A Cascading Delphi Method-Based FMEA Risk Assessment Framework for Surgical Instrument Design: A Case Study of a Fetoscope
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
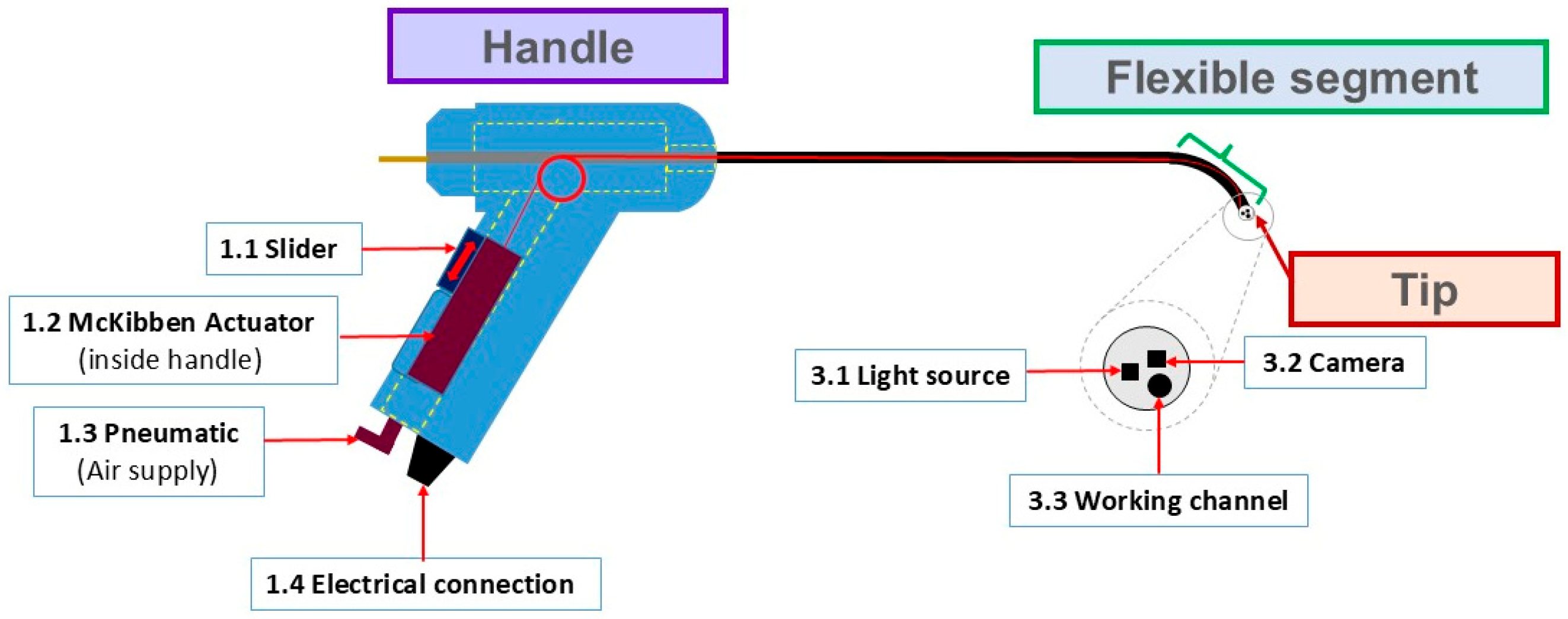
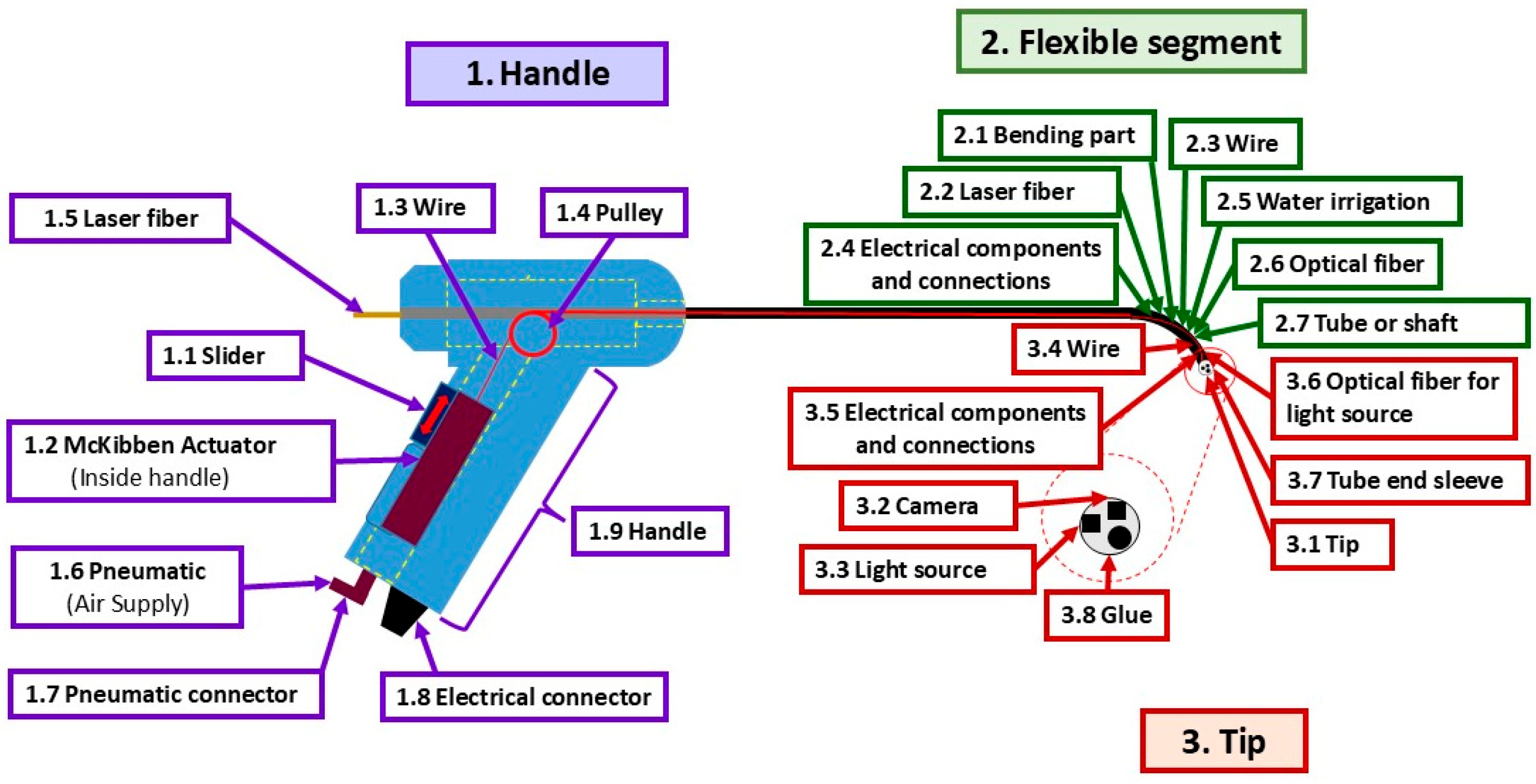
2.1. Flexible Fetoscope
2.2. FMEA Expert Team
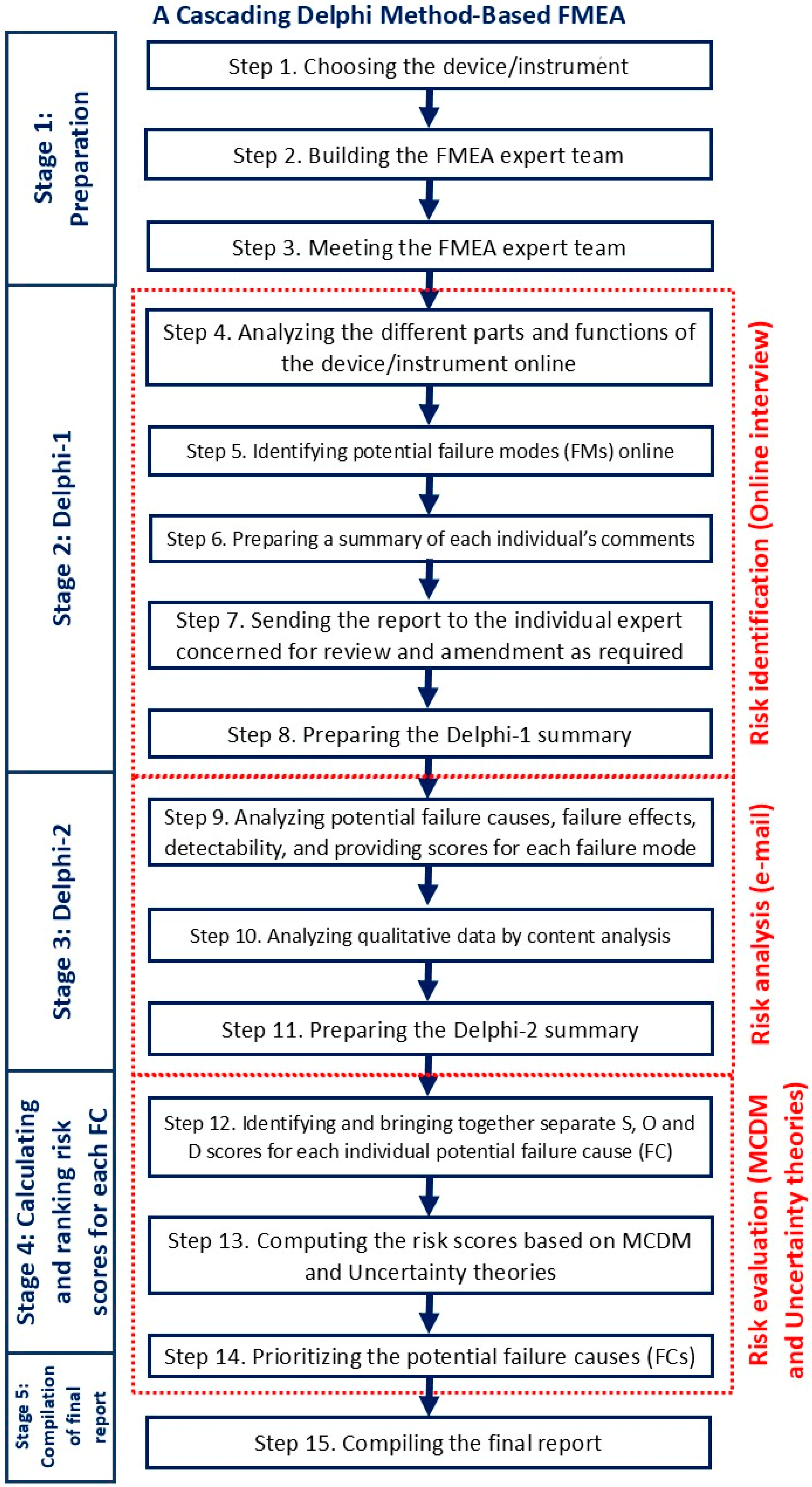
2.3. The Conceptual Framework of a Cascading Delphi Method
- Stage 1: Preparation: to set the scene for the experts
- Stage 2: Delphi-1: to identify the relevant failure modes (FMs)
- Stage 3: Delphi-2: to analyze causes, effects, and detectability and provide scores for S, O, and D
- Stage 4: Calculating RPN and prioritizing FCs: this stage was to process the numerical results as quantitative data for S, O, and D and to compute the risk scores. There are several methods of MCDM to improve the RPN calculation. This study applied hybrid MCDM methodology and uncertainty theories to overcome RPN calculation weaknesses. grey relational analysis (GRA) and Evaluation based on Distance from Average Solution (EDAS) are effective methods in MCDM for assessing and prioritizing risk factors in FMEA, particularly during the medical device design stage [12].
- (i)
- To form a multidisciplinary team of experts, identify potential failure modes, and assess the relevant evaluation criteria.
- (ii)
- To determine the distinct weights of the three FMEA factors—severity (S), probability of occurrence (O), and detection (D)—we applied the Entropy method. This technique addresses a key limitation of conventional FMEA, which assumes equal importance for all factors and thus overlooks their context-dependent influence on overall risk.
- (iii)
- To normalize the decision matrix using the vector normalization technique from the TOPSIS method to ensure comparability across criteria. After normalization, the Positive Ideal Solution (PIS) and Negative Ideal Solution (NIS) are derived by identifying the best and worst values for each criterion across all alternatives.
- (iv) To further assess the relationship between each alternative and the ideal/worst solutions, Grey Relational Coefficients (GRCs) are calculated. These coefficients reflect how closely each alternative aligns with PIS and NIS. The distance between each normalized value and the PIS/NIS is first computed. Then, using these distances, the GRCs for both the best (positive) and worst (negative) references are determined. This step allows for the partial incorporation of inter-criteria relationships and is especially useful under uncertainty.
- Stage 5: Compilation of final report: summarizing the report.
