Preparation of Nitrogen-Rich Tar by Co-Pyrolysis and Analysis of Nitrogen-Containing Compounds in Pyrolysis Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pyrolysis Processes
2.3. GC/MS Detection
2.4. Elemental Analysis
2.5. X-Ray Photoelectron Spectroscopy (XPS)
2.6. FTIR Analysis
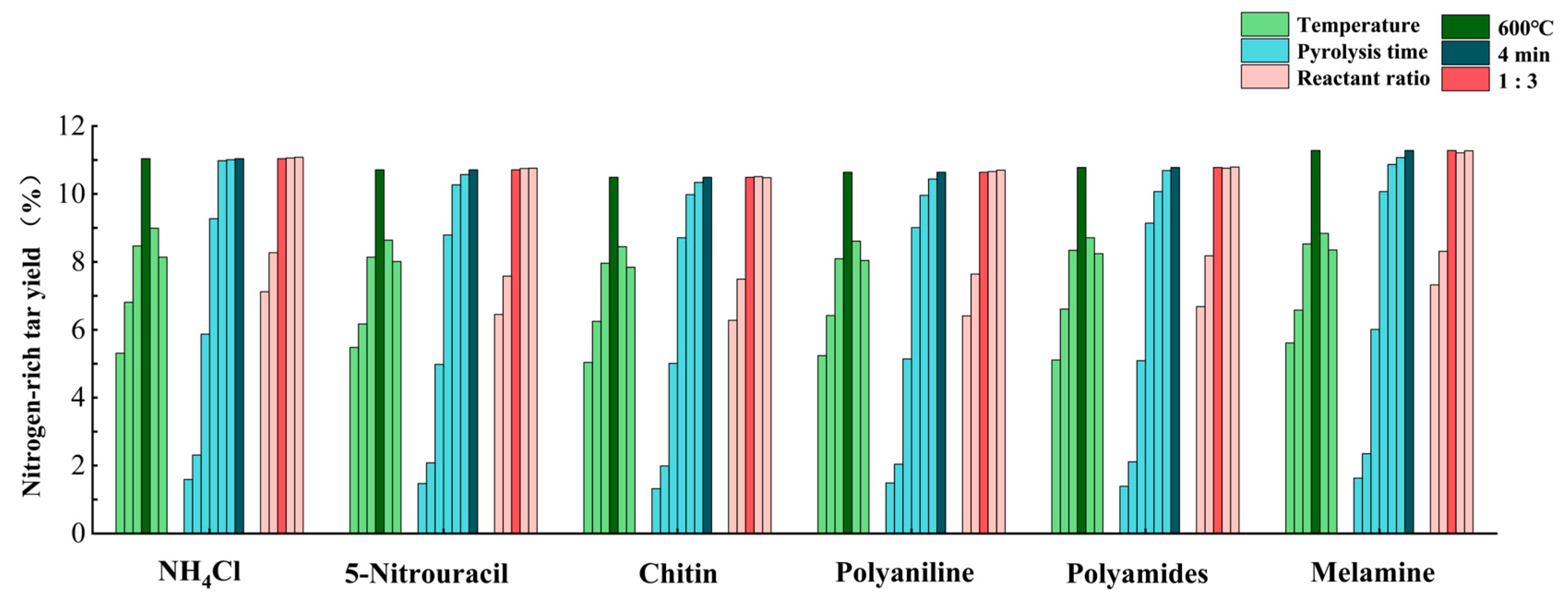
3. Results
3.1. Elemental Analysis of Char and Tar
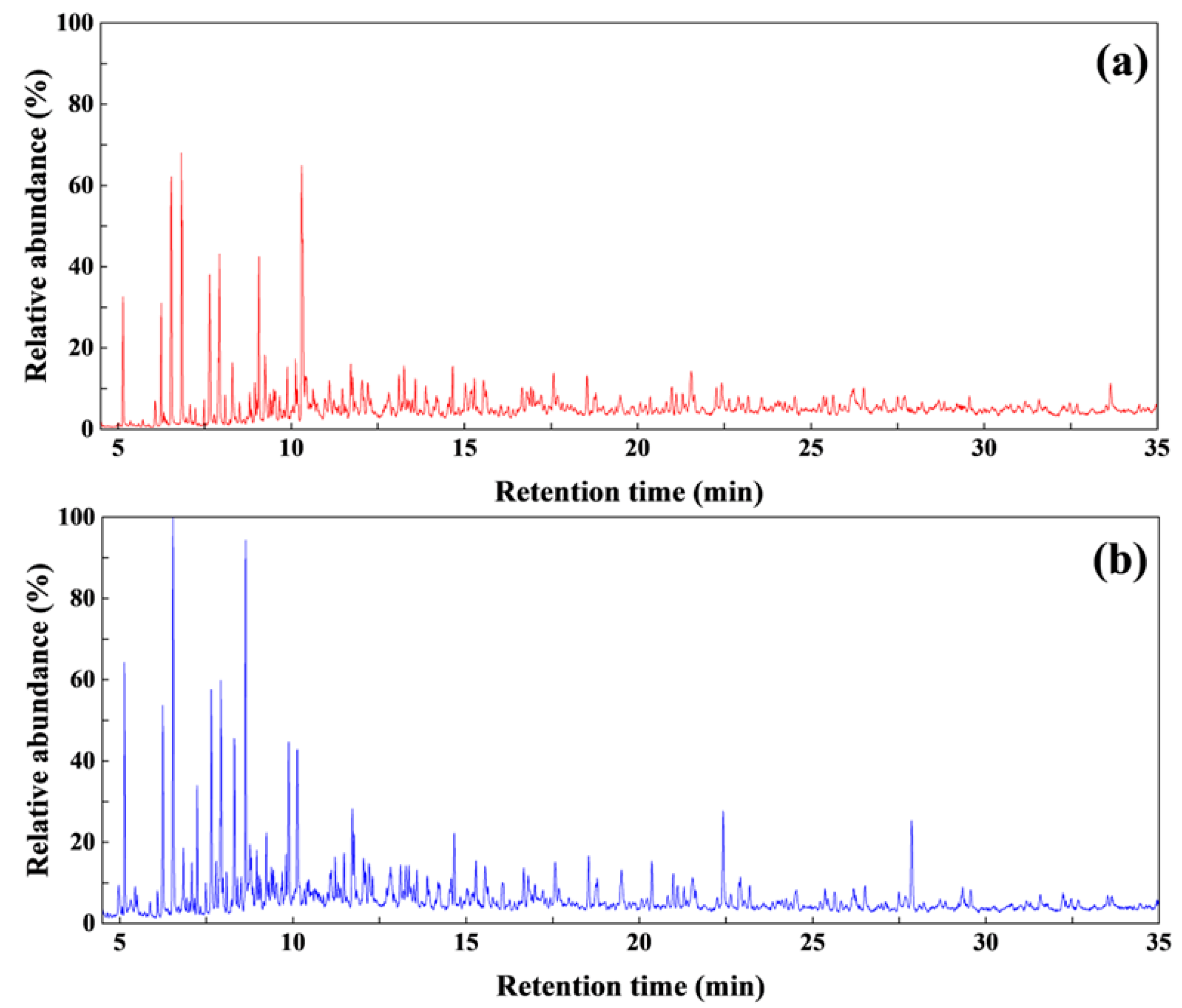
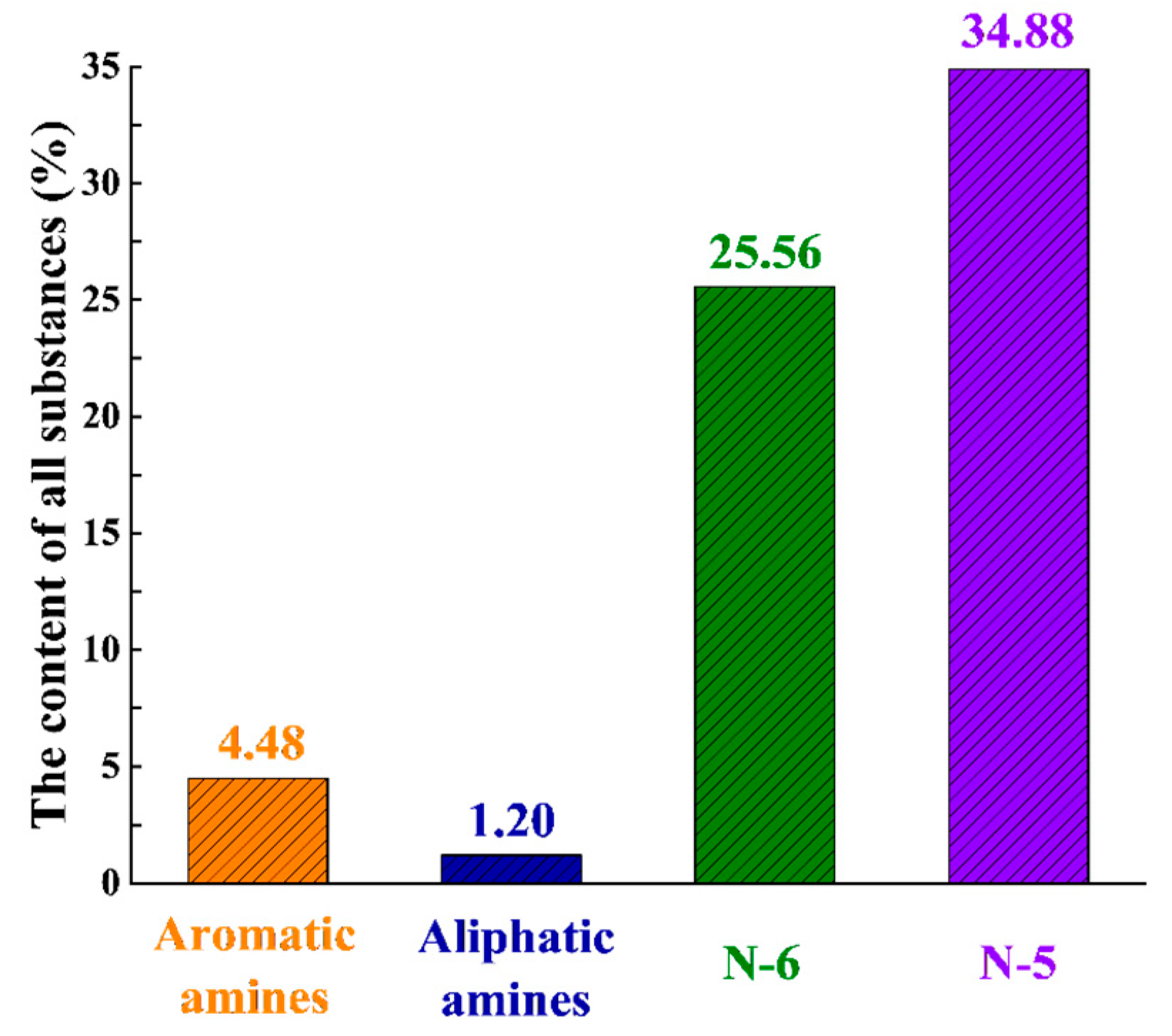
3.2. GC/MS Analysis
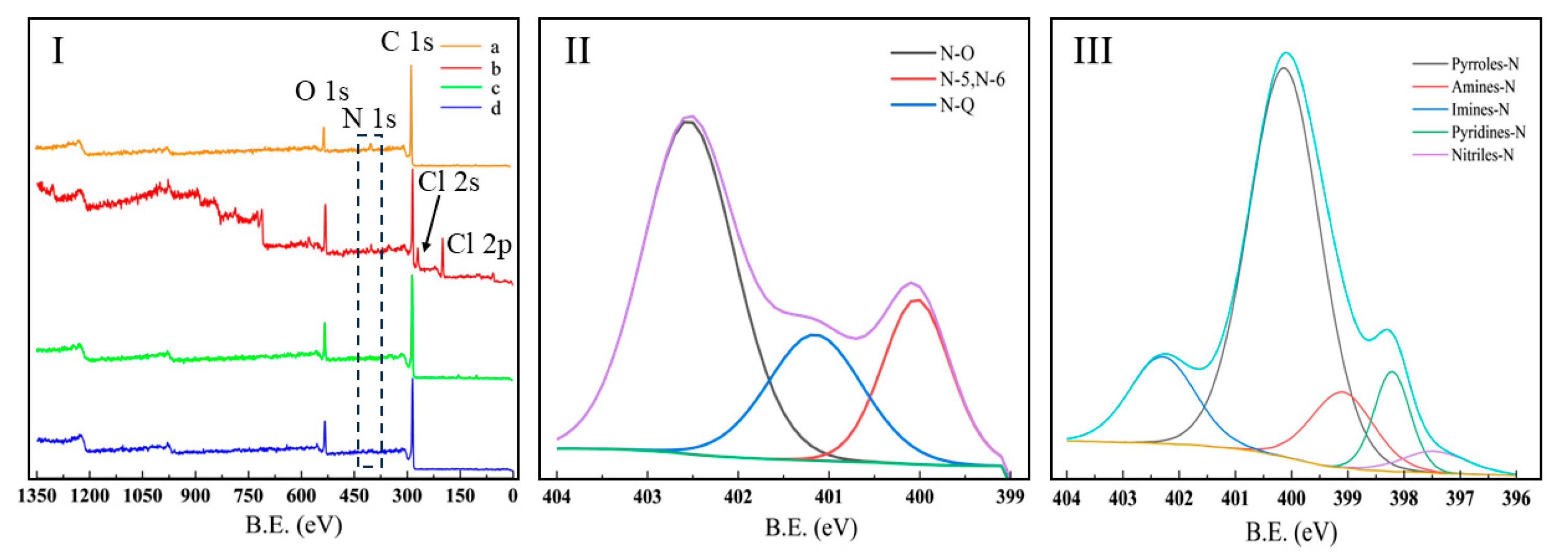
3.3. XPS Analysis
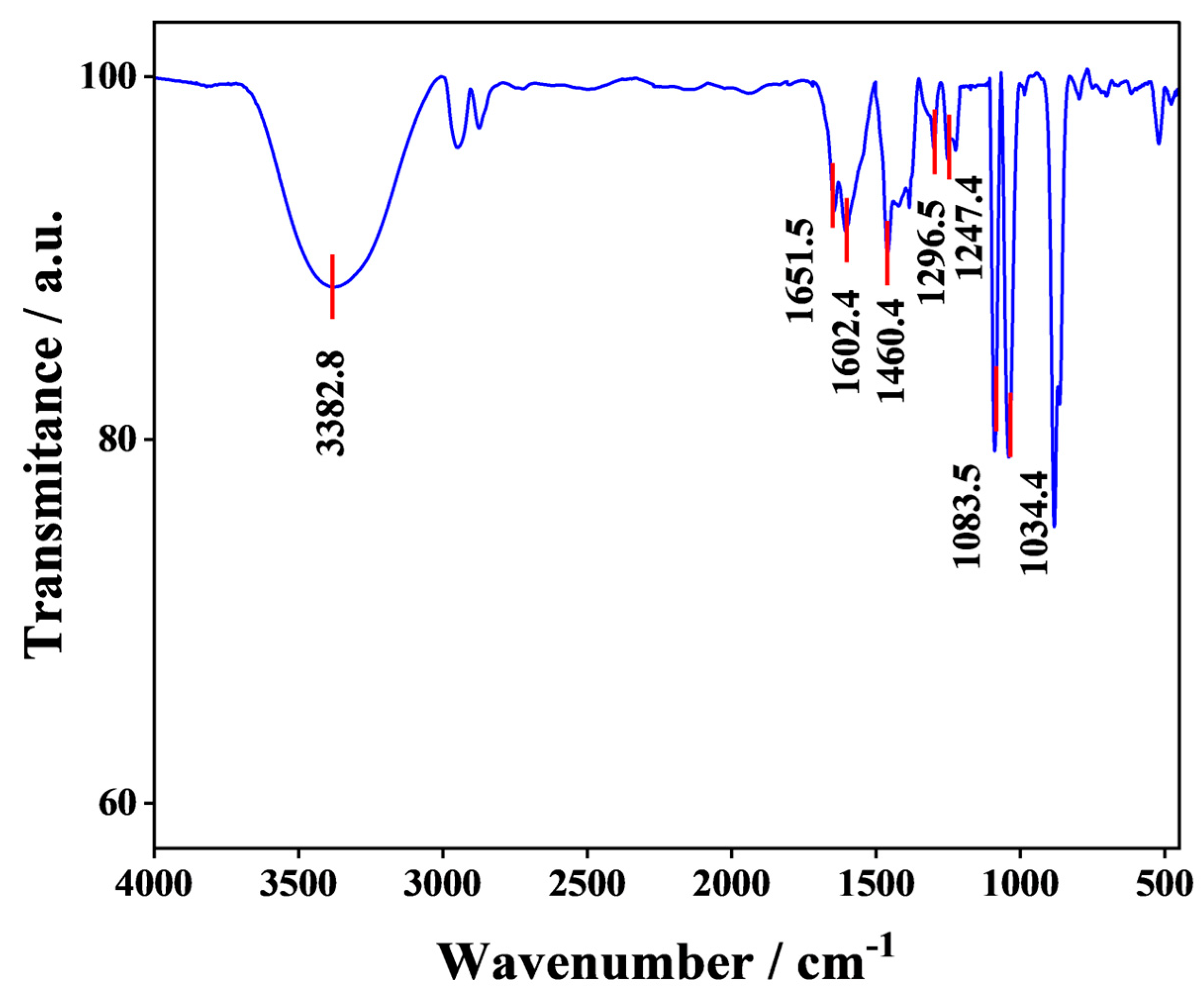
3.4. FTIR Analysis of Nitrogen-Rich Tar
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, J.; Ma, Y.; Li, S.; Shi, J.; He, L.; Li, J. Transformation of sulfur and nitrogen during Shenmu coal pyrolysis. Fuel 2018, 231, 134–144. [Google Scholar] [CrossRef]
- Han, D.; Yang, Q. China Coalfield Geology; China Coal Industry Publishing House: Beijing, China, 1979. [Google Scholar]
- Zhang, L.; Han, Z.; Shu, H.; Jia, Y.; Kuang, W.; Sun, Z. Basic characteristics of low-temperature pyrolysis of tar-rich coal in northern Shaanxi. Coal Eng. 2022, 54, 124–128. [Google Scholar]
- Zhao, N.; Liu, D.; Du, H.; Wang, C.; Wen, F.; Shi, N. Investigation on Component Separation and Structure Characterization of Medium-Low Temperature Coal Tar. Appl. Sci. 2019, 9, 4335. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, H.; Zhang, Y.; Ding, L.; Liu, X.; Wang, C.; Han, Z.; Jia, X.; Xu, G. Pyrolysis of coal and biomass in a fixed bed with internals to produce volatiles with respectively enriched light and heavy fractions. Fuel 2025, 397, 135365. [Google Scholar] [CrossRef]
