Investigation of In Situ and Ex Situ Passivation of Pyrophoric Uranium–Niobium Alloy Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Powder Production
2.2. Passivation
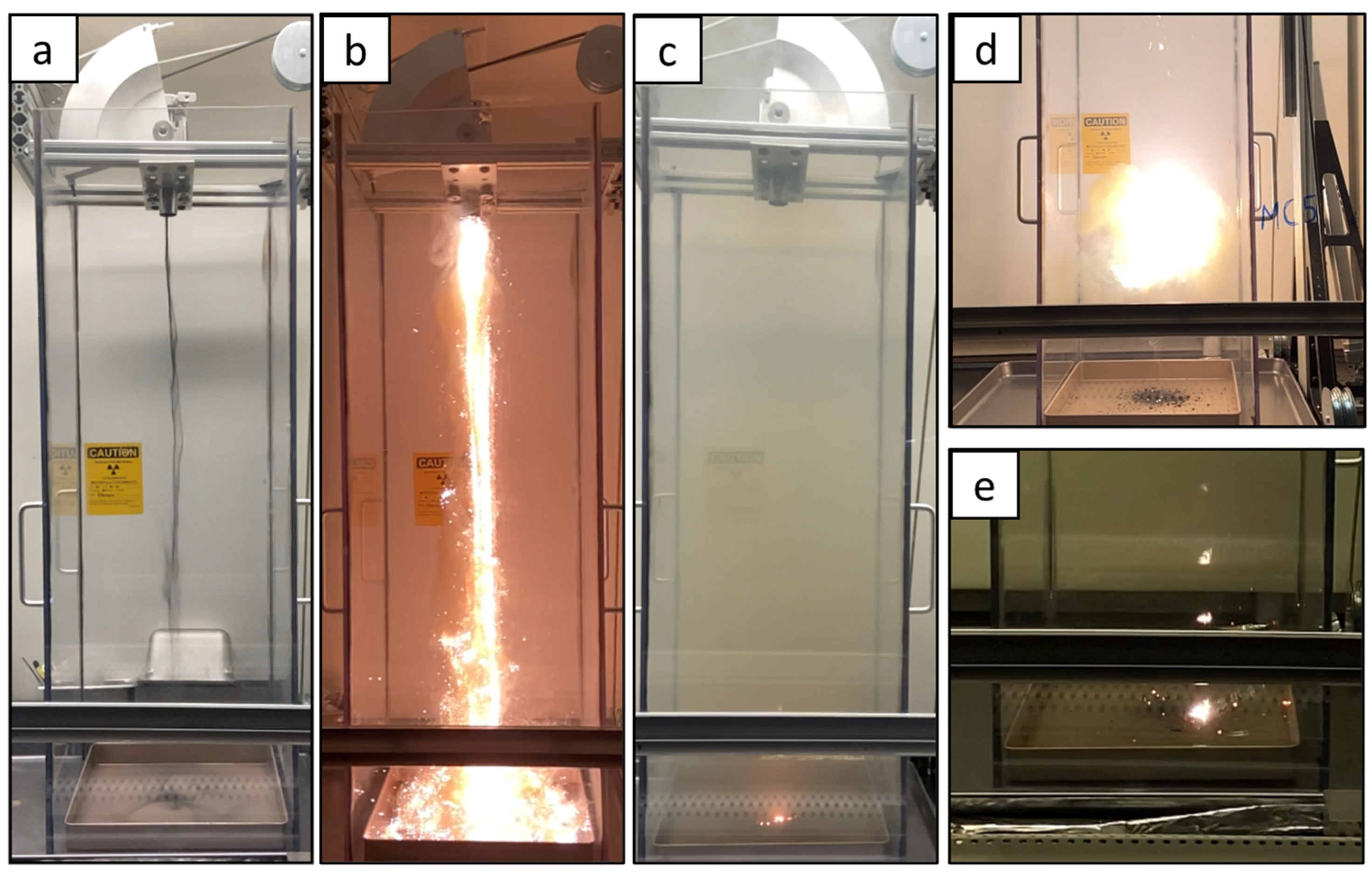
2.3. Pyrophoricity Testing (Sparkle Test)
2.4. Characterization
3. Results and Discussion
3.1. Pyrophoricity Results
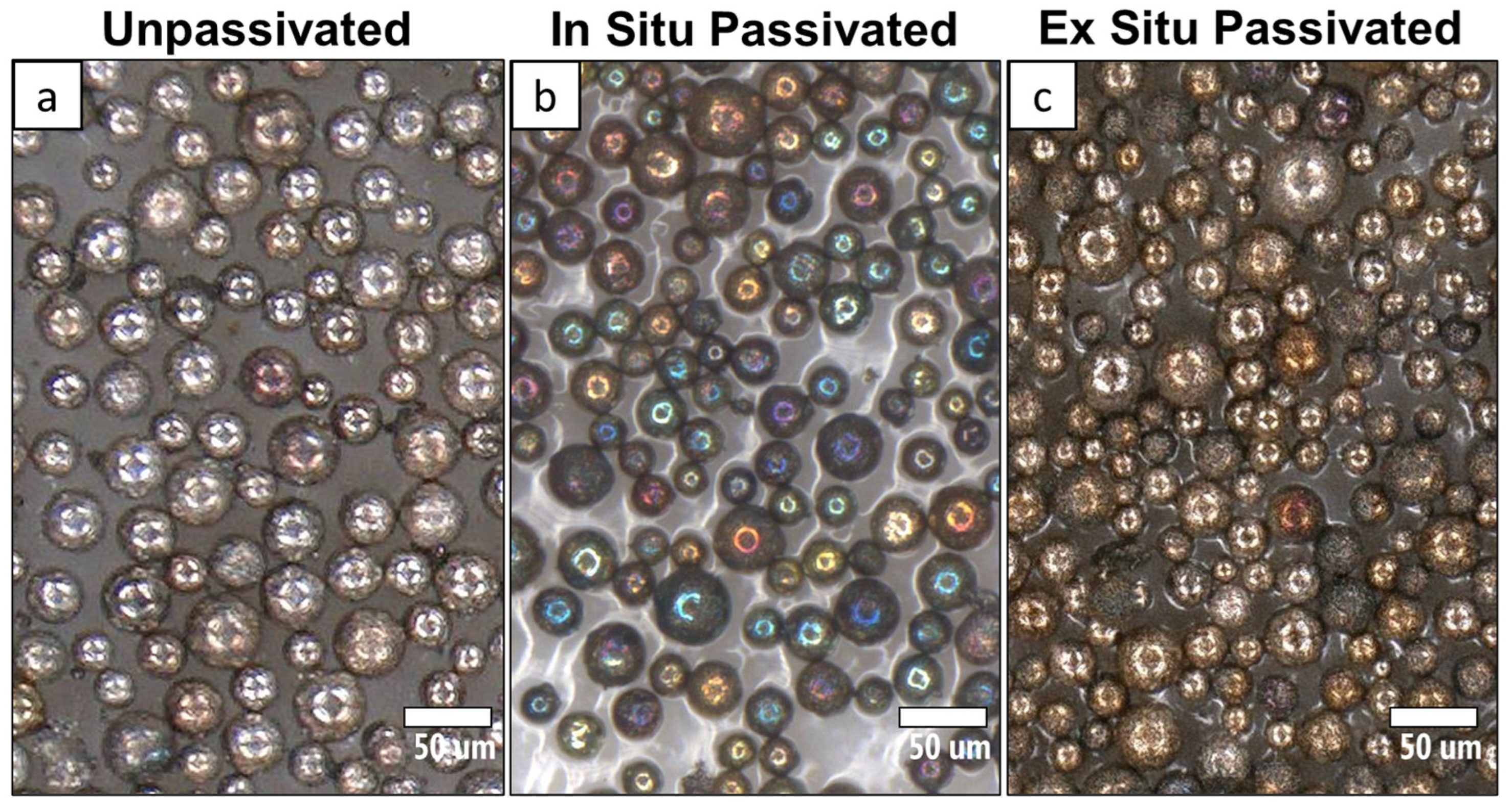
3.2. Optical Analysis and Color
3.3. TEM and Oxide Thickness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Zhang, J.; Jian, X.B.; Yan, F.; Ding, S.R.; Li, Y.M. Effects of Matrix Creep Properties on Effective Irradiation Swelling of U-10Mo/Zr Dispersion Nuclear Fuels. Front. Energy Res. 2022, 10, 851747. [Google Scholar] [CrossRef]
- McKeown, J.T.; Hsiung, L.L.; Ryu, H.J.; Park, J.M.; Turchi, P.E.A.; King, W.E. Rapidly solidified U–6wt%Nb powders for dispersion-type nuclear fuels. J. Nucl. Mater. 2014, 448, 72–79. [Google Scholar] [CrossRef]
- Susmikanti, M.; Sulistyo, J. Prediction of U-Mo Dispersion Nuclear Fuels with Al-Si Alloy Using Artificial Neural Network. Aip Conf. Proc. 2014, 1615, 175–181. [Google Scholar] [CrossRef]
- Singh, S.; Ramakrishna, S.; Singh, R. Material issues in additive manufacturing: A review. J. Manuf. Process. 2017, 25, 185–200. [Google Scholar] [CrossRef]
- He, J.L.; Hu, B.B.; Zhao, Y. Superaerophobic Electrode with Metal@Metal-Oxide Powder Catalyst for Oxygen Evolution Reaction. Adv. Funct. Mater. 2016, 26, 5998–6004. [Google Scholar] [CrossRef]
- Rayhan, U.; Do, J.H.; Arimura, T.; Yamato, T. Reduction of carbonyl compounds by Raney Ni-Al alloy and Al powder in the presence of noble metal catalysts in water. C. R. Chim. 2015, 18, 685–692. [Google Scholar] [CrossRef]
- Dewidar, M.M.; Yoon, H.C.; Lim, J.K. Mechanical properties of metals for biomedical applications using powder metallurgy process: A review. Met. Mater. Int. 2006, 12, 193–206. [Google Scholar] [CrossRef]
- Joseph, G.; Team, C.S.B.H.I. Combustible dusts: A serious industrial hazard. J. Hazard. Mater. 2007, 142, 589–591. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Energy. Primer on Spontaneous Heating and Pyrophoricity; U.S. Department of Energy: Washington, DC, USA, 2020.