3. Results
3.1. Framework Results
3.2. Expert Data
3.3. Stage 2: Delphi-1 Result
3.4. Stage 3: Delphi-2 Result
3.5. Sensitivity Analysis of Scenario Weights and Rank Stability
- – Scenario 1: (0.2, 0.2, 0.6)—emphasis on detection;
- – Scenario 2: (0.2, 0.6, 0.2)—emphasis on occurrence;
- – Scenario 3: (0.33, 0.33, 0.33)—equal weighting;
- – Scenario 4: (0.6, 0.2, 0.2)—emphasis on severity.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Severity | |
| Occurrence | |
| Detectability | |
| Index referring to the alternative | |
| Index referring to the criteria | |
| Element of initial decision matrix | |
| Element of Entropy normalization-matrix | |
| Entropy | |
| Degrees of variation | |
| Weight of the criterion | |
| Element of TOPSIS normalization matrix | |
| Positive ideal solution | |
| Negative ideal solution | |
| Distance since element and relative | |
| Distance since element and relative | |
| Distinguishing coefficient | |
| Grey relational coefficient relative at | |
| Grey relational coefficient relative at | |
| Matrix of | |
| Matrix of | |
| Average value relative matrix | |
| Average value relative matrix | |
| Positive distance to average calculated on best solution | |
| Negative distance to average calculated on best solution | |
| Positive distance to average calculated on worst solution | |
| Negative distance to average calculated on worst solution | |
| Weighted sum of for each alternative | |
| Weighted sum of for each alternative | |
| Weighted sum of for each alternative | |
| Weighted sum of for each alternative | |
| Normalized | |
| Normalized | |
| Normalized | |
| Normalized | |
| Best-solution appraisal score | |
| Worst-solution appraisal score | |
| Overall appraisal score |
References
- Bitkina, O.V.; Kim, H.K.; Park, J. Usability and User Experience of Medical Devices: An Overview of the Current State, Analysis Methodologies, and Future Challenges. Int. J. Ind. Ergon. 2020, 76, 102932. [Google Scholar] [CrossRef]
- Garro, F.; McKinney, Z. Toward a Standard User-Centered Design Framework for Medical Applications of Brain-Computer Interfaces. In Proceedings of the 2020 IEEE International Conference on Human-Machine Systems, ICHMS 2020, Rome, Italy, 7–9 September 2020; pp. 4–6. [Google Scholar] [CrossRef]
- Marešová, P.; Klímová, B.; Honegr, J.; Kuča, K.; Ibrahim, W.N.H.; Selamat, A. Medical Device Development Process, and Associated Risks and Legislative Aspects-Systematic Review. Front. Public Health 2020, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Shukla, V.K. Latest Risk Management Guideline (ISO 14971:2019) & Environmental Aspects of Medical Device. Int. J. Drug Regul. Aff. 2020, 8, 15–24. [Google Scholar] [CrossRef]
