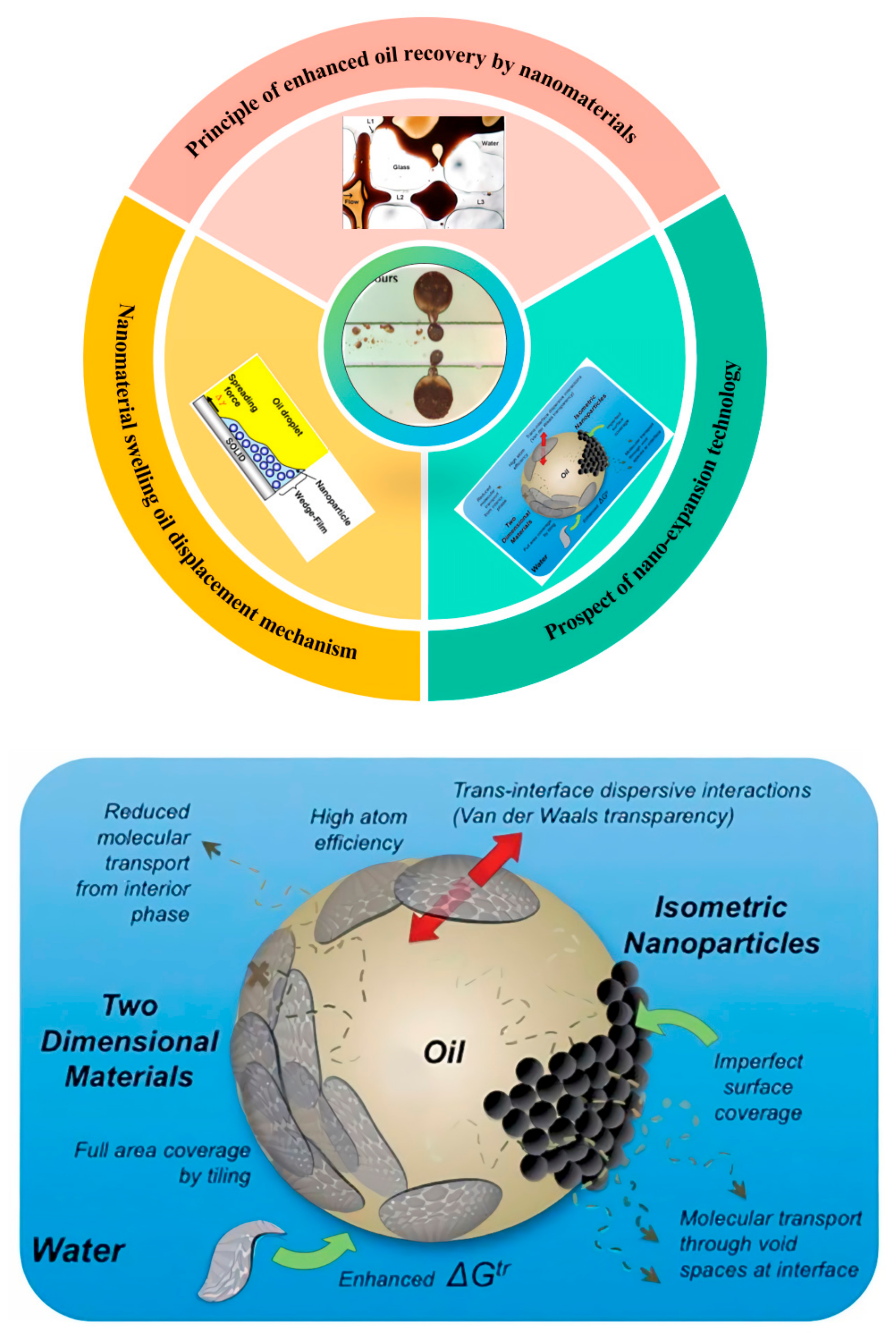
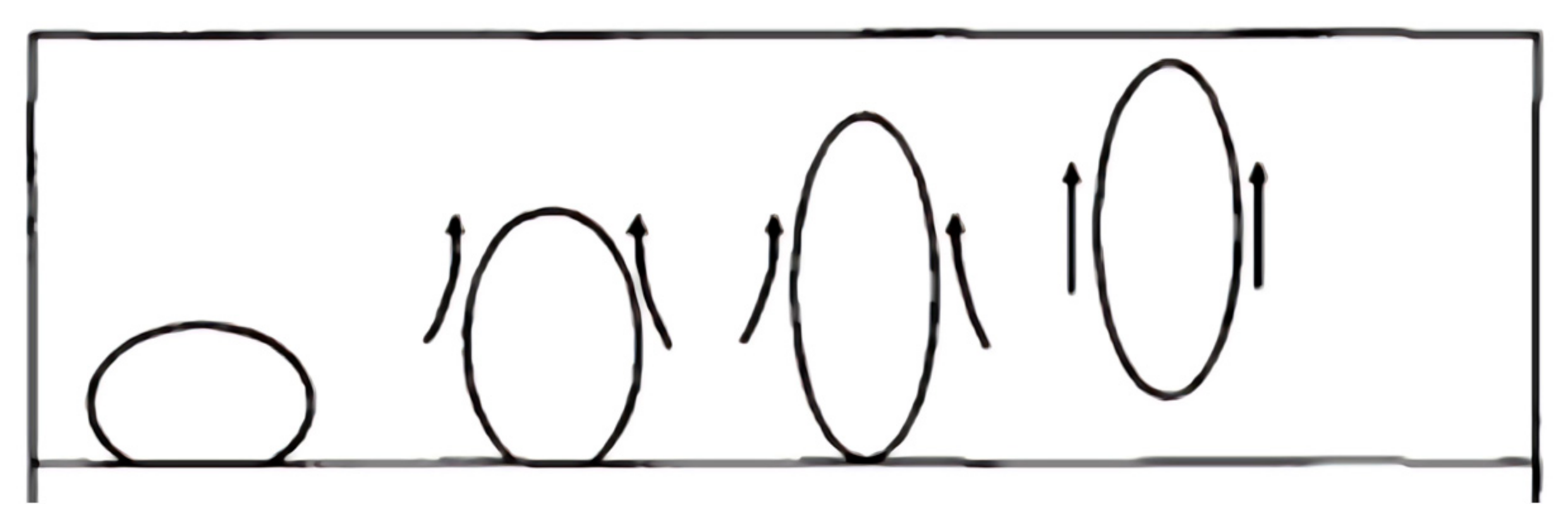1. Introduction
Nanotechnology focuses on the innovative applications of solid materials from 1 to 100 nanometers in the field of engineering [
1]. Since the beginning of the 21st century, this technology has gradually penetrated into the fields of medicine, food processing, electronic devices, and oil and gas development. In petroleum engineering operations, nanomaterials have enabled multifaceted integrated solutions, spanning wellbore cementation enhancement, drilling fluid rheology optimization, and chemical conformance control improvement [
2,
3]. It is worth noting that nanomaterials can greatly enhance the recovery efficiency in tertiary oil recovery processes through mechanisms such as reducing interfacial tension and altering rock wettability [
4]. Therefore, nanomaterials have become a research focus in the oil extraction industry [
5].
The application of nanofluids in the field of enhanced oil recovery (EOR) has undergone a comprehensive process, spanning from fundamental mechanism exploration to field validation. In 2013, Cheraghian et al. [
6] systematically investigated the role of nanoparticles in EOR. Their findings revealed that nanoparticles can significantly enhance oil recovery by altering rock wettability, reducing interfacial tension (IFT), and increasing fluid viscosity. This study established the theoretical foundation for nanofluid application in EOR. In 2018, Li et al. [
7], further validated that nanofluids improve oil recovery through the synergistic effects of IFT reduction and viscosity enhancement. That same year, Agista et al. [
8], building on previous research, highlighted that nanoparticle size, concentration, and surface properties also influence the effectiveness of oil displacement. Concurrently, Tong et al. [
9], through more in-depth research, elucidated multiple mechanisms by which nanofluids enhance oil recovery, including the generation of structural disjoining pressure and improvement of the mobility ratio. In 2019, Gbadamosi et al. [
10] indicated that nanomaterials like SiO
2 can stabilize fluid stability and improve flow characteristics through mechanisms such as adsorption and precipitation. Eltoum et al. [
11], in their 2020 review, examined the application of SiO
2 and ZnO nanoparticles in altering rock wettability and reducing IFT, emphasizing the crucial role of surface functionalization in nanofluid stability. In 2022, Al-Yaari et al. [
12] experimentally validated that SiO
2 and Al
2O
3 nanofluids significantly enhance oil recovery within porous media. They also noted that the thermal conductivity properties of these nanofluids can accelerate energy transfer. Dahham et al. [
13], in 2023, explored the potential of ionically stabilized nanofluids for use in heavy oil reservoirs. Wang et al. [
14] emphasized the application prospects of nanocomposites under complex geological conditions. The application of nanofluids in EOR has progressed beyond the initial exploration phase, with mechanisms becoming well-defined and technologies optimized. Subsequent research has continued to refine these applications. Extensive studies have demonstrated that certain nanomaterials exhibit excellent oil displacement performance while also demonstrating cost-effectiveness.
The content of impurities in silica is extremely low and the density is low, which makes it easier to disperse in the oil displacement process and means that it has less impact on the environment [
15]. At the same time, its preparation cost is relatively low, suitable for large-scale applications [
16]. Research has demonstrated that SiO
2 nanoparticles can attenuate interphase tension, generate multiphase emulsions, and induce wettability alteration, thereby substantially enhancing hydrocarbon displacement efficiency. For enhanced oil recovery (EOR) applications, Wu et al. [
17] developed a novel silica-based amphiphilic Janus nanofluid system. At a concentration of 100 mg/L, the incremental oil recovery factor reached 5.74%. Similarly, Yin et al. [
18] reported that carboxyl/alkyl composite silica hydrophilic Janus nanosheets achieved an 18.31% improvement in sweep efficiency. Lan et al. [
19] engineered water-in-oil (W/O) and oil-in-water (O/W) emulsion systems by tuning the hydrophilic–hydrophobic domain ratio of Janus particles. However, limited studies exist on Janus nanoparticle-stabilized O/W/O triphase Pickering emulsions and their EOR applications. Concurrently, mechanistic insights into silica nanoparticle-driven conformance control and capillary-driven oil mobilization remain underexplored.
Expansion oil recovery technology involves injecting nanomaterials into reservoirs to improve oil recovery efficiency. Due to the influence of nanomaterials, the expanded crude oil is more easily stripped and migrated in the pores. The application of nanomaterials provides possibilities for the further exploitation of remaining oil in low-permeability reservoirs. Current challenges in low-permeability reservoir development include escalating injector wellhead pressures and declining fluid intake capacities; producer wells exhibit suboptimal performance, significant reservoir pressure depletion, and acute productivity decline, leading to diminished waterflood rates, hydrocarbon yields, production velocities, and recovery factors [
20,
21,
22]. Concurrently, severe fracture-dominated water channeling and coning occur, while injectors distal to fracture networks demonstrate negligible sweep efficiency—summarized as “inaccessibility to injection, unviable production, and ineffective conformance control” [
23,
24,
25]. Throughout reservoir management evolution, a diversified innovation framework has emerged, integrating techniques such as microbial enhanced oil recovery (MEOR), polymer conformance flooding, composite chemical EOR, gas miscible injection, thermal stimulation [
26,
27], and swelling-based displacement technologies. After decades of field trials and refinements, these methods have gained broad acceptance across upstream operators, significantly elevating ultimate recovery rates. The expansion oil displacement technology injects special materials into the reservoir. Due to the influence of special materials, the expansion of crude oil is more likely to fall off from the rock wall or dead pores, and the material itself causes little harm to the environment [
28,
29].
As shown in
Figure 1, this paper summarizes the research results of nano-oil displacement technology in recent years from three aspects: the principle of using nanomaterials to enhance oil recovery, the mechanism of nanomaterial expansion and oil displacement, and the future research prospects of nanotechnology. The mechanism of nano-oil displacement technology to improve oil recovery is mainly introduced, and the related experimental research is deeply analyzed. At the same time, the mechanism of enhanced expansion of nano-silica oil droplets is deeply studied. The application status of nanomaterials in complex reservoirs is summarized, and its development trend is prospected. This study provides an important reference for the practical application and future development of nano-expansion profile control technology.
2. Principle of Enhanced Oil Recovery by Nanomaterials
In recent years, nanomaterials have been widely used in the field of oil exploitation to improve its efficiency. The main mechanisms whereby nanomaterials improve oil recovery include reducing interfacial tension [
31], changing wettability, and reducing crude oil viscosity [
32]. Considering the relationship between different mechanisms is the key factor to improving oil recovery. Adding surfactants to an oil displacement agent can effectively improve the wettability of the rock surface, thereby enhancing the emulsification. Reduction in interfacial tension is an important factor in improving oil recovery [
33]. A reduction in interfacial tension can improve the flow of crude oil in the pore throat so that oil droplets can pass through small pores more smoothly, thereby improving the oil recovery efficiency [
34]. Wettability is one of the important parameters in improving oil recovery. Changing the wettability can help the oil displacement fluid peel the crude oil from the rock gap to improve the oil recovery rate [
35].
