Machine and Deep Learning-Based Seizure Prediction: A Scoping Review on the Use of Temporal and Spectral Features
Abstract
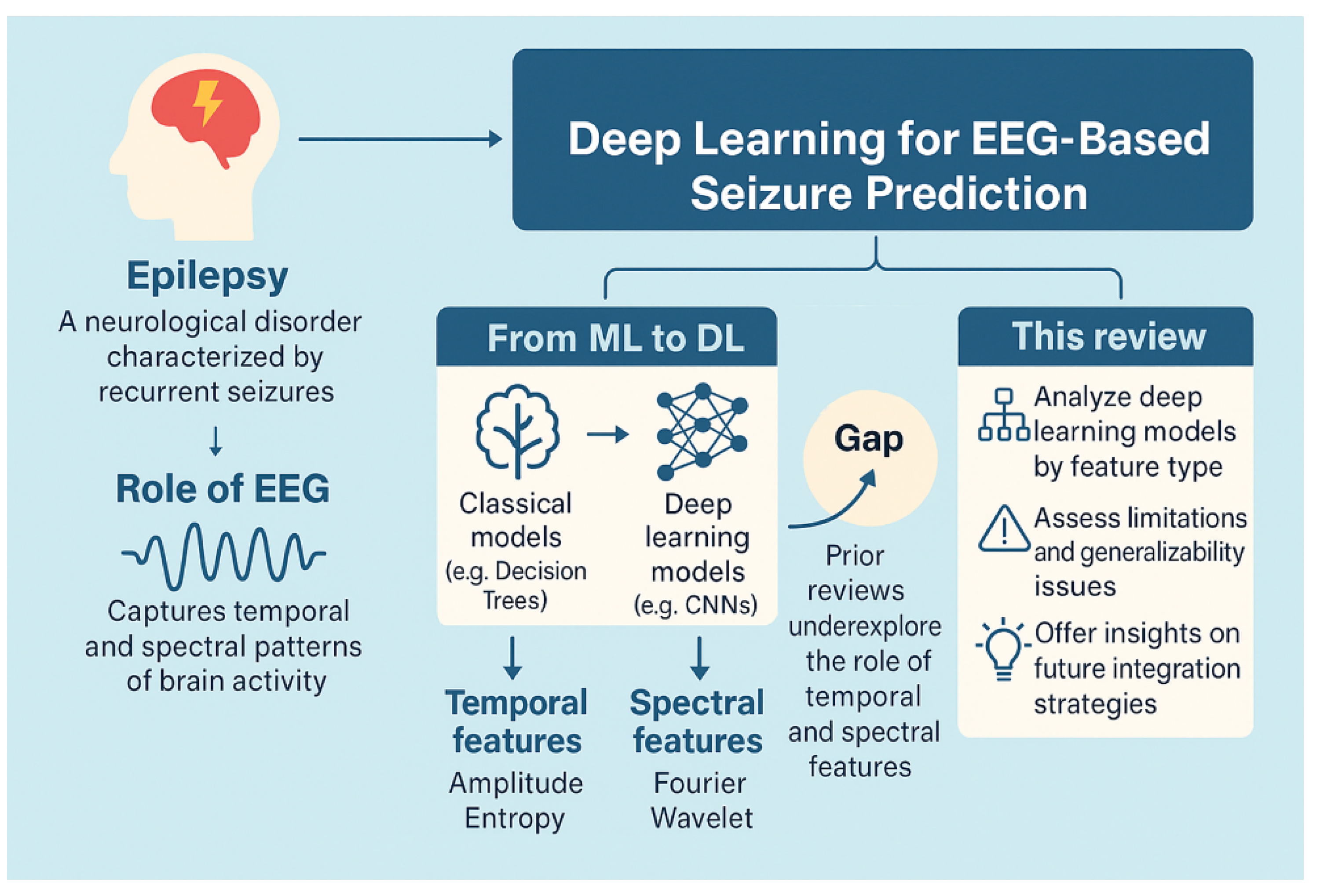
1. Introduction
- To systematically analyze deep learning models used for seizure prediction based on the nature of the temporal (e.g., amplitude, entropy, statistical time features) and spectral (e.g., Fourier, wavelet, or filter bank-based) features they utilize.
- To identify and critically assess common limitations in these approaches, including issues related to generalizability across datasets, the robustness of feature extraction methods, and computational complexity in clinical environments.
- To offer practical insights into deep feature-based classification strategies, highlighting how the integration of temporal and spectral representations can improve predictive accuracy and inform future directions in clinically viable seizure detection systems.
2. Related Reviews and Current Contribution
- This review categorizes seizure prediction approaches based on the type of deep features extracted, distinguishing between time-domain, frequency-domain, and hybrid feature representations.
- Unlike prior reviews that assess generic DL models, this study emphasizes CNN-based architectures, which have shown promising results for capturing spatial and temporal EEG patterns.
- Finally, this study outlines potential future research directions, particularly focusing on self-supervised learning, Transformer-based models, and interpretable DL approaches for seizure prediction.
3. Methodology of Literature Search and Selection
3.1. Search Strategy and Data Sources
- Disorder-related terms: “epilepsy”, “epileptic seizures”, “seizure detection”, and “seizure prediction”;
- Modeling techniques: “deep learning”, “CNN”, “convolutional neural network”, “LSTM”, “RNN”, “Transformer”, “graph neural network”, and “self-supervised learning”;
- Feature extraction approaches: “temporal features”, “spectral features”, “wavelet transform”, “STFT”, “Fourier transform”, “EEG spectrogram”, and “power spectral density”.
3.2. Inclusion and Exclusion Criteria
- The use of deep learning architectures (e.g., CNN, LSTM, RNN, Transformer) for seizure prediction (not merely detection);
- The use of EEG as the primary input modality;
- The incorporation of temporal, spectral, or hybrid features, either through explicit feature engineering or learned representations;
- Having reported the details of the experimental setup, including
- −
- Dataset source (e.g., CHB-MIT, Bonn, TUH);
- −
- Preprocessing steps (e.g., filtering, segmentation);
- −
- Evaluation strategy (e.g., cross-validation, patient-specific testing);
- −
- Performance metrics (e.g., accuracy, sensitivity, AUC).
- Studies that offer a comparative analysis of features or models.