- Miclăuş, T.; Valla, V.; Koukoura, A.; Nielsen, A.A.; Dahlerup, B.; Tsianos, G.I.; Vassiliadis, E. Impact of Design on Medical Device Safety. Ther. Innov. Regul. Sci. 2020, 54, 839–849. [Google Scholar] [CrossRef]
- Martinez-Marquez, D.; Terhaer, K.; Scheinemann, P.; Mirnajafizadeh, A.; Carty, C.P.; Stewart, R.A. Quality by Design for Industry Translation: Three-dimensional Risk Assessment Failure Mode, Effects, and Criticality Analysis for Additively Manufactured Patient-specific Implants. Eng. Rep. 2020, 2, e12113. [Google Scholar] [CrossRef]
- Frewen, H.; Brown, E.; Jenkins, M.; O’Donovan, A. Failure Mode and Effects Analysis in a Paperless Radiotherapy Department. J. Med. Imaging Radiat. Oncol. 2018, 62, 707–715. [Google Scholar] [CrossRef]
- DeRosier, J.; Stalhandske, E.; Bagian, J.P.; Nudell, T. Using Health Care Failure Mode and Effect Analysis: The VA National Center for Patient Safety’s Prospective Risk Analysis System. Jt. Comm. J. Qual. Improv. 2002, 28, 248–267. [Google Scholar] [CrossRef]
- Liu, H.C.; Zhang, L.J.; Ping, Y.J.; Wang, L. Failure Mode and Effects Analysis for Proactive Healthcare Risk Evaluation: A Systematic Literature Review. J. Eval. Clin. Pract. 2020, 26, 1320–1337. [Google Scholar] [CrossRef]
- Franklin, B.D.; Shebl, N.A.; Barber, N. Failure Mode and Effects Analysis: Too Little for Too Much? BMJ Qual. Saf. 2012, 21, 607–611. [Google Scholar] [CrossRef]
- Anjalee, J.A.L.; Rutter, V.; Samaranayake, N.R. Application of Failure Mode and Effect Analysis (FMEA) to Improve Medication Safety: A Systematic Review. Postgrad. Med. J. 2021, 97, 168–174. [Google Scholar] [CrossRef]
- Pintelon, L.; Di Nardo, M.; Murino, T.; Pileggi, G.; Vander Poorten, E. A New Hybrid MCDM Approach for RPN Evaluation for a Medical Device Prototype. Qual. Reliab. Eng. Int. 2021, 37, 2189–2213. [Google Scholar] [CrossRef]
- Southard, P.B.; Kumar, S.; Southard, C.A. A Modified Delphi Methodology to Conduct a Failure Modes Effects Analysis: A Patient-Centric Effort in a Clinical Medical Laboratory. Qual. Manag. Health Care 2011, 20, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.R.S.; Martinez, E.Z.; Simões, C.M.; Barach, P.R.; Carmona, M.J.C. Analyzing and Mitigating the Risks of Patient Harm during Operating Room to Intensive Care Unit Patient Handoffs. Int. J. Qual. Health Care 2025, 37, mzae114. [Google Scholar] [CrossRef] [PubMed]
- Parand, A.; Faiella, G.; Franklin, B.D.; Johnston, M.; Clemente, F.; Stanton, N.A.; Sevdalis, N. A Prospective Risk Assessment of Informal Carers’ Medication Administration Errors within the Domiciliary Setting. Ergonomics 2018, 61, 104–121. [Google Scholar] [CrossRef]
