Soil Mercury Pollution in Nature-Based Solutions Across Various Land Uses: A Review of Trends, Treatment Outcomes, and Future Directions
Abstract
1. Introduction
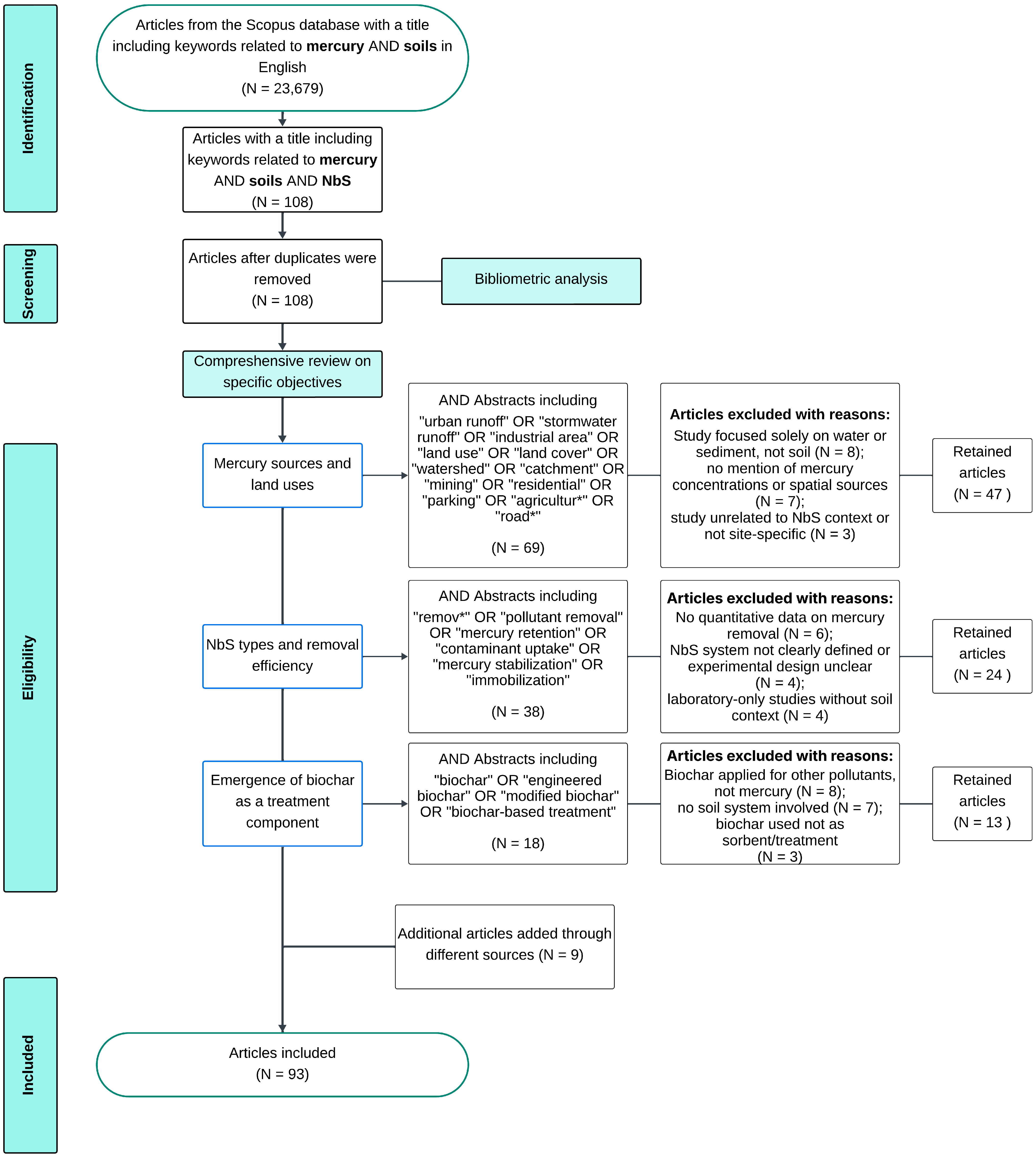
2. Materials and Methods
2.1. Systematic Review Process
Review Scope and Limitations
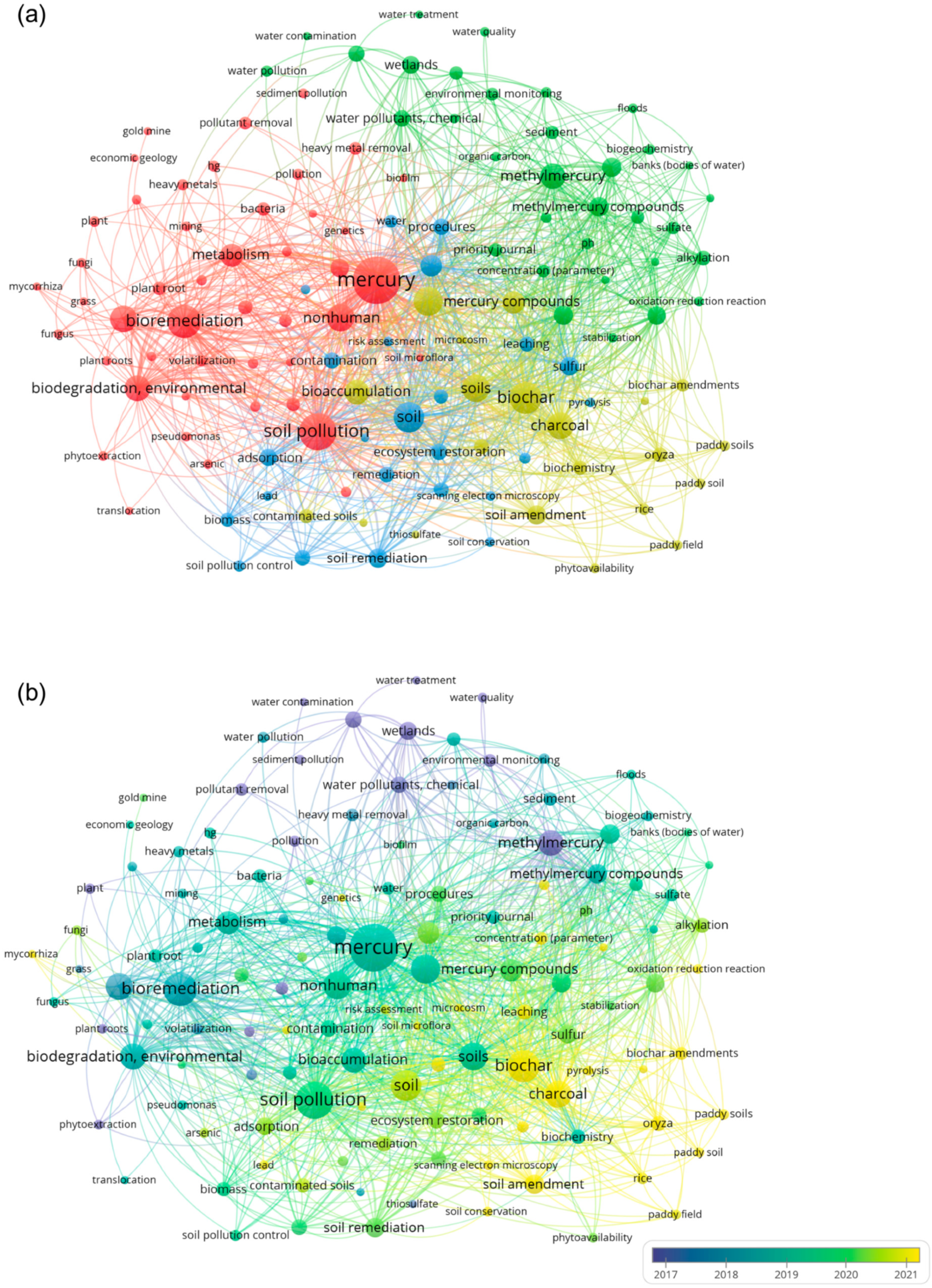
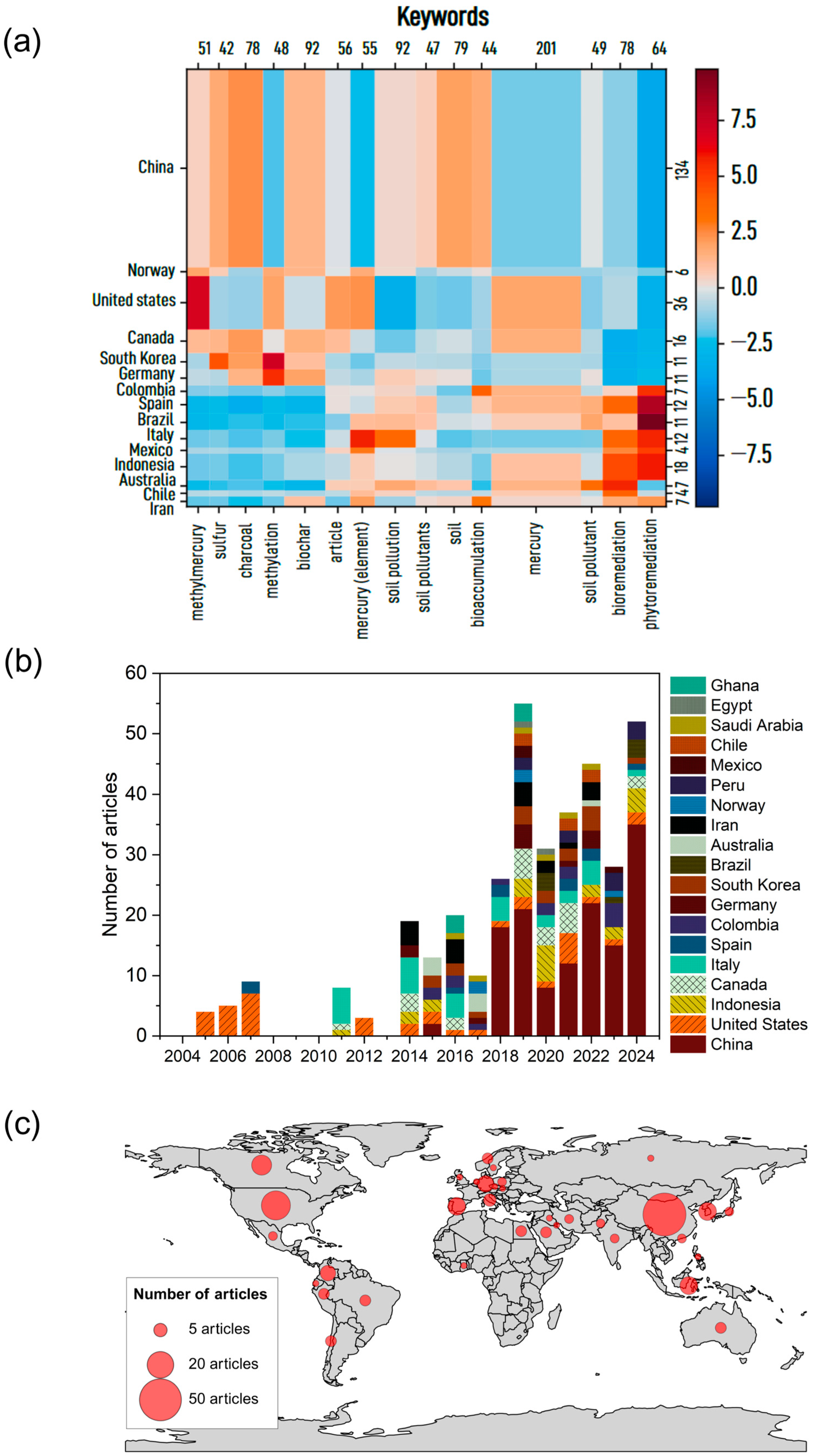
2.2. Bibliometric Analysis
3. Results
3.1. Keyword Co-Occurrence and Spatial Trends
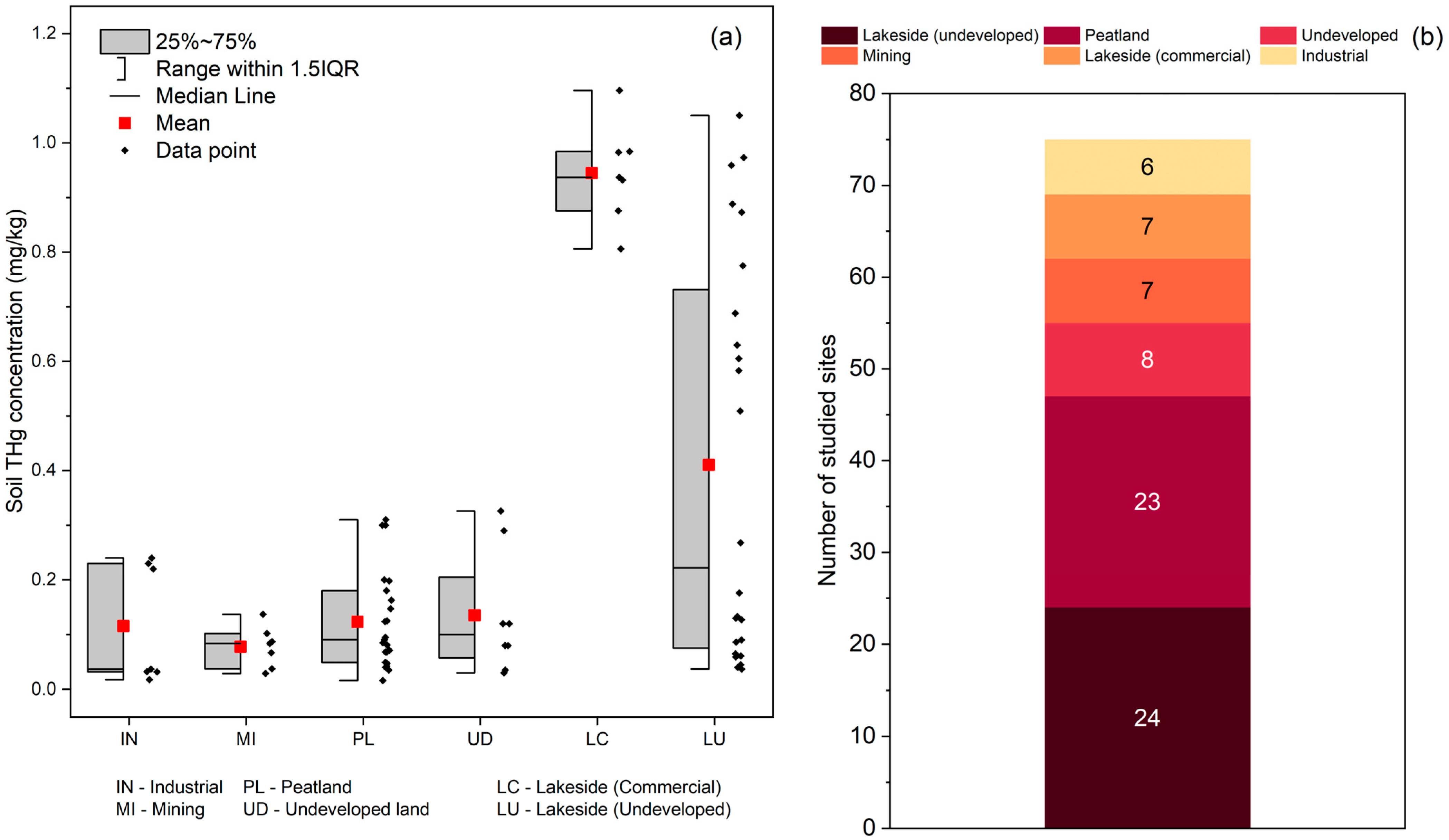
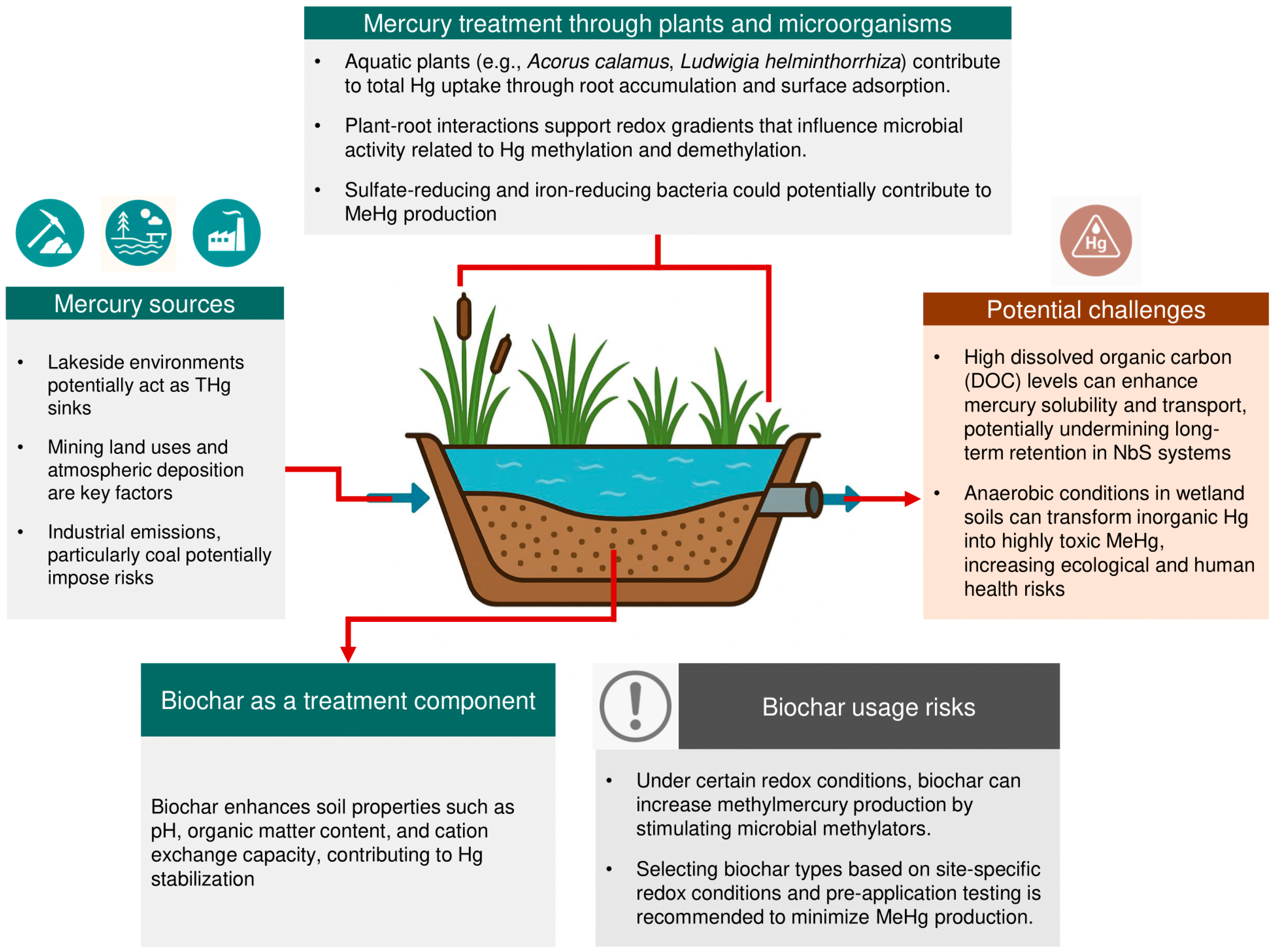
3.2. Sources of Mercury in Soils
| Country | Studied Land Uses | Results | Reference |
|---|---|---|---|
| Canada | Remote subarctic lake catchments with forested, wetland, and mixed terrain | Mercury concentrations in sediment were positively correlated with catchment characteristics such as dissolved organic carbon (DOC) input and forest cover. Forested and lower-elevation catchments had higher sediment THg. | [30] |
| China | Mining area in the Wanshan region, Guizhou Province | Hg detected in soils at all sites; no significant bioaccumulation from soil. Hg concentrations were highest in upper watershed areas near former mercury mines. Pollution source analysis identified mining as the main source of Hg in soils. | [22] |
| China | Peri-industrial and urbanized area in Gongzhuling, Northeast China | 24% of samples exceeded the provincial background value (0.04 mg/kg). | [31] |
| Finland and Sweden | Subarctic lake catchments along a climate–productivity gradient from pristine to intensive forestry | THg leaching from soils increases with forestry practices like peatland ditching. Higher THg baseline values found in eutrophic lakes with intensive land-use. Soil-related leaching of historical mercury due to land modification was a key factor. THg baseline positively correlated with catchment land use and the climate–productivity gradient. | [21] |
| Norway | Boreal forest and peatland soils, with undisturbed and disturbed catchments | Forest soils had higher THg due to canopy throughfall, while MeHg levels were similar across soil types. Soil disturbance from machinery increased MeHg and MeHg-to-THg ratios in topsoil. Peatlands had higher %MeHg in deeper layers, indicating more local production. Disturbed areas showed significantly elevated MeHg production compared to non-disturbed areas. | [20] |
| Slovenia | Nationwide study covering urban, rural, agricultural, and mining areas including Idrija and Litija | Hg concentrations were highest in western Slovenia due to mining. Urban and industrial areas showed elevated Hg. Hg values exceeded European averages; atmospheric and river sediment transport contributed to regional dispersion. | [23] |
| Sweden | Boreal coniferous forests in watersheds with clear-cut and mature Norway spruce | MeHg in organic topsoil increased after clear-cutting. Forest harvest adds ~6.6% of Sweden’s MeHg runoff. DOC-normalized MeHg levels also rose significantly after clear-cutting. | [19] |
| United States | Urban stormwater catchment treated by bioretention rain garden near San Francisco Bay | Hg and MeHg were concentrated in the top 100 mm of soil. Subdrainage helped reduce MeHg formation. Soil near inlets exceeded EPA residential screening levels, but not for Hg. Findings highlight surface soil as the main zone of Hg retention. | [32] |