- Finzel, T.G. Pyrophoric iron. I. Preparation and properties. J. Am. Chem. Soc. 1930, 52, 142–149. [Google Scholar] [CrossRef]
- Sundaram, D.; Yang, V.; Yetter, R.A. Metal-based nanoenergetic materials: Synthesis, properties, and applications. Prog. Energy Combust. Sci. 2017, 61, 293–365. [Google Scholar] [CrossRef]
- Le Guyadec, F.; Génin, X.; Bayle, J.P.; Dugne, O.; Duhart-Barone, A.; Ablitzer, C. Pyrophoric behaviour of uranium hydride and uranium powders. J. Nucl. Mater. 2010, 396, 294–302. [Google Scholar] [CrossRef]
- Le Guyadec, F.; Rado, C.; Joffre, S.; Coullomb, S.; Chatillon, C.; Blanquet, E. Thermodynamic and experimental study of UC powders ignition. J. Nucl. Mater. 2009, 393, 333–342. [Google Scholar] [CrossRef]
- Berthinier, C.; Coullomb, S.; Rado, C.; Blanquet, E.; Boichot, R.; Chatillon, C. Experimental study of uranium carbide pyrophoricity. Powder Technol. 2011, 208, 312–317. [Google Scholar] [CrossRef]
- Reding, N.S.; Shiflett, M.B. Metal Dust Explosion Hazards: A Technical Review. Ind. Eng. Chem. Res. 2018, 57, 11473–11482. [Google Scholar] [CrossRef]
- Gromov, A.; Il’in, A.; Teipel, U.; Pautova, J. Passivation of Metal Nanopowders. In Metal Nanopowders; Wiley: Hoboken, NJ, USA, 2014; pp. 133–152. [Google Scholar]
- Ablitzer, C.; Le Guyadec, F.; Raynal, J.; Génin, X.; Duhart-Barone, A. Influence of superficial oxidation on the pyrophoric behaviour of uranium hydride and uranium powders in air. J. Nucl. Mater. 2013, 432, 135–145. [Google Scholar] [CrossRef]
- Chesnokova, R.V.; Bykov, B.G.; Bondareva, A.A.; Sychkova, L.A.; Alekseev, A.M.; Vasilevich, A.A.; Sazonova, I.S. Formation of Oxide Layer in Passivation of Pyrophoric Nickel Methanation Catalysts. Kinet Catal. 1984, 25, 168–173. [Google Scholar]
- Litrico, G.; Proulx, P.; Gouriet, J.B.; Rambaud, P. Controlled oxidation of aluminum nanoparticles. Adv. Powder Technol. 2015, 26, 1–7. [Google Scholar] [CrossRef]
- Jeurgens, L.P.H.; Sloof, W.G.; Tichelaar, F.D.; Mittemeijer, E.J. Growth kinetics and mechanisms of aluminum-oxide films formed by thermal oxidation of aluminum. J. Appl. Phys. 2002, 92, 1649–1656. [Google Scholar] [CrossRef]
- Pollitt, A.A. The Causes and Prevention of Corrosion; Van Nostrand: New York, NY, USA, 1924. [Google Scholar]