2.1. Reduce Oil–Water Interfacial Tension
Water flooding will leave residual oil in pores, which will exist in the center of the pores in the form of oil droplets [
36].
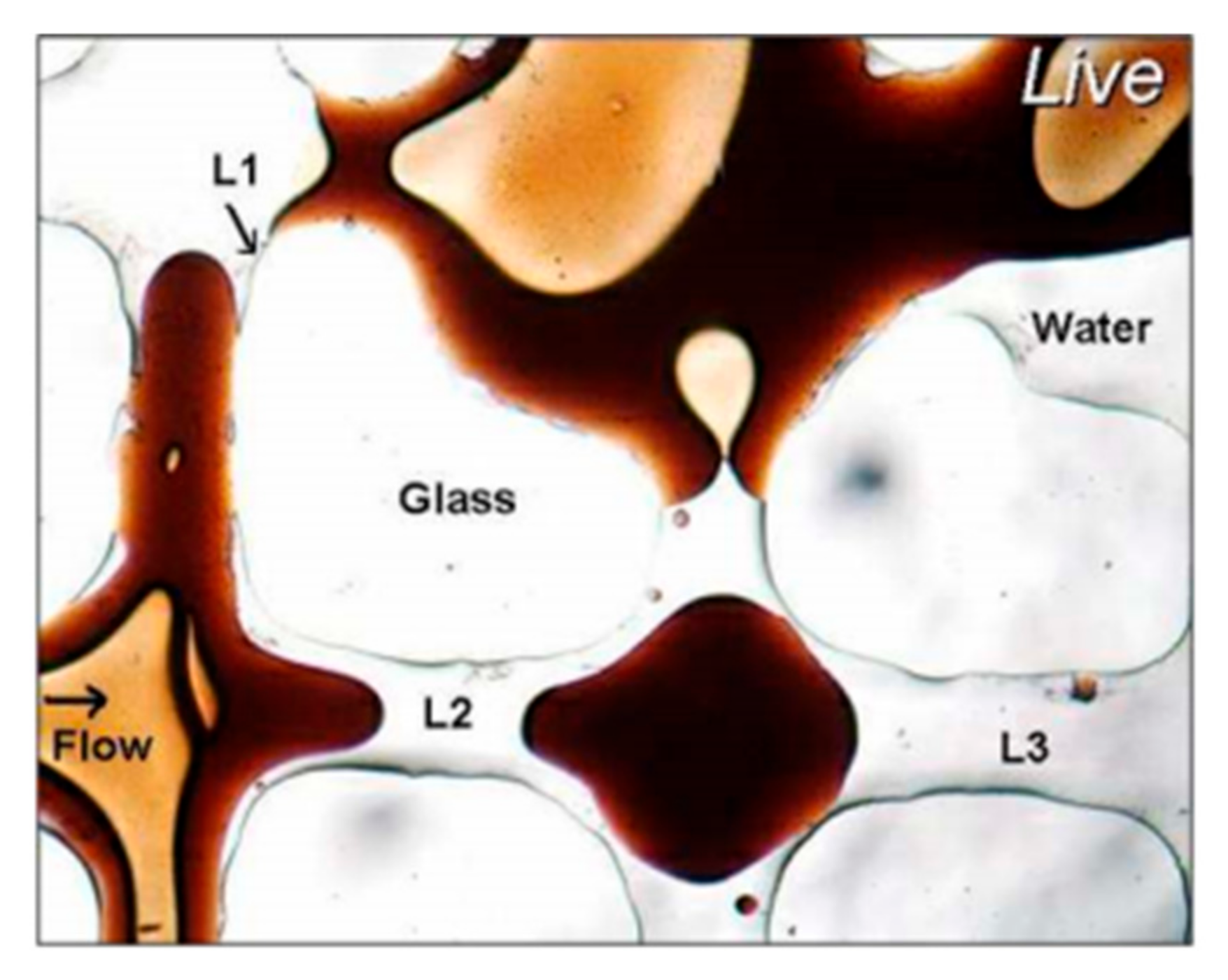
Figure 2 is a schematic diagram of oil droplets in a pore throat. The state of oil droplets in the pore throat is not the same under high oil–water interfacial tension and low oil–water interfacial tension. When the oil–water interfacial tension is high, the oil droplets cannot be retained through the pore throat; when the oil–water interfacial tension is low, the oil droplets deform and pass through the pore throat [
37]. As one of the core mechanisms of enhanced oil recovery, the attenuation of oil–water interphase tension will lead to a significant increase in the value of capillary force. This physical response mechanism lays a theoretical foundation for the optimization of oil displacement efficiency.
Gao et al. [
38] studied the effect of nanoparticles on interfacial tension through core displacement experiments. The experimental results show that when the mass fraction of Al
2O
3 nanofluids increases from 0.02% to 0.1%, the interfacial tension between crude oil and nanofluids decreases from 2.25 mN/m to 1.5 mN/m, and the interfacial tension decreases by 33.33%. Under the same experimental conditions, when the mass fraction of SiO2 nanofluids increases from 0.02% to 0.1%, the interfacial tension between crude oil and nanofluids decreases from 2.15 mN/m to 1.25 mN/m, and the interfacial tension decreases by 41.86%. The authors believe that nanofluids can reduce the interfacial tension, and the reduction in interfacial tension will also make oil droplets more prone to deformation, which further reduces the difficulty of their movement in pores. The viscous resistance of the pores to the oil droplets is reduced, making it easier for oil to flow for exploitation.
Raffizal et al. [
39] experimentally investigated the effect of aluminum nanoparticles coated with polyvinylpyrrolidone (PVP) on oil–water interfacial tension (IFT) in low-porosity sandstone and the enhanced oil recovery mechanism. It was found that when the injection volume was 0.52 pore volume (PV), the nanoparticles could reduce the oil–water interfacial tension from 92.68 N/m to 61.86 N/m and improve the fluidity. Nanomaterials enhance the migration ability of oil phases in porous media by destroying the adhesion between oil droplets and rock surfaces. This nanofluid system shows better oil displacement efficiency than traditional chemical flooding while maintaining lower injection costs, providing a new solution for tertiary oil recovery in high-salt reservoirs.
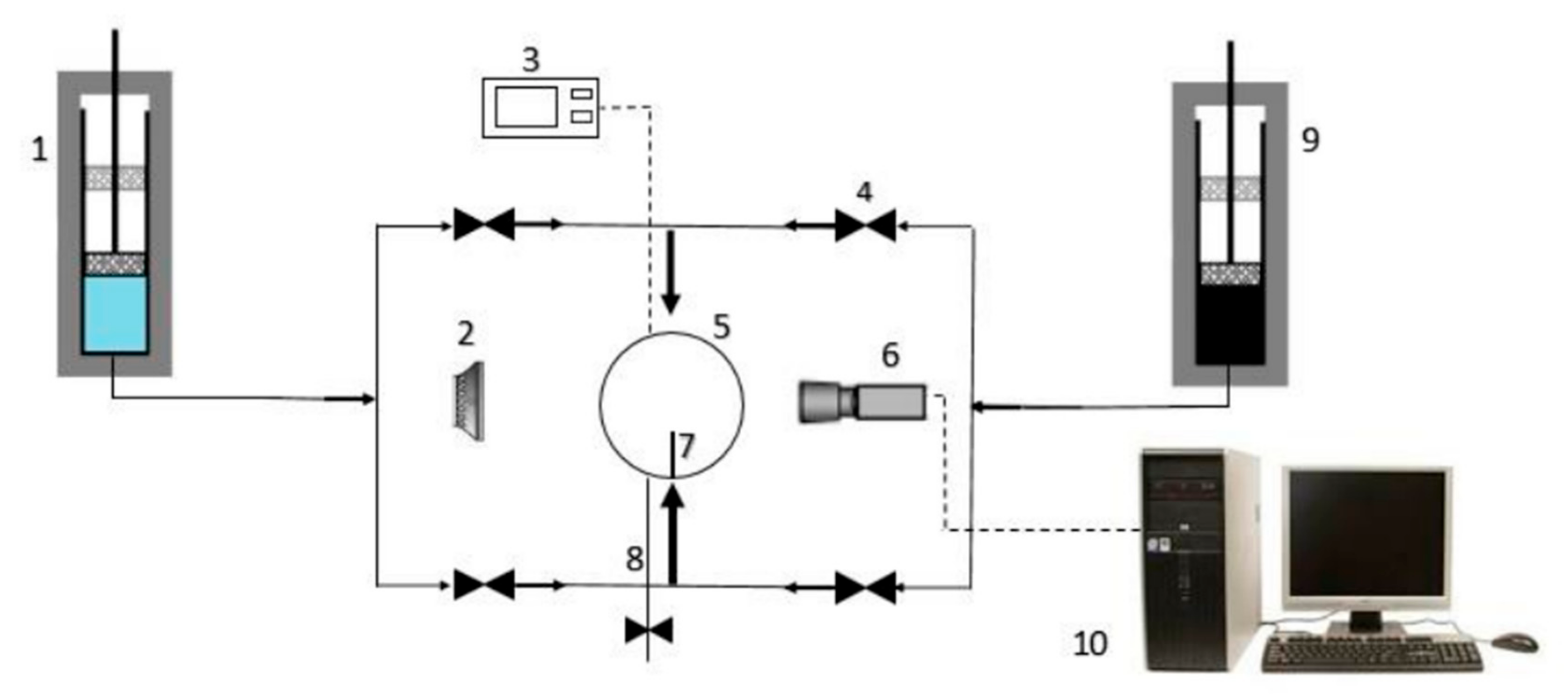
Manshad et al. [
40] used a high-pressure and high-temperature drop interfacial tension measuring instrument to measure the interfacial tension of oil droplets. A schematic diagram is shown in
Figure 3. Based on the integrated analysis of multi-source experimental data, this study systematically explored the mechanism of action between interfacial tension attenuation and enhanced oil recovery and finally reached the following key conclusions: Interfacial tension is an important index to evaluate the energy stability of water–oil two-phase interface separation. When the interfacial tension is at a low level, the two-phase interface is easier to separate. In addition, the authors also found that the addition of effective surfactants can make the two phases mix to form an emulsion phase, which further reduces the interfacial tension. That is to say, although a single nanomaterial can reduce the surface tension, its effect is not very significant. Therefore, nanomaterials and surfactants can be synergistically compounded to significantly reduce the interfacial tension between oil and water phases and effectively improve oil recovery.
The experiment of Liu et al. [
21] supports this view. Studies demonstrate that silica nanoparticles (ATNPs) and the nonionic surfactant lauroyl monoethanolamide (LMEA) co-assemble at the oil–water interface via hydrogen bonding to construct an elastic adsorbed layer. This stabilizes the formation of a thermodynamically unstable but kinetically stable Pickering emulsion, achieving in situ mobility control over a wide water–oil ratio (WOR) range. The surfactant LMEA preferentially adsorbs at the oil–water interface, reducing interfacial tension to the magnitude of 10
−2 mN/m. ATNPs enhance the strength of the interfacial film through a physical barrier, inhibiting emulsion coalescence and Ostwald ripening. Their synergy enables the emulsion viscosity to self-adjust with WOR, maintaining a value higher than the crude oil viscosity. Furthermore, ATNPs and LMEA synergistically modify the rock surface through physical adsorption, transforming oil-wet rock to weakly water-wet. This reduces oil film adsorption energy, enhances the capillary number, and synergistically displaces residual oil via the “Jamin effect” and interfacial effects.