| United States | Green roofs and conventional gravel roofs on campus and commercial buildings | Hg concentrations in runoff were significantly higher from green roofs than gravel roofs, despite runoff reduction benefits from green roofs. Long-term accumulation or remobilization of retained Hg remains uncertain. | [33] |
3.3. Mercury Removal Performance of NbSs
3.3.1. Hg Removal and MeHg Changes in NbSs
3.3.2. Hg Removal Through Plant Uptake
3.3.3. Microbial Dynamics and MeHg Risk in NbS Soils
3.4. Biochar as an NbS Component for Treating Mercury
| Biochar Type | System Type | Experimental Description | THg Removal | Statistical Significance | MeHg Change | Redox Conditions | Key Findings | Reference |
|---|---|---|---|---|---|---|---|---|
| Oak woodchip + Fe biochar | Constructed wetland column | Biochar and iron amendments tested in column-type constructed wetland | ~90% | p < 0.05 | Increased | Controlled aerobic | Combined amendment reduced THg and MeHg synergistically | [59] |
| Fe-modified rice straw | Surface flow constructed wetland mesocosm | Surface flow mesocosms tested with Fe-doped biochar | ~98% | p < 0.05 | Increased | Oxic–anoxic gradient | Iron doping enhanced Hg immobilization and suppressed MeHg | [63] |
| Maize stalk | Simulated constructed wetland substrate | Static pots with maize biochar and Hg-spiked solutions | 57.6% | p < 0.01 | Not studied | Anoxic | Biochar adsorbed Hg, effectiveness varied with pH and DOC | [62] |
| Rice straw | Paddy soil pots | Rice biochar added to flooded soil pots with rice plants | ~30% | Not reported | Increased | Anaerobic, flooded | Biochar increased MeHg due to microbial methylation activation | [60] |
| Rice husk biochar | Soils from Hg mining area | Soil microcosms | Adsorbed, not quantified | Not reported | Increased | Fluctuating redox | Biochar increased MeHg under reducing conditions; THg adsorbed | [61] |
| Pine wood | Anaerobic sediment microcosms | Lab-scale microcosms with biochar–sediment mixture | Significantly reduced; but no specific value | p < 0.05 | Increased | Anaerobic | Biochar reduced THg and MeHg in porewater through sorption and redox buffering | [64] |
| Aged rice husk | Field wetland mesocosms | Field mesocosms with continuous flow and aged biochar amendment | Not specified | - | Increased | Fluctuating/anoxic | Biochar increased MeHg production; shifted microbial composition | [58] |
4. Discussion and Future Perspective
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pochodyła-Ducka, E.; Glińska-Lewczuk, K.; Jaszczak, A. Changes in Stormwater Quality and Heavy Metals Content along the Rainfall–Runoff Process in an Urban Catchment. Water 2023, 15, 3505. [Google Scholar] [CrossRef]
- Reddy, K.R.; Xie, T.; Dastgheibi, S. Removal of Heavy Metals from Urban Stormwater Runoff Using Different Filter Materials. J. Environ. Chem. Eng. 2014, 2, 282–292. [Google Scholar] [CrossRef]
- Elwaleed, A.; Jeong, H.H.; Abdelbagi, A.H.; Quynh, N.T.; Agusa, T.; Ishibashi, Y.; Arizono, K. Human Health Risk Assessment from Mercury-Contaminated Soil and Water in Abu Hamad Mining Market, Sudan. Toxics 2024, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Addai-Arhin, S.; Novirsa, R.; Jeong, H.H.; Phan, Q.D.; Hirota, N.; Ishibashi, Y.; Shiratsuchi, H.; Arizono, K. The Human Health Risks Assessment of Mercury in Soils and Plantains from Farms in Selected Artisanal and Small-Scale Gold Mining Communities around Obuasi, Ghana. J. Appl. Toxicol. 2022, 42, 258–273. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Jahan, S.A. A Review on the Distribution of Hg in the Environment and Its Human Health Impacts. J. Hazard. Mater. 2016, 306, 376–385. [Google Scholar] [CrossRef]
