancer, and colon cancer, liquid biopsies can reveal the presence of specific variants or gene fusions that are indicative of malignancy. AI can then classify these alterations, even in their early stages, providing critical information for early-stage diagnosis and allowing clinicians to intervene before the disease becomes clinically significant [
102]. The ability to detect MRD or early relapse is another benefit of using AI in the context of liquid biopsies. This allows clinicians to monitor ctDNA levels over time, offering insights into how the disease is responding to therapy and whether the patient is at risk of recurrence. By tracking these molecular signals in real time, AI tools are helping oncologists make more informed decisions about when to adjust or intensify treatments (
Figure 5) [
102].
In addition to early detection, AI-driven tools are also crucial in the continuous monitoring of cancer progression and the detection of resistance mechanisms that may arise during treatment. Tumors are dynamic entities that evolve over time, often developing resistance to therapies, especially in cancers like lung, breast, and colorectal cancers, where resistance to common drugs (e.g., chemotherapy and targeted therapies) is a major challenge [
86].
AI plays an important role in monitoring these evolutionary changes. AI algorithms can detect variants or genetic alterations that signify tumor resistance to specific therapies, such as changes in the tumor’s drug targets, which can indicate the need for a change in the treatment approach. For instance, in cases of NSCLC, patients may initially respond to targeted therapies like EGFR inhibitors, but, over time, the tumor may develop EGFR T790M variants, leading to resistance [
54]. AI can help identify these variants through ctDNA analysis, allowing clinicians to switch to a more appropriate second-line therapy (e.g., osimertinib) before clinical relapse occurs. AI’s ability to track resistance mutations in real time provides critical insights for personalizing treatment, ensuring that the approach remains effective as the tumor adapts [
103].
Furthermore, AI is enhancing the ability to monitor tumor heterogeneity, the existence of multiple genetic variations within a single tumor. This heterogeneity can complicate treatment because different subpopulations of tumor cells may respond differently to therapies. By analyzing longitudinal data from liquid biopsies or imaging, AI models can detect shifts in tumor composition, helping to identify emerging clones that may be resistant to current treatment regimens [
104].
7.7. Supporting Clinical Decision Making
AI has become an invaluable tool in clinical decision making, supporting clinicians by processing vast amounts of clinical data, genomic information, and patient histories. By incorporating ML algorithms, these systems can analyze patterns in data that might not be immediately apparent to healthcare providers. AI can then recommend optimal treatment strategies based on the latest clinical guidelines, clinical trial data, and the patient’s individual genomic profile [
105].
For example, AI systems can help in identifying the most appropriate targeted therapies for cancers driven by specific genetic variants. In cases of breast cancer, AI might analyze a patient’s genetic profile to recommend treatment with HER2-targeted therapies for patients with overexpressed HER2 [
106]. Similarly, in lung cancer, AI can help in determining whether EGFR inhibitors or immunotherapy would be more effective for a patient based on their tumor’s molecular makeup [
107,
108].
Beyond drug recommendations, AI can also help clinicians decide the best treatment regimen, considering factors like drug interactions, tolerability, and side effect profiles. This personalized approach improves clinical outcomes, as therapies are optimized to the patient’s unique cancer characteristics, reducing errors and increasing the probability of success.
One of the most significant benefits of AI in clinical decision making is its ability to reduce clinician workload through automated data integration. Oncology requires clinicians to evaluate a huge amount of data from diverse sources: genomic reports, imaging scans, pathology results, electronic health records (EHRs), and many more. The enormous volume of information can be overwhelming and time-consuming, leading to overload of information and slower decision making. By improving data integration and reducing the manual effort required for data analysis, AI enables clinicians to focus on what they do best, such as making informed decisions and interacting with patients [
109]. Furthermore, AI can provide real-time alerts about changes in a patient’s condition or new research findings relevant to their treatment plan. These proactive, automated systems ensure that clinicians are always equipped with the most up-to-date information. The integration of AI-driven decision support systems has the potential to significantly improve patient care by providing clinicians with better tools to make personalized, data-driven decisions. To illustrate the growing adoption and regulatory acceptance of AI in clinical practice,
Table 7 provides a comprehensive overview of selected AI/machine-learning-based medical devices and software that have received FDA clearance in oncology and related diagnostic fields. These approvals underscore the robust validation and clinical utility of AI tools, marking a significant milestone in their integration into routine healthcare workflows.