- Ordabaeva, A.; Muldakhmetov, Z.; Meiramov, M.; Kim, S.; Sagintaeva, Z. Production of Pitch from Coal Tar of the Coke Chemical Production “Qarmet”. Molecules 2025, 30, 1441. [Google Scholar] [CrossRef]
- Correia, C.; Silva, A.; Silva, V. The Role of Flow Chemistry on the Synthesis of Pyrazoles, Pyrazolines and Pyrazole-Fused Scaffolds. Molecules 2025, 30, 1582. [Google Scholar] [CrossRef]
- Ren, X.; Feng, X.; Cao, J.; Tang, W.; Wang, Z.; Yang, Z.; Zhao, J.-P.; Zhang, L.-Y.; Wang, Y.-J.; Zhao, X.-Y. Catalytic Conversion of Coal and Biomass Volatiles: A Review. Energy Fuels 2020, 34, 10307–10363. [Google Scholar] [CrossRef]
- Roser, B.P.; Korsch, R.J. Provenance signatures of sandstone-mudstone suites determined using discriminant function analysis of major-element data. Chem. Geol. 1988, 67, 119–139. [Google Scholar] [CrossRef]
- Liu, Q.; Meng, W.; Qiu, Y.; Chen, L.; Song, Z.; Qi, Z. Multilevel screening and mechanism analysis of ionic liquids for separating pyridine from coal pyrolysis model oil. Sep. Purif. Technol. 2025, 362, 131856. [Google Scholar] [CrossRef]
- Wu, L.; Wei, Z.; Li, W.; Jin, K.; Zheng, Z.; Wang, D.; Wang, Q.-P.; Hou, Y.-T.; Ye, C.-Y.; Cheng, X.-Y.; et al. Experimental and kinetic study on the co-oxidation of pyridine and ammonia as a model compound of coal-ammonia co-firing. Combust. Flame 2025, 277, 114211. [Google Scholar] [CrossRef]