- Anderson, V.; Gough, W.A. A Typology of Nature-Based Solutions for Sustainable Development: An Analysis of Form, Function, Nomenclature, and Associated Applications. Land 2022, 11, 1072. [Google Scholar] [CrossRef]
- Sun, C.; Rao, Q.; Chen, B.; Liu, X.; Ikram, R.M.A.; Li, J.; Wang, M.; Zhang, D. Mechanisms and Applications of Nature-Based Solutions for Stormwater Control in the Context of Climate Change: A Review. Atmosphere 2024, 15, 403. [Google Scholar] [CrossRef]
- Wang, L.; Rinklebe, J.; Tack, F.M.G.; Hou, D. A Review of Green Remediation Strategies for Heavy Metal Contaminated Soil. Soil Use Manag. 2021, 37, 936–963. [Google Scholar] [CrossRef]
- Jacklin, D.M.; Brink, I.C.; Jacobs, S.M. Urban Stormwater Nutrient and Metal Removal in Small-Scale Green Infrastructure: Exploring Engineered Plant Biofilter Media Optimisation. Water Sci. Technol. 2021, 84, 1715–1731. [Google Scholar] [CrossRef]
- Piwowarska, D.; Kiedrzyńska, E.; Jaszczyszyn, K. A Global Perspective on the Nature and Fate of Heavy Metals Polluting Water Ecosystems, and Their Impact and Remediation. Crit. Rev. Environ. Sci. Technol. 2024, 54, 1436–1458. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, V.; Chawla, M.; Thakur, M.; Bhardwaj, R.; Wang, J.; O’Connor, D.; Hou, D.; Rinklebe, J. Bioremediation of Mercury Contaminated Soil and Water: A Review. Land Degrad. Dev. 2024, 35, 1261–1283. [Google Scholar] [CrossRef]
- Jatobá-Junior, A.A.; Hatje, V.; Masque, P.; de Rezende, C.E.; Cabral, A.; Yau, Y.Y.Y.; Barreira, J.; Reithmaier, G.M.S.; Santos, I.R. Carbon and Mercury Burial in Mangrove Soils across an Anthropogenic Gradient. Estuar. Coast. Shelf Sci. 2025, 318, 109226. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Tang, W.; Wu, M.; Chen, M.; He, H.; Lei, P.; Zhong, H. Mercury in Wetlands over 60 Years: Research Progress and Emerging Trends. Sci. Total Environ. 2023, 874, 161862. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.; Hou, D.; Ok, Y.S.; Mulder, J.; Duan, L.; Wu, Q.; Wang, S.; Tack, F.M.G.; Rinklebe, J. Mercury Speciation, Transformation, and Transportation in Soils, Atmospheric Flux, and Implications for Risk Management: A Critical Review. Environ. Int. 2019, 126, 747–761. [Google Scholar] [CrossRef]
- Zong, J.; Zhang, H.; Li, X.; Bai, X.; Hu, Y.; Cui, D.; Wang, Z.; Zhang, G. Process of Mercury Accumulation in Urban Strip River Artificial Wetland Ecosystems: A Case Study of Changchun, a Typical Industrial City in Northeast China. Front. Plant Sci. 2024, 15, 1392904. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Software Survey: VOSviewer, a Computer Program for Bibliometric Mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Breucker, P.; Cointet, J.; Hannud Abdo, A.; Orsal, G.; de Quatrebarbes, C.; Duong, T.; Martinez, C.; Ospina Delgado, J.; Medina Zuluaga, L.; Gómez Peña, D.; et al. CorTexT Manager. 2016. Available online: https://docs.cortext.net (accessed on 26 April 2025).
- Hu, H.; Jin, Q.; Kavan, P. A Study of Heavy Metal Pollution in China: Current Status, Pollution-Control Policies and Countermeasures. Sustainability 2014, 6, 5820–5838. [Google Scholar] [CrossRef]