- Tamura, H. The role of rusts in corrosion and corrosion protection of iron and steel. Corros. Sci. 2008, 50, 1872–1883. [Google Scholar] [CrossRef]
- Wegman, R.F.; Van Twisk, J. 7—Other Metals. In Surface Preparation Techniques for Adhesive Bonding, 2nd ed.; Wegman, R.F., Van Twisk, J., Eds.; William Andrew Publishing: Norwich, NY, USA, 2013; pp. 105–114. [Google Scholar]
- Watkins, E.B.; Kruk, I.; Majewski, J.; Allred, D.D. Oxide structure of air-passivated U-6Nb alloy thin films. J. Nucl. Mater. 2020, 539, 152356. [Google Scholar] [CrossRef]
- Landa, A.; Söderlind, P.; Wu, A. Phase Stability in U-6Nb Alloy Doped with Ti from the First Principles Theory. Appl. Sci. 2020, 10, 3417. [Google Scholar] [CrossRef]
- Cathcart, J.V. Gaseous Oxidation of Uranium Alloys. In Proceedings of the Physical Metallurgy of Uranium Alloys Conference, Oak Ridge, TN, USA, 12 February 1974. [Google Scholar]
- Eckelmeyer, K.H.; Romig, A.D.; Weirick, L.J. The effect of quench rate on the microstructure, mechanical properties, and corrosion behavior of U-6 wt pct Nb. Metall. Trans. A 1984, 15, 1319–1330. [Google Scholar] [CrossRef]
- Huang, K.; Elton, E.S.; Henderson, H.B.; Harward, N.K.; Clarke, E.M.; Winston, L.D.; Sedillo, E.M.; Rosas, D.L.; Schaeffer-Cuellar, C.A.; Simpson, S.A.; et al. The hydride-dehydride process combined with plasma spheroidization: An alternative route for producing spherical metallic powders of U-6 wt.% Nb. Adv. Powder Technol. 2023, 34, 103992. [Google Scholar] [CrossRef]
- Wu, A.S.; Gallegos, G.F.; Wraith, M.W.; Burke, S.C.; Elmer, J.W.; Brown, D.W.; Clausen, B.; Alexander, P.A.; Iniguez, M.R.; Ancheta, D.S.; et al. Additive Manufacturing of a y0-Tetragonal Uranium-Niobium Alloy; Lawrence Livermore National Lab. (LLNL): Livermore, CA, USA, 2018. [Google Scholar]
- Wu, A.S.; Wraith, M.W.; Burke, S.C.; Hamza, A.V.; Brown, D.W.; Clausen, B.; Hsiung, L.L.; McKeown, J.T.; Lindvall, R.E.; Sedillo, E.M.; et al. Mechanical Behavior of Additively Manufactured Uranium-6 wt. pct. Niobium; Lawrence Livermore National Lab. (LLNL): Livermore, CA, USA, 2017. [Google Scholar]