- Chen, X.; Zuo, P.; Min, R.; Zhang, G.; Zhao, S. Macromolecular pore model construction and theoretical study of Inner Mongolia Long-flame coal. Colloids Surf. A Physicochem. Eng. Asp. 2025, 717, 136772. [Google Scholar] [CrossRef]
- Meng, F.; Liu, Y.; Shi, K.; Zhou, J.; Yan, F.; Wang, J. Structural Characteristics of the Yunnan Deashed Lignite and Construction of the Macromolecular Structure Model. ACS Omega 2024, 9, 33594–33605. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hong, D.; Zhao, W.; Zheng, F.; Lyu, W.; Lu, J. Transformation simulation of N-containing functional groups in coal pyrolysis and combustion processes by using ReaxFF. Chem. Eng. Sci. 2024, 287, 119709. [Google Scholar] [CrossRef]
- Zhao, K.; Tao, S.; Yan, L.; Li, P.; Zhao, Y.; Li, X.; Wang, M.; Wang, J.; Chang, L.; Bao, W. Experimental and simulated analysis for nitrogen migration behavior during coal rapid pyrolysis under Ar/CO2 atmospheres using a free-fall reactor. J. Anal. Appl. Pyrolysis 2025, 186, 106982. [Google Scholar] [CrossRef]
- Wang, Z.; Meng, X.; Yang, J.; Chen, M.; Leng, L.; Li, H.; Zhan, H. Co-combustion of brewery spent grain and coal: Optimization strategies and synergistic effects. Energy 2025, 327, 136494. [Google Scholar] [CrossRef]
- Paula, S.; Chagas, B.; Pereira, M.; Rangel, A.; Sassi, C.; Borba, L.; Santos, E.S.; Asevedo, E.A.; Camara, F.R.A.; Araujo, R.M. Pyrolysis-GCMS of Spirulina platensis: Evaluation of biomasses cultivated under autotrophic and mixotrophic conditions. PLoS ONE 2022, 17, e0276317. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Y.; Li, C.; Yang, Y.; Zhang, W.; Zhao, C.; Wang, S. N-doping of biomass by ammonia (NH3) torrefaction pretreatment for the production of renewable N-containing chemicals by fast pyrolysis. Bioresour. Technol. 2019, 292, 122034. [Google Scholar] [CrossRef]
