Effect of Heating Rate on the Properties and Mechanism of Nanocomposite Ceramic Coatings Prepared by Slurry Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.2.1. Aggregate Pretreatment
2.2.2. Substrate Pretreatment
2.2.3. Preparation of Coating
2.2.4. Organizational Structure and Performance Test
3. Results and Discussion
3.1. Microscopic Morphology and Organizational Structure
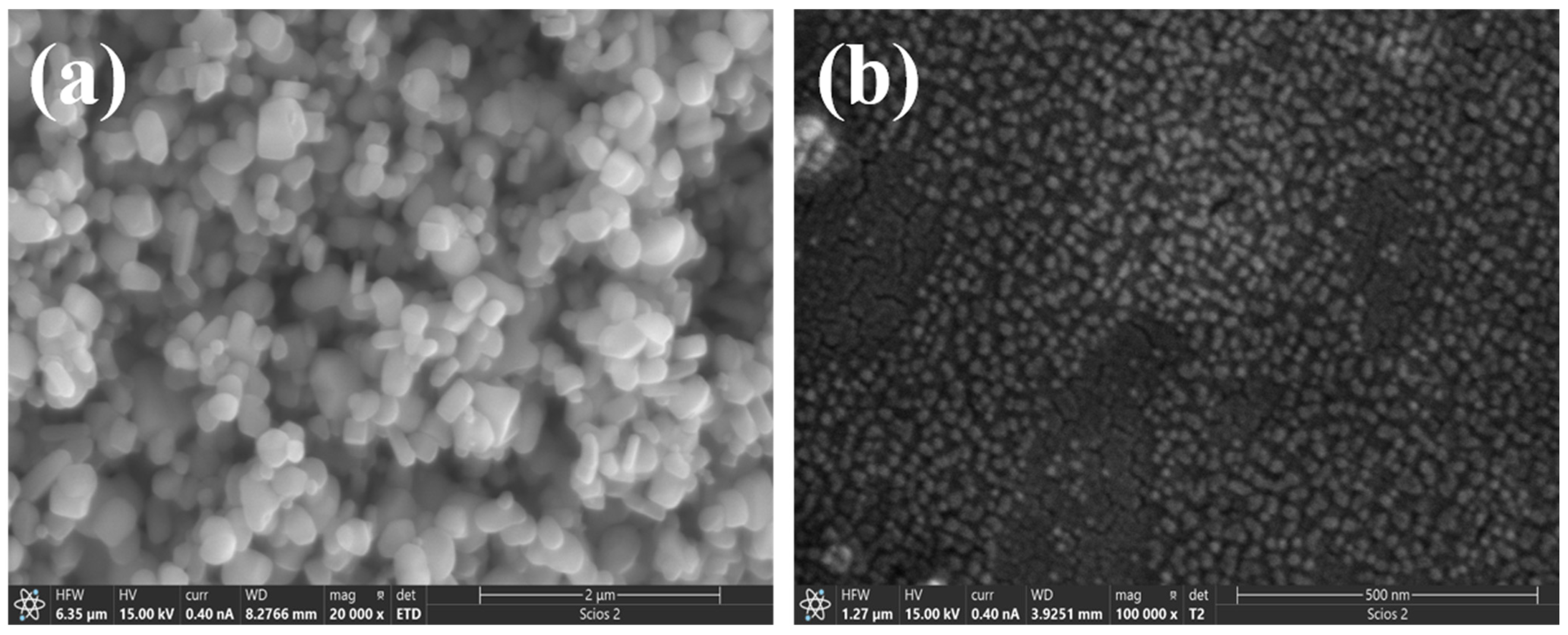
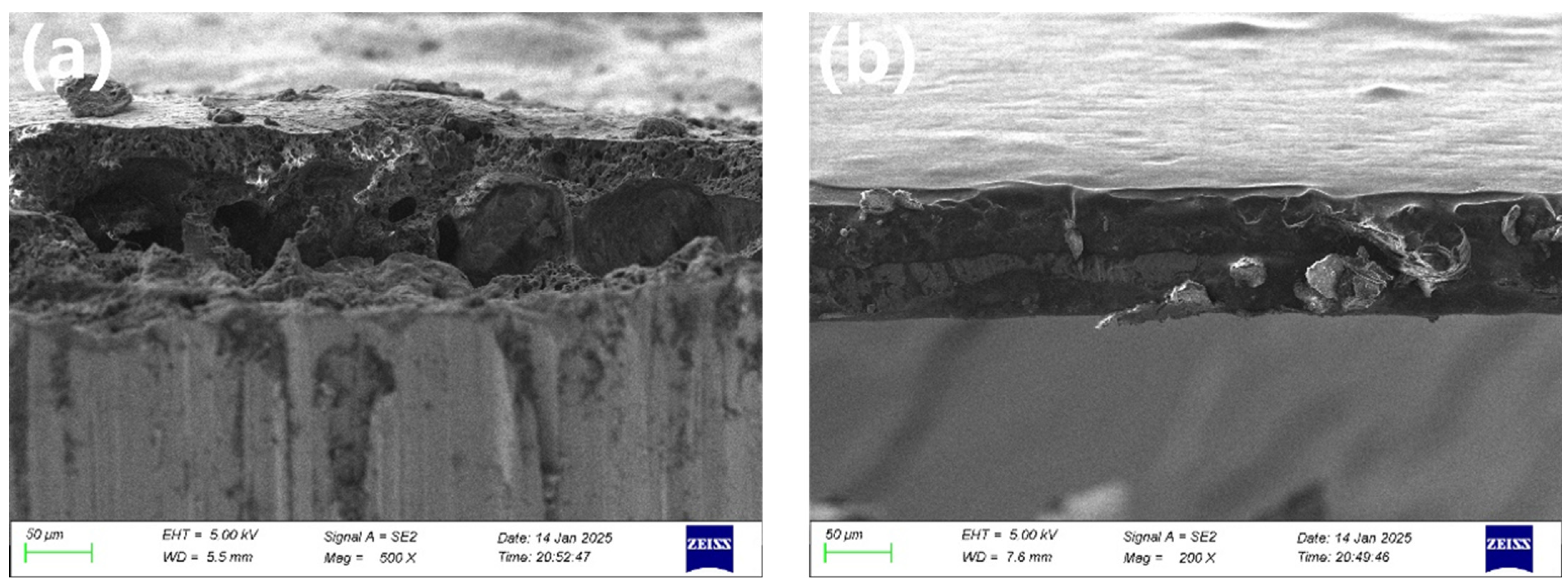