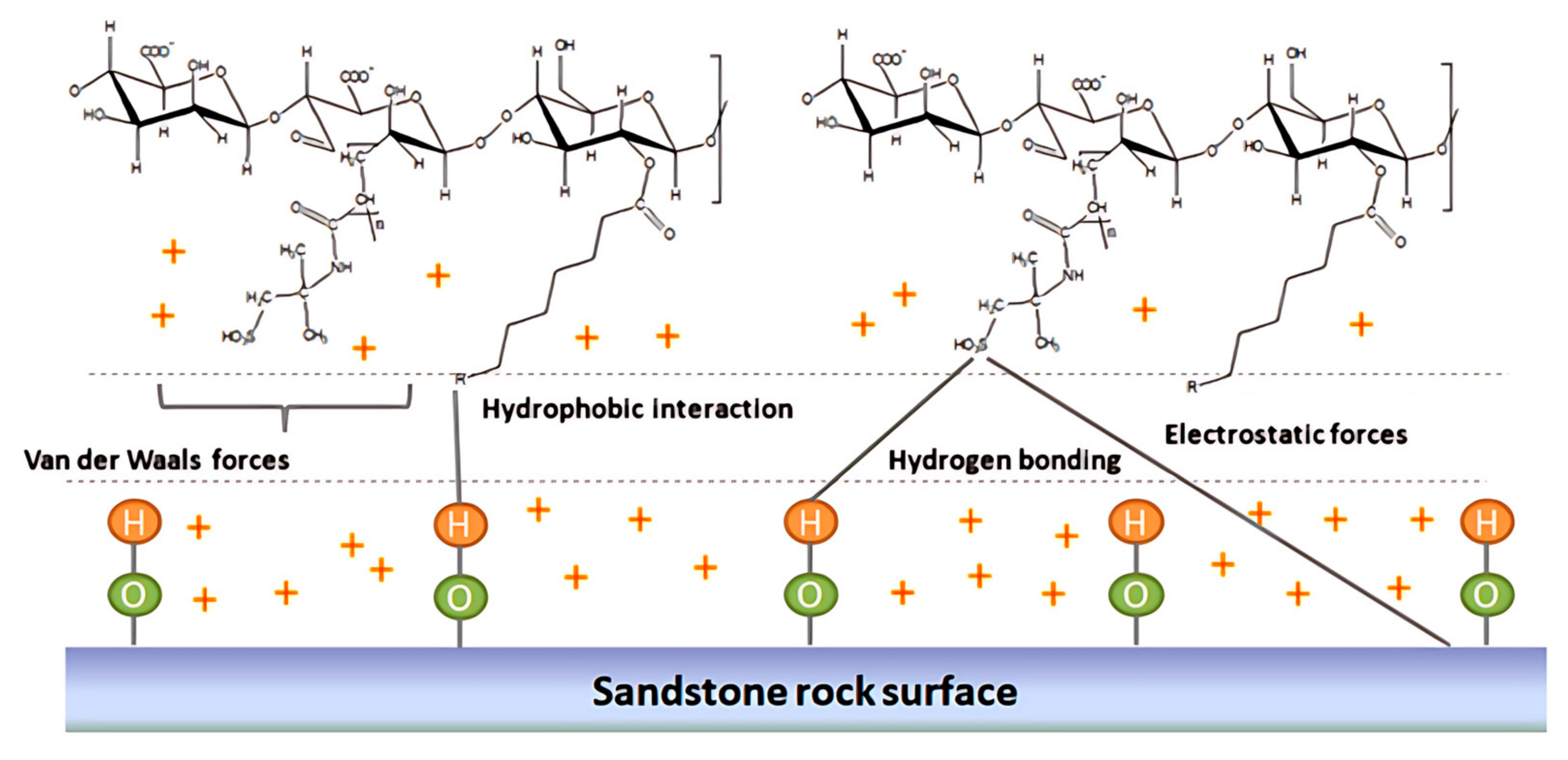
Essentially, nanoparticles form a nanolayer by adsorbing at the oil–water interface. This nanolayer replaces the pure oil–water interface, alters the properties of the oil–water interface, and thus reduces the interfacial tension between oil and water [
41]. When surfactants are added to the nanofluid, the ability of the nanofluid to reduce the interfacial tension between oil and water is further enhanced. Sharma et al. [
42] focused on the performance of silica nanofluid with and without sodium dodecyl sulfate (SDS). By measuring the interfacial tension (IFT) between the fluid and paraffin-based crude oil, it was found that the nanofluid containing SDS had a stronger ability to reduce IFT compared with the single nanofluid, with a reduction of 43.02%. This study shows that nanofluids containing surfactants have more promising application potential in the field of enhanced oil recovery.
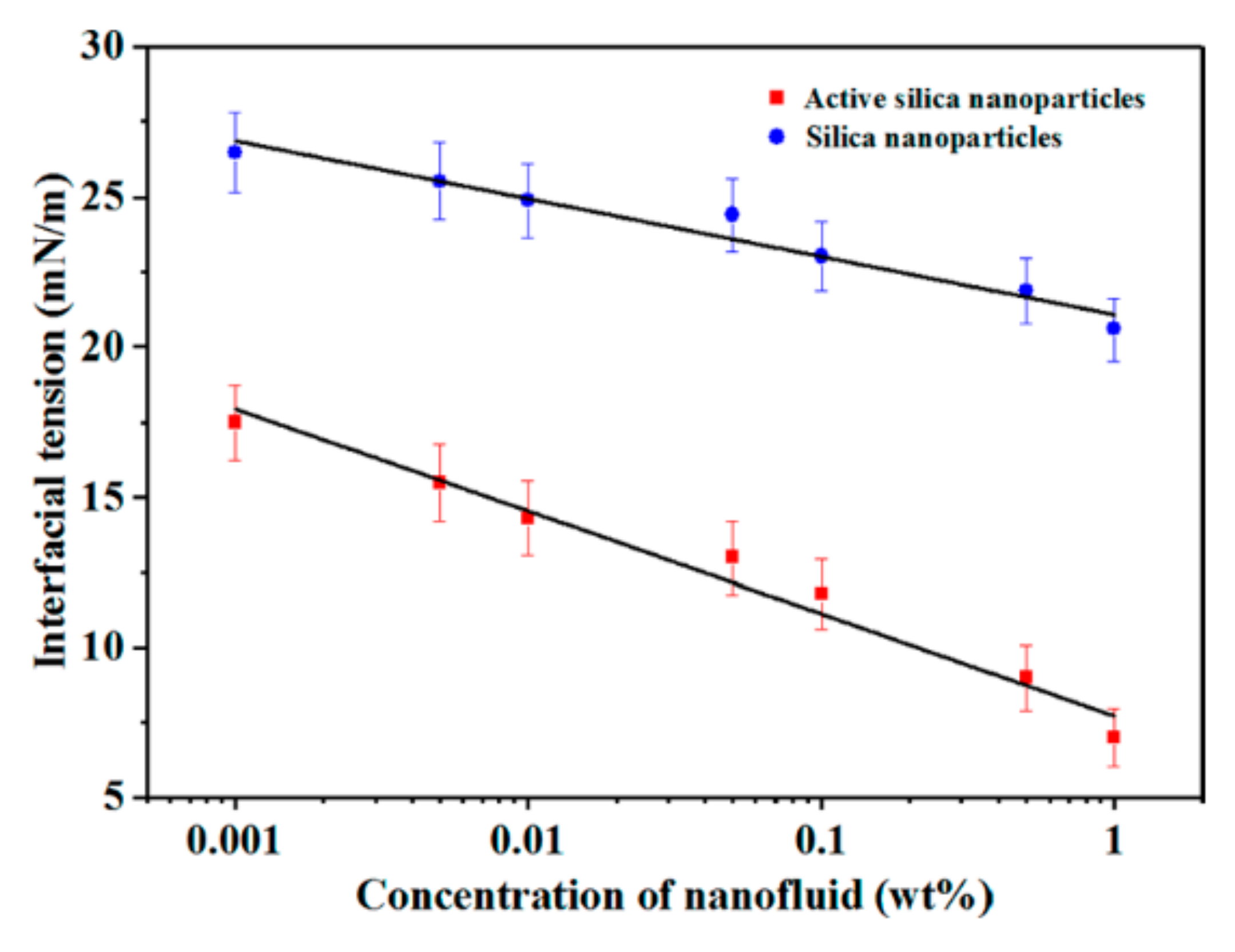
Li et al. [
42] formed water-soluble active silica nanoparticles by condensation of adipic acid with hydroxyl groups of silica particles, the introduction of carboxyl groups on the surface of the particles, and neutralization of carboxylates by NaOH. The authors studied the physical and chemical properties of active silica nanoparticles by transmission electron microscopy and Fourier transform infrared spectroscopy. The interfacial activity was confirmed by the determination of interfacial tension and interfacial expansion moduli. As shown in
Figure 4 below, the authors demonstrated the influence of silica nanofluids and active silica nanofluids at different concentrations on the oil–water interfacial tension. The oil–water interfacial tension was measured to be 26.8 mN/m at 60 °C. When 1 wt% silica nanofluid was added, the interfacial tension decreased to 20.6 mN/m, with a decrease of about 23.13%. When 1 wt% active silica nanofluid was added, the interfacial tension was greatly reduced to 7.0 mN/m, a decrease of 73.8%. This shows that the surface modification of active nanoparticles gives them higher interfacial adsorption efficiency, and the interfacial tension can be reduced more effectively at the same concentration. It can be seen that nanofluids can reduce interfacial tension, and active nanofluids have a more significant effect in terms of reducing interfacial tension.
Luo et al. [
44] proposed an ultralow-concentration nanofluid based on graphene-based amphiphilic Janus nanosheets to improve the efficiency of secondary oil recovery through the interface regulation mechanism. Experiments show that at a concentration of 0.005 wt%, the amphiphilic structure formed by surface functionalization of the nanofluid can self-assemble to form an elastic film at the oil–water interface, reducing the contact angle of the oil droplets from 150° to 79° and significantly reducing the interfacial tension to 1.0 mN/m. The unique two-dimensional nanostructure not only maintains fluidity in the rock pores but also promotes the stripping of crude oil from the rock surface through the synergistic effect of wettability reversal and interfacial film strengthening. Compared with traditional tertiary oil recovery, this method can achieve 7.5% recovery improvement in the secondary oil recovery stage, and it has the advantages of reducing the amount of chemical agent required, reducing water consumption and increasing production rate.
Tian et al. [
45] synthesized a modified Janus nano-calcium carbonate (JNC-12) with a particle size of 30–150 nm and prepared an in situ-emulsified nanofluid (ISNE). Experimental studies have shown that the addition of ISNE can reduce the oil–water interfacial tension to 0.01–0.1 mN/m. The verification results of core displacement experiments show that compared with the initial water flooding oil recovery rate, the oil recovery rate is increased by 17.6% after adding ISNE.
It can be seen from
Table 1 that under the same experimental conditions, the effects of different mass fractions of nanofluids on surface tension are different, and their ability to reduce interfacial tension will increase with the increase in the fluid mass fraction within a certain range. Although the experimental conditions used by different scholars were different, the data obtained show a certain similarity. Specifically, a single nanofluid can usually maintain a level of about 30% in reducing interfacial tension, while it is roughly maintained at about 10% in improving oil recovery.
2.2. Improve Rock Wettability
The wettability of the reservoir rock wall plays a leading role in the flow and distribution of fluid in a reservoir and is the key factor to improve oil recovery [
46]. As its core physical property, the wetting property of a material is mainly controlled by the synergistic regulation of its micro-morphology and chemical composition [
47]. Hu et al. [
48] pointed out in their study of high permeability core flooding that SiO
2 nanoparticles can form a wedge structure to generate structural separation pressure and promote the diffusion of crude oil. At the same time, its adsorption on the rock surface makes the wettability change from oil-wet to strong water-wet, so that the contact angle decreases from 110° to 18°, reducing the adsorption of crude oil. The synergistic effect of the two enhances oil recovery.
Karimi et al. [
32] verified the adsorption of ZrO
2 nanoparticles on rocks and the formation of nanostructures on rock surfaces. The surfactant molecules in the nano-oil displacement agent are adsorbed on the wet surface of the rock oil and are finally combined with the pollutant molecules in the crude oil to peel it off. By removing the contaminated layer on the rock surface, the original water-wet rock surface is exposed to achieve wettability transformation [
49]. Ju et al. [
50] showed through experimental research that polycrystalline silicon nanomaterials can effectively regulate the surface characteristics of porous media through interface modification, induce the wetting behavior of rock to change from a hydrophobic state to a hydrophilic state, and then significantly improve the relative permeability characteristics and water injection recovery efficiency [
51]. Rostami et al. [
52]. simulated the penetration process of nanofluids through a microfluidic chip and found that the time-dependent wettability alteration effects exhibited nonlinear characteristics.
