Biofabricating Three-Dimensional Bacterial Cellulose Composites Using Waste-Derived Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Scoby Culture
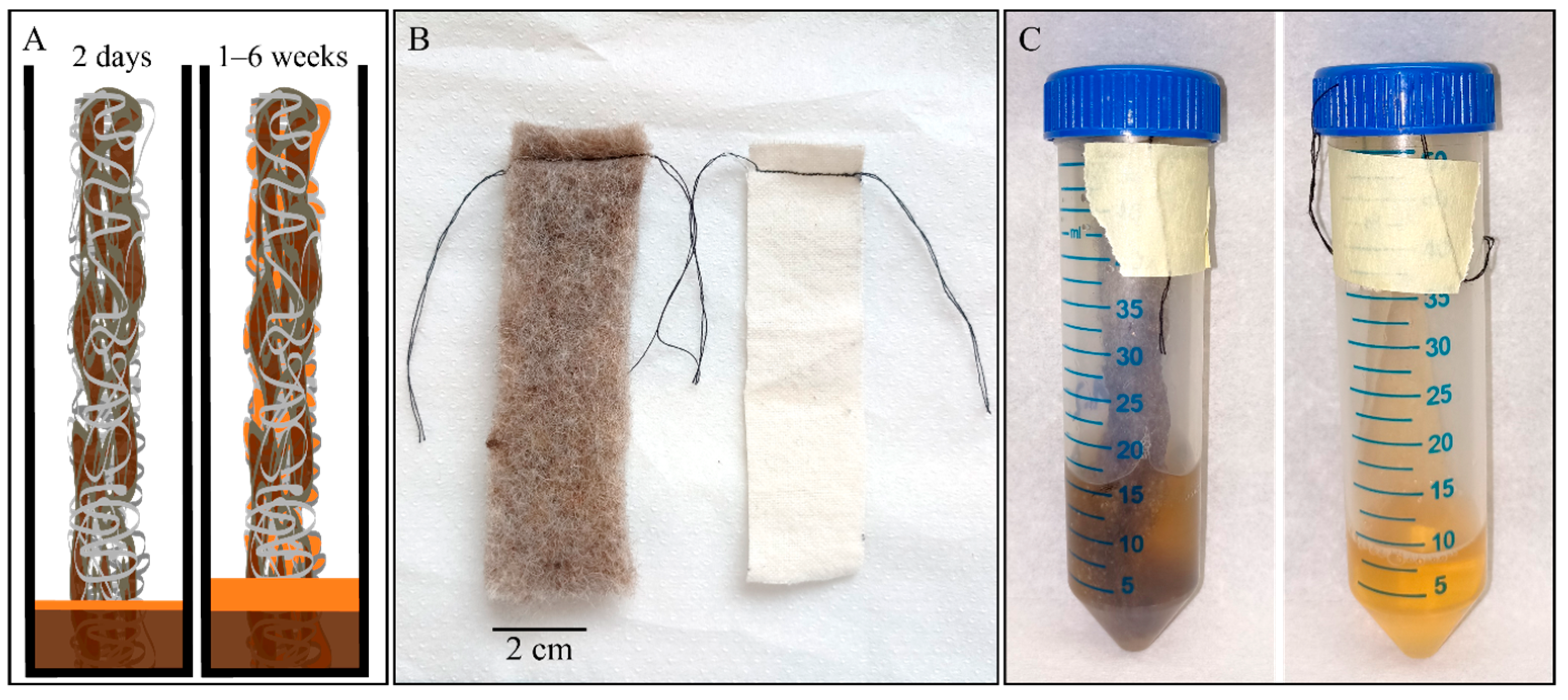
2.2. Growing Using Scaffolds
2.3. Visual Analysis