- Zhu, H.; Cheng, M.; Xu, J.; Chen, S.; Niu, F.; Yu, D. Nitrogen migration and transformation from ammonia to char during ammonia-coal/char co-pyrolysis. Int. J. Hydrogen Energy 2024, 49, 137–148. [Google Scholar] [CrossRef]
- Hong, D.; Yuan, L.; Wang, C.; Wang, H. Insight into the Competitive and Synergistic Effects during Coal/NH3 Cofiring via Reactive Molecular Dynamics Simulations. Energy Fuels 2023, 37, 3071–3082. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Z.; Zhao, J. Subcritical Extraction of Coal Tar Slag and Analysis of Extracts and Raffinates. Appl. Sci. 2025, 15, 2694. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Z.; Li, X. Analysis of the Organic Chemical Fractions of Three Coal Extracts. Appl. Sci. 2024, 14, 8933. [Google Scholar] [CrossRef]
- Muñoz-Castells, R.; Sánchez-Suárez, F.; Moreno, J.; Alvarez-Gil, J.; Moreno-García, J. The Effects of Flocculant Yeast or Spontaneous Fermentation Strategies Supplemented with an Organic Nitrogen-Rich Additive on the Volatilome and Organoleptic Profile of Wines from a Neutral Grape Variety. Appl. Sci. 2025, 15, 4196. [Google Scholar] [CrossRef]
- Li, Q.; Song, X.; Cao, X.; Wang, H.; Zhang, J.; Wang, S. Interaction between surfactants and hydrophobic associative polymers for oilfield applications. J. Petrochem. Univ. 2013, 26, 52–56. [Google Scholar]