- Kronberg, R.M.; Drott, A.; Jiskra, M.; Wiederhold, J.G.; Björn, E.; Skyllberg, U. Forest Harvest Contribution to Boreal Freshwater Methyl Mercury Load. Glob. Biogeochem. Cycles 2016, 30, 825–843. [Google Scholar] [CrossRef]
- Braaten, H.F.V.; de Wit, H.A. Effects of Disturbance and Vegetation Type on Total and Methylmercury in Boreal Peatland and Forest Soils. Environ. Pollut. 2016, 218, 140–149. [Google Scholar] [CrossRef]
- Kozak, N.; Ahonen, S.A.; Keva, O.; Østbye, K.; Taipale, S.J.; Hayden, B.; Kahilainen, K.K. Environmental and Biological Factors Are Joint Drivers of Mercury Biomagnification in Subarctic Lake Food Webs along a Climate and Productivity Gradient. Sci. Total Environ. 2021, 779, 146261. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Z.; Chen, F.; Li, B.; Yang, C. Non-Lethal Sampling of Phalanges for Heavy Metal Bioaccumulation in Pelophylax Nigromaculatus from Historical Mercury Mining Areas in Southwestern China. Sci. Rep. 2024, 14, 30809. [Google Scholar] [CrossRef] [PubMed]
- Gosar, M.; Šajn, R.; Teršič, T. Distribution Pattern of Mercury in the Slovenian Soil: Geochemical Mapping Based on Multiple Geochemical Datasets. J. Geochem. Explor. 2016, 167, 38–48. [Google Scholar] [CrossRef]
- Yang, H.; Turner, S.; Rose, N.L. Mercury Pollution in the Lake Sediments and Catchment Soils of Anthropogenically-Disturbed Sites across England. Environ. Pollut. 2016, 219, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Leng, M.; Ge, X.; Wu, X.; Liu, H.; Lin, G.; Huang, Z.; Chen, Y. Characterization and Risk Assessment of Nutrient and Heavy Metal Pollution in Surface Sediments of Representative Lakes in Yangxin County, China. Sustainability 2024, 16, 2252. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, B.; Jiang, X.; Zhao, Z.; Zhan, Y.; Yi, F.; Ren, J. Spatial Distribution and Pollution Assessment of Mercury in Sediments of Lake Taihu, China. J. Environ. Sci. 2013, 25, 316–325. [Google Scholar] [CrossRef]
- Miller, C.L.; Watson, D.B.; Lester, B.P.; Lowe, K.A.; Pierce, E.M.; Liang, L. Characterization of Soils from an Industrial Complex Contaminated with Elemental Mercury. Environ. Res. 2013, 125, 20–29. [Google Scholar] [CrossRef]
- Osterwalder, S.; Huang, J.H.; Shetaya, W.H.; Agnan, Y.; Frossard, A.; Frey, B.; Alewell, C.; Kretzschmar, R.; Biester, H.; Obrist, D. Mercury Emission from Industrially Contaminated Soils in Relation to Chemical, Microbial, and Meteorological Factors. Environ. Pollut. 2019, 250, 944–952. [Google Scholar] [CrossRef]
- Pei, P.; Sun, T.; Xu, Y.; Sun, Y. Soil Aggregate–Associated Mercury (Hg) and Organic Carbon Distribution and Microbial Community Characteristics under Typical Farmland–Use Types. Chemosphere 2021, 275, 129987. [Google Scholar] [CrossRef]
- Aqdam, M.M.; Baltzer, J.L.; Branfireun, B.A.; Low, G.; Low, M.; Laird, B.D.; Swanson, H.K. Factors and Mechanisms Driving Among-Lake Variability of Mercury Concentrations in a Benthivorous Fish in the Canadian Subarctic. Chemosphere 2025, 372, 144078. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, Y.; Wu, J.; Yin, S.; Huang, J.; Huang, W.; Huang, H.; Zhang, G. Characteristics and Risk Assessments of Mercury Pollution in a County Region Undergoing Industrialization in Northeastern China: A Case Study in Gongzhuling. Front. Environ. Sci. 2024, 12, 1440426. [Google Scholar] [CrossRef]