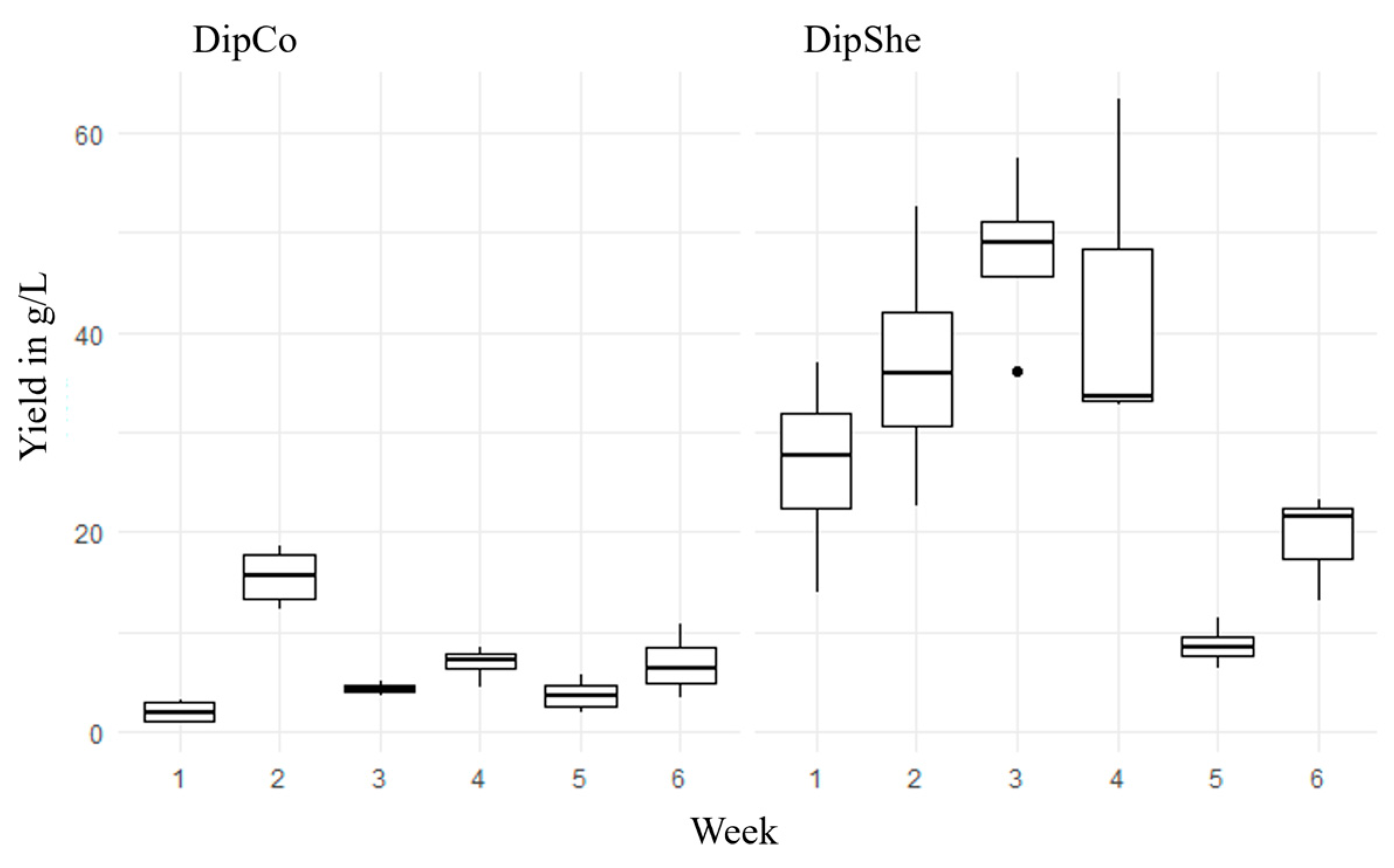
3. Results
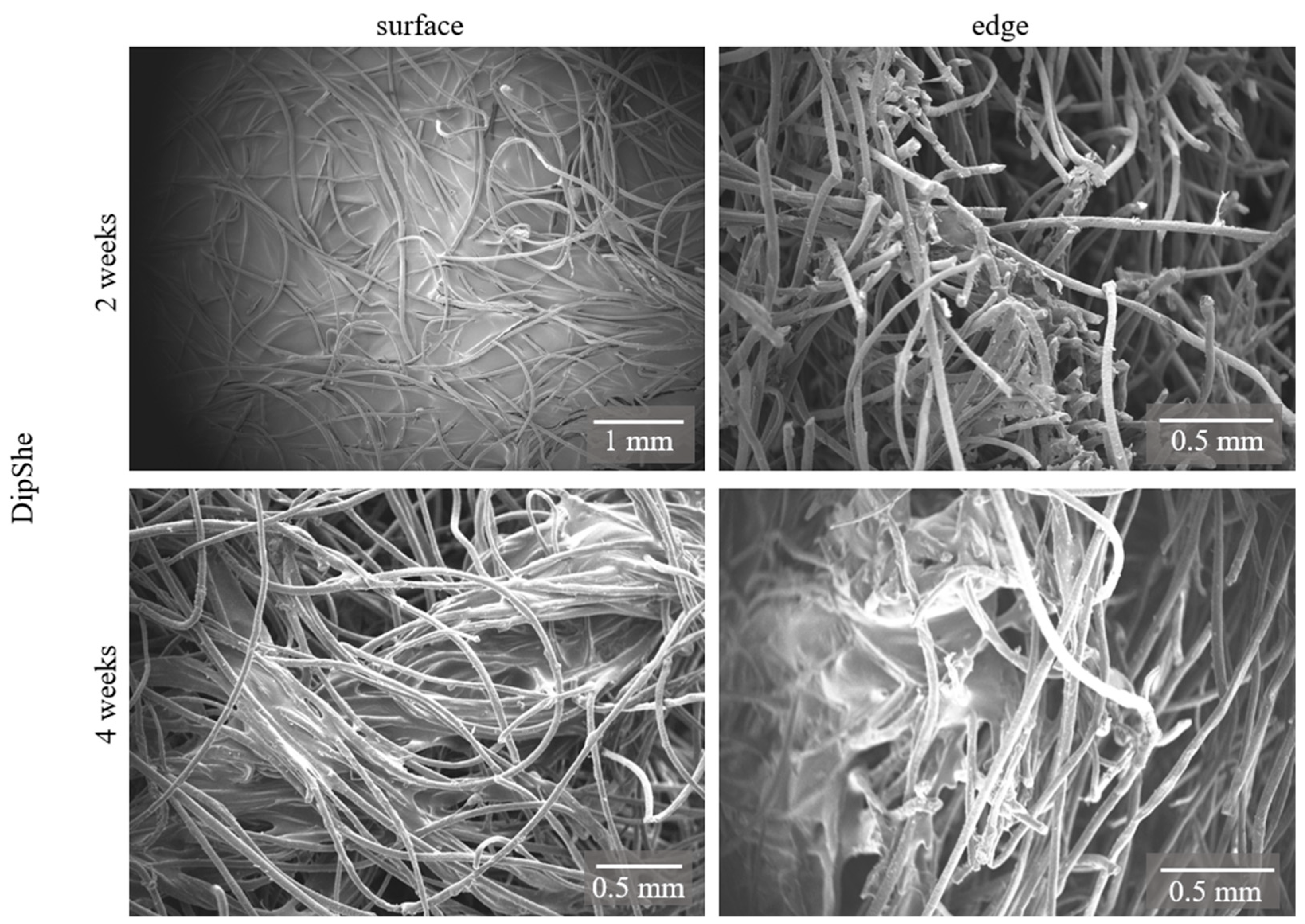
3.1. DipShe
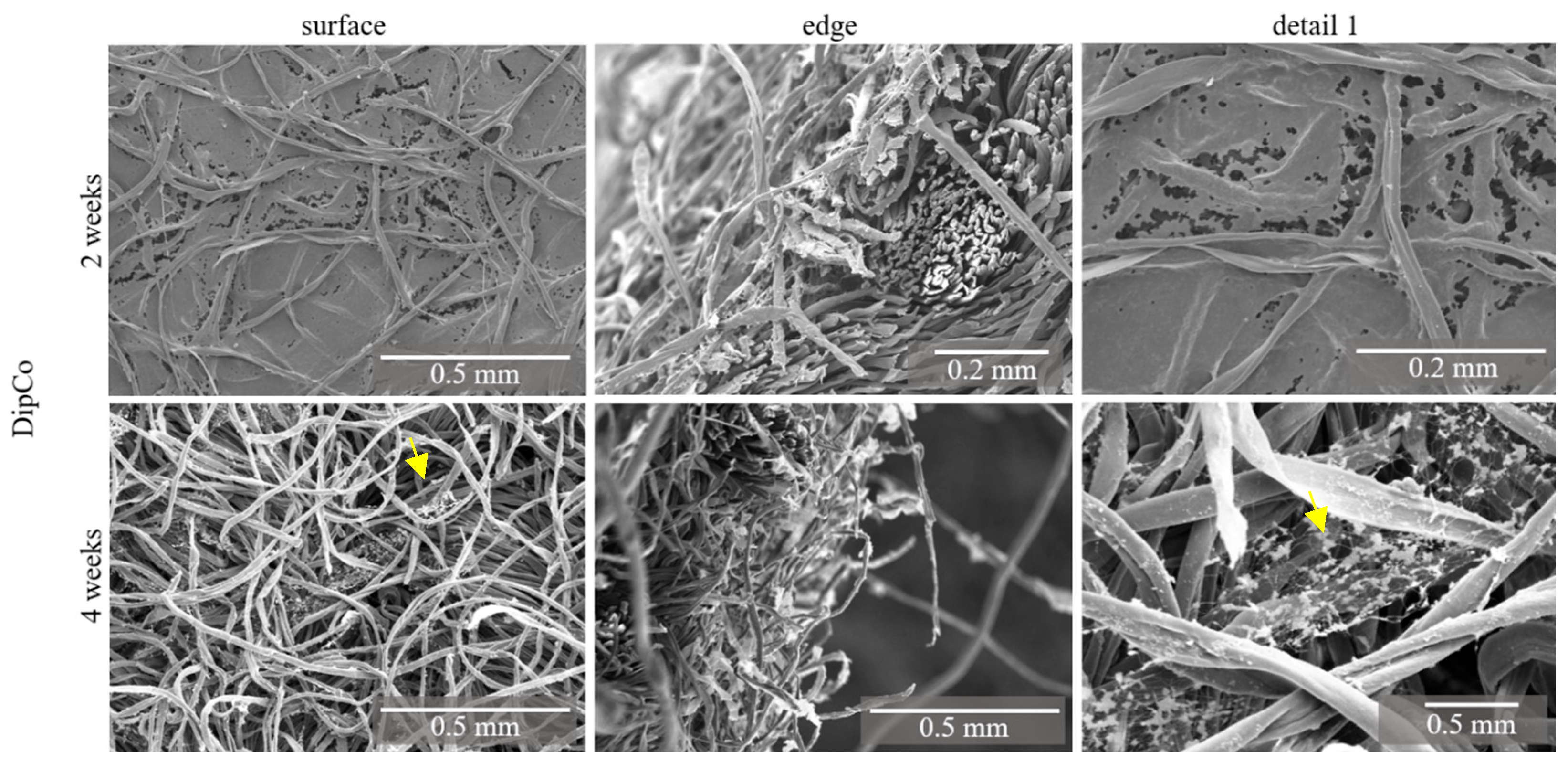
3.2. DipCo
4. Discussion
4.1. Biofilm Growth
4.2. SCOBY Growth in Scaffold Materials
4.3. Waste to Value—Design Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Bacterial Cellulose |
| DipCo | Dip-in cotton fabric samples |
| DipShe | Dip-in Sheep wool felt samples |
| LAI | Liquid–air interface |
| SCOBY | Symbiotic Culture of Bacteria and Yeast |
| SEM | Scanning electron microscope |
References
- Earth Overshoot Day. 2024. Available online: https://overshoot.footprintnetwork.org/newsroom/press-release-2024-english/ (accessed on 9 September 2024).