3.1.1. SEM Morphology Analysis of Ceramic Coatings
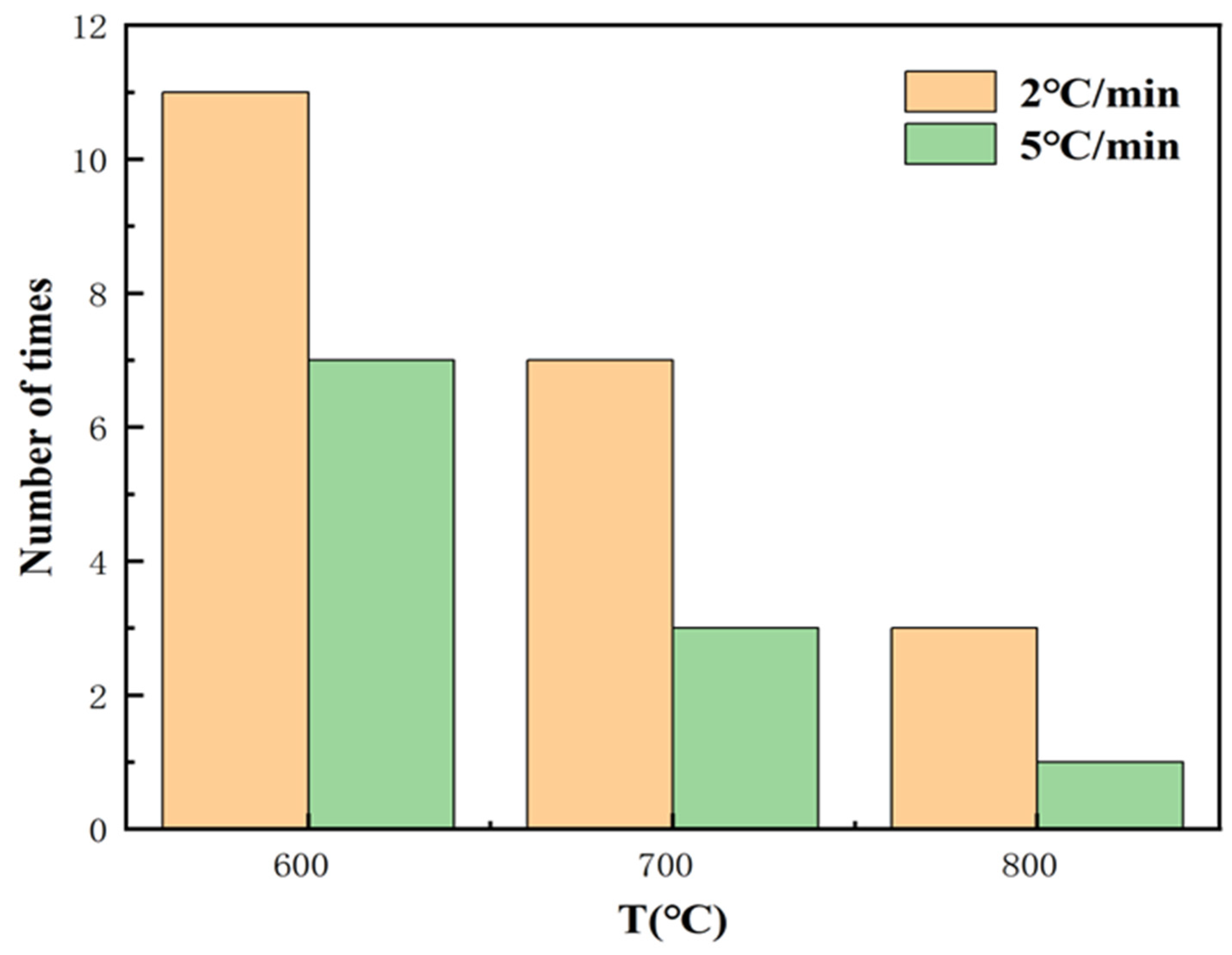
- (1)
- Insufficient room temperature drying time. After the coating is prepared, it needs to be dried at room temperature to remove the free moisture inside the coating. However, if the drying time is insufficient, the residual liquid moisture evaporates too quickly during the high-temperature curing process, making it easy to form air bubbles, which can create porosity inside and on the surface of the coating.