- Öhrn, A.; Ericsson, C.; Andersson, C.; Elfström, J. High Rate of Implementation of Proposed Actions for Improvement with the Healthcare Failure Mode Effect Analysis Method: Evaluation of 117 Analyses. J. Patient Saf. 2018, 14, 17–20. [Google Scholar] [CrossRef]
- Varndell, W.; Fry, M.; Elliott, D. Applying Real-Time Delphi Methods: Development of a Pain Management Survey in Emergency Nursing. BMC Nurs. 2021, 20, 149. [Google Scholar] [CrossRef]
- Ankam, V.S.S.; Hong, G.Y.; Fong, A.C. Design of a Mixed-Reality Application to Reduce Pediatric Medication Errors in Prehospital Emergency Care. Appl. Sci. 2024, 14, 8426. [Google Scholar] [CrossRef]
- Liu, H.C.; Liu, L.; Liu, N. Risk Evaluation Approaches in Failure Mode and Effects Analysis: A Literature Review. Expert Syst. Appl. 2013, 40, 828–838. [Google Scholar] [CrossRef]
- Shebl, N.A.; Franklin, B.D.; Barber, N. Failure Mode and Effects Analysis Outputs: Are They Valid? BMC Health Serv. Res. 2012, 12, 150. [Google Scholar] [CrossRef]
- Xu, A.Y.; Bhatnagar, J.; Bednarz, G.; Flickinger, J.; Arai, Y.; Vacsulka, J.; Feng, W.; Monaco, E.; Niranjan, A.; Lunsford, L.D.; et al. Failure Modes and Effects Analysis (FMEA) for Gamma Knife Radiosurgery. J. Appl. Clin. Med. Phys. 2017, 18, 152–168. [Google Scholar] [CrossRef]
- Liu, H.C.; Chen, X.Q.; Duan, C.Y.; Wang, Y.M. Failure Mode and Effect Analysis Using Multi-Criteria Decision Making Methods: A Systematic Literature Review. Comput. Ind. Eng. 2019, 135, 881–897. [Google Scholar] [CrossRef]
- Liu, H.C. FMEA Using Uncertainty Theories and MCDM Methods; Springer Nature: Singapore, 2016; ISBN 9789811014666. [Google Scholar]
- Legrand, J.; Javaux, A.; Ourak, M.; Wenmakers, D.; Vercauteren, T.; Deprest, J.; Ourselin, S.; Denis, K.; Vander Poorten, E. Handheld Active Add-on Control Unit for a Cable-Driven Flexible Endoscope. Front. Robot AI 2019, 6, 87. [Google Scholar] [CrossRef] [PubMed]
- Malshe, A.; Snowise, S.; Mann, L.K.; Boring, N.; Johnson, A.; Bebbington, M.W.; Moise, K.J.; Papanna, R. Preterm Delivery after Fetoscopic Laser Surgery for Twin–Twin Transfusion Syndrome: Etiology and Risk Factors. Ultrasound Obstet. Gynecol. 2017, 49, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Perry, H.; Duffy, J.; Reed, K.; Baschat, A.; Deprest, J.; Hecher, K.; Lewi, L.; Lopriore, E.; Oepkes, D. Twin-Twin Transfusion Syndrome: Study Protocol for Developing, Disseminating, and Implementing a Core Outcome Set. Trials 2017, 18, 325. [Google Scholar] [CrossRef]