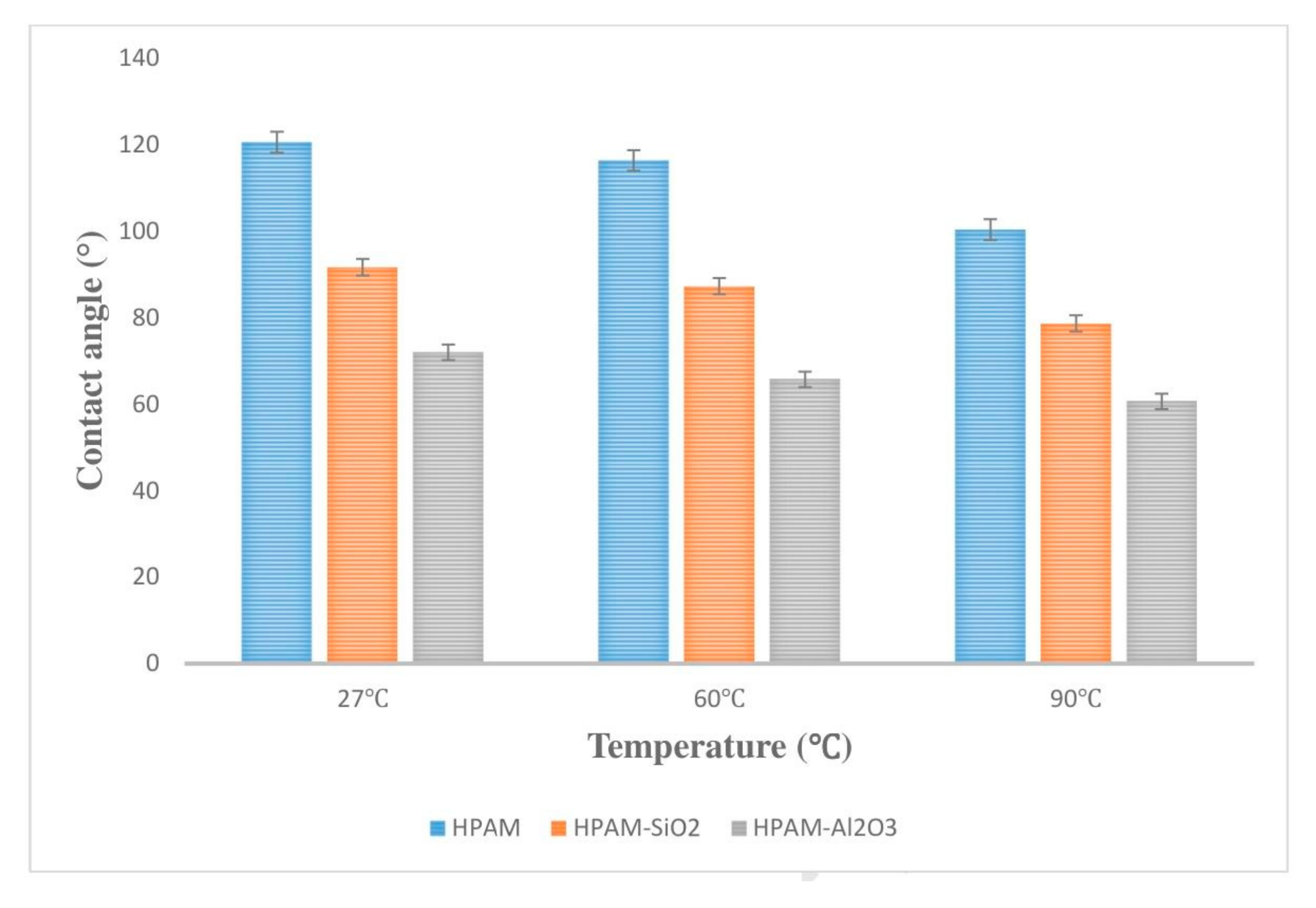
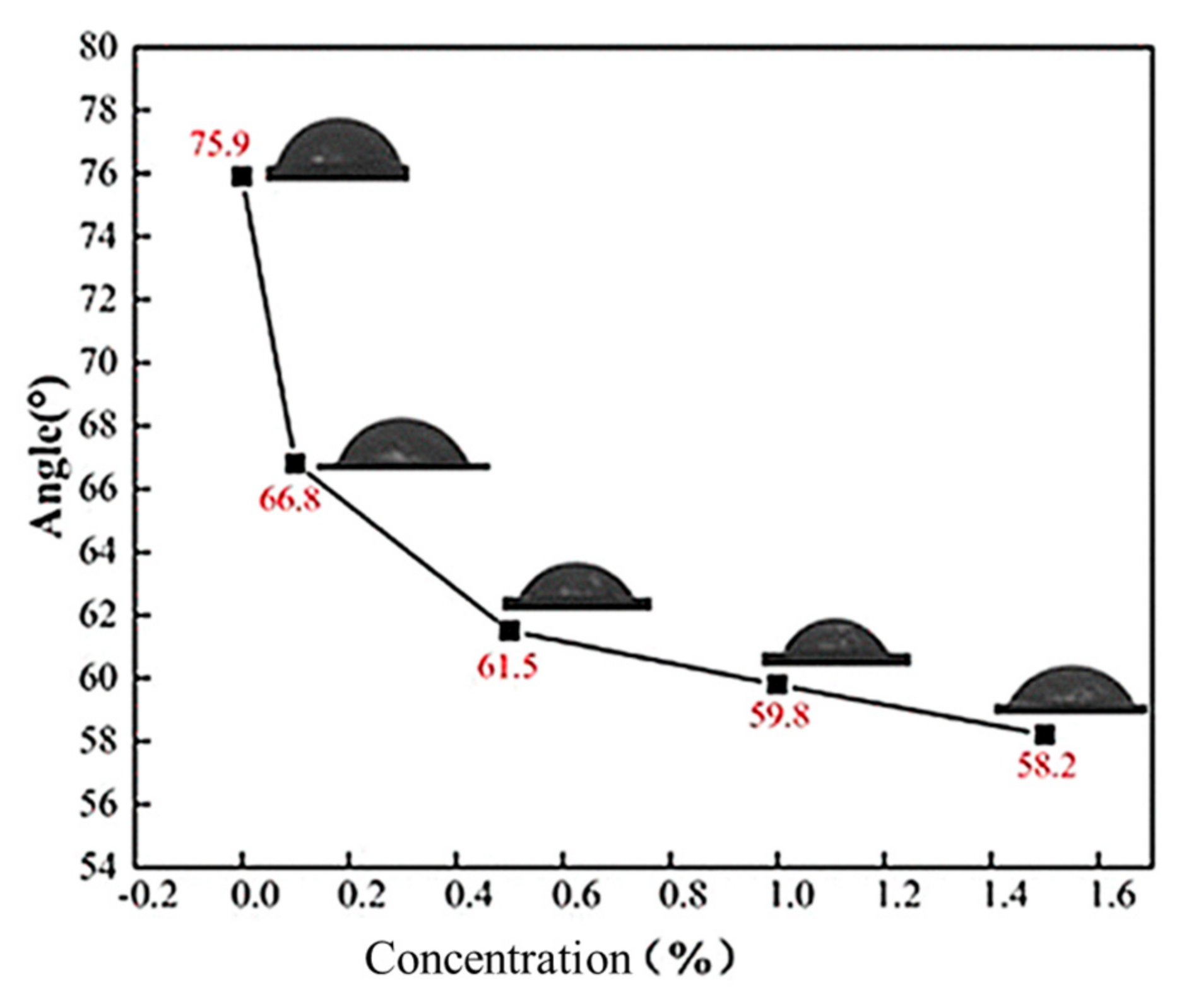
Gbadamosi et al. [
53] studied the effect of Al
2O
3 polymer nanofluids on the wettability of sandstone cores by contact angle measurement. Firstly, the sandstone core was treated as being in an oil-wet state, and the contact angle was 144.7°. Polyacrylamide (HPAM), SiO
2, and Al
2O
3 nanofluids were treated at 27–90 °C. The experimental results in
Figure 5 show that the contact angle decreases to 100.3°, that of SiO
2 nanofluids to 78.6°, and that of Al
2O
3 nanofluids to 60.6° after HPAM treatment at 90 °C. At this time, the wettability of the core is converted from oil-wet to water-wet, and the change in wettability is attributed to the adsorption of nanoparticles on the core surface. Nanoparticles replace the oil film by structural separation pressure and hydrogen bonding, and the increase in temperature can enhance the adhesion efficiency between the particles and the core surface.
Bayat et al. [
54] also reported that differences in capillary force will affect wettability and that measurement of the contact angle can determine the wettability of rock. The contact angle of limestone was measured to be about 90° at 26 °C, and the θ values in the presence of Al
2O
3, TiO
2, and SiO
2 nanofluids were 71°, 57°, and 26°, respectively. The recovery rates of Al
2O
3, TiO
2, and SiO
2 nanofluids were 52.6%, 50.9%, and 48.7%, respectively. The contact angles of Al
2O
3, TiO
2, and SiO
2 decrease with the increase in temperature. We can find that although SiO
2 has a higher adsorption capacity on limestone surfaces than Al
2O
3 and TiO
2 nanofluids (a strong adsorption capacity means a smaller contact angle), the effect of enhanced oil recovery is inferior to that of Al
2O
3 and TiO
2 nanofluids, that is to say, the oil displacement effect of nanofluids in strong hydrophilic reservoirs is not as good as that in neutral wetting reservoirs. This phenomenon underscores the critical relationship between contact angle and capillary forces: a reduction in contact angle directly lowers the capillary entry pressure according to the Young–Laplace equation. However, excessively low contact angles (e.g., <30°) in strongly water-wet systems may exacerbate water channeling and bypass residual oil, whereas neutral wettability (θ ≈ 90°) is minimized, maximizing oil droplet mobilization efficiency. Thus, the optimal contact angle range observed in Al
2O
3/TiO
2 nanofluids directly correlates with higher recovery rates by balancing capillary-driven displacement and fluid mobility control.
Ogolo et al.’s [
55] research can further illustrate this view. Studies have shown that some strategies to convert the wettability of rock from water to neutral can reduce the capillary force [
56] and significantly improve the recovery rate. Hydrophilic SiO
2 fluid can change sandstone from water-wet to strongly water-wet, and the contact angle decreases from 36° to 10°, which cannot improve oil recovery. However, the hydrophobic SiO
2 nanofluid can convert the wettability of the rock into neutral wettability, that is, the contact angle is about 90°, and the oil recovery rate is significantly improved. Therefore, we can conclude that the change in rock wettability will significantly affect oil recovery, and wettability can be used as an important parameter to improve oil recovery.
In summary, this section summarizes the changes in the contact angle, wettability, and recovery rate of rock treated by nanofluids, as shown in
Table 2 below. Nanofluids change the surface wettability of rock by spreading and adsorbing on the surface of a reservoir rock mass. The wettability of rock can be expressed by measuring the contact angle. A small contact angle means stronger wettability. A large number of studies have shown from the perspective of physical bases that neutral and wet rock surface conditions can reduce the increase in surface water film thickness, thereby effectively reducing the impact of pore throat channel stenosis on crude oil extraction. The wettability can be used as one of the important criteria to improve the recovery rate of crude oil. The study of this parameter is helpful in improving the recovery efficiency of crude oil in practical engineering.
2.3. Lower Oil Viscosity
Regulating the rheological properties of crude oil is one of the core mechanisms of enhanced oil recovery [
57]. The mechanism is the spatial dispersion of nanoparticles, which can effectively inhibit the formation of macromolecular viscoelastic networks, thereby significantly weakening the fluid flow resistance [
58]. Li et al. [
59] pointed out that the hydroxyl groups in nanoparticles such as silicone interact with the polar groups in asphaltenes, destroy the hydrogen bonds between molecules, reduce the size of aggregates, and thus reduce the viscosity of heavy oil. In addition, metal nanoparticles (such as Fe) can catalyze the cleavage of chemical bonds in heavy oil aggregates at high temperatures, which promotes the cracking of macromolecules and further reduces the viscosity.
Taborda et al. [
60] studied the effects of SiO
2 nanoparticles, Al
2O
3 nanoparticles, and acidic SiO
2 nanoparticles on the viscosity of heavy oil by adsorption and aggregation experiments on n-C7 asphaltene. Through experimental research, they found that acidic SiO
2 can reduce the viscosity of heavy oil well, and there is a relationship between the viscosity reduction (DVR) of heavy oil and the addition of nanofluids, as shown in the Equation below, where
and
represent the viscosity before and after adding nanoparticles, respectively.