- (2)
- Influence of temperature rise rate. If the initial temperature rise rate is too fast during the curing process, it will easily lead to the formation of pores and cracks. The main reason for this is that the free water that is not completely removed from the coating is violently evaporated during the rapid temperature rise, resulting in the formation of tiny pores within the coating. To avoid this problem, a lower temperature is usually used to slowly increase the temperature, so that the coating is fully cured in the low-temperature stage, thus reducing the generation of pores and improving the densification of the coating.
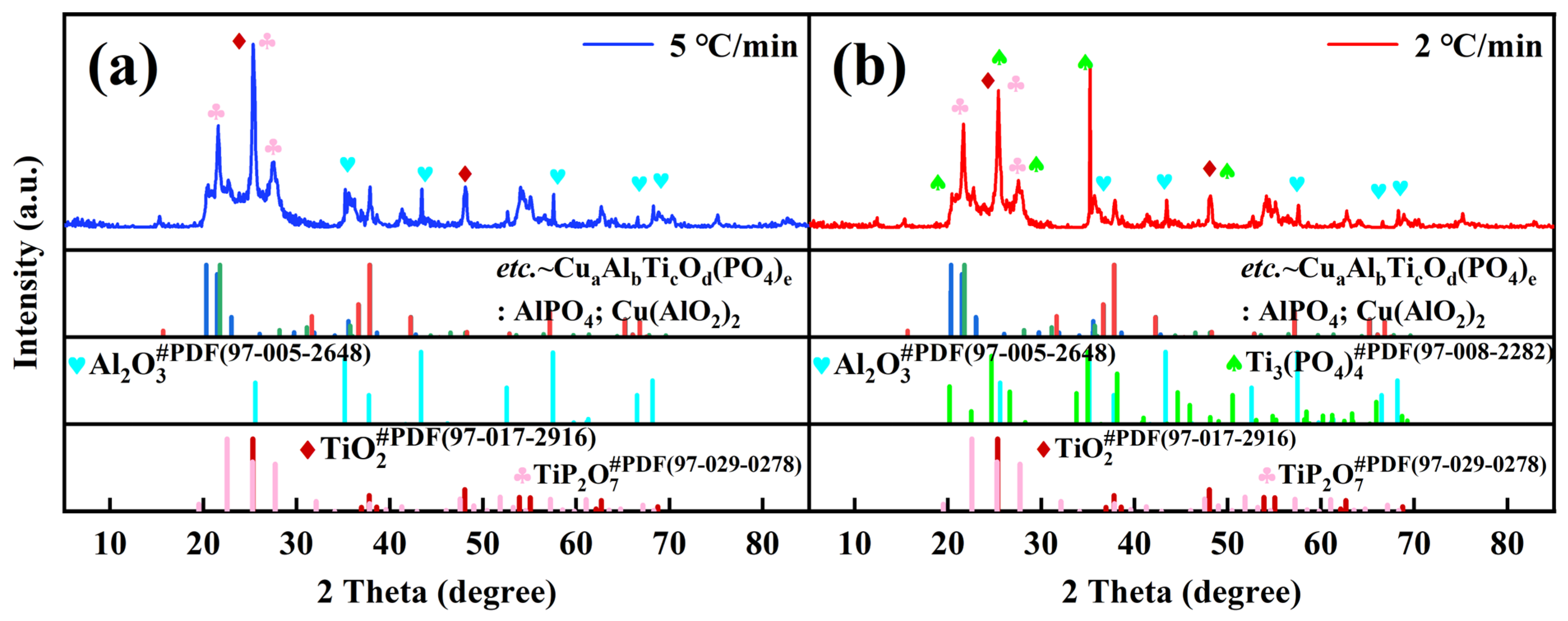
3.1.2. X-Ray Analysis of Ceramic Coatings
3.2. Analysis of Coating Performance Test
3.2.1. Coating Densification Analysis
3.2.2. Coating Abrasive Wear Test
3.2.3. Coating Shear Strength Analysis
3.2.4. Coating Thermal Shock Resistance Test
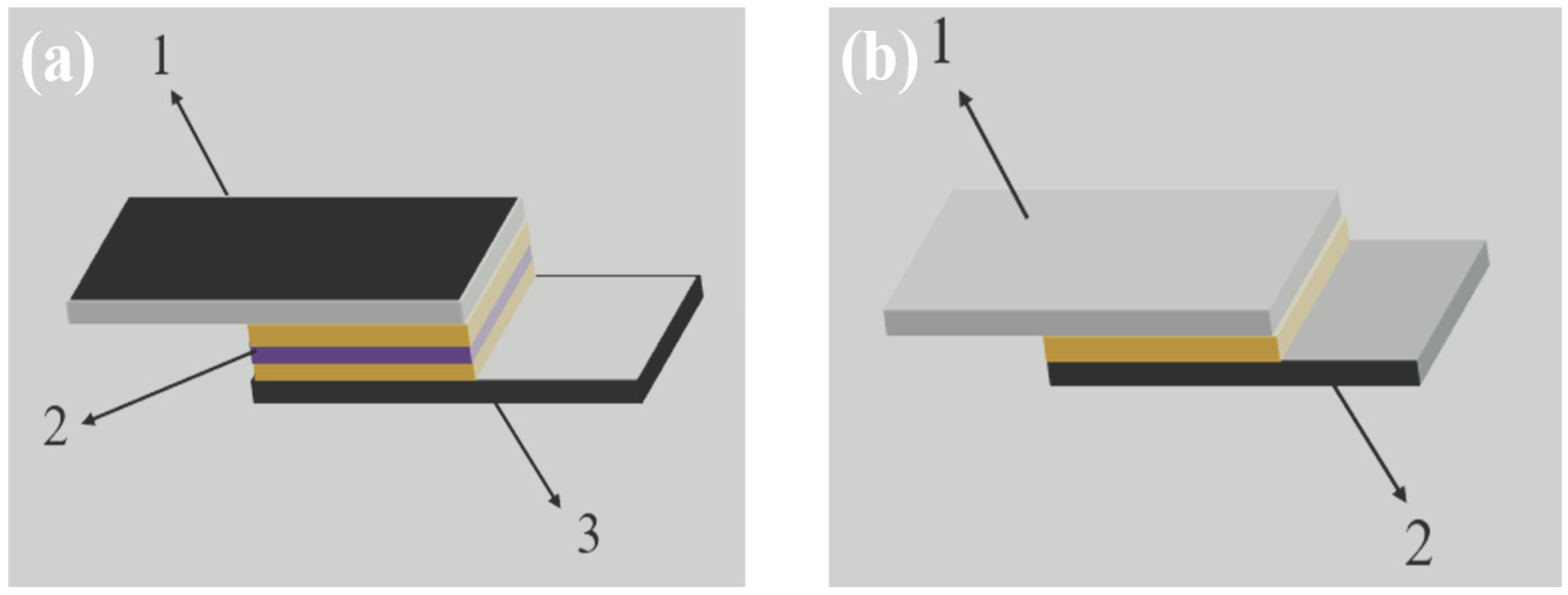
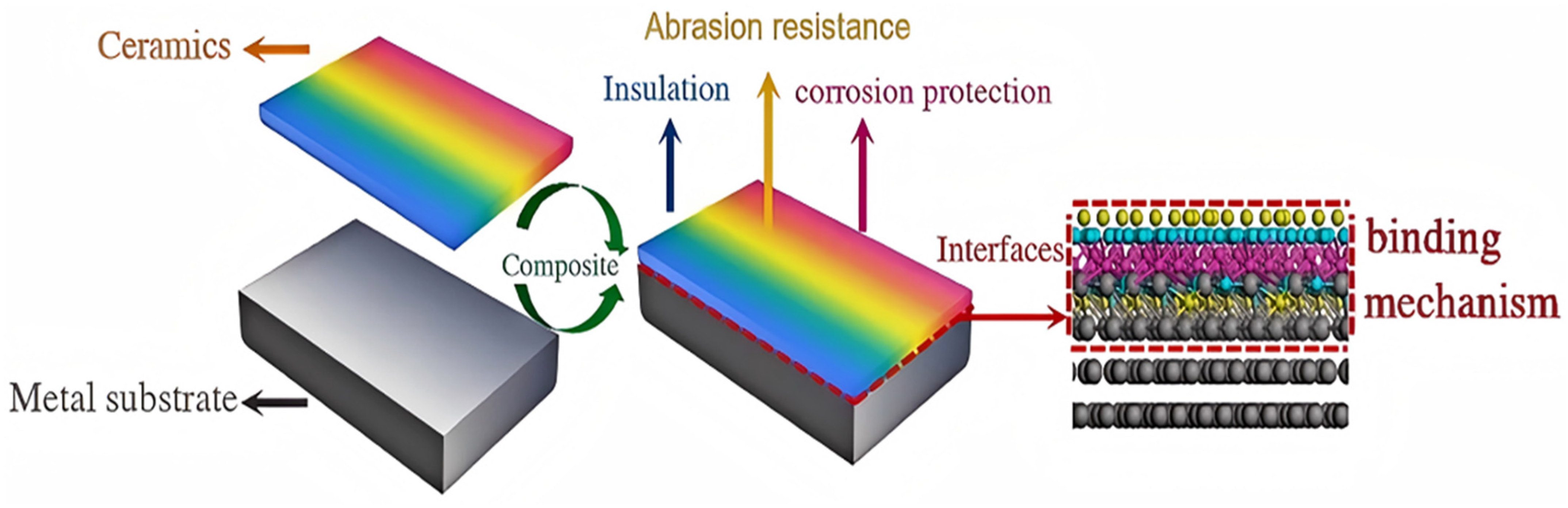
3.3. Ceramic-Metal Interface Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salman, S.; Köse, R.; Urtekin, L.; Findik, F. An investigation of different ceramic coating thermal properties. Mater. Des. 2006, 27, 585–590. [Google Scholar] [CrossRef]
- Zang, D.M.; Xun, X.W. Ceramics Coated Metallic Materials: Methods, Properties and Applications. In Advanced Ceramic Materials; Intech Open: London, UK, 2020. [Google Scholar] [CrossRef]
- Li, C.; Shi, J.; Li, Y.H.; Li, Y.F.; Gao, Y.M.; Li, B.; Goei, R.; Cheng, C.Y.; Zhao, S.Y.; Tok, A.I.Y. Interfacial bonding and abrasive wear behaviours of the iron matrix composites. Mater. Sci. Technol. 2022, 38, 965–976. [Google Scholar] [CrossRef]
- Fedrizzi, L.; Rossi, S.; Cristel, R.; Bonor, P.L. Corrosion and wear behaviour of HVOF cermet coatings used to replace hard chromium. Electrochim. Acta 2004, 49, 2803–2814. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, W.; Zhang, T.; Yang, Y. Microstructure, spallation and corrosion of plasma sprayed Al2O3–13%TiO2 coatings. Corros. Sci. 2009, 51, 2824–2931. [Google Scholar] [CrossRef]
- Yu, J.J.; Liu, S.Y.; Li, F.; Wang, T.H. Na2SiO3/Al2O3 composite coatings on 304 stainless steels for enhanced high temperature oxidation inhibition and chloride-induced corrosion resistance. Surf. Coat. Technol. 2017, 309, 1089–1098. [Google Scholar] [CrossRef]
- Qin, D.C.; Xu, G.Y.; Yang, Y.; Chen, S. Multiphase ceramic coatings with high hardness and wear resistance on 5052 aluminum alloy by a microarc oxidation method. ACS Sustain. Chem. Eng. 2018, 6, 2431–2437. [Google Scholar] [CrossRef]