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. World Bank Group Waste a Waste 2.0. 2018. Available online: http://hdl.handle.net/10986/30317 (accessed on 9 September 2024).
- Leonarski, E.; Guimarães, A.C.; Cesca, K.; Poletto, P. Production process and characteristics of kombucha fermented from alternative raw materials. Food Biosci. 2022, 49, 101841. [Google Scholar] [CrossRef]
- Nguyen, T.K.T.; Dao, Q.K.; Tanaka, D.; Nghiem, L.H.T.; Nguyen, M.V.; Nguyen, Z.H.; Pham, T.T. Flexible, affordable and environmentally sustainable solar vapor generation based on ferric tannate/bacterial cellulose composite for efficient desalination solutions. RSC Adv. 2021, 11, 31641–31649. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Patel, P.; Shukla, A.; Wong, J.W.C.; Varjani, S.; Gosai, H. Sustainable Bioconversion of Industrial Wastes into Bacterial Cellulose for Diverse Applications: A Way Towards Pollution Control and Abatement. Curr. Pollut. Rep. 2023, 9, 226–242. [Google Scholar] [CrossRef]
- Quijano, L.; Rodrigues, R.; Fischer, D.; Tovar-Castro, J.D.; Payne, A.; Navone, L.; Hu, Y.; Yan, H.; Pinmanee, P.; Poon, E.; et al. Bacterial Cellulose Cookbook: A Systematic Review on Sustainable and Cost-Effective Substrates. J. Bioresour. Bioprod. 2024, 9, 379–409. [Google Scholar] [CrossRef]
- Takayama, G. Structure−Property Relationship of Bacterial Cellulose Nanofiber Networks Leading to Precise Material Design. Kyushu University Institutional Repository Structure-Property. 2023. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4482800 (accessed on 9 September 2024).
- Klemm, D.; Schumann, D.; Kramer, F.; Heßler, N.; Hornung, M.; Schmauder, H.P.; Marsch, S. Nanocelluloses as innovative polymers in research and application. Adv. Polym. Sci. 2006, 205, 49–96. [Google Scholar]
- Huang, K.; Maltais, A.; Wang, Y. Enhancing water resistance of regenerated cellulose films with organosilanes and cellulose nanocrystals for food packaging. Carbohydr. Polym. Technol. Appl. 2023, 6, 100391. [Google Scholar] [CrossRef]
- Marcuello, C.; Foulon, L.; Chabbert, B.; Aguié-Béghin, V.; Molinari, M. Atomic force microscopy reveals how relative humidity impacts the Young’s modulus of lignocellulosic polymers and their adhesion with cellulose nanocrystals at the nanoscale. Int. J. Biol. Macromol. 2020, 147, 1064–1075. [Google Scholar] [CrossRef]
- Dutta, H.; Paul, S.K. Kombucha Drink: Production, Quality, and Safety Aspects. In Production and Management of Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 259–288. [Google Scholar]
- Laavanya, D.; Shirkole, S.; Balasubramanian, P. Current challenges, applications and future perspectives of SCOBY cellulose of Kombucha fermentation. J. Clean. Prod. 2021, 295, 126454. [Google Scholar] [CrossRef]
- Sievers, M.; Lanini, C.; Weber, A.; Schuler-Schmid, U.; Teuber, M. Microbiology and Fermentation Balance in a Kombucha Beverage Obtained from a Tea Fungus Fermentation. Syst. Appl. Microbiol. 1995, 18, 590–594. [Google Scholar] [CrossRef]