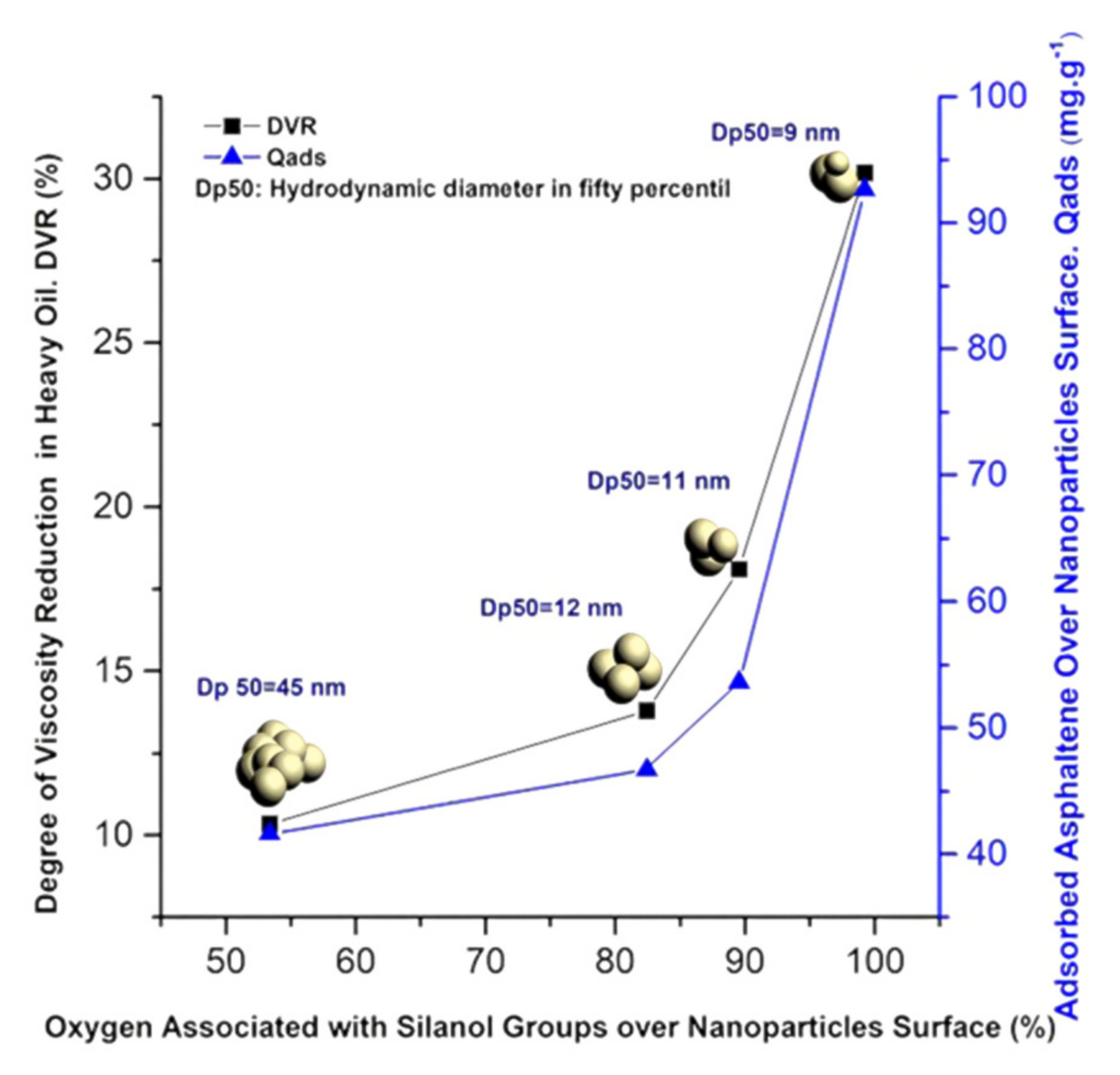
Although this relationship was not fully confirmed in this study, the research of Montes et al. [
61] provides strong support for this view. They successfully prepared four kinds of silica nanofluids by combining silane precursors with them. The hydrodynamic diameters of these fluids were measured to be between 9 and 45 nm. In subsequent experiments, these four nanofluids were injected into heavy oil for simulation experiments. The results reveal that the number of siloxane and silanol groups on the surface of SiO
2 nanoparticles has a significant positive correlation with the adsorption capacity of asphaltene and DVR (residual oil saturation reduction rate), which is visually demonstrated in
Figure 6. It can be clearly seen from the diagram that the maximum value of DVR reaches 30%.
It can be concluded that the interaction between the active silicon hydroxyl group in the nano-silica fluid and asphaltene is an important factor leading to the decrease in oil viscosity, and the degree of adsorption of the nanoparticles to asphaltene is an important manifestation of their ability to reduce the viscosity of crude oil.
García-Duarte et al. [
62] studied the effect of alumina-based nanofluids (AlNPs) on improving oil recovery by reducing viscosity in cyclic steam stimulation. The results show that the adsorption efficiency of alumina nanoparticles on asphaltene is 48 mg/g at below 250 °C, and the complete conversion time of the catalytic decomposition of asphaltene is only 90 min, which is better than that of cerium dioxide and silica nanoparticles. When the concentration of nanoparticles and surfactants in the nanofluids was 0.05 wt%, the oil viscosity decreased by 90%. Dynamic tests showed that when steam injection was combined with naphtha and nanofluids, the oil recovery rates were 64% and 75%, respectively. The API weight of the recovered oil increased from 11.9° to 34°, and the viscosity decreased to less than 100 cP. The mechanism of action includes the destruction of the viscoelastic network formed by asphaltene–resin by nanoparticles, the catalytic cracking of heavy components to generate light compounds, and the enhancement of the heat transfer efficiency of the injected fluid to achieve efficient heat transfer, thereby effectively reducing oil viscosity and improving oil quality, showing the application potential of alumina-based nanofluids in heavy oil recovery and upgrading.
Cheraghian et al. [
63] confirmed that a nano-ceramic added to the HPAM solution has a better effect on the viscosity of the aqueous phase. The entry of nanomaterials in the oil displacement agent reduces the viscosity of the oil phase mainly by adsorption. The mechanism destroys the connection structure between crude oils and reduces the viscosity between crude oils. Nanofluids increase the viscosity of the aqueous phase, increase the mobility ratio between oil and water, increase the sweep efficiency, and achieve the purpose of improving the efficiency of oil production by means of physical and chemical combination.
In the process of heavy oil reservoir exploitation, an effective way to maintain a favorable mobility ratio is to reduce the viscosity of crude oil. One of the principles of using a nano-oil displacement agent based on metal nanomaterials to improve oil recovery is to increase the viscosity of displacement fluid. Experiments have confirmed that the rheological properties of the nano-oil displacement system are significantly regulated by multi-physical field coupling effects such as the concentration gradient, thermodynamic conditions, shear rate, and salinity gradient. These key parameters directly affect the reservoir seepage dynamics by changing the fluid microstructure. Therefore, the establishment of a quantitative response model of oil displacement agent viscosity–seepage efficiency has important theoretical guidance value for the intelligent optimization of heavy oil recovery process parameters [
64].
The mobility ratio (M) is a function of the relative permeability and viscosity of the displacement phase and the displaced phase, which is used to represent the difference in the flow capacity between the oil displacement agent and the crude oil and is related to the wettability of the rock and the interfacial tension. The formula for mobility ratio (M) is
In the formula: M—flow ratio;
λw, λo—water and oil mobility, D/(mPa·s);
Kw, Ko—water- and oil-phase permeability, D;
μo, μw—viscosity of oil and water, mPa·s.
In the case of a high mobility ratio (M > 1), it is easy to cause the displacement phase to point to the inside of the crude oil [
65]. There will be viscosity in the residual oil at the front end of the injected fluid, which often occurs in the process of enhanced oil recovery, such as water flooding and CO
2 flooding, which is not conducive to crude oil recovery. The high-viscosity characteristics of nano-oil displacement agents help to maintain a low mobility ratio during the oil displacement process, enhance the flow capacity of the displacement phase, and expand the degree of oil washing, thereby improving oil recovery [
31].
In summary, nanomaterials can be uniformly dispersed in heavy oil, which greatly inhibits the formation of large-area viscoelastic networks. Thus, the viscosity of heavy oil is reduced. These nanoparticles include silica, iron oxide, alumina, nano-ceramics, etc. Nanoparticles affect the viscosity of crude oil by affecting the chemical bonds between substances or changing the structure of substances. The degree of adsorption of nanoparticles on asphaltene is an important manifestation of their ability to reduce the viscosity of crude oil. Nanomaterials reduce the viscosity of heavy oil and thus reduce the difficulty of oil start and improve the efficiency of oil production.
3. Nanomaterial Swelling Oil Displacement Mechanism
Traditional methods such as water flooding mainly rely on osmotic pressure to drive water molecules into crude oil, but the effect is limited. In addition, polymer flooding reduces the flow ratio by increasing fluid viscosity, but its effect is affected by high-temperature and high-salt environments [
66]. Nanoparticles show advantages in the field of chemical flooding due to their special physical and chemical properties. Nanoparticles usually have smaller particle sizes and larger specific surface areas [
67]. In addition, the hydroxyl groups on the surface can adsorb metal cations and organic matter. Based on this principle, in the oil–water system, nanoparticles can adhere to the oil–water interface, prompting the surface active substances originally present on the oil film to enter the oil phase in the form of reverse micelles, and the nanoparticles themselves will be adsorbed on the oil–water interface [
68]. According to this principle, the volume of crude oil will be expanded to a certain extent.
3.1. Nano-Silica Oil Droplets Enhanced Expansion
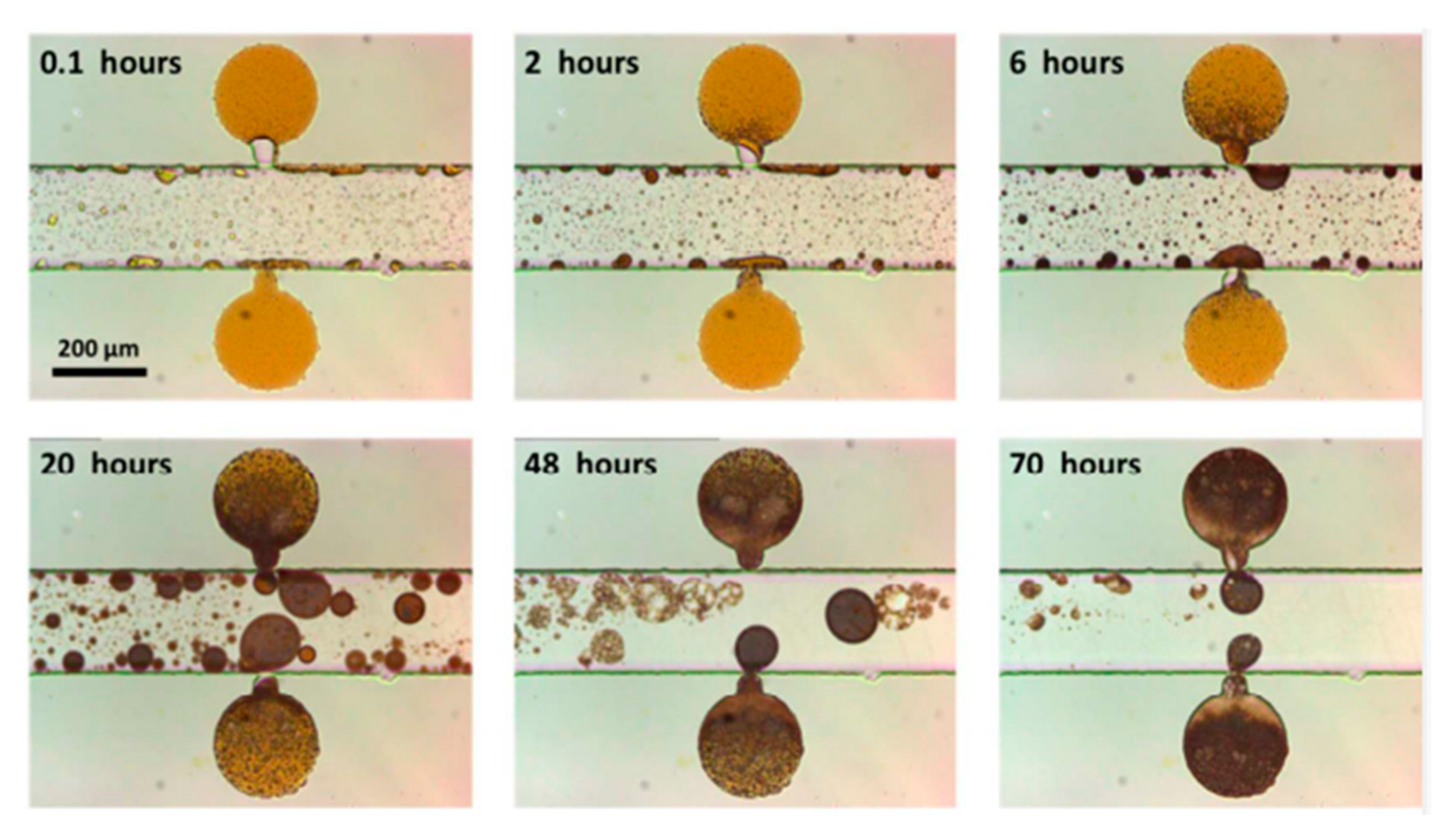
Xu et al. [
69] successfully prepared a new type of nanocomposite with the function of crude oil interface regulation and analyzed its molecular-level dynamic response mechanism with a hydrocarbon fluid based on a microfluidic chip platform system. The experimental results show that the hydrophilic silica (NPS) will cause the crude oil to expand sharply under the water-phase condition, as shown in
Figure 7. This expansion effect is very significant: even the small oil droplets on the surface of the main channel will expand by nearly a thousand times. The swelling ratio increases with the decrease in salinity and the increase in NPS concentration in the environment. The expansion of crude oil droplets increases the flow resistance in the scanning area, and the improvement of scanning efficiency increases the recovery rate by about 11%. Negatively charged hydrophilic nanoparticles drive the polar components of crude oil into the oil phase through competitive adsorption. Low-salinity water drives water to diffuse to form spontaneous emulsification, causing the oil phase to expand and dewet, changing the relative permeability of oil and water, reducing the mobility ratio, and improving the oil sweeping efficiency.