- Wang, M.; Jin, L.; Zhao, H.; Yang, X.; Li, Y.; Hu, H.; Bai, Z. In-situ catalytic upgrading of coal pyrolysis tar over activated carbon supported nickel in CO2 reforming of methane. Fuel 2019, 250, 203–210. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Jin, L.; Hu, H. Integrated Process of Coal Pyrolysis with Steam Reforming of Ethane for Improving the Tar Yield. Energy Fuels 2019, 32, 12268–12276. [Google Scholar] [CrossRef]
- Kuroki, S.; Nabae, Y.; Chokai, M.; Kakimoto, M.; Miyata, S. Oxygen reduction activity of pyrolyzed polypyrroles studied by 15N solid-state NMR and XPS with principal component analysis. Carbon 2012, 50, 153–162. [Google Scholar] [CrossRef]
- Tan, Z.; Ye, Z.; Zhang, L.; Huang, Q. Application of the 15N tracer method to study the effect of pyrolysis temperature and atmosphere on the distribution of biochar nitrogen in the biomass-biochar-plant system. Sci. Total Environ. 2018, 622, 79–87. [Google Scholar] [CrossRef]
- Ye, Z.; Liu, L.; Tan, Z.; Zhang, L.; Huang, Q. Effects of pyrolysis conditions on migration and distribution of biochar nitrogen in the soil-plant-atmosphere system. Sci. Total Environ. 2020, 723, 138006. [Google Scholar] [CrossRef]
- Pietrzak, R. XPS study and physico-chemical properties of nitrogen-enriched microporous activated carbon from high volatile bituminous coal. Fuel 2009, 88, 1871–1877. [Google Scholar] [CrossRef]
- May, H. Pyrolysis of melamine. J. Appl. Chem. 1959, 9, 340–344. [Google Scholar] [CrossRef]
- He, Y.; Chen, F.; Han, X.; Zhang, N. Conversion of methyl cinnamic acid into nitrogenous compounds during pyrolysis revealed using 15N isotopic labeling. J. Anal. Appl. Pyrolysis 2024, 178, 106408. [Google Scholar] [CrossRef]