- Gilbreath, A.; McKee, L.; Shimabuku, I.; Lin, D.; Werbowski, L.M.; Zhu, X.; Grbic, J.; Rochman, C. Multiyear Water Quality Performance and Mass Accumulation of PCBs, Mercury, Methylmercury, Copper, and Microplastics in a Bioretention Rain Garden. J. Sustain. Water Built Environ. 2019, 5. [Google Scholar] [CrossRef]
- Malcolm, E.G.; Reese, M.L.; Schaus, M.H.; Ozmon, I.M.; Tran, L.M. Measurements of Nutrients and Mercury in Green Roof and Gravel Roof Runoff. Ecol. Eng. 2014, 73, 705–712. [Google Scholar] [CrossRef]
- Chen, J.; Guo, F.; Wu, F.; Bryan, B.A. Costs and Benefits of Constructed Wetlands for Meeting New Water Quality Standards from China’s Wastewater Treatment Plants. Resour. Conserv. Recycl. 2023, 199, 107248. [Google Scholar] [CrossRef]
- Pillay, A.E.; Yaghi, B.; Williams, J.R.; Al-Kindy, S. Mercury Pollution from Irrigation with Treated Sewage Water (TSW). J. Water Health 2007, 5, 315–322. [Google Scholar] [CrossRef]
- Zheng, Y.M.; Liu, Y.R.; Hu, H.Q.; He, J.Z. Mercury in Soils of Three Agricultural Experimental Stations with Long-Term Fertilization in China. Chemosphere 2008, 72, 1274–1278. [Google Scholar] [CrossRef]
- Salvato, L.A.; Marvin-DiPasquale, M.; Fleck, J.A.; McCord, S.A.; Linquist, B.A. Influence of Irrigation Water and Soil on Annual Mercury Dynamics in Sacramento Valley Rice Fields. J. Environ. Qual. 2024, 53, 327–339. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, Y.; Dong, J.; Ma, H.; Guo, Z.; Wu, H.; Hu, Z.; Xie, H.; Zhang, J. Synergetic Effect of Pyrrhotite and Zero-Valent Iron on Hg(Ⅱ) Removal in Constructed Wetland: Mechanisms of Electron Transfer and Microbial Reaction. J. Hazard. Mater. 2024, 480, 136041. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, G.; Wang, Y.; Dai, W.; Luan, Y. Mercury Uptake and Transport by Plants in Aquatic Environments: A Meta-Analysis. Appl. Sci. 2021, 11, 8829. [Google Scholar] [CrossRef]
- Bachand, P.A.M.; Kraus, T.E.C.; Stumpner, E.B.; Bachand, S.M.; Stern, D.; Liang, Y.L.; Horwath, W.R. Mercury Sequestration and Transformation in Chemically Enhanced Treatment Wetlands. Chemosphere 2019, 217, 496–506. [Google Scholar] [CrossRef]
- King, J.K.; Harmon, S.M.; Fu, T.T.; Gladden, J.B. Mercury Removal, Methylmercury Formation, and Sulfate-Reducing Bacteria Profiles in Wetland Mesocosms. Chemosphere 2002, 46, 859–870. [Google Scholar] [CrossRef]
- Compeau, G.C.; Bartha, R. Sulfate-Reducing Bacteria: Principal Methylators of Mercury in Anoxic Estuarine Sediment. Appl. Environ. Microbiol. 1985, 50, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, C.C.; Riedel, G.S.; Ederington, M.C.; Bell, J.T.; Benoit, J.M.; Gill, G.A.; Stordal, M.C. Methylmercury Concentrations and Production Rates across a Trophic Gradient in the Northern Everglades. Biogeochemistry 1998, 40, 327–345. [Google Scholar] [CrossRef]
- Marrugo-Negrete, J.; Enamorado-Montes, G.; Durango-Hernández, J.; Pinedo-Hernández, J.; Díez, S. Removal of Mercury from Gold Mine Effluents Using Limnocharis Flava in Constructed Wetlands. Chemosphere 2017, 167, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Siswanti, D.U.; Ayuningtyas, D.; Nugraheni, S.N.; Nurhanifah, T.; Petrus, H.T.B.M.; Suyono, E.A.; Daryono, B.S. Mercury Removal by Aquarius Palifolius (Nees & Mart.) Christenh. & Byng: Isotherms Model, Superoxide Dismutase Activity, and Chlorophyll Content. Watershed Ecol. Environ. 2024, 6, 227–233. [Google Scholar] [CrossRef]