In Qin et al.’s study [
70], hydrophobic SiO
2 nanoparticles were treated at different pH levels and temperatures for different lengths of time, and the hydrophobicity of the nanoparticles was determined by the Washburn method [
71]. It was concluded that the external environment had a great influence on the surface lipophilicity of SiO
2 nanoparticles. Hydrophobic SiO
2 nanoparticles have high salt tolerance. When the lipophilicity of hydrophobic SiO
2 nanoparticles is more important for the improvement of reservoir exploitation efficiency, it is more suitable for use under high-temperature and high-alkaline conditions, but the use time should not be too long.
Wen et al. [
72] studied the effect of nanofluid application on enhanced oil recovery of Berea sandstone cores. Silica, alumina, nickel oxide, and titanium oxide were used as nanoparticles, and they were added to different concentrations of water to form aqueous solutions. When deionized water entered the emulsion droplets, they formed reverse micelles, and emulsified water droplets gradually entered the original water droplets (W2), increasing the volume of the crude oil droplets. In the experiment, the emulsion droplets were wrapped by nanofluids to form W2/O/W1. A schematic diagram is shown in the following
Figure 8. The double emulsion, in which the oil-soluble surfactant Span80 acts as a carrier, is the main factor for water transport in the double emulsion. The emulsion has the effect of expanding the swept volume to improve the harvesting efficiency [
73].
The expansion of emulsion droplets is due to the following: Deionized water has a higher chemical potential than water in emulsion droplets. The molecular migration caused by chemical potential difference changes the volume and morphology of oil droplets. When they contact with each other, deionized water diffuses into emulsion droplets, so that part of the Span80 molecules in the emulsion droplets are adsorbed on the surface of water molecules, and the Span80 molecules are pushed closer to the original water droplets, which eventually increases the volume of the emulsion droplets; after adding negatively charged nanoparticles, while deionized water enters the interior of the emulsion droplet, due to adsorption, nanoparticles will be adsorbed on the nanofluid interface of the emulsion droplet, and more oil-soluble Span80 molecules will be topped to the interior of the droplet. The amphiphilicity of the Span80 molecule makes it adsorb on the surface of water molecules in the form of reverse micelles and form small water droplets. The formation of water droplets in oil droplets is accelerated, and the volume of droplets increases rapidly, which accelerates the apparent expansion of emulsion droplets.
In recent years, ordinary nanoparticles have exposed deficiencies in interfacial activities and maintenance of emulsion stability [
75]. In order to overcome this problem, academia and industry have focused their research on Janus nanofluids [
76]. Studies have shown that amphiphilic Janus nanosheets have higher interfacial activity and better stability than traditional nanoparticles, which is mainly due to their larger desorption energy and surface area [
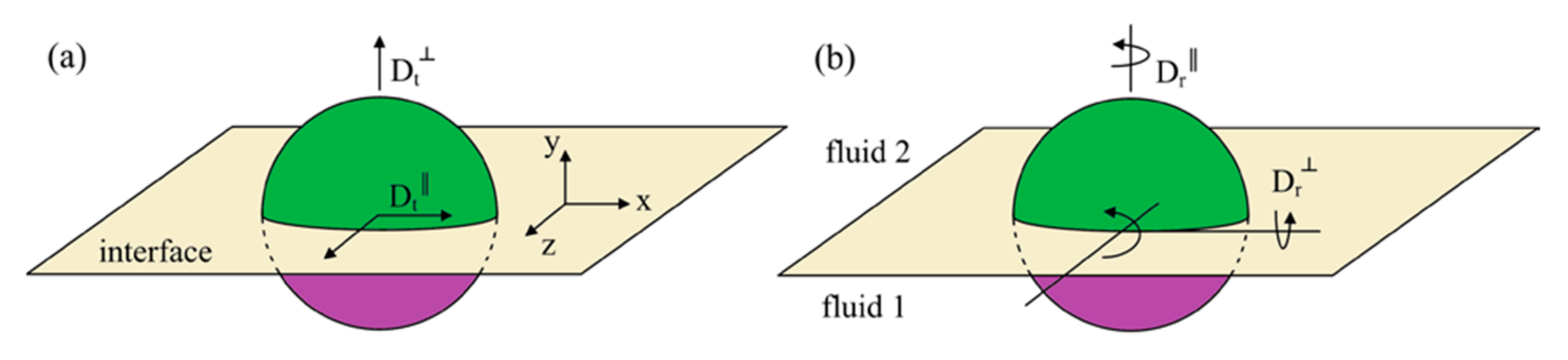
77]. In addition, the Janus particles exhibit unique characteristics of translational and rotational diffusion at the liquid interface, as shown in
Figure 9. These properties enable Janus nanosheets to significantly improve the efficiency of crude oil extraction at very low concentrations. Through their unique asymmetric structure and surface activity, Janus nanomaterials can effectively regulate the physical and chemical properties of the oil–water interface, promote the expansion of emulsion droplets, and thus improve oil recovery. Jia et al. [
78] studied the emulsification mechanism of amphiphilic Janus–SiO
2 nanoparticles (Janus-C12) in O/W, O/W/O, and W/O emulsions. They systematically analyzed the effects of nanoparticle concentration, water–oil volume ratio, and NaCl concentration on the emulsification effect. It was observed that the water droplets expanded to different degrees under different conditions. This phenomenon indicates that amphiphilic Janus–SiO
2 nanoparticles can theoretically expand crude oil droplets, thereby improving the exploitation efficiency of unconventional oil and gas fields.
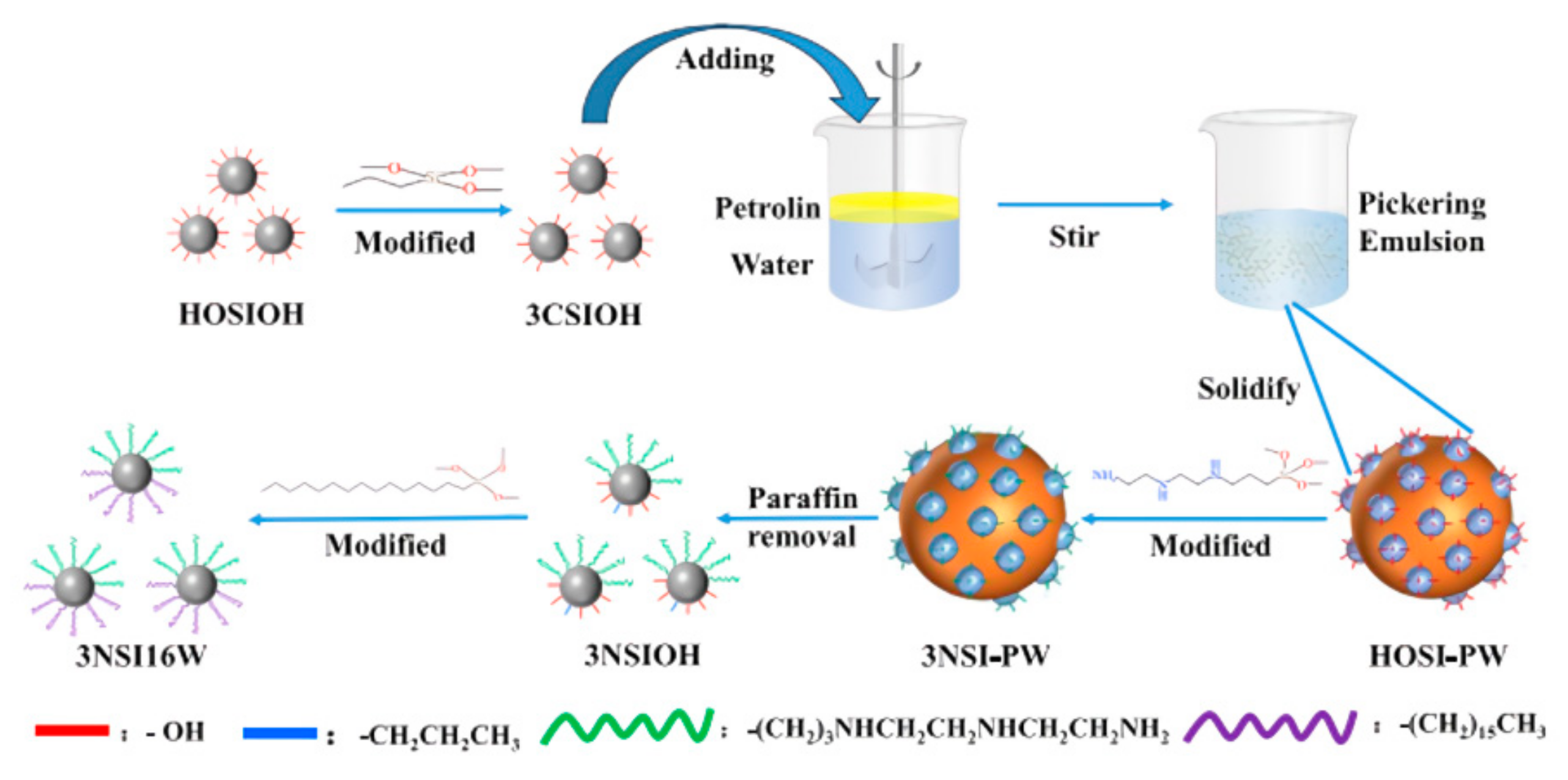
The usual preparation of Janus nanosheets is shown in
Figure 10. Ye [
80] proposed a new strategy for the preparation of amphiphilic Janus nanosheets, using cetyltrimethoxysilane and 3-aminopropyltrimethoxysilane (APTMS) as modifiers and thermally expandable microspheres (TEMS) as templates. Firstly, 3-aminopropyltrimethoxysilane (APTMS) was adsorbed on the surface of thermally expanded microspheres (TEMSs), and Janus spheres (TEMS @ SiO
2) were obtained by sol–gel treatment. Then, TEMS @ SiO
2 were modified by hexadecyltrimethoxysilane (HDTMS). TEMSs were heated and expanded to break the shell of the Janus spheres; thus, amphiphilic Janus nanosheets were prepared. The amphiphilic Janus nanosheets were analyzed by FT-IR, TG, and TEM. Amphiphilic Janus nanosheets were dispersed into simulated formation water to prepare nanofluids, and their stability, oil–water interfacial tension, emulsification ability, and enhanced oil recovery ability were evaluated. The results showed that the nanofluid (0.1%, mass fraction) did not appear stratified after 10 days of storage, and its Zeta potential was 31.2 mV at pH = 7, showing good stability. The emulsification index of the nanofluid (0.1%) for 10 days was 100%, indicating its excellent emulsification ability. The nanofluid (0.1%) can reduce the oil–water interfacial tension to 3.2 mN/m. Nanofluid flooding (0.1%) can significantly improve the recovery of low-permeability reservoirs. When the permeability is 8.9 × 10
−3 μm
2, the recovery can be increased by 21.5%. This shows that Janus nanosheets have better performance than traditional homogeneous nanoparticles and have great application potential in various fields [
19,
45].
Oil droplets can expand in water under the emulsification state, and the fundamental reason is that there is a chemical potential difference between the surrounding environment and the oil droplets. Due to the relatively high chemical potential of the environment, this potential energy difference drives the increase in oil droplet volume, which in turn leads to the expansion phenomenon. The promoting effect of nanoparticles on oil droplet expansion is due to their unique interface behavior. Nanoparticles change the distribution of surface-active substances by adsorbing at the oil–water interface. This leads to the movement of the active material inside the oil droplets, which increases the volume of the oil droplets and causes the oil droplets to expand. Further studies have shown that amphiphilic Janus nanomaterials can also expand oil droplets and exhibit better stability than ordinary nanofluids. The characteristics of nanofluids make it possible to promote the expansion of crude oil, and the study of nanofluid expansion oil displacement technology is of great significance for improving the efficiency of crude oil exploitation in practical engineering.
3.2. Pressure Oil Displacement by Structural Separation
The research on wetting phenomena is generally based on the famous Young equation [
81] and its modified model for rough surfaces [
82]. However, the Young equation is based on the following assumptions: the shape of the droplet is spherical, the thickness of the liquid at the contact line between the droplet and the solid phase is 0, and the spherical contour of the droplet is always not deformed. However, experiments show that the wetting phenomenon of nanofluids on solid surfaces contradicts this assumption, making the Young equation no longer applicable to describe the wetting phenomenon of nanofluids on solid surfaces [
83].
The concept of disjoining pressure was first proposed by Derjaguin in 1936 [
84]. However, Starov pointed out in a 2010 study that the naming of the term is somewhat misleading because it not only contains the separation effect but also involves the binding effect. In view of this, Starov suggested the use of the term “Derjaguin’s pressure”, which can not only reflect the original contribution of the concept but also avoid the misunderstanding caused by the literal meaning of the term. However, considering the historical background of the term’s long-term use in the academic field and the continuity of the literature, this paper still adopts the traditional name of “separation pressure”. It is worth noting that a large number of scholars’ studies have further confirmed the key role of separation pressure in the nanofluid displacement mechanism [
85].