- Studies focused solely on seizure detection without predictive modeling;
- Non-EEG-based studies or multimodal approaches where EEG was not the core signal;
- Articles lacking sufficient methodological transparency or evaluation rigor;
- Review papers, editorials, non-English texts, or studies without accessible full-text.
3.3. Screening Workflow and Reviewer Agreement
3.4. Data Charting and Extraction
- Study metadata: authorship, year, publication type;
- Model type and architecture;
- EEG features (temporal, spectral, hybrid);
- Dataset(s) used and data size;
- Preprocessing pipeline;
- Evaluation metrics and validation strategy;
- Key findings and comparative insights.
3.5. Classification and Synthesis Strategy
- Horizontal comparisons: the performance of different architectures using similar features;
- Vertical comparisons: the impact of different feature types within the same model family.
4. DL Models
5. ML Model with Time-Domain Input
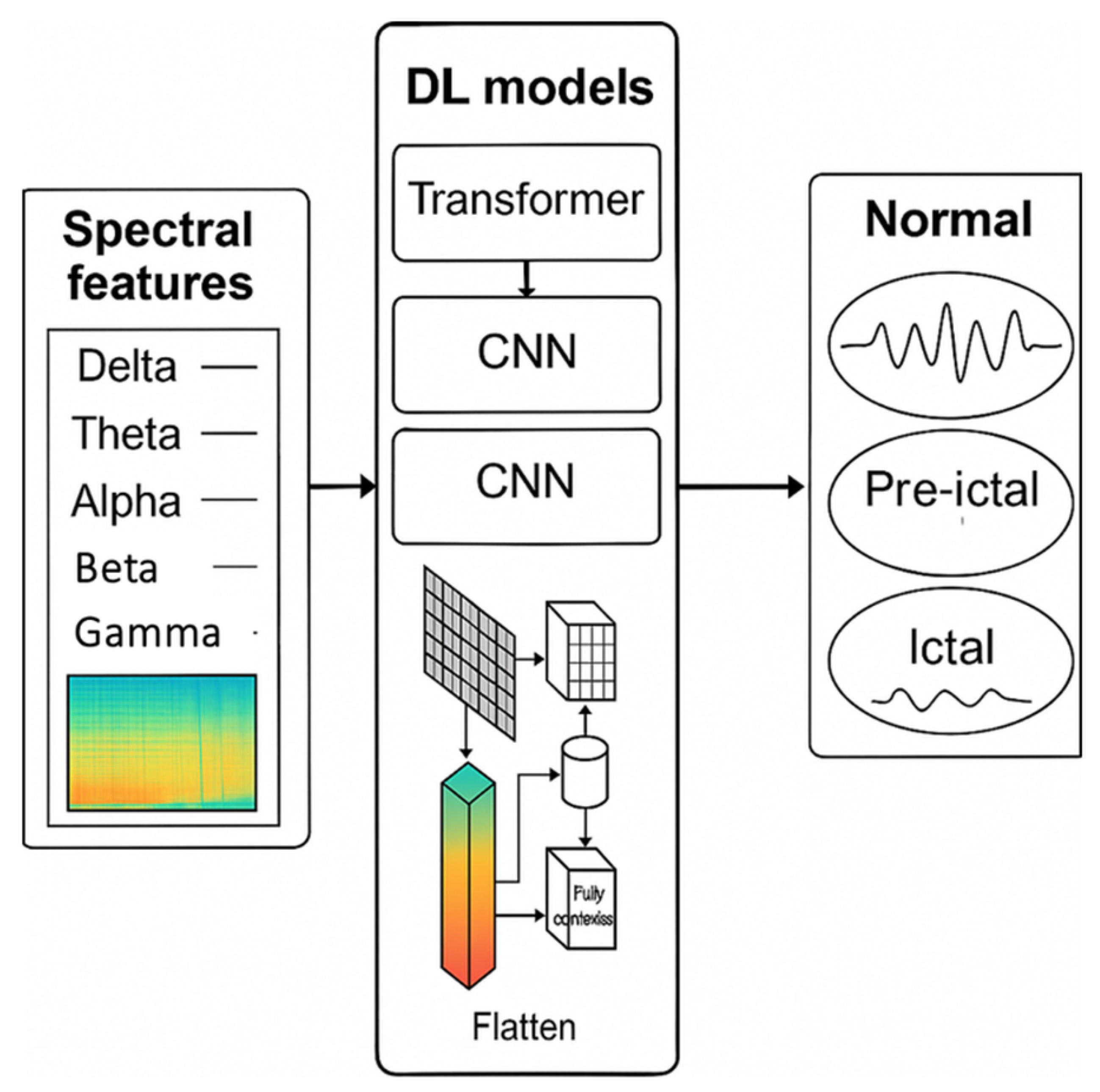
6. DL Models with Spectral-Domain Input
7. Discussion
7.1. Impact of Feature Representation on Prediction Performance
7.2. Challenges and Limitations
7.3. Future Directions
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix A.1. PRISMA
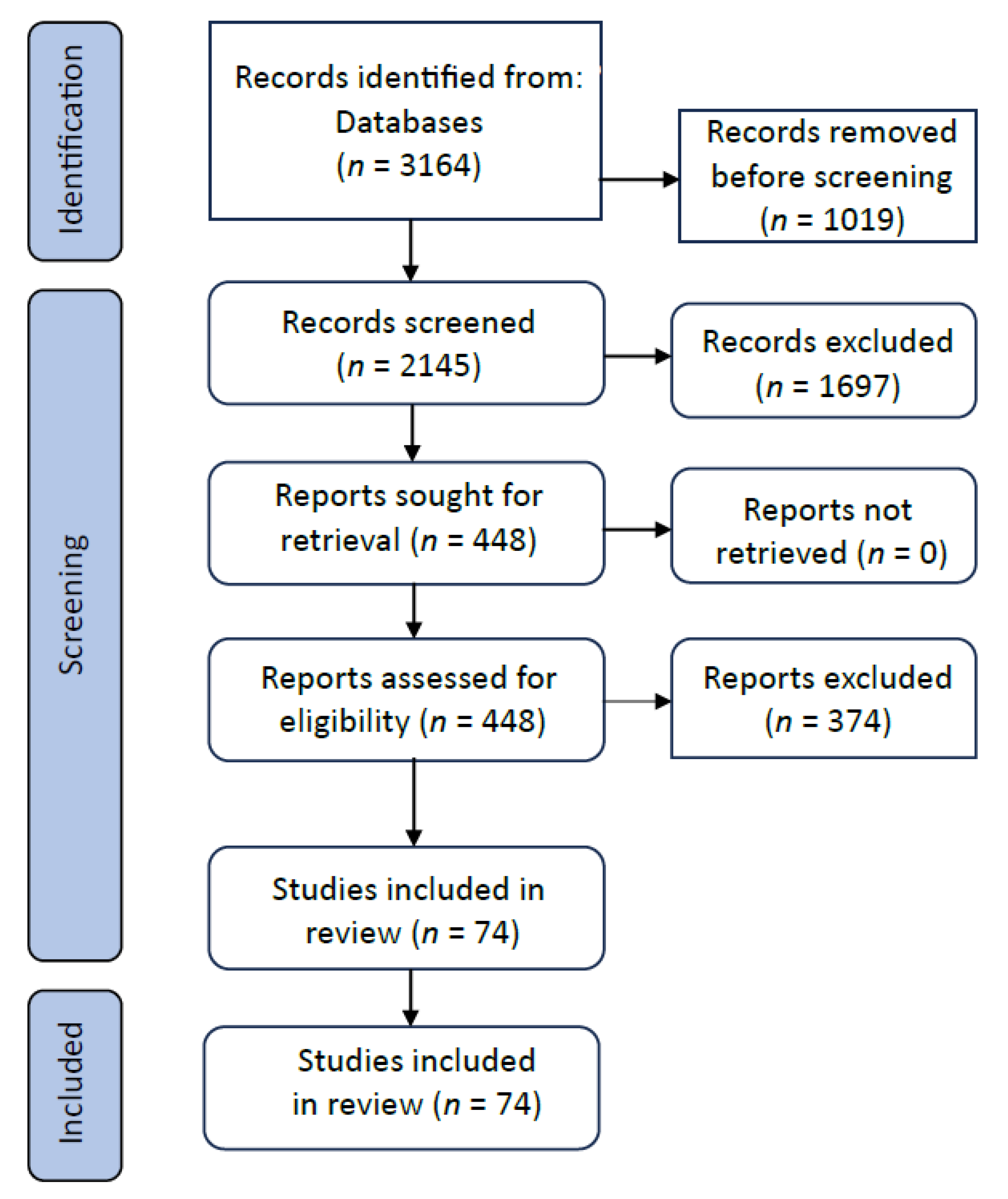
Appendix A.2. Search Strategy
- Databases Searched:
- PubMed;
- Scopus;
- IEEE Xplore;
- Web of Science;
- Google Scholar.
- Search Period: January 2000–April 2025.
- Search Terms Included
- Disorder-related terms: “epilepsy”, “seizure prediction”, “epileptic seizures”, “ictal”, “preictal”;
- ML/DL modeling terms: “machine learning”, “deep learning”, “CNN”, “LSTM”, “RNN”, “transformer”, “self-attention”, “graph neural network”;
- Feature engineering terms: “temporal features”, “spectral features”, “EEG spectrogram”, “wavelet”, “Fourier transform”, “STFT”.
Appendix A.3. Inclusion Criteria
- Studies employing deep or machine learning for seizure prediction or detection;
- The use of EEG as the primary modality for input signals;
- Clear usage of temporal and/or spectral features;
- Defined datasets (e.g., CHB-MIT, TUH, Bonn, EPILEPSIAE) with declared methodology;
- Published in English.
Appendix A.4. Exclusion Criteria
- Reviews, editorials, or studies without experimental EEG-based ML/DL frameworks;
- Lack of feature representation clarity (e.g., vague input types);
- Non-EEG modalities without EEG integration;
- Insufficient methodological detail or evaluation.
Appendix A.5. Study Categorization
- Type of feature representation (temporal, spectral, or hybrid);
- Deep learning model architecture (e.g., CNN-based, RNN-based, Transformer-based);
- Clinical applicability and experimental design (e.g., preictal/ictal classification, seizure onset forecast);
- Dataset and evaluation methodology.
References
- Sirven, J.I. Epilepsy: A spectrum disorder. Cold Spring Harb. Perspect. Med. 2015, 5, a022848. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Galanopoulou, A.S. Epilepsy and epileptic syndrome. Adv. Exp. Med. Biol. 2012, 724, 99–113. [Google Scholar] [PubMed]
- Laxer, K.D.; Trinka, E.; Hirsch, L.J.; Cendes, F.; Langfitt, J.; Delanty, N.; Benbadis, S.R. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014, 37, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Light, G.A.; Williams, L.E.; Minow, F.; Sprock, J.; Rissling, A.; Sharp, R.; Braff, D.L. Electroencephalography (EEG) and event-related potentials (ERPs) with human participants. Curr. Protoc. Neurosci. 2010, 52, 6–25. [Google Scholar] [CrossRef]
- Shoeibi, A.; Khodatars, M.; Ghassemi, N.; Jafari, M.; Moridian, P.; Alizadehsani, R.; Acharya, U.R. Epileptic seizures detection using deep learning techniques: A review. Int. J. Environ. Res. Public Health 2021, 18, 5780. [Google Scholar] [CrossRef]