- Gomez, F.H.; Collivignarelli, M.C.; Masoud, A.M.N.; Carnevale Miino, M.; Torres, K.C.; Quintero, J.A.; Sorlini, S.; Vaccari, M. Mercury Removal from Mining Wastewater by Phytoaccumulation in Autochthonous Aquatic Plant Species. Clean Technol. 2023, 5, 839–851. [Google Scholar] [CrossRef]
- Feng, S.; Ai, Z.; Zheng, S.; Gu, B.; Li, Y. Effects of Dryout and Inflow Water Quality on Mercury Methylation in a Constructed Wetland. Water Air Soil Pollut. 2014, 225, 1929. [Google Scholar] [CrossRef]
- Wang, T.; Du, B.; Forbrich, I.; Zhou, J.; Polen, J.; Sunderland, E.M.; Balcom, P.H.; Chen, C.; Obrist, D. Above- and Belowground Plant Mercury Dynamics in a Salt Marsh Estuary in Massachusetts, USA. Biogeosciences 2024, 21, 1461–1476. [Google Scholar] [CrossRef]
- Sitarska, M.; Traczewska, T.; Hołtra, A.; Zamorska-Wojdyła, D.; Filarowska, W.; Hanus-Lorenz, B. Removal of Mercury from Water by Phytoremediation Process with Salvinia natans(L.) All. Environ. Sci. Pollut. Res. 2023, 30, 85494–85507. [Google Scholar] [CrossRef]
- Sitarska, M.; Traczewska, T.; Filarowska, W.; Hołtra, A.; Zamorska-Wojdyła, D.; Hanus-Lorenz, B. Phytoremediation of Mercury from Water by Monocultures and Mixed Cultures Pleustophytes. J. Water Process Eng. 2023, 52, 103529. [Google Scholar] [CrossRef]
- Molisani, M.M.; Rocha, R.; Machado, W.; Barreto, R.C.; Lacerda, L.D. Mercury Contents in Aquatic Macrophytes from Two Reservoirs in the Paraíba do Sul: Guandú River System, SE Brazil. Braz. J. Biol. 2006, 66, 101–107. [Google Scholar] [CrossRef]
- Frey, B.; Rast, B.M.; Qi, W.; Stierli, B.; Brunner, I. Long-Term Mercury Contamination Does Not Affect the Microbial Gene Potential for C and N Cycling in Soils but Enhances Detoxification Gene Abundance. Front. Microbiol. 2022, 13, 1034138. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, M.; Harrison, M.C.; Storck, V.; Planas, D.; Amyot, M.; Walsh, D.A. Microbial Diversity and Mercury Methylation Activity in Periphytic Biofilms at a Run-of-River Hydroelectric Dam and Constructed Wetlands. mSphere 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Chavan, P.V.; Dennett, K.E.; Marchand, E.A.; Gustin, M.S. Evaluation of Small-Scale Constructed Wetland for Water Quality and Hg Transformation. J. Hazard. Mater. 2007, 149, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Peng, D.; Deng, S.; Chen, J.; Duan, C. Efficient Treatment of Mercury(Ⅱ)-Containing Wastewater in Aerated Constructed Wetland Microcosms Packed with Biochar. Chemosphere 2022, 290, 133302. [Google Scholar] [CrossRef]
- Du, J.; Ren, Y.; Li, J.; Zhang, S.; Huang, H.; Liu, J. The Study on the Effect of Mercury Pollution on Soil Microorganisms around Mercury Mining Area. Sci. Rep. 2023, 13, 21605. [Google Scholar] [CrossRef]
- Li, Z.; Tong, Y.; Wu, Z.; Liao, B.; Liu, G.; Xia, L.; Liu, C.; Zhao, L. Management Strategies to Reduce Microbial Mercury Methylation in Constructed Wetlands: Potential Routes and Future Challenges. J. Hazard. Mater. 2025, 445, 138009. [Google Scholar] [CrossRef]
- Boie, F.; Ducey, T.F.; Xing, Y.; Wang, J.; Rinklebe, J. Field-Aged Rice Hull Biochar Stimulated the Methylation of Mercury and Altered the Microbial Community in a Paddy Soil under Controlled Redox Condition Changes. J. Hazard. Mater. 2024, 472, 134446. [Google Scholar] [CrossRef]