- Markov, S.; Jerinic, V.; Cvetkovic, D.; Malbasa, R. Kombuha—Funkcionalni Napitak: Sastav, Karakteristike I Proces Biotransformacije. Hem. Ind. 2003, 57, 456–462. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, S.; Li, J.; Wang, B.; Jin, M.; Liang, Q.; Zhang, D.; Han, Z.; Deng, L.; Qu, X.; et al. Insights into Hierarchical Structure–Property–Application Relationships of Advanced Bacterial Cellulose Materials. Adv. Funct. Mater. 2023, 33, 2214327. [Google Scholar] [CrossRef]
- Soares, M.G.; de Lima, M.; Schmidt, V.C.R. Technological aspects of kombucha, its applications and the symbiotic culture (SCOBY), and extraction of compounds of interest: A literature review. Trends Food Sci. Technol. 2021, 110, 539–550. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Taillandier, P. Understanding Kombucha Tea Fermentation: A Review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.; Kim, M.J. A comprehensive review on kombucha biofilms: A promising candidate for sustainable food product development. Trends Food Sci. Technol. 2024, 144, 104325. [Google Scholar] [CrossRef]
- Machfidho, A.; Ismayati, M.; Sholikhah, K.W.; Kusumawati, A.N.; Salsabila, D.I.; Fatriasari, W.; Thangunpai, K.; Enomae, T.; Ihsanpuro, S.I.; Gabriel, A.A.; et al. Characteristics of bacterial nanocellulose composite and its application as self-cooling material. Carbohydr. Polym. Technol. Appl. 2023, 6, 100371. [Google Scholar] [CrossRef]
- Xiong, C.; Zhao, M.; Wang, T.; Han, J.; Zhang, Y.; Zhang, Z.; Ji, X.; Xiong, Q.; Ni, Y. Recent advances in multidimensional (1D, 2D, and 3D) Joule heating devices based on cellulose: Design, structure, application, and perspective. J. Mater. Sci. Technol. 2025, 205, 53–78. [Google Scholar] [CrossRef]
- Mai, H.H.; Dao, Q.K.; Nguyen, M.N.; Pham, H.M. Random lasing from kombucha bacterial cellulose—ZnO bionanocomposite foam. J. Phys. D Appl. Phys. 2023, 56, 495107. [Google Scholar] [CrossRef]
- Jones, R.J.; Srubar, W.V. Biomineralization of Symbiotic Cultures of Bacteria and Yeast (SCOBY) Cellulose Aerogels. Adv. Eng. Mater. 2022, 24, 2200681. [Google Scholar] [CrossRef]
- Hahn, J. Ganni Unveils Faux Leather Jacket Made Using Bacteria Instead of Cowhide. In: Dezeen. 2023. Available online: https://www.dezeen.com/2023/07/04/ganni-bacterial-cellulose-leather-jacket-polybion/ (accessed on 9 September 2024).
- Hahn, J. Public School Creates Compostable Sneaker from Kombucha Waste. In: Dezeen. 2021. Available online: https://www.dezeen.com/2021/03/10/public-school-compostable-trainers-theanne-schiros-onexone/ (accessed on 9 September 2024).
- Brogan, C. Plastic-Free Vegan Leather that Dyes Itself Grown from Bacteria|Imperial News|Imperial College London. In: Imperial. 2024. Available online: https://www.imperial.ac.uk/news/252494/plastic-free-vegan-leather-that-dyes-itself/ (accessed on 9 September 2024).
- Crook, L. Imperfect Perfection Lighting Range is Made Using Bacteria. In: Dezeen. 2020. Available online: https://www.dezeen.com/2020/04/28/imperfect-perfection-studio-lionne-van-deursen-ventura-projects-vdf/ (accessed on 9 September 2024).
- Modern Synthesis. Crafting the Next Generation of Materials. 2024. Available online: https://www.modernsynthesis.com/#tech (accessed on 9 September 2024).
- Hitti, N. Elena Amato Creates Sustainable Cosmetics Packaging from Bacteria. In: Dezeen. 2019. Available online: https://www.dezeen.com/2019/02/28/elena-amato-bacteria-packaging-design/ (accessed on 9 September 2024).