- Papanna, R.; Block-Abraham, D.; Mann, L.K.; Buhimschi, I.A.; Bebbington, M.; Garcia, E.; Kahlek, N.; Harman, C.; Johnson, A.; Baschat, A.; et al. Risk Factors Associated with Preterm Delivery after Fetoscopic Laser Ablation for Twin-Twin Transfusion Syndrome. Ultrasound Obstet. Gynecol. 2014, 43, 48–53. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Ourak, M.; Gruijthuijsen, C.; Legrand, J.; Vercauteren, T.; Deprest, J.; Ourselin, S.; Poorten, E. Vander Design and Shared Control of a Flexible Endoscope with Autonomous Distal Tip Alignment. In Proceedings of the 2019 19th International Conference on Advanced Robotics, ICAR 2019, Belo Horizonte, Brazil, 2–6 December 2019; pp. 647–653. [Google Scholar]
- Thia, E.; Thain, S.; Yeo, G.S.H. Fetoscopic Laser Photocoagulation in Twin-to-Twin Transfusion Syndrome: Experience from a Single Institution. Singapore Med. J. 2017, 58, 321–326. [Google Scholar] [CrossRef]
- Winkler, S.M.; Harrison, M.R.; Messersmith, P.B. Biomaterials in Fetal Surgery. Biomater. Sci. 2019, 7, 3092–3109. [Google Scholar] [CrossRef]
- Sobaih, A.E.E.; Ritchie, C.; Jones, E. Consulting the Oracle? Applications of Modified Delphi Technique to Qualitative Research in the Hospitality Industry. Int. J. Contemp. Hosp. Manag. 2012, 24, 886–906. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Guldentops, R. Development of a Framework for Efficient Risk Assessment of Surgical Robots and Advanced Surgical Instrumentation. Ph.D. Thesis, Katholieke Universiteit Leuven, Leuven, Belgium, 2017. [Google Scholar]
- Spencer, R.; Ambler, G.; Brodszki, J.; Diemert, A.; Figueras, F.; Gratacós, E.; Hansson, S.R.; Hecher, K.; Huertas-Ceballos, A.; Marlow, N.; et al. EVERREST Prospective Study: A 6-Year Prospective Study to Define the Clinical and Biological Characteristics of Pregnancies Affected by Severe Early Onset Fetal Growth Restriction. BMC Pregnancy Childbirth 2017, 17, 4–11. [Google Scholar] [CrossRef]
- Ford Motor Company. FMEA Handbook Version 4.1; Ford Motor Company: Dearborn, MI, USA, 2004. [Google Scholar]
- Kendall, M.G. A New Measure of Rank Correlation. Biometrika 1938, 30, 81. [Google Scholar] [CrossRef]
- Nathan, R.; Bhandari, S. Risk Assessment in Clinical Practice: A Framework for Decision-Making in Real-World Complex Systems. BJPsych Adv. 2024, 30, 53–63. [Google Scholar] [CrossRef]
- Subriadi, A.P.; Najwa, N.F. The Consistency Analysis of Failure Mode and Effect Analysis (FMEA) in Information Technology Risk Assessment. Heliyon 2020, 6, e03161. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Shen, M.; Geng, Y. Risk Assessment Based on Failure Mode and Effects Analysis (FMEA) and WASPAS Methods under Probabilistic Double Hierarchy Linguistic Term Sets. Comput. Ind. Eng. 2023, 186, 109758. [Google Scholar] [CrossRef]