- Goñez-Rodríguez, L.; Johs, A.; Lowe, K.A.; Carter, K.E.; Löffler, F.E.; Mayes, M.A. Evaluation of Engineered Sorbents for the Sorption of Mercury from Contaminated Bank Soils: A Column Study. Environ. Sci. Pollut. Res. 2021, 28, 22651–22663. [Google Scholar] [CrossRef]
- Man, Y.; Wang, B.; Wang, J.; Slaný, M.; Yan, H.; Li, P.; El-Naggar, A.; Shaheen, S.M.; Rinklebe, J.; Feng, X. Use of Biochar to Reduce Mercury Accumulation in Oryza Sativa L: A Trial for Sustainable Management of Historically Polluted Farmlands. Environ. Int. 2021, 153, 106527. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, J.; Kinder, C.E.S.; Yang, X.; Slaný, M.; Wang, B.; Song, H.; Shaheen, S.M.; Leinweber, P.; Rinklebe, J. Rice Hull Biochar Enhances the Mobilization and Methylation of Mercury in a Soil under Changing Redox Conditions: Implication for Hg Risks Management in Paddy Fields. Environ. Int. 2022, 168, 107484. [Google Scholar] [CrossRef]
- Liu, P.; Ptacek, C.J.; Blowes, D.W.; Gould, W.D. Control of Mercury and Methylmercury in Contaminated Sediments Using Biochars: A Long-Term Microcosm Study. Appl. Geochem. 2018, 92, 30–44. [Google Scholar] [CrossRef]
- Li, H.; Yang, L.; Mao, Q.; Zhou, H.; Guo, P.; Agathokleous, E.; Wang, S. Modified Biochar Enhances Soil Fertility and Nutrient Uptake and Yield of Rice in Mercury-Contaminated Soil. Environ. Technol. Innov. 2023, 32, 103435. [Google Scholar] [CrossRef]
- Beckers, F.; Awad, Y.M.; Beiyuan, J.; Abrigata, J.; Mothes, S.; Tsang, D.C.W.; Ok, Y.S.; Rinklebe, J. Impact of Biochar on Mobilization, Methylation, and Ethylation of Mercury under Dynamic Redox Conditions in a Contaminated Floodplain Soil. Environ. Int. 2019, 127, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Randall, P.M.; Chattopadhyay, S. Mercury Contaminated Sediment Sites-An Evaluation of Remedial Options. Environ. Res. 2013, 125, 131–149. [Google Scholar] [CrossRef]
- Wang, L.; Hou, D.; Cao, Y.; Ok, Y.S.; Tack, F.M.G.; Rinklebe, J.; O’Connor, D. Remediation of Mercury Contaminated Soil, Water, and Air: A Review of Emerging Materials and Innovative Technologies. Environ. Int. 2020, 134, 105281. [Google Scholar] [CrossRef]
- Ganguly, M.; Dib, S.; Ariya, P.A. Fast, Cost-Effective and Energy Efficient Mercury Removal-Recycling Technology. Sci. Rep. 2018, 8, 16255. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, D.; Adhityan, A.; Ng, W.J.; Dong, J.; Tan, S.K. Assessing Cost-Effectiveness of Bioretention on Stormwater in Response to Climate Change and Urbanization for Future Scenarios. J. Hydrol. 2016, 543, 423–432. [Google Scholar] [CrossRef]
- Chui, T.F.M.; Liu, X.; Zhan, W. Assessing Cost-Effectiveness of Specific LID Practice Designs in Response to Large Storm Events. J. Hydrol. 2016, 533, 353–364. [Google Scholar] [CrossRef]
- Gwenzi, W.; Chaukura, N.; Noubactep, C.; Mukome, F.N.D. Biochar-Based Water Treatment Systems as a Potential Low-Cost and Sustainable Technology for Clean Water Provision. J. Environ. Manag. 2017, 197, 732–749. [Google Scholar] [CrossRef]
- Campion, L.; Bekchanova, M.; Malina, R.; Kuppens, T. The Costs and Benefits of Biochar Production and Use: A Systematic Review. J. Clean. Prod. 2023, 414, 137138. [Google Scholar] [CrossRef]
- Evers, D.C.; Keane, S.E.; Basu, N.; Buck, D. Evaluating the Effectiveness of the Minamata Convention on Mercury: Principles and Recommendations for Next Steps. Sci. Total Environ. 2016, 569–570, 888–903. [Google Scholar] [CrossRef] [PubMed]