| Sample | N Analysis (wt %) | |
|---|---|---|
| Char | Tar | |
| Rich-oil coal | 0.77 | - |
| Rich-oil coal pyrolysis | 0.74 | 1.83 |
| Rich-oil coal and NH4Cl co-pyrolysis | 2.38 | 6.49 |
| Rich-oil coal and 5-nitrouracil co-pyrolysis | 2.19 | 5.16 |
| Rich-oil coal and chitin co-pyrolysis | 2.46 | 4.06 |
| Rich-oil coal and polyaniline co-pyrolysis | 5.74 | 6.03 |
| Rich-oil coal and polyamides co-pyrolysis | 4.65 | 5.77 |
| Rich-oil coal and melamine co-pyrolysis | 11.61 | 7.04 |
| Type of Compound | Method | Aliphatic Alkane | Aromatic Alkane | Phenol | Nitrogen Compound | Others |
|---|---|---|---|---|---|---|
| Rt (%) | Py-GC/MS | 11.47 | 60.21 | 10.96 | 5.85 | 11.51 |
| GC/MS | 17.97 | 50.18 | 12.84 | 6.98 | 12.03 |
| Rt (%) | |||||
|---|---|---|---|---|---|
| Aliphatic Alkane | Aromatic Hydrocarbon | Phenol | Nitrogen Compound | Other Compounds | |
| By NIST | 4.41 | 12.48 | 7.76 | 65.19 | 10.16 |
| By isotopic labeling | 4.52 | 12.48 | 7.76 | 66.28 | 8.96 |
| Most Likely Nitrogenous Compounds | Retention Time | Relative Abundance a | Relative Content b |
|---|---|---|---|
| 3-(tert-butyl)-1-methyl-1H-pyrrole | 4.901 | 23.14 | 2.57 |
| 2,4,6-trimethylpyridine | 5.343 | 4.41 | 0.59 |
| 2,2,5,5-Tetramethyl-4-ethyl-3-imidazoline-1-oxyl | 5.564 | 93.90 | 11.70 |
| 3-hydroxy-2,5-dimethylisonicotinaldehyde | 6.810 | 8.36 | 0.97 |
| 4-methylbenzonitrile | 6.870 | 1.28 | 0.81 |
| 2-methyl-5-(prop-1-en-2-yl)pyridine | 7.091 | 2.72 | 0.33 |