- Kumar, Y.; Koul, A.; Singla, R.; Ijaz, M.F. Artificial intelligence in disease diagnosis: A systematic literature review, synthesizing framework and future research agenda. J. Ambient. Intell. Humaniz. Comput. 2023, 14, 8459–8486. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Singh, R.P.; Suman, R.; Rab, S. Significance of machine learning in healthcare: Features, pillars and applications. Int. J. Intell. Netw. 2022, 3, 58–73. [Google Scholar] [CrossRef]
- Ahsan, M.M.; Luna, S.A.; Siddique, Z. Machine-learning-based disease diagnosis: A comprehensive review. Healthcare 2022, 10, 541. [Google Scholar] [CrossRef]
- Polat, K.; Güneş, S. Classification of epileptiform EEG using a hybrid system based on decision tree classifier and fast Fourier transform. Appl. Math. Comput. 2007, 187, 1017–1026. [Google Scholar] [CrossRef]
- Arunkumar, N.; Ram Kumar, K.; Venkataraman, V. Automatic detection of epileptic seizures using permutation entropy, Tsallis entropy and Kolmogorov complexity. J. Med. Imaging Health Inform. 2016, 6, 526–531. [Google Scholar] [CrossRef]
- Nicolaou, N.; Georgiou, J. Detection of epileptic electroencephalogram based on permutation entropy and support vector machines. Expert Syst. Appl. 2012, 39, 202–209. [Google Scholar] [CrossRef]
- Li, K.; Zhang, X.; Du, Y. A SVM based classification of EEG for predicting the movement intent of human body. In Proceedings of the 2013 10th International Conference on Ubiquitous Robots and Ambient Intelligence (URAI), Jeju, Republic of Korea, 30 October–2 November 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 402–406. [Google Scholar]
- Wang, S.; Chaovalitwongse, W.A.; Wong, S. Online seizure prediction using an adaptive learning approach. IEEE Trans. Knowl. Data Eng. 2013, 25, 2854–2866. [Google Scholar] [CrossRef]
- Wang, G.; Deng, Z.; Choi, K.S. Detection of epileptic seizures in EEG signals with rule-based interpretation by random forest approach. In Proceedings of the Advanced Intelligent Computing Theories and Applications: 11th International Conference, ICIC 2015, Fuzhou, China, 20–23 August 2015; pp. 738–744. [Google Scholar]
- Hu, X.; Yuan, S.; Xu, F.; Leng, Y.; Yuan, K.; Yuan, Q. Scalp EEG classification using deep Bi-LSTM network for seizure detection. Comput. Biol. Med. 2020, 124, 103919. [Google Scholar] [CrossRef]
- Hussein, R.; Palangi, H.; Ward, R.K.; Wang, Z.J. Optimized deep neural network architecture for robust detection of epileptic seizures using EEG signals. Clin. Neurophysiol. 2019, 130, 25–37. [Google Scholar] [CrossRef]
- Mohseni, H.R.; Maghsoudi, A.; Shamsollahi, M.B. Seizure detection in EEG signals: A comparison of different approaches. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 6724–6727. [Google Scholar]
- Kamini, K.P.; Arthi, R. Epileptic Seizure Prediction: A Review. In Proceedings of the 2021 10th International Conference on System Modeling & Advancement in Research Trends (SMART), Moradabad, India, 10–11 December 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 733–738. [Google Scholar]
- Xu, Y.; Yang, J.; Sawan, M. Trends and challenges of processing measurements from wearable devices intended for epileptic seizure prediction. J. Signal Process. Syst. 2021, 94, 527–542. [Google Scholar] [CrossRef]
- Craik, A.; He, Y.; Contreras-Vidal, J.L. Deep learning for electroencephalogram (EEG) classification tasks: A review. J. Neural Eng. 2019, 16, 031001. [Google Scholar] [CrossRef]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Kural, M.A.; Duez, L.; Sejer Hansen, V.; Larsson, P.G.; Rampp, S.; Schulz, R.; Beniczky, S. Criteria for defining interictal epileptiform discharges in EEG: A clinical validation study. Neurology 2020, 94, e2139–e2147. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Eswaran, C.; Sriraam, A.N. Artificial neural network based epileptic detection using time-domain and frequency-domain features. J. Med. Syst. 2005, 29, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Theeranaew, W.; McDonald, J.; Zonjy, B.; Kaffashi, F.; Moseley, B.D.; Friedman, D.; Loparo, K.A. Automated detection of postictal generalized EEG suppression. IEEE Trans. Biomed. Eng. 2017, 65, 371–377. [Google Scholar] [CrossRef]