- Cheng, W.J.; Wang, J.J.; Ma, X.; Liu, P.; Liaw, P.K.; Li, W. A review on microstructures and mechanical properties of protective nano-multilayered films or coatings. J. Mater. Res. Technol. 2023, 27, 2413–2442. [Google Scholar] [CrossRef]
- Zhao, D.; Gutmark, E.; De Goey, P. A review of cavity-based trapped vortex, ultra-compact, high-g, inter-turbine combustors. Prog. Energy Combust. Sci. 2018, 66, 42–82. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, J.; Deng, X.; Liu, Y.; Sun, D.; Zhang, Y. Research and analysis of a thermal optimisation design method for aluminum alloy pistons in diesel engines. Case Stud. Therm. Eng. 2023, 52, 103667. [Google Scholar] [CrossRef]
- Qin, B.B.; Ma, L.Q.; Wang, S.C.; Hou, C.J.; Zhou, S.G. Construction of a sandwich-like Gr/Ni composite coating on AZ91D magnesium alloy to achieve excellent corrosion and wear resistances in the seawater. Diam. Relat. Mater. 2022, 130, 109400. [Google Scholar] [CrossRef]
- Pei, W.L.; Pei, X.L.; Xie, Z.Z.; Wang, J.M. Research progress of marine anti-corrosion and wear-resistant coating. Tribol. Int. 2024, 198, 109864. [Google Scholar] [CrossRef]
- Song, X.; Liu, F.; Yan, Y.; Wang, W.; Wang, Y.; Sun, W.; Cui, J. Influence of surface roughness of thermal barrier coating on the cooling performance of a film-cooled turbine vane. Case Stud. Therm. Eng. 2025, 65, 105698. [Google Scholar] [CrossRef]
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef]
- Chen, D.C.; He, L.P.; Shang, S.P. Study on aluminum phosphate binder and related Al2O3-SiC ceramic coating. Mater Sci. Eng. A-Struct Mater Prop Microstruct. Process 2003, 348, 29–35. [Google Scholar] [CrossRef]
- Guo, R.F.; Shen, P.; Li, S.X.; Shaga, A.; Jiang, Q.C. High compressive strength in nacre-inspired Al-7Si-5Cu/Al2O3-ZrO2 composites at room and elevated temperatures by regulating interfacial reaction. Ceram. Int. 2017, 43, 7369–7373. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, J.; Zhou, J.; Zhu, J.; Wang, A.Z.; Zhou, Q. Research progress on preparation technology and failure mechanism of metal/ceramic laminated composites. J. Aeronaut. Mater. 2020, 40, 33–44. [Google Scholar] [CrossRef]
- Chen, B.S.; Meng, J.S.; Wang, Y.; Shi, X.P. Research progress on preparation technology and coating of nano-powder by plasma spraying. Heat Treat. Met. 2023, 48, 223–236. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiong, J.; Guo, Z.X.; Liu, J.B.; Zhou, L.M.; Ye, J.L.; Zhao, W. Microstructures and properties of PVD TiAlN coating deposited on ceramets with different Ti (C, N) grain size. J. Cent. South Univ. 2020, 27, 721–735. [Google Scholar] [CrossRef]
- Geng, M.Z.; Wang, W.Q.; Zhang, X.G. Microstructures and Properties of Ni/Ti(C,N) Composite Cermet Coating Prepared by Electro-spark Deposition. Surf. Technol. 2020, 49, 222–229. [Google Scholar] [CrossRef]