Kondiparty et al. [
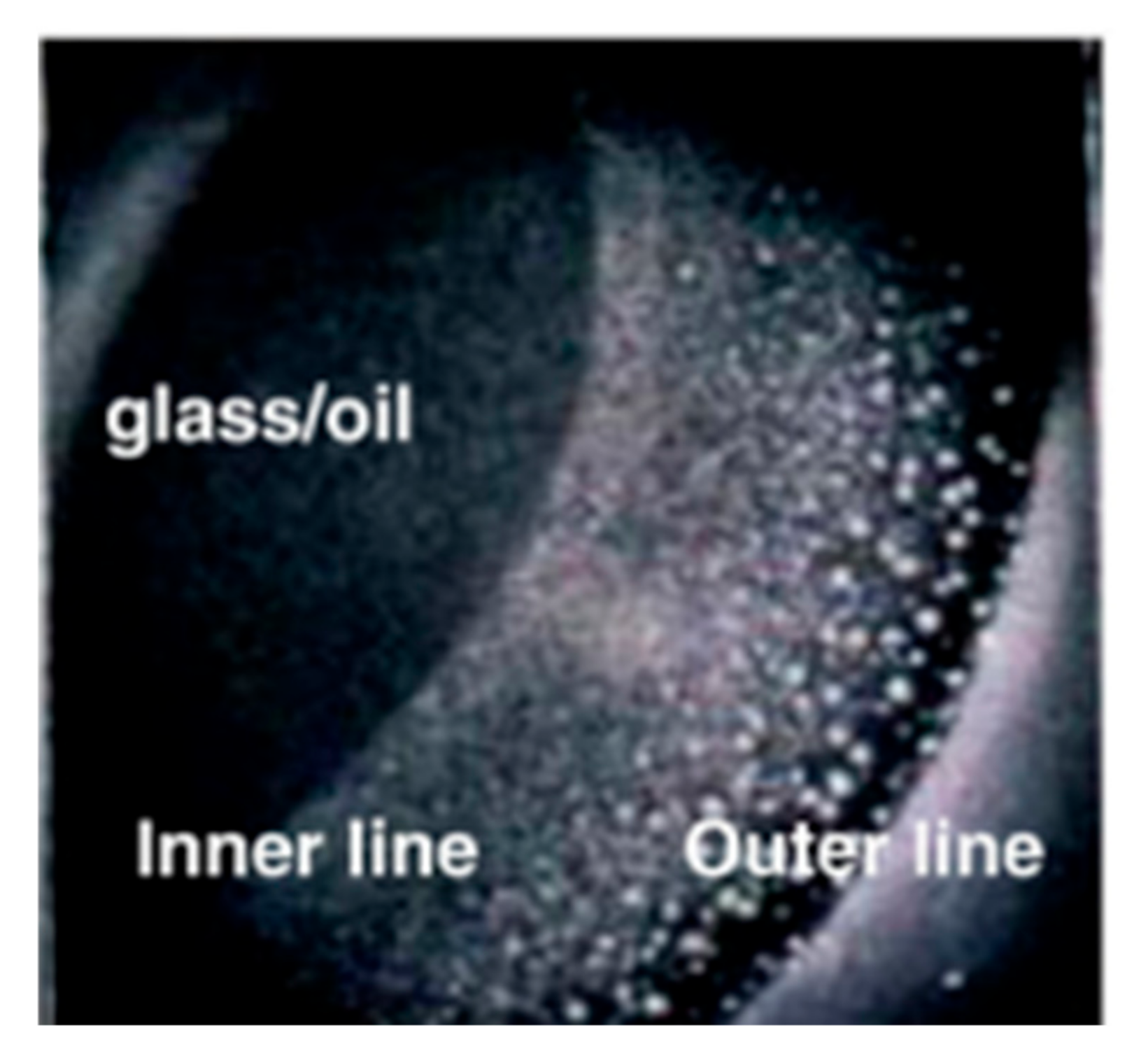
86] studied the effect of structural separation pressure on the diffusion rate of nanofluids by regulating the size of oil droplets and the content of nanofluids. It can be seen from the observations during the experiment that when the oil droplets on the surface of the glass sheet started to form, two obvious contact lines could be observed [
87], as shown in
Figure 11. One of them is defined as the outer contact line, and the other is defined as the outer contact line. This contact mainly refers to the contact between oil droplets, solids, and oil–water interfaces. The thickness of the interface film of the inner contact line will increase with the increase in time, which leads to an increase in the shrinkage rate of the inner contact line, which will eventually substantially increase the stripping efficiency of the oil droplets from the solid surface so as to improve the oil recovery efficiency.
Liang et al. [
88] clarified the oil-washing efficiency of modified sheet nanofluids through oil sand-washing experiments. The experimental results show that under the condition of simulating the actual engineering environment, the oil film shrinkage rate of the solid interface is relatively slow, and there is no wedge area at this time. Under the same experimental conditions, when the modified nanofluids are added, the oil film at the solid interface will have a more obvious wedge-shaped area during the shrinkage process, and two more obvious contact lines will appear. At this time, the shrinkage rate of the outer contact line is 8.5817 × 10
−5 cm/s, and the shrinkage rate of the inner contact line is 0.6617 × 10
−5 cm/s. Through experimental verification, it can be concluded that the oil washing efficiency of the oil sands can be as high as 95.7% after adding the modified nanosheet fluid.
Zhang et al. [
89] observed the displacement of oil droplets by nanofluids through a reflection interference microscope and studied the diffusion law of hydrophilic nano-silica particles on a solid surface. The experimental results show that the displacement of oil droplets by ordinary salt water did not change the oil droplets, that is, the contact line did not shrink obviously. However, when nanofluids were used to displace oil droplets, the contact line contracted and the contraction rate was faster. That is to say, the wedge-film diffused under the action of structural pressure.
Wasan et al. [
87] observed the self-assembly phenomenon of nanoparticles in the three-phase contact wedge region formed on a nanofluid–bubble–solid surface using a reflective light digital video microscope. On this basis, the dynamic process of nanofluid displacement of oil droplets on the glass surface was further studied. Two contact lines were also formed during the displacement process. One was the outer line formed between the oil droplets, the solid surface, and the micelle solution, and the other was the inner line formed between the oil droplets, the solid surface, and the liquid film. The thickness of the liquid film increased with the extension of the diffusion time, and the shrinkage rate of the inner contact line was greater than that of the outer contact line. Finally, the oil droplets were completely detached from the glass surface under the action of a ripple-like water-based film [
90]. After research, Wasan et al. [
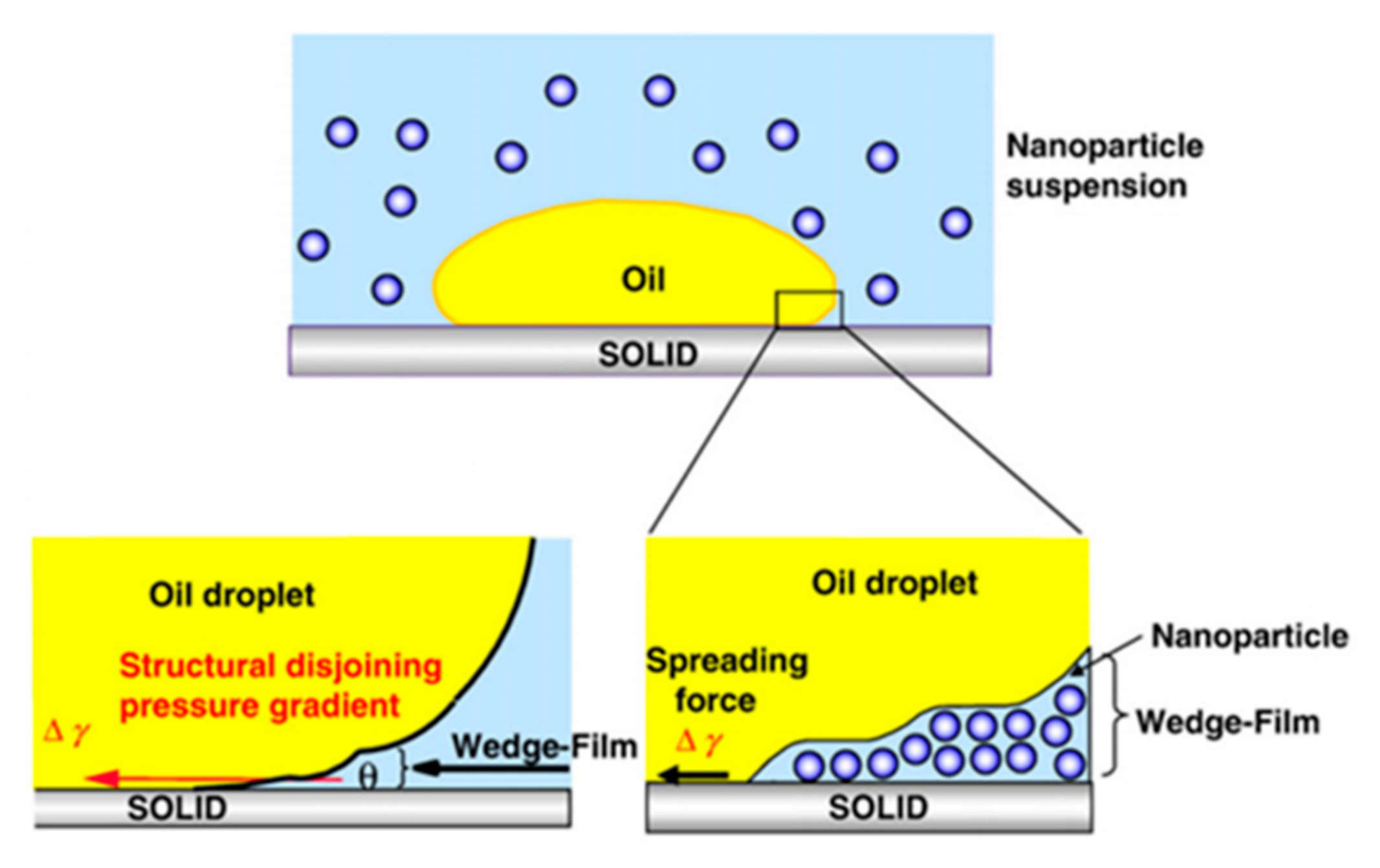
87] found that nanoparticles form a certain structure under the action of wedge constraints and have a high tension at the apex of the film. When the tension of the membrane increases towards the wedge vertex, the nanofluid will move in the three-phase contact line, which leads to the enhancement of the dynamic diffusion behavior of the nanofluid. The nanoparticle structure in the wedge membrane leads to the structural separation pressure gradient at the wedge apex, as shown in
Figure 12. With the injection of nanofluids, the size of the wedge-film will gradually increase. At the same time, the wedge membrane will continue to move forward under the action of the driving force, and the separation pressure will be generated under this movement. In addition, Anoop Chengara et al. [
91] used a theoretical model to verify that changes in the physical and chemical properties of nanoparticles, such as size, temperature, and concentration, will have a certain effect on the separation pressure.
In summary, due to the existence of electrostatic repulsion and Brownian motion between molecules, nanofluids will lead to the formation of a wedge-shaped, film-like structure at the contact surface between the three phases of oil, water, and solid, and the wedge-shaped film structure will continue to move forward due to the presence of the driving force, resulting in separation pressure and improving the wettability of the rock surface. Through the discussion of the Principle of Enhanced Oil Recovery by Nanomaterials section of this paper, we know that the change in wettability can greatly affect the efficiency of oil exploitation.
The structure separation pressure displacement mechanism is one of the important mechanisms of nanofluid flooding, involving the pressure of the wedge area of the contact line, and this mechanism also provides a new explanation of the microscopic process of crude oil stripping. The combination of the structural pressure separation mechanism and the reduction in interfacial tension and wettability conversion is an important factor affecting mining efficiency. In the process of nanofluid flooding, the generation of structural pressure is closely related to the behavior of nanofluids at the interface between oil and water phases. Nanoparticles can change the nature of the interface between oil and water, so they can affect the stripping and movement of crude oil droplets. The mechanism of structural separation pressure flooding involves the adsorption and spreading of nanoparticles at the interface and also involves the structure and dynamic behavior of nanoparticles affecting the oil–water interface. By affecting the interface and the micro level, nanofluids can affect the fluidity of crude oil and thus greatly affect the efficiency of oil exploitation.
In a word, the study of the mechanism of structural separation pressure flooding is of great significance in improving the efficiency of oil exploitation by nanofluid flooding. Nanoparticles will change the micro-level structure and dynamics of the oil–water interface, thus affecting the peeling efficiency and fluidity of oil droplets, thus affecting the efficiency of oil exploitation. The research on the mechanism of structural separation pressure is of great significance for further optimizing nanofluid flooding technology in engineering and improving recovery efficiency.