- Jacquin, A.; Causevic, E.; John, E.R. Automatic identification of spike-wave events and non-convulsive seizures with a reduced set of electrodes. IEEE Eng. Med. Biol. Soc. 2007, 65, 1928–1931. [Google Scholar]
- Srinivasan, V.; Eswaran, C.; Sriraam, N. Approximate entropy-based epileptic EEG detection using artificial neural networks. IEEE Trans. Inf. Technol. Biomed. 2007, 11, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Ahammad, N.; Fathima, T.; Joseph, P. Detection of epileptic seizure event and onset using EEG. Biomed Res. Int. 2014, 2014, 450573. [Google Scholar] [CrossRef] [PubMed]
- Martis, R.J.; Acharya, U.R.; Tan, J.H.; Petznick, A.; Tong, L.; Chua, C.K.; Ng, E.Y.K. Application of intrinsic time-scale decomposition (ITD) to EEG signals for automated seizure prediction. Int. J. Neural Syst. 2013, 23, 1350023. [Google Scholar] [CrossRef]
- Fadlallah, B.; Chen, B.; Keil, A.; Príncipe, J. Weighted-permutation entropy: A complexity measure for time series incorporating amplitude information. Phys. Rev. E 2013, 87, 022911. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, B.; Zhang, Y.; Duan, M.; Liu, S.; Zhang, Y.; Feng, X.; Tan, R.; Huang, L.; Zhou, F. Selection of features for patient-independent detection of seizure events using scalp EEG signals. Comput. Biol. Med. 2020, 119, 103671. [Google Scholar] [CrossRef]
- Li, Q.; Gao, J.; Huang, Q.; Wu, Y.; Xu, B. Distinguishing epileptiform discharges from normal electroencephalograms using scale-dependent Lyapunov exponent. Front. Bioeng. Biotechnol. 2020, 8, 1006. [Google Scholar] [CrossRef]
- Brari, Z.; Belghith, S. A novel Machine Learning approach for epilepsy diagnosis using EEG signals based on Correlation Dimension. IFAC-PapersOnLine 2021, 54, 7–11. [Google Scholar] [CrossRef]
- Soomro, M.H.; Musavi, S.H.A.; Pandey, B. Canonical correlation analysis and neural network (CCA-NN) based method to detect epileptic seizures from EEG signals. Int. J. Bio-Sci. Bio-Technol. 2016, 8, 11–20. [Google Scholar] [CrossRef]
- Abbaszadeh, B.; Teixeira, C.A.D.; Yagoub, M.C.E. Feature selection techniques for the analysis of discriminative features in temporal and frontal lobe epilepsy: A comparative study. Open Biomed. Eng. J. 2021, 15, 1–15. [Google Scholar] [CrossRef]
- Memarian, N.; Kim, S.; Dewar, S.; Engel, J., Jr.; Staba, R.J. Multimodal data and machine learning for surgery outcome prediction in complicated cases of mesial temporal lobe epilepsy. Comput. Biol. Med. 2015, 64, 67–78. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Yang, P.; Chen, W.; Lo, B. Epilepsy seizure prediction on EEG using common spatial pattern and convolutional neural network. IEEE J. Biomed. Health Inform. 2019, 24, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhou, L.; Chen, Z.; Zhang, L.; Zhou, Y. Automatic seizure detection using three-dimensional CNN based on multi-channel EEG. BMC Med. Inform. Decis. Mak. 2018, 18, 111. [Google Scholar] [CrossRef] [PubMed]
- Xun, G.; Jia, X.; Zhang, A. Detecting epileptic seizures with electroencephalogram via a context-learning model. BMC Med. Inform. Decis. Mak. 2016, 16, 70. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, J.; Sun, Q.; Lu, J.; Ma, X. An effective dual self-attention residual network for seizure prediction. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1604–1613. [Google Scholar] [CrossRef]
- Lih, O.S.; Jahmunah, V.; Palmer, E.E.; Barua, P.D.; Dogan, S.; Tuncer, T.; García, S.; Molinari, F.; Acharya, U.R. EpilepsyNet: Novel automated detection of epilepsy using transformer model with EEG signals from 121 patient population. Comput. Biol. Med. 2023, 164, 107312. [Google Scholar] [CrossRef]
- Saadoon, Y.A.; Khalil, M.; Battikh, D. Predicting epileptic seizures using EfficientNet-B0 and SVMs: A deep learning methodology for EEG analysis. Bioengineering 2025, 12, 109. [Google Scholar] [CrossRef]