| Score | Scale | Description |
|---|---|---|
| 1 | Grade I | Without the need for pharmacological treatment |
| 2 | Grade II | Requiring pharmacological treatment |
| 3 | Grade IIIa | Intervention not under general anesthesia |
| 4 | Grade IIIb | Intervention under general anesthesia |
| 5 | Grade IVa | Single organ dysfunction (including dialysis) |
| 6 | Grade IVb | Multiorgan dysfunction |
| 7 | Grade V | Death of the patient |
| Score | Scale | Description |
|---|---|---|
| 1 | Low danger | Does not endanger the fetuses in any way. |
| 2 | Moderate danger | Harm to a fetal organ |
| 3 | Dangerous | Harm to a fetal organ which requires future intervention |
| 4 | Very dangerous | Harm to a fetal organ which is life-threatening |
| 5 | Extremely dangerous | Fetal death |
| Score | Scale | Description |
|---|---|---|
| 1 | Very low | Failure happens very infrequently (1/10,000–fewer) |
| 2 | Low | Failure happens rarely (1/1000–1/10,000) |
| 3 | Moderate | Failure happens occasionally (1/100–1/1000) |
| 4 | High | Failure happens often (1/10–1/100) |
| 5 | Very high | Failure is unavoidable or very possible (1–1/10) |
| Score | Scale | Description |
|---|---|---|
| 1 | Very high | Failure is easily detected |
| 2 | High | Failure is detected frequently |
| 3 | Moderate | Failure is detected occasionally by double checking |
| 4 | Low | Failure is rarely detected |
| 5 | Very low | Failure is almost never detected |
| Topic | Number |
|---|---|
| 1. Occupation | 11 (100%) |
| Engineer from KU Leuven, Belgium (producer) | 6 (55%) |
| Obstetric surgeon from University in Thailand (User) | 5 (45%) |
| 2. Position | 11 (100%) |
| Medical teacher | 5 (45%) |
| PhD student | 4 (37%) |
| Research engineer | 1 (9%) |
| Postdoctoral researcher | 1 (9%) |
| 3. Specialization | 11 (100%) |
| Maternal–fetal medicine | 5 (46%) |
| Minimally invasive surgery | 2 (18%) |
| Electromechanical engineering | 1 (9%) |
| Medical robotic and control | 2 (18%) |
| Mechanical engineering | 1 (9%) |
| 4. Experience | 11 (100%) |
| >5 years | 5 (44%) |
| 2–5 years | 3 (28%) |
| ≤2 years | 3 (28%) |
| ID | Potential Failure Modes (26) | Number (%) * |
|---|---|---|
| 1 | Handle—6 (23%) | |
| 1.1.1 | Slider breaks | 4 (36%) |
| 1.2.1 | Applying excessive force on wire | 8 (73%) |
| 1.3.1 | Wire suddenly snaps | 4 (36%) |
| 1.4.1 | Excessive air pressure | 3 (27%) |
| 1.4.2 | Air leaks within the handle | 6 (55%) |
| 1.4.3 | Unfiltered or unclean air supply | 1 (9%) |