3.3. The “Rolling Up” and “Diffusion” Phenomena
The early nanofluids used for oil displacement were mainly surfactant solutions with nano-scale micelles [
92,
93]. In 1988, Kao et al. [
94] first revealed the oil displacement mechanism of a surfactant micellar solution. They used a reflected light differential interference microscope to observe in detail the process of oil droplet separation from the solid surface under the action of a surfactant micelle solution. For the first time, they found two obvious contact lines in the three-phase contact zone formed on an oil droplet–surfactant micelle solution–solid surface during oil droplet separation. One of them was a conventional contact line, called the outer line; the other was the contact line formed by the diffusion of water and surfactant molecules between the oil phase and the solid phase, which was located in front of the conventional contact line, called the inner line. They believed that the surfactant inhibits the surface tension to cause the oil droplets to roll up and the water and the surfactant micelle solution to diffuse between the oil and solid phases in front of the contact line. “Rolling-up” and “diffusion” occur together in the dual oil displacement mechanism. At the same time, the decrease in the contact angle during the oil displacement process is due to the existence of the roll-up mechanism. The second contact line exists because of the role of the diffusion mechanism. The principle is shown in
Figure 13, below.
In the process of crude oil exploitation, the study of the oil droplet migration law has always been the core scientific problem in this field. At present, the two main displacement mechanisms have been identified as interfacial stripping migration and the diffusion mass transfer process. In the aqueous-phase displacement environment, the process of oil droplet detachment and migration is mainly controlled by the interfacial wetting characteristics [
96]. When the oil droplets contact with the surface of the hydrophilic rock, the higher interface energy of the oil–water interface will hinder the oil droplets from leaving the rock surface. It is worth noting that the introduction of a nanofluid system can significantly improve the interfacial wetting characteristics: by regulating the concentration of nanoparticles, the contact angle of oil droplets can be effectively reduced (as shown in
Figure 14), thereby promoting the detachment and migration of oil droplets from the rock surface and ultimately improving oil recovery [
97]. Research on the diffusion mass transfer mechanism mainly focuses on the diffusion behavior of fluid in porous media during gas flooding. In the aqueous-phase environment, the mass transfer efficiency of gas in crude oil can be enhanced by diffusion. In particular, when there is initial water saturation in porous media, this mechanism will exhibit nonlinear characteristics due to the complex interaction between multi-component displacement agents and crude oil. Studies have shown that nanofluids can further enhance the diffusion and mass transfer process by improving the stability and fluidity of crude oil systems [
98]. There is a significant synergistic effect between the two displacement mechanisms [
99]. For example, in a nanofluid system containing surfactants, the decrease in the contact angle can promote the detachment and migration of oil droplets, and the addition of nanofluids can accelerate the diffusion rate of the displacement fluid. The coupling effect of the two can significantly improve the overall displacement efficiency.
According to the discussion in the Principle of Enhanced Oil Recovery by Nanomaterials section, we know that nanoparticles will have a directional arrangement at the oil-water interface due to the differences in the physical and chemical properties of the different parts. Nanoparticles can replace the original interface between oil and water, reduce the friction between the oil phase and the water phase, and further reduce the interfacial tension. The study of Bing Wei et al. [
101] also shows that the presence of nanofluids will lead to changes in the morphology of rock surfaces.
Figure 15, below, is a schematic diagram of the adsorption of nanoparticles on rock surfaces. The presence of nanofluids will cause the wedge-shaped film structure to form at the contact part of the oil–water and rock interface. The existence of the wedge-shaped structure separation pressure will cause the oil droplets on the rock to peel off, thereby rolling up the oil film.
Based on the analysis results presented in the Principle of Enhanced Oil Recovery by Nanomaterials section, it can be seen that the nanofluid system has a significant synergistic effect in regulating the interfacial tension and improving the wettability of the rock surface. This synergistic effect is mainly achieved by changing the direction of the capillary force, thus providing kinetic advantages for the migration and diffusion of oil droplets [
102]. Chen et al. [
103] found through experiments that amphiphilic-modified graphene oxide nanomaterials have excellent interfacial activity characteristics, and the nano-adsorption layer formed by them can reduce the oil–water interfacial tension to the order of 10 1 mN/m. This system can not only construct a super-hydrophilic interface on the surface of reservoir rock but also effectively control the wettability through the formation of nano-scale interfacial film. This multi-mechanism coupling provides a new technical path for enhancing oil recovery. The relevant research results have laid a theoretical foundation for the engineering application of nanofluids in the field of tertiary oil recovery.
The mechanism of nanofluids’ synergistic microscopic oil displacement effect can be summarized in three key elements: structural separation pressure, interfacial tension control, and rock wettability optimization. Among them, the presence of surfactants is the core condition that triggers the detachment of oil droplets. These substances promote the interfacial behavior of crude oil particles adsorbed on the pore surface by reducing the contact angle of the oil–rock interface. When the contact angle is less than the critical value, the oil droplets undergo morphological reconstruction under the action of capillary force to form larger aggregates, which is the “rolling-up” phenomenon of oil droplets [
104]. The synergistic effect of the diffusion mechanism is reflected in the dynamic regulation of oil–water interfacial tension. The microbubble diffusion effect formed by the Brownian motion of nanoparticles can significantly reduce the interfacial tension between crude oil and displacement fluid, thereby improving the fluidity of crude oil. It is worth noting that there is a significant interaction between the two mechanisms: the large-sized oil droplets formed in the “roll-up” process provide a larger interface for diffusion, and the microbubbles generated by diffusion further promote the detachment of oil droplets by enhancing the interface disturbance. This dynamic synergistic effect makes nanofluids show better recovery efficiency than traditional oil displacement agents in porous media [
105].
In oil exploitation engineering, entrainment and the diffusion effect constitute the core mechanism to improve the efficiency of crude oil migration. The entrainment effect significantly improves the flow performance of crude oil by changing the droplet shape parameters. The nanolayer formed by the diffusion effect enhances the migration efficiency of droplets in the pore throat by regulating the interaction between bubbles and fluids. The synergistic effect of these two mechanisms has a significant role in promoting oil recovery and provides important theoretical support for the optimization and upgrading of oil exploitation technology.
4. Prospects of Nano-Expansion Technology
Although nanomaterials have shown significant potential in the field of enhanced oil recovery, their application still faces multiple challenges: the current nano-oil displacement system has stability bottlenecks, such as interface deactivation, phase separation, and mechanical degradation in extreme reservoir environments, and the interface activity and emulsion stability of ordinary nanoparticles are insufficient. At the same time, the multi-scale transmission law and interface interaction mechanism of nanofluids in porous media have not been fully clarified, and the lack of intelligent optimization models restricts the precise regulation of engineering applications. In addition, biodegradability, low-cost preparation, and recycling technology for environmentally friendly nanomaterials still need to be achieved. These shortcomings need to be solved by material structure innovation, multidisciplinary cross-integration, and engineering verification system improvement. Based on this, this paper puts forward the following prospects.
Discussion of Scalability, Economic Feasibility, and Practical Implementation: Nanomaterials enhance oil recovery by altering rock wettability, reducing interfacial tension, and improving foam stability, making them adaptable to various reservoir conditions. Furthermore, nanomaterials can be combined with traditional chemical agents to form nano-composite fluids, further optimizing oil displacement efficiency. However, their application is constrained by the need to match the diverse morphologies of nanomaterials with reservoir complexity. Future research should further evaluate the compatibility mechanisms between nanomaterials and reservoir conditions. Simultaneously, the low-cost and high-surface-area characteristics of nanomaterials provide cost advantages, but significant resources are still required for large-scale preparation and characterization techniques. Additionally, the stability issues of nanomaterials under extreme conditions, such as high temperature, high pressure, and high salinity, increase development costs. Although some studies propose reducing complexity through simple material modifications, practical applications still require balancing performance with cost. The main challenges in the practical application of nanomaterials lie in their controllability, environmental impact, and long-term effectiveness assessment. Moreover, the risks of environmental release and the long-term ecological impacts of nanomaterials are not yet fully understood. Future research needs to focus on green synthesis methods, the design of smart-responsive materials, and field trial validation to advance the commercial-scale application of nano-EOR technology.
Multi-Physics Coupling Seepage Mechanisms and Intelligent Prediction Model Development: It is critical to advance research on the cross-scale transport dynamics of nano-flooding systems in porous media. By integrating microfluidic experiments, molecular dynamics simulations, and reservoir-scale numerical modeling, the dynamic transport characteristics of nanoparticles in heterogeneous pore networks and their interfacial interaction mechanisms can be systematically elucidated. Incorporating artificial intelligence algorithms to develop intelligent decision-making systems will enable dynamic optimization of key parameters such as nanofluid concentration gradients and injection rates, achieving visualized prediction and adaptive control of the displacement process.
Development of Stable Nano-Systems for Extreme Reservoir Environments: To address the challenges of deep and ultra-deep reservoir development, there is an urgent need to design nano-flooding systems with high-temperature resistance (>150 °C), salt tolerance (TDS > 30 × 104 mg/L), and high-pressure stability (>70 MPa). Functional Janus-structured nanomaterials, organic–inorganic core–shell composites, and surface-charge-tunable nanoformulations can effectively mitigate issues such as interfacial deactivation, phase separation, and mechanical degradation under harsh conditions. For instance, zirconia-coated mesoporous silica composites exhibit enhanced structural stability in high-temperature, high-salinity environments.
Research on Environmentally Friendly Nano-Flooding Technologies: Establishing a green and sustainable nano-flooding system requires breakthroughs in the following areas: (1) developing biodegradable nanomaterials from bio-based raw materials such as lignin and chitosan; (2) optimizing low-environmental-risk formulations by replacing traditional organic solvents with supercritical fluid synthesis techniques; (3) constructing a recycling system for spent nanomaterials through magnetic separation and surface regeneration technologies to improve material utilization efficiency. Carbon footprint assessment models should guide R&D processes to ensure lifecycle environmental management of the technology.
Potential Integration with Other EOR Technologies: Innovative synergistic technologies combining nanofluids with gas flooding, bio-enzyme flooding, and electromagnetic-assisted methods should be explored. Key applications include (1) designing nanoemulsion–supercritical CO2 composite systems to synergistically reduce oil viscosity and enhance microscopic sweep efficiency in tight reservoirs and (2) developing hybrid schemes integrating magnetic nanoparticles with low-frequency pulse waves to improve oil mobilization in fracture networks. A multidimensional techno-economic evaluation framework is recommended to validate feasibility through field trials and establish industrial standards for nano-flooding applications.
5. Conclusions
This paper systematically reviews the progress in the application of nanomaterials in oil displacement, with a focus on mechanisms for enhanced oil recovery, nano-swelling oil displacement mechanisms, and technical challenges. Through in-depth analysis of interfacial regulation, wettability alteration, and crude oil rheology optimization mechanisms, it provides theoretical support for the engineering application of nano-displacement technology. The key conclusions are as follows:
Nanomaterials synergistically enhance oil recovery through three core mechanisms: significantly reducing oil–water interfacial tension, regulating rock wettability, and decreasing crude oil viscosity. Experiments demonstrate that active silica nanofluids can reduce interfacial tension by up to 73.8%. Optimal wettability regulation occurs under neutral–wet conditions, achieving a maximum recovery enhancement of 52.6%. Acidic silica nanoparticles reduce heavy oil viscosity by 30%. Nanoparticles form interfacial nanolayers that replace pure interfaces, and their combination with surfactants further enhances emulsification and oil stripping efficiency.
- 2.
Unique Mechanisms and Performance Advantages of Nano-Swelling Displacement
Hydrophilic silica nanomaterials induce drastic expansion of crude oil droplets through interfacial adsorption, with volume expansion of nearly a thousand-fold. Their structural disjoining pressure forms wedge-shaped films at oil–water–solid three-phase regions, driving contact line contraction. The inner contact line contraction rate reaches 0.6617 × 10−5 cm/s, significantly improving oil stripping efficiency, with modified nanosheet fluids achieving up to 95.7% displacement efficiency. Amphiphilic Janus nanomaterials reduce interfacial tension to 0.01–0.1 mN/m and enhance recovery by 21.5% at ultralow concentrations (0.005 wt%).
- 3.
Technical Challenges and Future Development Directions
Current technology faces challenges, including insufficient stability in extreme reservoir environments, unclear mass transfer mechanisms in porous media, and the high costs of eco-friendly materials. Future efforts should focus on developing functionalized Janus materials and core–shell composites resistant to high-temperature/high-salinity environments, optimizing displacement parameters using artificial intelligence, advancing bio-based biodegradable nanomaterials, and implementing composite technologies combining supercritical CO2 with nanoemulsions.
The core contribution of this work lies in revealing how nanomaterials enhance oil recovery through synergistic mechanisms of interfacial tension reduction, directional wettability control, and structural disjoining pressure. It innovatively proposes theoretical models of droplet dynamic expansion and wedge-film diffusion in nano-swelling displacement. The key findings include the following: neutral–wet conditions maximize recovery enhancement; Janus nanomaterials achieve orders-of-magnitude interfacial tension reduction at ultralow concentrations; structural disjoining pressure is the core physical mechanism for microscopic oil stripping. Material innovation and intelligent modeling will drive future advancements toward efficient and environmentally sustainable technologies.