- Qiu, S.; Cheng, K.; Zhou, T.; Tahir, R.; Ting, L. An EEG signal recognition algorithm during epileptic seizure based on distributed edge computing. Int. J. Interact. Multimed. Artif. Intell. 2022, 7, 6–13. [Google Scholar] [CrossRef]
- Salafian, B.; Ben-Knaan, E.F.; Shlezinger, N.; De Ribaupierre, S.; Farsad, N. MICAL: Mutual information-based CNN-aided learned factor graphs for seizure detection from EEG signals. IEEE Access 2023, 11, 23085–23096. [Google Scholar] [CrossRef]
- Zhou, W.; Zheng, W.; Feng, Y.; Li, X. LMA-EEGNet: A lightweight multi-attention network for neonatal seizure detection using EEG signals. Electronics 2024, 13, 2354. [Google Scholar] [CrossRef]
- Cui, X.; Cao, J.; Lai, X.; Jiang, T.; Gao, F. Cluster embedding joint-probability-discrepancy transfer for cross-subject seizure detection. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 593–605. [Google Scholar] [CrossRef]
- Darvishi-Bayazi, M.J.; Ghaemi, M.S.; Lesort, T.; Arefin, M.R.; Faubert, J.; Rish, I. Amplifying pathological detection in EEG signaling pathways through cross-dataset transfer learning. Comput. Biol. Med. 2024, 169, 107893. [Google Scholar] [CrossRef] [PubMed]
- Zarei Eskikand, P.; Soto-Breceda, A.; Cook, M.J.; Burkitt, A.N.; Grayden, D.B. Neural dynamics and seizure correlations: Insights from neural mass models in a Tetanus Toxin rat model of epilepsy. Neural Netw. 2024, 180, 106746. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Ma, S.; Zhao, W.; Wang, H.; Fu, Q.; Wang, J. A novel method for optimizing epilepsy detection features through multi-domain feature fusion and selection. Front. Comput. Neurosci. 2024, 18, 1416838. [Google Scholar] [CrossRef]
- Qiao, W.; Bi, X.; Han, L.; Zhang, Y. Epilepsy prediction and detection using Attention-CssCDBN with dual-task learning. Sensors 2025, 25, 51. [Google Scholar] [CrossRef]
- Xiang, J.; Li, Y.; Wu, X.; Dong, Y.; Wen, X.; Niu, Y. Synchronization-based graph spatio-temporal attention network for seizure prediction. Sci. Rep. 2025, 15, 88492. [Google Scholar] [CrossRef]
- Wei, B.; Xu, L.; Zhang, J. A compact graph convolutional network with adaptive functional connectivity for seizure prediction. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 32, 3531–3542. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, Y.; Li, S. Weighted directed graph-based automatic seizure detection with effective brain connectivity for EEG signals. Signal Image Video Process. 2024, 18, 899–909. [Google Scholar] [CrossRef]
- Aldana, Y.R.; Macías, F.S.; Reyes, E.J.M.; Rodríguez, V.R.; Van Huffel, S.; Hunyadi, B. Using partial least squares for nonconvulsive epileptic seizure detection. Rev. Cuba. Cienc. Informáticas 2019, 13, 1–13. [Google Scholar]
- Dümpelmann, M.; Jacobs, J.; Schulze-Bonhage, A. Temporal and spatial characteristics of high frequency oscillations as a new biomarker in epilepsy. Epilepsia 2015, 56, 197–206. [Google Scholar] [CrossRef]
- Wei, B.; Zhao, X.; Shi, L.; Xu, L.; Liu, T.; Zhang, J. A deep learning framework with multi-perspective fusion for interictal epileptiform discharges detection in scalp electroencephalogram. J. Neural Eng. 2021, 18, 046001. [Google Scholar] [CrossRef]
- Bundy, D.T.; Pahwa, M.; Szrama, N.; Leuthardt, E.C. Decoding three-dimensional reaching movements using electrocorticographic signals in humans. J. Neural Eng. 2016, 13, 026021. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, K.V.N.; Ashok, S.; Imoize, A.L.; Ojo, S.; Selvan, K.S.; Ahanger, T.A.; Alhassan, M. On the use of wavelet domain and machine learning for the analysis of epileptic seizure detection from EEG signals. J. Healthc. Eng. 2022, 2022, 8928021. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, B.; Teixeira, C.A.D.; Yagoub, M.C.E. Online seizure prediction system: A novel probabilistic approach for efficient prediction of epileptic seizure with iEEG signal. Open Biomed. Eng. J. 2022, 16. [Google Scholar] [CrossRef]