- Yang, J.H.; Zhang, W.; Gao, X.M.; Zhang, Q.L. Study of Fe-Ni(Cr) Alloy and Nano-Al2O3 Particles Composite Coating Prepared by Laser Cladding. Solid State Phenom. 2006, 118, 585–590. [Google Scholar] [CrossRef]
- Tian, Z.J.; Gao, Y.W.; Wang, D.S.; Shen, L.D.; Liu, Z.D.; Gao, X.S.; Huang, Y.H. Hot Corrosion Behavior of Laser-remelted Nanostructured Ceramic Coatings on TiAl Alloy Surface. Appl. Laser 2010, 30, 73–79. [Google Scholar] [CrossRef]
- Xiao, K.; Xue, W.; Li, Z.L.; Wang, J.R.; Li, X.M.; Dong, C.F.; Wu, J.S.; Li, X.G.; Wei, D. Effect of sintering temperature on the microstructure and performance of a ceramic coating obtained by the slurry method. Ceram. Int. 2018, 44, 11180–11186. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Chen, H.; Wang, Y.Q.; Wang, G.; Li, L.H.; Zhong, M.; Bai, H. Effect of sodium silicate binder on the performance of ceramic coatings on copper prepared by the slurry method. Surf. Coat. Technol. 2022, 448, 128868. [Google Scholar] [CrossRef]
- Wang, J.Q.; Yuan, Y.C.; Chi, Z.H.; Zhang, G.X. High-temperature sulfur corrosion behavior of h-BN-based ceramic coating prepared by slurry method. Mater. Chem. Phys. 2018, 206, 186–192. [Google Scholar] [CrossRef]
- Chen, Q.; Kang, S.H.; Li, Z.P.; Tang, J.G.; Deng, Y.L.; Chen, M.A. Effect of changes in organo-silicon electrolyte concentration and composition on SiO2 ceramic coating prepared by plasma electrolytic oxidation on 6061 aluminum alloy. Ceram. Int. 2024, 50, 29920–29936. [Google Scholar] [CrossRef]
- Yang, Q.B.; Sang, S.B.; Yang, F.Y.; Li, Y.W.; Zhu, T.B.; Wang, H. Influence of Al2O3 content on microstructure and oxidation resistance of glass-ceramic coatings for 06Ni9DR alloy steel. Ceram. Int. 2024, 50, 48788–48798. [Google Scholar] [CrossRef]
- Jiang, J.P.; Chen, Y.N.; Jiang, C.P.; Zhang, Y.; Zhao, Q.Y.; Zhang, Z.; Yang, Z.H.; Wang, S.P.; Li, H.Z. Composite ceramic coating with enhanced thermal shock resistance formed by the in-situ synthesis of nano-ZrO2. Ceram. Int. 2022, 48, 10629–10637. [Google Scholar] [CrossRef]
- Peng, W.D.; Chen, Z.; Yan, W.; Chen, J.F.; Li, G.Q.; Li, Y.W. Microstructure and properties of ceramic filter containing porous MgO coating and its filtration of Al2O3 inclusions in molten steel. Ceram. Int. 2024, 50, 218–229. [Google Scholar] [CrossRef]
- Wu, H.B.; Zhang, X.Y.; Geng, Z.H.; Yin, Y.; Hang, R.Q.; Huang, X.B.; Yao, X.H.; Tang, B. Preparation, antibacterial effects and corrosion resistant of porous Cu–TiO2 coatings. Appl. Surf. Sci. 2014, 308, 43–49. [Google Scholar] [CrossRef]
- Huang, L.J.; Jing, S.L.; Zhuo, O.; Meng, X.F.; Wang, X.Z. Surface hydrophilicity and antifungal properties of TiO2 films coated on a Co-Cr substrate. BioMed Res. Int. 2017, 2017, 2054723. [Google Scholar] [CrossRef]