- United Nations. Recommendations on the Transport of Dangerous Goods: Tests and Criteria, 7th revised ed.; United Nations: New York, NY, USA; Geneva, Switzerland, 2019. [Google Scholar]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Thompson, N.B.A.; O’Sullivan, S.E.; Howell, R.J.; Bailey, D.J.; Gilbert, M.R.; Hyatt, N.C. Objective colour analysis from digital images as a nuclear forensic tool. Forensic Sci. Int. 2021, 319, 110678. [Google Scholar] [CrossRef] [PubMed]
- Young, T. XIV. An account of some cases of the production of colours, not hitherto described. Philos. Trans. R. Soc. Lond. 1802, 92, 387–397. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Del Curto, B.; Pedeferri, M. Interference colors of thin oxide layers on titanium. Color. Res. Appl. 2008, 33, 221–228. [Google Scholar] [CrossRef]
- Qiu, S.R.; Amrhein, C.; Hunt, M.L.; Pfeffer, R.; Yakshinskiy, B.; Zhang, L.; Madey, T.E.; Yarmoff, J.A. Characterization of uranium oxide thin films grown from solution onto Fe surfaces. Appl. Surf. Sci. 2001, 181, 211–224. [Google Scholar] [CrossRef]
- Larson, D.T.; Lott, L.A.; Cash, D.L. Surface Film Thickness Determination by Reflectance Measurements. Appl. Opt. 1973, 12, 1271–1275. [Google Scholar] [CrossRef]
- Komatsu, I.; Aoki, H.; Ebisawa, M.; Kuroda, A.; Kuroda, K.; Maeda, S. Color change mechanism of niobium oxide thin film with incidental light angle and applied voltage. Thin Solid Films 2016, 603, 180–186. [Google Scholar] [CrossRef]
- Awasthi, A.; Kumar, D.; Marla, D. Understanding the role of oxide layers on color generation and surface characteristics in nanosecond laser color marking of stainless steel. Opt. Laser Technol. 2024, 171, 110469. [Google Scholar] [CrossRef]
- Grossmann, K.; Arnold, T.; Steudtner, R.; Weiss, S.; Bernhard, G. Visualizing different uranium oxidation states during the surface alteration of uraninite and uranium tetrachloride. Naturwissenschaften 2009, 96, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, G.; Desgranges, L.; Charlot, F.; Millot, N.; Nièpce, J.C.; Pijolat, M.; Valdivieso, F.; Baldinozzi, G.; Bérar, J.F. A detailed study of UO2 to U3O8 oxidation phases and the associated rate-limiting steps. J. Nucl. Mater. 2006, 355, 10–20. [Google Scholar] [CrossRef]