- Truong, N.D.; Nguyen, A.D.; Kuhlmann, L.; Bonyadi, M.R.; Yang, J.; Ippolito, S.; Kavehei, O. Convolutional neural networks for seizure prediction using intracranial and scalp electroencephalogram. Neural Netw. 2018, 105, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Ghosh-Dastidar, S.; Adeli, H.; Dadmehr, N. Mixed-band wavelet-chaos-neural network methodology for epilepsy and epileptic seizure detection. IEEE Trans. Biomed. Eng. 2007, 54, 1545–1551. [Google Scholar] [CrossRef]
- Vidyaratne, L.S.; Iftekharuddin, K.M. Real-time epileptic seizure detection using EEG. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 2146–2156. [Google Scholar] [CrossRef]
- Orosco, L.; Correa, A.G.; Diez, P.; Laciar, E. Patient non-specific algorithm for seizures detection in scalp EEG. Comput. Biol. Med. 2016, 71, 128–134. [Google Scholar] [CrossRef]
- Chandel, G.; Upadhyaya, P.; Farooq, O.; Khan, Y.U. Detection of seizure event and its onset/offset using orthonormal triadic wavelet based features. IRBM 2019, 40, 103–112. [Google Scholar] [CrossRef]
- Truong, N.D.; Nguyen, A.D.; Kuhlmann, L.; Bonyadi, M.R.; Yang, J.; Kavehei, O. A generalised seizure prediction with convolutional neural networks for intracranial and scalp electroencephalogram data analysis. arXiv 2017, arXiv:1707.01976. [Google Scholar]
- Hu, W.; Cao, J.; Lai, X.; Liu, J. Mean amplitude spectrum based epileptic state classification for seizure prediction using convolutional neural networks. J. Ambient. Intell. Humaniz. Comput. 2019, 14, 15485–15495. [Google Scholar] [CrossRef]
- Ramos-Aguilar, R.; Olvera-López, J.A.; Olmos-Pineda, I.; Sánchez-Urrieta, S. Feature extraction from EEG spectrograms for epileptic seizure detection. Pattern Recognit. Lett. 2020, 133, 202–209. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, J.; Sawan, M. A novel multi-scale dilated 3D CNN for epileptic seizure prediction. In Proceedings of the 2021 IEEE 3rd International Conference on Artificial Intelligence Circuits and Systems (AICAS), Washington, DC, USA, 6–9 June 2021; pp. 1–4. [Google Scholar]
- Jahankhani, P.; Revett, K.; Kodogiannis, V. Data mining an EEG dataset with an emphasis on dimensionality reduction. In Proceedings of the 2007 IEEE Symposium on Computational Intelligence and Data Mining, Honolulu, HI, USA, 1 March–5 April 2007; IEEE: Piscataway, NJ, USA, 2007; pp. 405–412. [Google Scholar]
- Kaleem, M.; Guergachi, A.; Krishnan, S. Patient-specific seizure detection in long-term EEG using wavelet decomposition. Biomed. Signal Process. Control 2018, 46, 157–165. [Google Scholar] [CrossRef]
- Pérez-Elvira, R.; Oltra-Cucarella, J.; Carrobles, J.A.; Teodoru, M.; Bacila, C.; Neamtu, B. Individual alpha peak frequency, an important biomarker for live z-score training neurofeedback in adolescents with learning disabilities. Brain Sci. 2021, 11, 167. [Google Scholar] [CrossRef]
- Tsipouras, M.G. Spectral information of EEG signals with respect to epilepsy classification. EURASIP J. Adv. Signal Process. 2019, 2019, 10. [Google Scholar] [CrossRef]
- Usman, S.M.; Khalid, S.; Aslam, M.H. Epileptic seizures prediction using deep learning techniques. IEEE Access 2020, 8, 39998–40007. [Google Scholar] [CrossRef]
- Liu, C.L.; Xiao, B.; Hsaio, W.H.; Tseng, V.S. Epileptic seizure prediction with multi-view convolutional neural networks. IEEE Access 2019, 7, 170352–170361. [Google Scholar] [CrossRef]
- Singh, Y.P.; Lobiyal, D.K. Automatic prediction of epileptic seizure using hybrid deep ResNet-LSTM model. AI Commun. 2023, 36, 57–62. [Google Scholar] [CrossRef]
- Wang, G.; Wang, D.; Du, C.; Li, K.; Zhang, J.; Liu, Z.; Yan, X. Seizure prediction using directed transfer function and convolution neural network on intracranial EEG. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2711–2720. [Google Scholar] [CrossRef]
- Romney, A.; Manian, V. Comparison of frontal-temporal channels in epilepsy seizure prediction based on EEMD-reliefF and DNN. Computers 2020, 9, 78. [Google Scholar] [CrossRef]