- Krishna, D.S.R.; Sun, Y. Thermally oxidized rutile-TiO2 coating on stainless steel for tribological properties and corrosion resistance enhancement. Appl. Surf. Sci. 2005, 252, 1107–1116. [Google Scholar] [CrossRef]
- Procopio, A.M.S.; Carvalho, J.D.L.; Silveira, T.H.R.; Valério, A.L.; Vaz, I.C.F.; Cabral, A.C.T.; Freitas, M.R.; Silva, M.C.; Filho, F.M. CeO2 thin film supported on TiO2 porous ceramics. Mater. Lett. 2020, 276, 128224. [Google Scholar] [CrossRef]
- Emarati, S.M.; Mozammel, M. Efficient one-step fabrication of superhydrophobic nano-TiO2/TMPSi ceramic composite coating with enhanced corrosion resistance on 316L. Ceram. Int. 2020, 46, 1652–1661. [Google Scholar] [CrossRef]
- Lakkimsetty, N.R.; Mai, A.A.; Joy, V.M.; Karunya, S.; Shaik, F. Anti-corrosion behaviour of TiO2-nanocomposites coated stainless steel. Mater. Today Proc. 2023, 80, 641–647. [Google Scholar] [CrossRef]
- Haewon, B.; Sunil, J. Exploring the novel environmentally friendly highly hydrophobic TiO2 coating for enhanced anti-corrosion performance of steel in potential industrial applications. Results Chem. 2025, 14, 102087. [Google Scholar] [CrossRef]
- Jéhanno, P.; Heilmaier, M.; Kestler, H. Characterization of an industrially processed Mo-based silicide alloy. Intermetallics 2004, 12, 1005–1009. [Google Scholar] [CrossRef]
- Abdulagatov, A.I.; Yan, Y.; Cooper, J.R.; Zhang, Y.; Gibbs, Z.M.; Cavanagh, A.S.; Yang, R.G.; Lee, Y.C.; George, S.M. Al2O3 and TiO2 atomic layer deposition on copper for water corrosion resistance. ACS Appllied Mater. Interfaces 2011, 3, 4593–4601. [Google Scholar] [CrossRef]
- Pinzón, A.V.; Urrego, K.J.; González-Hernández, A.; Ortiza, M.R.; Galvis, F.V. Corrosion protection of carbon steel by alumina-titania ceramic coatings used for industrial applications. Ceram. Int. 2018, 44, 21765–21773. [Google Scholar] [CrossRef]
- Zhang, J.X.; He, J.N.; Dong, Y.H.; Li, X.Z.; Yan, D.R. Microstructure and properties of Al2O3-13%TiO2 coatings sprayed using nanostructured powders. Rare Met. 2007, 26, 391–397. [Google Scholar] [CrossRef]
- Ahn, J.; Hwang, B.C.; Song, E.P.; Lee, S.H.; Kim, N.J. Correlation of microstructure and wear resistance of Al2O3-TiO2 coatings plasma sprayed with nanopowders. Metall. Mater. Trans. A 2006, 37, 1851–1861. [Google Scholar] [CrossRef]
- Li, F.J.; Chen, M.H.; Wang, Q.C.; Wang, F.H. Effect of Al2O3 content on microstructure and oxidation behavior of silicate enamel coatings on a Ni-based superalloy at 1000 °C. Ceram. Int. 2022, 48, 25445–25457. [Google Scholar] [CrossRef]
- TB 700-2006; Carbon Structural Steel. China Standard Press: Beijing, China, 2006.