| 2 | Flexible segment—7 (27%) | |
| 2.1.1 | Bending or tip positioning issues | 4 (36%) |
| 2.1.2 | Permanent plastic deformation due to excessive forces | 1 (9%) |
| 2.2.1 | Laser fiber snaps—during ablation | 3 (27%) |
| 2.2.2 | Preventing ablation of laser fiber | 2 (18%) |
| 2.3.1 | Wire suddenly snaps | 1 (9%) |
| 2.4.1 | Electrical wire snaps/ disconnects (due to repetitive large bending) | 1 (9%) |
| 2.5.1 | Leakage of irrigation fluid and contact with bare electrical wire | 1 (9%) |
| 3 | Tip—13 (50%) | |
| 3.1.1 | The tip breaks during use | 1 (9%) |
| 3.2.1 | Damage of the camera | 6 (55%) |
| 3.2.2 | Camera detachment | 2 (18%) |
| 3.3.1 | Light-source failure | 3 (27%) |
| 3.3.2 | Heating or thermal injury | 1 (9%) |
| 3.4.1 | Wire suddenly snaps | 1 (9%) |
| 3.5.1 | Fluid contact with bare electrical wires | 2 (18%) |
| 3.6.1 | Loosening of the tube end sleeve | 1 (9%) |
| 3.7.1 | Cracking of the glue | 1 (9%) |
| 3.7.2 | Loss of particles (debris) during use inside the patient | 1 (9%) |
| 3.7.3 | Dislodging of the glue | 2 (18%) |
| 3.7.4 | Heating, glue melting | 6 (55%) |
| 3.8.1 | Contamination of the fetoscope | 1 (9%) |
| ID | Potential Failure Modes | Main Effects | Potential Failure Effects (S) | S Score | Potential Failure Causes (O) | O Score | Detectability (D) | D Score |
|---|---|---|---|---|---|---|---|---|
| 1. Handle | ||||||||
| 1.1 Slider | ||||||||
| 1.1.1 | Slider breaks | |||||||
| 1.1.1A1 | Mother | Device configuration issues | 1 | Slider or handle mechanism issues | 1 | Visual observation | 1 | |
| 1.1.1B1 | Fetuses | Device configuration issues | 2 | Slider or handle mechanism issues | 2 | Visual observation | 1 | |
| 1.1.1A2 | Mother | Device configuration issues | 1 | User errors or misuse | 2 | Visual observation | 1 | |
| 1.1.1B2 | Fetuses | Device configuration issues | 2 | User errors or misuse | 2 | Visual observation | 1 | |
| Criteria | Weights |
|---|---|
| Severity | 0.265981 |
| Occurrence | 0.233806 |
| Detectability | 0.500213 |
| Total | 1 |
| Rank | ID | S | O | D | Risk Score | Rank | ID | S | O | D | Risk Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.8.1A3 | 7 | 1 | 5 | 0.927 | 53 | 2.5.1B9 | 3 | 1 | 5 | 0.737 |
| 2 | 3.7.2A1 | 4 | 2 | 5 | 0.896 | 54 | 3.5.1B10 | 3 | 1 | 5 | 0.737 |
| 3 | 3.7.2B6 | 4 | 2 | 5 | 0.896 | 55 | 3.5.1B12 | 3 | 1 | 5 | 0.737 |
| 4 | 3.7.3A1 | 4 | 2 | 5 | 0.896 | 56 | 3.5.1B13 | 3 | 1 | 5 | 0.737 |
| 5 | 3.7.3B5 | 4 | 2 | 5 | 0.896 | 57 | 3.5.1B14 | 3 | 1 | 5 | 0.737 |
| 6 | 3.8.1A1 | 4.333 | 2.333 | 4.333 | 0.849 | 58 | 3.5.1B15 | 3 | 1 | 5 | 0.737 |