- Qi, N.; Piao, Y.; Yu, P.; Tan, B. Predicting epileptic seizures based on EEG signals using spatial depth features of a 3D-2D hybrid CNN. Med. Biol. Eng. Comput. 2023, 61, 1845–1856. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, B.; Chen, Q.; Liu, J.; Zhang, Y. Deep convolutional neural network-based epileptic electroencephalogram (EEG) signal classification. Front. Neurol. 2020, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Assali, I.; Blaiech, A.G.; Abdallah, A.B.; Khalifa, K.B.; Carrère, M.; Bedoui, M.H. CNN-based classification of epileptic states for seizure prediction using combined temporal and spectral features. Biomed. Signal Process. Control 2023, 82, 104519. [Google Scholar] [CrossRef]
- Zhu, R.; Pan, W.X.; Liu, J.X.; Shang, J.L. Epileptic seizure prediction via multidimensional transformer and recurrent neural network fusion. J. Transl. Med. 2024, 22, 895. [Google Scholar] [CrossRef]
- Li, J.; Feng, G.; Lv, J.; Chen, Y.; Chen, R.; Chen, F.; Zhang, S.; Vai, M.I.; Pun, S.H.; Mak, P.U. A lightweight multi-mental disorders detection method using entropy-based matrix from single-channel EEG signals. Brain Sci. 2024, 14, 987. [Google Scholar] [CrossRef]
- Urbina Fredes, S.; Dehghan Firoozabadi, A.; Adasme, P.; Zabala-Blanco, D.; Palacios Játiva, P.; Azurdia-Meza, C. Enhanced epileptic seizure detection through wavelet-based analysis of EEG signal processing. Appl. Sci. 2024, 14, 5783. [Google Scholar] [CrossRef]
- Amer, N.S.; Belhaouari, S.B. Exploring new horizons in neuroscience disease detection through innovative visual signal analysis. Sci. Rep. 2024, 14, 4217. [Google Scholar] [CrossRef]
- Li, C.; Huang, X.; Song, R.; Qian, R.; Liu, X.; Chen, X. EEG-based seizure prediction via Transformer guided CNN. Measurement 2022, 203, 111948. [Google Scholar] [CrossRef]
- Hermawan, A.T.; Zaeni, I.A.E.; Wibawa, A.P.; Gunawan, G.; Hendrawan, W.H.; Kristian, Y. A multi-representation deep learning approach for epileptic seizure detection. J. Robot. Control (JRC) 2024, 5, 187–204. [Google Scholar] [CrossRef]
- Xu, M.; Jie, J.; Zhou, W.; Zhou, H.; Jin, S. Synthetic epileptic brain activities with TripleGAN. Comput. Math. Methods Med. 2022, 2022, 2841228. [Google Scholar] [CrossRef]
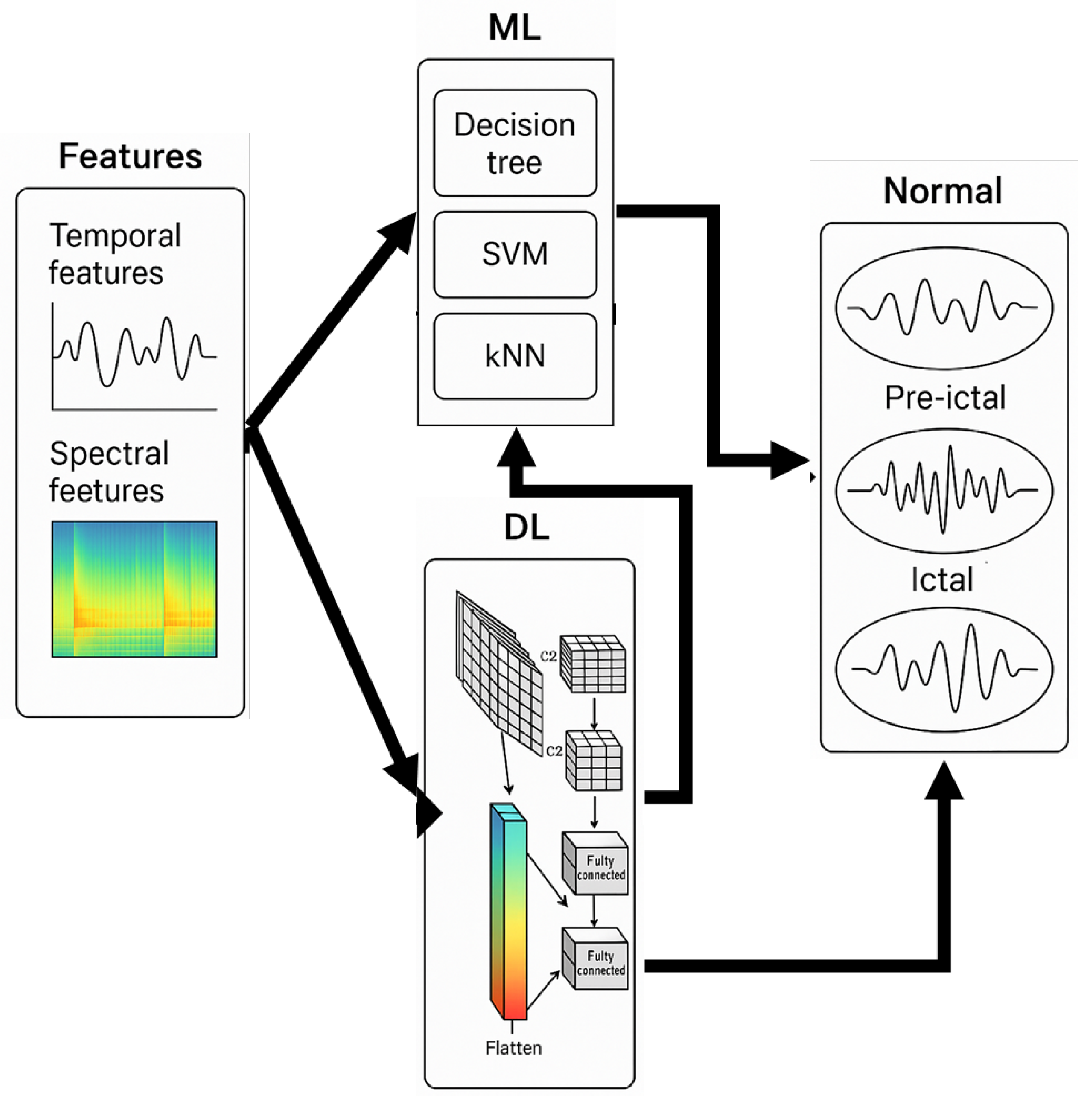
| Ref | Temporal Feature Representation | Model Type | Dataset | Performance |
|---|---|---|---|---|
| Srinivasan et al. [26] | Approximate Entropy (ApEn) | Elman NN, Probabilistic NN | EEG | 100% overall accuracy |
| Ahammad et al. [27] | Energy, SEN, IQR, MAD | Statistical Classifiers | CHB-MIT | 84.2% overall accuracy |
| Martis et al. [28] | Energy, Fractal Dim., Sample Entropy (via ITD) | Decision Tree | EEG | 95.67% average classification accuracy |
| Fadlallah et al. [29] | Weighted Permutation Entropy (WPE) | Not specified | EEG | Not specified |
| Yang et al. [30] | Min, Max, Mean, Variance, Skewness, Kurtosis, RMS | Supervised Model | EEG | Not specified |
| Zhang et al. [36] | Wavelet-decomposed CSP Time Features | CNN | EEG | 90.2% sensitivity, 0.096/h FPR |
| Li et al. [31] | Scale-Dependent Lyapunov Exponents (SDLE) | SVM, Random Forest | EEG | 92.2% sensitivity, 0.12/h FPR |
| Brari [32] | Correlation Dimension (CD) | Simplified Classifier | EEG | Accuracy 100% |
| Soomro et al. [33] | CCA-based features | MLPNN | EEG | Accuracy 92.583% |
| Abbaszadeh et al. [34] | IQR, Kruskal-Wallis | Supervised ML | EEG | - |
| Memarian et al. [35] | mRMR, mutual info | ML classifiers | Multimodal EEG | Accuracy 95% |
| Wei et al. [37] | Raw EEG (Temporal Modeling) | 3D-CNN | EEG | Accuracy > 90% |
| Xun et al. [38] | Temporal Autoencoding | Sparse Autoencoder | EEG | Error rate 22.93% |
| Yang et al. [39] | Temporal Dependency | CNN-LSTM, Self-attention | EEG | Accuracy 92.07% |
| Bundy et al. [56] | Time-domain EEG | PLS Regression | EEG | Sensitivity > 99% |
| Dümpelmann et al. [54] | Temporal HFOs | Kruskal-Wallis test | EEG | - |
| Wei et al. [55] | IED classification | DL Temporal Models | EEG | Accuracy 95.1% |