- Li, L.F.; Lu, F.T.; Ma, X.; Wang, E.Z. Study on the effect of curing agent on the curing behavior of phosphate binder. J. Artif. Cryst. 2014, 43, 1311–1316. [Google Scholar]
- Zeng, X.G.; Yu, L.Y.; Li, X.Y.; Zheng, X.W. Preparation and study of new high temperature phosphate adhesives. Surf. Technol. 2010, 39, 103–105. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Yang, L.; Tang, S.Y.; Fan, S.; Liu, J.F.; Jiang, W.M.; Fan, Z.T. Effect of Cr transition layer on the Al2O3/Fe(ceramic/metal) interface microstructure based on binder jetting. Ceram. Int. 2024, 50, 39318–39329. [Google Scholar] [CrossRef]
- Tang, S.L.; Gao, Y.M.; Li, Y.F.; Zhen, Q.L. Preparation and interface investigation of Fe/Al2O3 composite activated by Ni and Ti. Adv. Eng. Mater. 2016, 18, 1913–1920. [Google Scholar] [CrossRef]
- Lan, W.; Chen, Z.H.; Zhang, X.; Bi, W.X.; Liu, W.H. Corrosion resistance analysis of Al2O3-TiO2 composite ceramic coatings on carbon steel pipe surfaces. Alex. Eng. J. 2025, 110, 377–385. [Google Scholar] [CrossRef]
- Shi, P.Y.; Sun, H.W.; Yi, G.W.; Wang, W.Z.; Wan, S.H.; Yu, Y.; Wang, Q.H. Tribological behavior and mechanical properties of thermal sprayed TiO2–ZnO and TiOx ceramic coatings. Ceram. Int. 2023, 49, 18662–18670. [Google Scholar] [CrossRef]
- Li, H.P.; Liu, S.Q.; Zheng, Z.Y.; Fan, G.F.; Yan, Y.W.; Wang, X.Y. One-step fabrication of AlPO4 nanosheet reinforced ceramic coatings with improved fracture toughness and hydrogen resistance. J. Mater. Process. Technol. 2024, 333, 118597. [Google Scholar] [CrossRef]
- Li, C.M.; Ilyas, N.; Wang, J.Y.; Li, Y.A.; Luo, H.L.; Li, D.Y.; Gu, D.E.; Liu, F.C.; Jiang, Y.D.; Li, W. Nanostructured CuAlO2@ZnO optoelectronic device for artificial synaptic applications. Appl. Surf. Sci. 2023, 611, 155682. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Yu, B.H.; Cui, W.F.; Sun, Y.M.; Ma, M. Multi-dimensional carbon modification strategy for lithium-ion battery anodes: Carbon-covered TiP2O7 supported by 3D carbon skeleton. J. Power Sources 2024, 622, 235363. [Google Scholar] [CrossRef]
- Wang, X.D.; Zhou, M.M.; Deng, Y.R.; Liu, Z.J.; Dong, H.Y.; Yan, P.; Liu, R.P. Dual functional Ti3(PO4)4-coated NCM811 cathode enables highly stable sulfide-based all-solid-state lithium batteries. Chin. Chem. Lett. 2024, 110307. [Google Scholar] [CrossRef]
- Liang, B.; Ding, C.X. Thermal shock resistances of nanostructured and conventional zirconia coatings deposited by atmospheric plasma spraying. Surf. Coat. Technol. 2005, 197, 185–192. [Google Scholar] [CrossRef]
- Li, J.F.; Liao, H.; Wang, X.Y.; Normand, B.; Ji, V.; Ding, C.X.; Coddet, C. Improvement in wear resistance of plasma sprayed yttria stabilized zirconia coating using nanostructured powder. Tribol. Int. 2004, 37, 77–84. [Google Scholar] [CrossRef]
- Fang, J.J.; Li, Z.X.; Shi, Y.W.; Jiang, J.M. Effect of nanostructured ceramic powder on particles characteristics and coatings properties. Trans. China Weld. Inst. 2007, 28, 41–44, 48. [Google Scholar] [CrossRef]
- Lathabai, S.; Ottmüller, M.; Fernandez, I. Solid particle erosion behaviour of thermal sprayed ceramic, metallic and polymer coatings. Wear 1998, 221, 93–108. [Google Scholar] [CrossRef]
- McPherson, R. A review of microstructure and properties of plasma sprayed ceramic coatings. Surf. Coat. Technol. 1989, 39, 173–181. [Google Scholar] [CrossRef]
- Rico, A.; Poza, P.; Rodríguez, J. High temperature tribological behavior of nanostructured and conventional plasma sprayed alumina-titania coatings. Vacuum 2013, 88, 149–154. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Zhang, S.W.; Wang, D.S.; Chen, W.C.; Wu, H.S.; Zhan, Y.W.; Lu, X.G. An innovative interlayer improving the interfacial connection of FeCoNiCrTi coating on copper surface. Surf. Coat. Technol. 2024, 494, 131541. [Google Scholar] [CrossRef]