7.8. Addressing Challenges
While AI holds immense potential in cancer genomics, its integration into clinical practice comes with a range of challenges that must be addressed to ensure effective, ethical, and equitable use. These challenges primarily focus on transparency, bias, and ethical considerations, each of which plays a crucial role in maximizing the potential of AI-driven technologies while minimizing risks.
One of the key challenges in AI implementation in oncology is ensuring that AI models are transparent and comprehensible. In clinical settings, particularly in oncology, decisions based on AI-generated insights must be easily understandable by healthcare providers to build trust and confidence. Black-box models, where the decision making process of the AI is not transparent, can be problematic because clinicians and patients may not understand how a particular conclusion was obtained. This lack of transparency can cause problems around the reliability of AI systems, especially when they are used in important decisions like cancer treatment. To address this issue, there is a growing need for explainable AI (XAI), which focuses on developing algorithms whose decisions can be easily interpreted and explained by humans [
110]. Examples of XAI methods include LIME (Local Interpretable Model–agnostic Explanations) and SHAP (SHapley Additive exPlanations), which provide local explanations for individual predictions, as well as saliency maps in deep learning, which highlight input features most relevant to a model’s output [
111]. By integrating interpretability into AI systems, clinicians can better understand why a particular treatment recommendation was made or how a diagnostic result was reached. Crucially, without proper XAI analyses and demonstrable interpretability, the widespread application of AI into routine clinical practice should be approached with extreme caution, as the lack of transparency can impede clinical trust, accountability, and the ability to ensure patient safety and equitable care. This helps in ensuring that AI becomes a useful tool for clinicians rather than a source of confusion or mistrust, thereby improving its clinical adoption. Moreover, explainable AI also supports the legal and ethical responsibility of healthcare providers when utilizing AI tools. By understanding AI decisions, clinicians can make informed choices about whether to accept or challenge an AI recommendation, fostering a more collaborative and transparent decision making process.
Another significant challenge in the integration of AI in cancer genomics is bias in datasets [
112]. AI algorithms are only as good as the data they are trained on, and if the training data are biased, the AI model can extend or even exacerbate existing healthcare disparities. For example, if genomic data or clinical records predominantly represent certain populations, such as individuals of European descent, AI models may be less accurate for people from other ethnic groups [
113]. This can lead to disparities in diagnosis, treatment recommendations, and patient outcomes, as the AI system may fail to account for the genetic variations or healthcare needs of diverse populations.
To mitigate this risk, it is crucial to diversify datasets used for training AI models [
114]. This includes ensuring that datasets are representative of various ethnicities, age groups, and other demographic factors. In addition, AI systems should undergo rigorous testing and validation across different populations to ensure that their predictions are robust and equitable. This requires collaboration between clinicians, data scientists, and policy makers to establish guidelines and best practices for bias mitigation in AI.
The use of AI in cancer genomics also raises important ethical considerations, particularly those related to data privacy and patient consent [
112]. As AI systems rely heavily on large datasets, including genomic and clinical data, protecting the privacy and security of patient information is paramount. Misuse or breaches of sensitive data could undermine public trust in these technologies, hindering their widespread adoption.