- Maha, M.M.; Matsuyama, A.; Arima, T.; Sainoki, A. Assessment of Total Mercury Levels Emitted from ASGM into Soil and Groundwater in Chami Town, Mauritania. Sustainability 2024, 16, 7992. [Google Scholar] [CrossRef]
- Ansah, E.; Bak, J.L.; Sørensen, P.; Darko, G. Modelling Mercury Concentration in Ghanaian Soil. Chemosphere 2022, 307, 135553. [Google Scholar] [CrossRef] [PubMed]
- Mulenga, M.; Ouma, K.O.; Monde, C.; Syampungani, S. Aquatic Mercury Pollution from Artisanal and Small-Scale Gold Mining in Sub-Saharan Africa: Status, Impacts, and Interventions. Water 2024, 16, 756. [Google Scholar] [CrossRef]
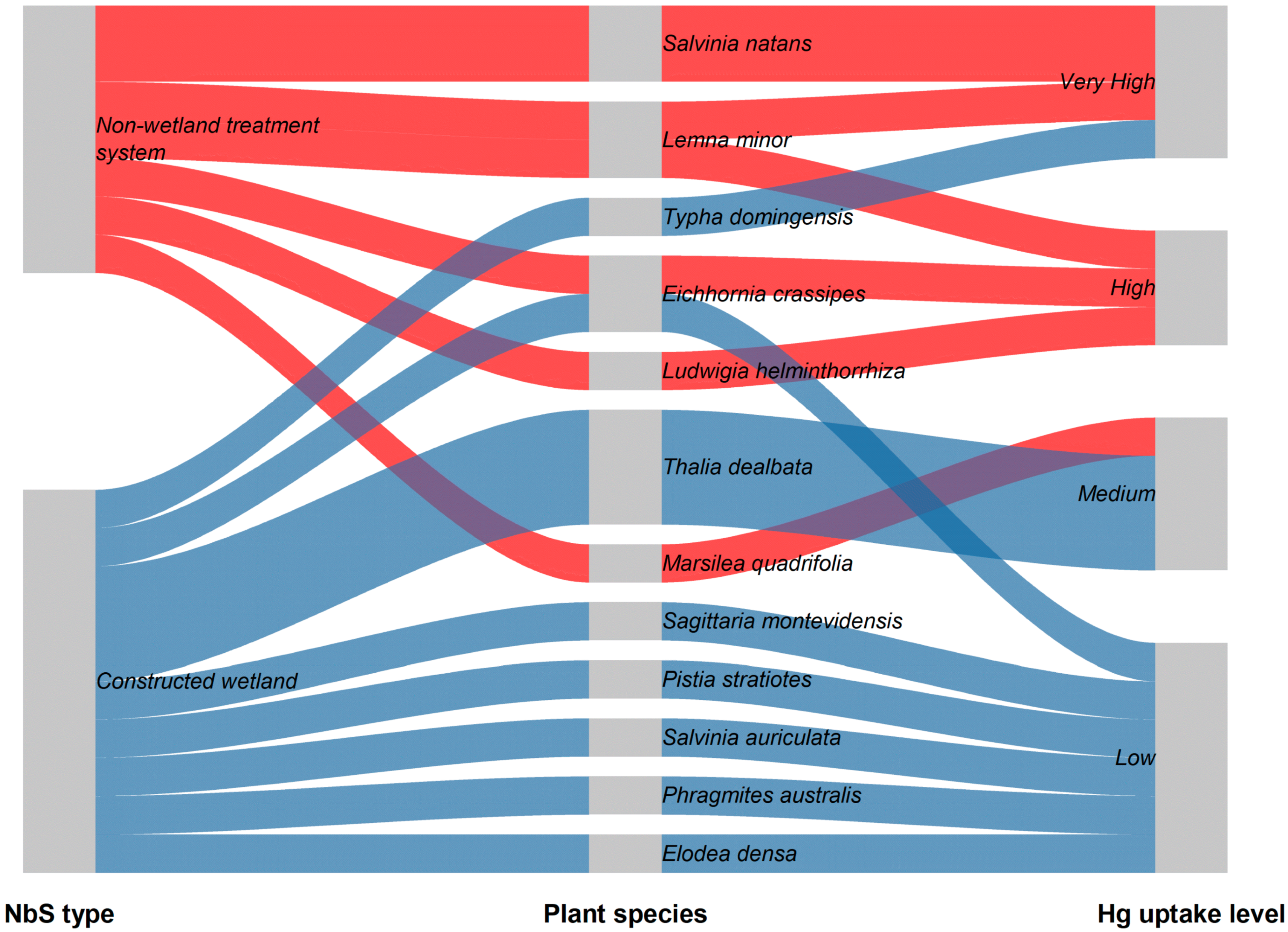
| NbS Type | THg Removal Efficiency | MeHg Change | Statistical Significance | Reference |
|---|---|---|---|---|
| Free water surface flow constructed wetland | 86.3 | Not reported | Not reported | [44] |
| Free water surface flow constructed wetland with amendments | 98.9 | Decreased | p < 0.01 | [38] |
| Constructed wetland mesocosm | 50 | Increased | p = 0.0001 | [40] |
| Free water surface flow constructed wetland | 91.8 | Not reported | Not reported | [45] |
| Biochar cells | 63 | Increased | p < 0.05 | [41] |
| Biochar mesocosms | 92 | Decreased | p < 0.05 | [39] |
| Floating wetland | 78 | Not reported | Not reported | [46] |
| NbS Type | Plant Species | Removal Efficiency | Reference |
|---|---|---|---|
| Constructed wetland | T. latifolia, T. angustifolia, T. domingensis | 14% removal of THg; 11% increase in TMeHg | [40] |
| Pilot-scale constructed wetland | Limnocharis flava | 90% | [44] |
| Constructed wetland with pyrrhotite and zero-valent iron | Acorus calamus | 98.9% | [38] |
| Free water surface constructed wetland | Aquarius palifolius | 91.8% | [45] |
| Pilot-scale constructed wetland treatment cell | Scirpus californicus; Potamogeton pusillus | ~50% THg removal; 8% increase in TMeHg | [41] |
| NbS Type | Relative Capital Costs | Hg Removal Efficiency | Advantages | Drawbacks/Risks |
|---|---|---|---|---|
| Constructed wetland | 50–120 (installation) [68] | 30–98% |
|
|
| Bioretention cells | 100–200 (installation) [69] | 24–78% |
|
|
| Biochar-amended soils | 300–500 (per ton, production cost) [70,71] | 57–95% |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robles, M.E.; Oh, Y.; Haque, M.T.; Jeon, M.; Kim, L.-H. Soil Mercury Pollution in Nature-Based Solutions Across Various Land Uses: A Review of Trends, Treatment Outcomes, and Future Directions. Appl. Sci. 2025, 15, 6502. https://doi.org/10.3390/app15126502
Robles ME, Oh Y, Haque MT, Jeon M, Kim L-H. Soil Mercury Pollution in Nature-Based Solutions Across Various Land Uses: A Review of Trends, Treatment Outcomes, and Future Directions. Applied Sciences. 2025; 15(12):6502. https://doi.org/10.3390/app15126502
Chicago/Turabian StyleRobles, Miguel Enrico, Yugyeong Oh, Md Tashdedul Haque, Minsu Jeon, and Lee-Hyung Kim. 2025. "Soil Mercury Pollution in Nature-Based Solutions Across Various Land Uses: A Review of Trends, Treatment Outcomes, and Future Directions" Applied Sciences 15, no. 12: 6502. https://doi.org/10.3390/app15126502
APA StyleRobles, M. E., Oh, Y., Haque, M. T., Jeon, M., & Kim, L.-H. (2025). Soil Mercury Pollution in Nature-Based Solutions Across Various Land Uses: A Review of Trends, Treatment Outcomes, and Future Directions. Applied Sciences, 15(12), 6502. https://doi.org/10.3390/app15126502