- Kelly, D.; Lillard, J.A.; Manner, W.L.; Hanrahan, R.; Paffett, M.T. Surface characterization of oxidative corrosion of U-Nb alloys. J. Vac. Sci. Technol. A-Vac. Surf. Film. 2001, 19, 1959–1964. [Google Scholar] [CrossRef]
- Luo, L.; Yang, J.; Zhou, P. Study on thermo-oxide layers of uranium-niobium alloy. At. Energy Sci. Technol. 2010, 44, 1047–1053. [Google Scholar]
- Mcgillivray, G.W.; Geeson, D.A.; Greenwood, R.C. Studies of the Kinetics and Mechanism of the Oxidation of Uranium by Dry and Moist Air—A Model for Determining the Oxidation Rate over a Wide-Range of Temperatures and Water-Vapor Pressures. J. Nucl. Mater. 1994, 208, 81–97. [Google Scholar] [CrossRef]
- Manner, W.L.; Lloyd, J.A.; Hanrahan, R.J.; Paffett, M.T. An examination of the initial oxidation of a uranium-base alloy (U–14.1 at.% Nb) by O2 and D2O using surface-sensitive techniques. Appl. Surf. Sci. 1999, 150, 73–88. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clarke, E.M.; Henderson, H.B.; Elton, E.S.; Li, T.T.; Winston, L.D.; Crystal, I.R.; Long, O.G.; Harris, S.L.; Stillwell, R.L.; Jeffries, J.R.; et al. Investigation of In Situ and Ex Situ Passivation of Pyrophoric Uranium–Niobium Alloy Powder. Appl. Sci. 2025, 15, 6431. https://doi.org/10.3390/app15126431
Clarke EM, Henderson HB, Elton ES, Li TT, Winston LD, Crystal IR, Long OG, Harris SL, Stillwell RL, Jeffries JR, et al. Investigation of In Situ and Ex Situ Passivation of Pyrophoric Uranium–Niobium Alloy Powder. Applied Sciences. 2025; 15(12):6431. https://doi.org/10.3390/app15126431
Chicago/Turabian StyleClarke, Evan M., Hunter B. Henderson, Eric S. Elton, Tian T. Li, Logan D. Winston, Isabel R. Crystal, Olivia G. Long, Sharee L. Harris, Ryan L. Stillwell, Jason R. Jeffries, and et al. 2025. "Investigation of In Situ and Ex Situ Passivation of Pyrophoric Uranium–Niobium Alloy Powder" Applied Sciences 15, no. 12: 6431. https://doi.org/10.3390/app15126431
APA StyleClarke, E. M., Henderson, H. B., Elton, E. S., Li, T. T., Winston, L. D., Crystal, I. R., Long, O. G., Harris, S. L., Stillwell, R. L., Jeffries, J. R., Kuntz, J. D., & Huang, K. (2025). Investigation of In Situ and Ex Situ Passivation of Pyrophoric Uranium–Niobium Alloy Powder. Applied Sciences, 15(12), 6431. https://doi.org/10.3390/app15126431