To safeguard patient privacy, it is essential to implement robust data protection protocols, such as encryption and secure data storage, to ensure that patient data are not accessed or shared without proper authorization. Data encryption involves transforming data into a coded format to prevent unauthorized access. Common types include symmetric encryption (using a single key for both encryption and decryption) and asymmetric encryption (using a pair of public and private keys) [
115]. Given the advent of quantum computing, the field is also exploring post-quantum encryption methods, which are cryptographic algorithms designed to be secure against attacks by quantum computers, ensuring long-term data protection in the face of evolving technological threats [
116]. Additionally, clear and transparent informed consent procedures should be established so that patients are fully aware of how their data will be used in AI-driven research and decision making processes. One promising approach is Federated Learning, which enables multiple institutions to train AI models on decentralized genomic datasets without transferring sensitive patient data. In Federated Learning, instead of centralizing all patient data for training, the AI model is distributed to local data silos (e.g., individual hospitals or research centers). Each local model is trained on its respective sensitive dataset, and only aggregated model updates (e.g., changes in model parameters or weights), not the raw patient data, are sent back to a central server. These updates are then combined to improve a global model, which is subsequently shared back with the local participants for further refinement [
117]. This iterative process allows for collaborative model training across diverse datasets while strictly preserving patient privacy and data locality. This method enhances collaboration across research centers while maintaining patient privacy. Recent studies have demonstrated how Federated Learning can improve predictive modeling in genomics by aggregating diverse datasets securely [
118].
Furthermore, ethical concerns related to algorithmic fairness and the potential for discriminatory outcomes in AI-driven decisions must be addressed. Ensuring that AI models are designed to respect human dignity and autonomy is critical. This involves ensuring that AI systems do not disseminate harmful stereotypes or lead to unfair decisions based on non-medical factors, such as a patient’s socio-economic status or gender.
8. Conclusions
AI has profoundly transformed the field of clinical genomics and oncology, significantly enhancing the precision, efficiency, and personalization of cancer care. From its early roots in the 1950s to today’s powerful ML and deep learning algorithms, AI has evolved into an indispensable assistant in interpreting complex genomic data and translating data into actionable insights. These innovations have greatly advanced our capabilities in early detection, prognosis, and personalized therapies tailored to individual genetic profiles.
Throughout this review, we have systematically explored the extensive contributions of genomics and AI across the cancer care continuum, encompassing improved cancer diagnosis, more precise risk assessment, the development of targeted therapies and immunotherapies, and enhanced early detection and prognostication. We have highlighted how the synergy between high-throughput genomic technologies and advanced AI models is revolutionizing personalized medicine by enabling the identification of key genetic alterations, predicting treatment responses, and facilitating drug repurposing. However, as discussed within each specific section, the full realization of this potential is contingent on addressing several critical challenges. These include the necessity for data standardization and harmonization across diverse datasets, ensuring the interpretability and transparency of complex AI algorithms, mitigating inherent biases within training data to ensure equitable outcomes, and navigating the intricate ethical considerations surrounding data privacy and patient consent.
As the global population continues to age, chronic degenerative diseases and cancer are increasingly challenging the sustainability of worldwide healthcare systems. The growing burden of these diseases demands not only innovative treatments but also more efficient and equitable healthcare strategies. In this context, the integration of genomics and AI holds transformative potential. By enabling earlier diagnosis, more accurate risk prediction, and the development of tailored therapeutic approaches, these technologies can improve clinical outcomes while optimizing the use of healthcare resources. Achieving this goal, however, requires rethinking academic education. A new generation of healthcare professionals must be trained, one that combines traditional clinical expertise with direct competencies in AI and advanced molecular medicine. In this new paradigm, AI becomes the modern physician’s stethoscope as a powerful, indispensable tool that applies the most sophisticated developments in computer science in the service of medicine.
In the near future, genomics and AI could become key tools for ensuring that advanced, effective cancer care remains accessible to all, helping healthcare systems to provide better care for more people without compromising sustainability. Their integration will not only reshape the practice of oncology but also redefine the skills and tools of the physicians of tomorrow.