| 2,2,6,6-tetramethylpiperidin-4-one | 7.211 | 100.00 | 10.97 |
| 4-hydroxy-1,6-dimethylpyridin-2(1H)-one | 7.563 | 28.64 | 3.00 |
| 5-β,8-β-Epoxy-3,5,8,8a-tetrahydro-1H-2-benzopyran | 7.964 | 1.78 | 0.13 |
| 3,5-Dimethylbenzaldehyde thiocarbamoylhydrazone | 8.497 | 1.87 | 0.19 |
| 3-methylcyclohex-2-en-1-one O-methyl oxime | 8.738 | 2.39 | 0.42 |
| 4-((isopropylimino)methyl)-N,N-dimethylaniline | 9.180 | 2.36 | 0.20 |
| 2,2,6,6-tetramethylpiperidin-4-one O-acetyl oxime | 9.451 | 9.10 | 1.24 |
| 5-(piperidin-1-yl)furan-2-carbaldehyde | 9.692 | 11.68 | 1.26 |
| 3-Ethyl-5,6,7,8-tetrahydroquinoline | 9.973 | 2.61 | 0.24 |
| 7-[2,2-dimethoxy-1-propyl]-pyrrolizin-1-one | 10.074 | 4.06 | 0.38 |
| 4-(2,6,6-Trimethyl-cyclohex-1-enyl)-but-3-en-2-one oxime | 11.098 | 5.48 | 1.04 |
| 2-Acetyldecahydroisoquinoline-3-carbonitril | 11.972 | 1.95 | 0.10 |
| 8-ethyl-2-methyl-4-quinolinol | 12.534 | 1.91 | 0.14 |
| 12-Azabicyclo [9.2.2]pentadeca-1(14),11(15)-dien-13-one | 12.936 | 12.13 | 2.47 |
| 6,7,8,9-Tetrahydro-5-methylisothiazolo [5,4-c]isoquinolin-1(2H)-one | 13.066 | 4.82 | 1.14 |
| 1-[3-(3,3-Dimethyl-but-1-ynyl)-2,2-dimethyl-cyclopropyl]-piperazine | 13.187 | 5.23 | 1.66 |
| 9-Amino-1,8-Dimethyl-3,6-diazahomoadamantane | 13.639 | 6.37 | 1.12 |
| 2-[2-(1-piperidyl)ethyl]-2,4-benzoxazin-1-one | 16.672 | 16.68 | 2.74 |
| 2-Methyl-a-Pyrrolidinopropiophenone | 18.459 | 6.63 | 1.38 |
| 6,7-dichloro-5-[(1-ethylpyrrolidin-2-yl)methylamino]-1,3-dimethyl- pyrido [2,3-d]pyrimidine-2,4(1H,3H)-dione | 19.645 | 96.92 | 18.30 |
| Nitrogen Compound | Rt (%) | ||||
|---|---|---|---|---|---|