| Kavitha et al. [57], Abbaszadeh et al. [58] | Peak-to-peak, Variance, IQR, Energy | SVM, DT, KNN, RF | Bonn, Senthil | Alert 75 min before seizure |
| Qiao et al. [49], Xiang et al. [50] | Temporal-Spatial Features | GCN, STGAT | Multichannel EEG | Sensitivity 98.5% |
| Wei et al. [51], Sun et al. [52] | Time-domain Connectivity | Directed Graph Models | EEG | Accuracy 98.15% |
| Qiu et al. [42] | Real-time EEG Monitoring | Cloud/Edge DL | EEG | Accuracy 93.4% |
| Salafian et al. [43] | Real-time EEG | Federated, TSK-Fuzzy | EEG | Accuracy 91.43% |
| Zhou et al. [44] | Neonatal EEG Temporal | LMA-EEGNet | EEG (Neonatal) | Accuracy 95.71% |
| Cui et al. [45], Darvishi-Bayazi et al. [46] | Mean amplitude, std, median, kurtosis, skewness of 0–40 Hz WPD | CEJT Transfer | Cross-subject EEG | Accuracy 86.31% |
| Zarei Eskikand et al. [47] | Neural Mass Time-Domain | Neural Models | EEG | 1 hr before seizure |
| Kong et al. [48] | PSO, Correlation | Optimized ML Models | EEG | Accuracy 99.32% |
| Lih et al. [40] | Temporal Transformers | EpilepsyNet | EEG | Accuracy 85% |
| Saadoon et al. [41] | EEG Variability | EfficientNet-B0 + SVM | EEG | Accuracy 96.12% |
| Ref | Feature Representation | Model Type | Dataset | Performance Metrics |
|---|---|---|---|---|
| Truong et al. [59,64] | STFT (spectrogram) | CNN | CHB-MIT | 81.4% Sensitivity, FPR 0.06/h |
| Wang et al. [67] | STFT (3D Tensor) | 3D CNN with Dilated Conv | CHB-MIT | 85.8% Sensitivity, 80.5% Accuracy |
| Ramos-Aguilar et al. [66] | Spectrogram + Descriptors | MLP, K-means, LTP | Bonn | 100% Accuracy |
| Usman et al. [72] | STFT | CNN-SVM | CHB-MIT | 92.7% Sensitivity, 90.8% Specificity |
| Hu et al. [65] | Mean Amplitude Spectrum (MAS) | CNN-SVM | CHB-MIT | 86.25% Accuracy |
| Yang et al. [39] | STFT | Self-attentive Residual CNN | CHB-MIT | 92.07% Accuracy, 89.33% Sensitivity, 93.02% Specificity |
| Liu et al. [73] | Temporal + Spectral | Multi-view CNN | CHB-MIT | 93% Sensitivity, 71% Specificity |
| Singh & Lobiyal [74] | Spectrogram | CNN-LSTM | CHB-MIT | 94.5% Accuracy, F1-score 0.9376, FPR 0.055/h |
| Wang et al. [75] | DTF (Spectral Flow) | CNN + Moving Avg | iEEG | 90.8% Sensitivity, FPR 0.08/h |
| Romney et al. [76] | EEMD | Neural Networks | CHB-MIT | 86.7% Sensitivity, 89.5% Specificity |
| Qi et al. [77] | Spectral Depth | 3D+2D HyCNN | CHB-MIT | 98.43% Accuracy, 98.58% Sensitivity, 96.86% Specificity |
| Lih et al. [40] | PCC | Transformer | EEG | 85% Accuracy |
| Zhu et al. [80] | Spectral Features | Transformer + LSTM/GRU | CHB-MIT | 98.24% Sensitivity |
| Li et al. [84] | STFT | TGCNN | CHB-MIT | 91.5% Sensitivity, AUC 93.5%, FPR 0.145/h |
| Assali et al. [79] | STFT + SI | CNN | CHB-MIT | Accuracy 90.1%–94.5% |
| Li et al. [81] | Entropy Spectral Features | SVM, Decision Trees | EEG | High Accuracy, Low Data Requirements |
| Urbina Fredes et al. [82] | Alpha/Beta Bands | SVM | EEG | High Accuracy, Real-Time Suitability |
| Amer & Belhouari [83] | FBFT | CNN | EEG | High Accuracy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saadoon, Y.A.; Khalil, M.; Battikh, D. Machine and Deep Learning-Based Seizure Prediction: A Scoping Review on the Use of Temporal and Spectral Features. Appl. Sci. 2025, 15, 6279. https://doi.org/10.3390/app15116279
Saadoon YA, Khalil M, Battikh D. Machine and Deep Learning-Based Seizure Prediction: A Scoping Review on the Use of Temporal and Spectral Features. Applied Sciences. 2025; 15(11):6279. https://doi.org/10.3390/app15116279
Chicago/Turabian StyleSaadoon, Yousif A., Mohamad Khalil, and Dalia Battikh. 2025. "Machine and Deep Learning-Based Seizure Prediction: A Scoping Review on the Use of Temporal and Spectral Features" Applied Sciences 15, no. 11: 6279. https://doi.org/10.3390/app15116279
APA StyleSaadoon, Y. A., Khalil, M., & Battikh, D. (2025). Machine and Deep Learning-Based Seizure Prediction: A Scoping Review on the Use of Temporal and Spectral Features. Applied Sciences, 15(11), 6279. https://doi.org/10.3390/app15116279