- Massoud, P.; AbouSeada, N.; M.Saada, A.; Zolfakkar, M. Creating sustainable and flexible architectural skin with microbial cellulose-based material: Synthesis and mechanical characterization. J. Umm Al-Qura Univ. Eng. Arch. 2024, 15, 455–466. [Google Scholar] [CrossRef]
- Wang, K.; Hazra, R.S.; Ma, Q.; Khan, R.H.; Al Hoque, A.; Jiang, L.; Quadir, M.; Zhang, Y.; Wang, S.; Han, G. Robust biocompatible bacterial cellulose/silk nonwoven fabric/silk sericin sandwich membrane with strong UV-blocking and antioxidant properties. Cellulose 2023, 30, 3973–3993. [Google Scholar] [CrossRef]
- Lakshmanan, A. Physical and chemical properties of wool fibers. Wool Fiber Reinf. Polym. Compos. 2022, 4, 49–71. [Google Scholar]
- Emergenresearch. Bacterial Cellulose Market Size, Trend, Demand Analysis Till 2032. 2022. Available online: https://www.emergenresearch.com/industry-report/bacterial-cellulose-market (accessed on 21 June 2024).
- Sharma, C.; Bhardwaj, N.K. Biotransformation of fermented black tea into bacterial nanocellulose via symbiotic interplay of microorganisms. Int. J. Biol. Macromol. 2019, 132, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Amorim, L.F.A.; Li, L.; Gomes, A.P.; Fangueiro, R.; Gouveia, I.C. Sustainable bacterial cellulose production by low cost feedstock: Evaluation of apple and tea by-products as alternative sources of nutrients. Cellulose 2023, 30, 5589–5606. [Google Scholar] [CrossRef]
- Khan, S.; Ul-Islam, M.; Fatima, A.; Manan, S.; Khattak, W.A.; Limited, E.Y.S.W.C.; WajidUllah, M.W.; Yang, G. Potential of Food and Agro-Industrial Wastes for Cost-Effective Bacterial Cellulose Production: An Updated Review of Literature. ES Food Agrofor. 2023, 13, 905. [Google Scholar] [CrossRef]
- Priyadharshini, T.; Nageshwari, K.; Vimaladhasan, S.; Parag Prakash, S.; Balasubramanian, P. Machine learning prediction of SCOBY cellulose yield from Kombucha tea fermentation. Bioresour. Technol. Rep. 2022, 18, 101027. [Google Scholar] [CrossRef]
- Tapias, Y.A.R.; Di Monte, M.V.; Peltzer, M.A.; Salvay, A.G. Bacterial cellulose films production by Kombucha symbiotic community cultured on different herbal infusions. Food Chem. 2022, 372, 131346. [Google Scholar] [CrossRef]
- Esa, F.; Tasirin, S.M.; Rahman, N.A. Overview of Bacterial Cellulose Production and Application. Agric. Agric. Sci. Procedia 2014, 2, 113–119. [Google Scholar] [CrossRef]
- Domskiene, J.; Sederaviciute, F.; Simonaityte, J. Kombucha bacterial cellulose for sustainable fashion. Int. J. Cloth. Sci. Technol. 2019, 31, 644–652. [Google Scholar] [CrossRef]
- Shavyrkina, N.A.; Skiba, E.A.; Kazantseva, A.E.; Gladysheva, E.K.; Budaeva, V.V.; Bychin, N.V.; Gismatulina, Y.A.; Kashcheyeva, E.I.; Mironova, G.F.; Korchagina, A.A.; et al. Static culture combined with aeration in biosynthesis of bacterial cellulose. Polymers 2021, 13, 4241. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Praharaj, P.T. Principal component analysis revealed the key influencing factors of kombucha bacterial cellulose yield and productivity. Technol. Rep. 2023, 23, 101539. [Google Scholar] [CrossRef]
- Biagiotti, J.; Puglia, D.; Kenny, J.M. A review on natural fibre-based composites—Part I: Structure, processing and properties of vegetable fibres. J. Nat. Fibers 2004, 1, 37–68. [Google Scholar] [CrossRef]