| Aliphatic Alkane | Aromatic Hydrocarbon | Phenol | Nitrogen Compound | Others | |
| NH4Cl | 4.41 | 12.48 | 7.76 | 65.19 | 10.16 |
| 5-Nitrouracil | 4.82 | 9.21 | 13.26 | 41.73 | 30.98 |
| chitin | 5.28 | 11.74 | 20.26 | 30.96 | 31.76 |
| polyaniline | 5.02 | 25.26 | 7.43 | 54.21 | 8.08 |
| polyamides | 5.13 | 11.08 | 15.65 | 31.85 | 36.29 |
| melamine | 4.32 | 12.34 | 6.98 | 70.48 | 5.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Li, G.; Shao, J.; Bai, B.; Hu, J.; Han, X.; Zhou, A.; Wang, Q.; Chen, F. Preparation of Nitrogen-Rich Tar by Co-Pyrolysis and Analysis of Nitrogen-Containing Compounds in Pyrolysis Products. Appl. Sci. 2025, 15, 6284. https://doi.org/10.3390/app15116284
Chen P, Li G, Shao J, Bai B, Hu J, Han X, Zhou A, Wang Q, Chen F. Preparation of Nitrogen-Rich Tar by Co-Pyrolysis and Analysis of Nitrogen-Containing Compounds in Pyrolysis Products. Applied Sciences. 2025; 15(11):6284. https://doi.org/10.3390/app15116284
Chicago/Turabian StyleChen, Peiqi, Gang Li, Jie Shao, Baoping Bai, Jie Hu, Xiang Han, Anning Zhou, Qiuhong Wang, and Fuxin Chen. 2025. "Preparation of Nitrogen-Rich Tar by Co-Pyrolysis and Analysis of Nitrogen-Containing Compounds in Pyrolysis Products" Applied Sciences 15, no. 11: 6284. https://doi.org/10.3390/app15116284
APA StyleChen, P., Li, G., Shao, J., Bai, B., Hu, J., Han, X., Zhou, A., Wang, Q., & Chen, F. (2025). Preparation of Nitrogen-Rich Tar by Co-Pyrolysis and Analysis of Nitrogen-Containing Compounds in Pyrolysis Products. Applied Sciences, 15(11), 6284. https://doi.org/10.3390/app15116284