- Farooq, S.A.; Raina, A.; Mohan, S.; Singh, R.A.; Jayalakshmi, S.; UI Haq, M.I. Nanostructured Coatings: Review on Processing Techniques, Corrosion Behaviour and Tribological Performance. Nanomaterials 2022, 12, 1323. [Google Scholar] [CrossRef]
- Silva, L.F.M.; Ferreira, N.M.A.J.; Richter-Trummer, V.; Marques, E.A.S. Effect of grooves on the strength of adhesively bonded joints. Int. J. Adhes. Adhes. 2010, 30, 735–743. [Google Scholar] [CrossRef]
- da Silva, L.F.M.; Rodrigues, T.N.S.S.; Figueiredo, M.A.V.; de Moura, M.F.S.F.; Chousal, J.A.G. Effect of adhesive type and thickness on the lap shear strength. J. Adhes. 2006, 82, 1091–1115. [Google Scholar] [CrossRef]
- Wang, M.C.; Zhang, J.; Wei, T.; Zhou, Q.J.; Li, Z.P. Effect of Al:P ratio on bonding performance of high-temperature resistant aluminum phosphate adhesive. Int. J. Adhes. Adhes. 2020, 100, 102627–102633. [Google Scholar] [CrossRef]
- Rudawska, A. Selected aspects of the effect of mechanical treatment on surface roughness and adhesive joint strength of steel sheets. Int. J. Adhes. Adhes. 2014, 50, 235–243. [Google Scholar] [CrossRef]
- Nieto-Maestre, J.; Muñoz-Sánchez, B.; Fernández, A.G.; Faik, A.; Grosu, Y.; García-Romero, A. Compatibility of container materials for concentrated solar power with a solar salt and alumina based nanofluid: A study under dynamic conditions. Renew. Energy 2020, 146, 384–396. [Google Scholar] [CrossRef]


| Ingredient | Nano-TiO2 | Al2O3 | Nano-CuO |
|---|---|---|---|
| Mass fraction | 65% | 30% | 5% |
| Element | Carbon (C) | Silicon (Si) | Manganese (Mn) | Phosphorus (P) | Sulfur (S) | Iron (Fe) |
|---|---|---|---|---|---|---|
| Content (wt.%) | ≤0.22 | ≤0.35 | 0.30~1.40 | ≤0.045 | ≤0.050 | Balance |
| Sample | Compactness |
|---|---|
| 2 °C/min ceramic coating | 20 min diffusion |
| 5 °C/min ceramic coating | 8 min diffusion |
| Sample | Wear Weight Loss Per Unit Area (g/m−2) | Relative Wear Resistance ε |
|---|---|---|
| Q235 | 168 | 1.00 |
| 5 °C/min ceramic coating | 123 | 1.36 |
| 2 °C/min ceramic coating | 89 | 1.89 |
| Test item | Sample | Heating Rate | Shearing Strength (MPa) |
|---|---|---|---|
| Shear strength test of coating and substrate | 5 °C/min ceramic coating | 5 °C/min | 2.36 |
| 2 °C/min ceramic coating | 2 °C/min | 4.13 | |
| Shear strength of the coating itself | 5 °C/min ceramic coating | 5 °C/min | 0.39 |
| 2 °C/min ceramic coating | 2 °C/min | 0.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, Y.; Cao, J.; Ma, S.; Chen, G.; Duan, K.; Liu, J. Effect of Heating Rate on the Properties and Mechanism of Nanocomposite Ceramic Coatings Prepared by Slurry Method. Appl. Sci. 2025, 15, 6561. https://doi.org/10.3390/app15126561
Zhang Y, Li Y, Cao J, Ma S, Chen G, Duan K, Liu J. Effect of Heating Rate on the Properties and Mechanism of Nanocomposite Ceramic Coatings Prepared by Slurry Method. Applied Sciences. 2025; 15(12):6561. https://doi.org/10.3390/app15126561
Chicago/Turabian StyleZhang, Yuntian, Yinhui Li, Jiaqi Cao, Songyuchen Ma, Guangsong Chen, Kunquan Duan, and Jie Liu. 2025. "Effect of Heating Rate on the Properties and Mechanism of Nanocomposite Ceramic Coatings Prepared by Slurry Method" Applied Sciences 15, no. 12: 6561. https://doi.org/10.3390/app15126561
APA StyleZhang, Y., Li, Y., Cao, J., Ma, S., Chen, G., Duan, K., & Liu, J. (2025). Effect of Heating Rate on the Properties and Mechanism of Nanocomposite Ceramic Coatings Prepared by Slurry Method. Applied Sciences, 15(12), 6561. https://doi.org/10.3390/app15126561