- Popescu, C.; Wortmann, F.J. Wool-Structure, Mechanical Properties and Technical Products based on Animal Fibres. In Industrial Applications of Natural Fibres: Structure, Properties and Technical Applications; John Wiley & Sons, Ltd.: London, UK, 2010; pp. 255–266. [Google Scholar]
- Czaja, W.; Romanovicz, D.; Brown, R.M. Structural investigations of microbial cellulose produced in stationary and agitated culture. Cellulose 2004, 1, 403–411. [Google Scholar] [CrossRef]
- Phiri, R.; Rangappa, S.M.; Siengchin, S.; Oladijo, O.P.; Dhakal, H.N. Development of sustainable biopolymer-based composites for lightweight applications from agricultural waste biomass: A review. Adv. Ind. Eng. Polym. Res. 2023, 6, 436–450. [Google Scholar] [CrossRef]
- Mujtaba, M.; Fraceto, L.F.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; de Medeiros, G.A.; Pereira, A.D.E.S.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic biomass from agricultural waste to the circular economy: A review with focus on biofuels, biocomposites and bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Vea, E.B.; Romeo, D.; Thomsen, M. Biowaste Valorisation in a Future Circular Bioeconomy. Procedia CIRP 2018, 69, 591–596. [Google Scholar] [CrossRef]
- Karana, E.; Blauwhoff, D.; Hultink, E.J.; Camere, S. When the material grows: A case study on designing (with) mycelium-based materials. Int. J. Des. 2018, 12, 119–136. [Google Scholar]
- Gundogdu, T.K.; Deniz, I.; Ariç, A.; Yilmazsoy, B.T.; Andic-Cakir, O.; Erdoğan, A.; Altun, D.A.; Tokuç, A.; Demirci, B.F.; Sendemir, A.; et al. Development of Ecological Biodesign Products by Bacterial Biocalcification. EJENS 2019, 3, 17–25. [Google Scholar]
- Gargey, I.A.; Indira, D.; Jayabalan, R.; Balasubramanian, P. Optimization of Etherification Reactions for Recycling of Tea Fungal Biomass Waste into Carboxymethylcellulose. In Green Buildings and Sustainable Engineering; Springer: Berlin/Heidelberg, Germany, 2019; pp. 337–346. [Google Scholar]
- Brugnoli, M.; La China, S.; Lasagni, F.; Romeo, F.V.; Pulvirenti, A.; Gullo, M. Acetic acid bacteria in agro-wastes: From cheese whey and olive mill wastewater to cellulose. Appl. Microbiol. Biotechnol. 2023, 107, 3729–3744. [Google Scholar] [CrossRef]
- Qiu, W.; Ren, H.; Wang, Q. Production of bacterial cellulose from enzymatic hydrolysate of kitchen waste by fermentation with kombucha. Biomass Convers. Biorefinery 2023, 13, 14485–14496. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kniep, J.; Thundathil, M.; Rezwan, K.; Nazmi, A.R. Biofabricating Three-Dimensional Bacterial Cellulose Composites Using Waste-Derived Scaffolds. Appl. Sci. 2025, 15, 6396. https://doi.org/10.3390/app15126396
Kniep J, Thundathil M, Rezwan K, Nazmi AR. Biofabricating Three-Dimensional Bacterial Cellulose Composites Using Waste-Derived Scaffolds. Applied Sciences. 2025; 15(12):6396. https://doi.org/10.3390/app15126396
Chicago/Turabian StyleKniep, Jula, Manu Thundathil, Kurosch Rezwan, and Ali Reza Nazmi. 2025. "Biofabricating Three-Dimensional Bacterial Cellulose Composites Using Waste-Derived Scaffolds" Applied Sciences 15, no. 12: 6396. https://doi.org/10.3390/app15126396
APA StyleKniep, J., Thundathil, M., Rezwan, K., & Nazmi, A. R. (2025). Biofabricating Three-Dimensional Bacterial Cellulose Composites Using Waste-Derived Scaffolds. Applied Sciences, 15(12), 6396. https://doi.org/10.3390/app15126396