| 7 | 3.7.1B3 | 3 | 2 | 5 | 0.848 | 59 | 3.5.1B16 | 3 | 1 | 5 | 0.737 |
| 8 | 3.7.1B6 | 3 | 2 | 5 | 0.848 | 60 | 3.5.1B17 | 3 | 1 | 5 | 0.737 |
| 9 | 3.7.2A5 | 3 | 2 | 5 | 0.848 | 61 | 3.5.1B3 | 5 | 2 | 3 | 0.722 |
| 10 | 3.7.3A4 | 3 | 2 | 5 | 0.848 | 62 | 3.7.1A1 | 5 | 2 | 3 | 0.722 |
| 11 | 3.7.2A4 | 4.143 | 1.714 | 4.714 | 0.844 | 63 | 3.8.1A2 | 4.25 | 1.5 | 3.75 | 0.720 |
| 12 | 1.4.3A2 | 4 | 3 | 4 | 0.838 | 64 | 3.7.3B4 | 2.444 | 1.889 | 4.333 | 0.717 |
| 13 | 3.8.1B5 | 4 | 3 | 4 | 0.838 | 65 | 3.7.2A3 | 5 | 1 | 4 | 0.708 |
| 14 | 3.8.1B4 | 5 | 1 | 5 | 0.826 | 66 | 2.5.1B6 | 3 | 1.667 | 4 | 0.707 |
| 15 | 3.5.1A2 | 7 | 2 | 3 | 0.823 | 67 | 1.2.1B2 | 3 | 5 | 2 | 0.696 |
| 16 | 1.4.3B2 | 3.5 | 3 | 4 | 0.818 | 68 | 3.5.1A8 | 4 | 1.5 | 3.5 | 0.686 |
| 17 | 3.8.1B1 | 3.5 | 2.333 | 4.333 | 0.815 | 69 | 2.2.2A3 | 4 | 2 | 3 | 0.681 |
| 18 | 3.7.3A3 | 3.889 | 1.889 | 4.222 | 0.79 | 70 | 3.3.2A2 | 4 | 2 | 3 | 0.681 |
| 19 | 3.7.1B5 | 2.2 | 2 | 5 | 0.787 | 71 | 3.3.2A5 | 4 | 2 | 3 | 0.681 |
| 20 | 2.5.1A10 | 4 | 1 | 5 | 0.785 | 72 | 3.3.2A6 | 4 | 2 | 3 | 0.681 |
| 21 | 2.5.1A5 | 4 | 1 | 5 | 0.785 | 73 | 3.7.1B1 | 4 | 2 | 3 | 0.681 |
| 22 | 2.5.1A7 | 4 | 1 | 5 | 0.785 | 74 | 3.7.3B3 | 4 | 2 | 3 | 0.681 |
| 23 | 2.5.1A8 | 4 | 1 | 5 | 0.785 | 75 | 2.5.1A3 | 5.5 | 2 | 2.5 | 0.679 |
| 24 | 2.5.1A9 | 4 | 1 | 5 | 0.785 | 76 | 2.5.1B7 | 4 | 1 | 4 | 0.668 |
| 25 | 3.5.1A11 | 4 | 1 | 5 | 0.785 | 77 | 3.5.1B11 | 4 | 1 | 4 | 0.668 |
| 26 | 3.5.1A12 | 4 | 1 | 5 | 0.785 | 78 | 3.5.1B5 | 4 | 1 | 4 | 0.668 |
| 27 | 3.5.1A13 | 4 | 1 | 5 | 0.785 | 79 | 3.7.2B3 | 4 | 1 | 4 | 0.668 |
| 28 | 3.5.1A14 | 4 | 1 | 5 | 0.785 | 80 | 3.5.1A3 | 2 | 2 | 4 | 0.654 |
| 29 | 3.5.1A15 | 4 | 1 | 5 | 0.785 | 81 | 3.5.1B2 | 2 | 2 | 4 | 0.654 |
| 30 | 3.5.1A16 | 4 | 1 | 5 | 0.785 | 82 | 3.5.1B7 | 2 | 2 | 4 | 0.654 |
| 31 | 3.5.1A9 | 4 | 1 | 5 | 0.785 | 83 | 3.6.1B6 | 2 | 2 | 4 | 0.654 |
| 32 | 3.8.1B2 | 4 | 1 | 5 | 0.785 | 84 | 3.2.2A4 | 5 | 1 | 3 | 0.610 |
| 33 | 3.7.1B2 | 2.667 | 2 | 4.667 | 0.78 | 85 | 1.2.1A3 | 4.5 | 1.75 | 2.25 | 0.575 |
| 34 | 3.7.2B4 | 2.857 | 1.714 | 4.714 | 0.78 | 86 | 2.2.1A12 | 5 | 1.5 | 2 | 0.526 |
| 35 | 3.7.2A7 | 4 | 2 | 4 | 0.779 | 87 | 1.2.1A1 | 5 | 1 | 2 | 0.458 |
| 36 | 3.7.2A8 | 4 | 2 | 4 | 0.779 | 88 | 3.1.1A11 | 5 | 1 | 2 | 0.458 |
| 37 | 3.7.2A9 | 4 | 2 | 4 | 0.779 | 89 | 2.2.1A10 | 5.333 | 1.333 | 1.667 | 0.457 |
| 38 | 1.4.3A1 | 2 | 2 | 5 | 0.771 | 90 | 3.3.2A7 | 5.5 | 1.5 | 1.5 | 0.453 |
| 39 | 3.7.1A2 | 2 | 2 | 5 | 0.771 | 91 | 3.3.2B8 | 4.5 | 1.5 | 1.5 | 0.411 |
| 40 | 3.7.1A5 | 2 | 2 | 5 | 0.771 | 92 | 3.4.1A6 | 4.4 | 1.2 | 1.2 | 0.307 |
| 41 | 3.7.1A6 | 2 | 2 | 5 | 0.771 | 93 | 1.4.2A1 | 6 | 1 | 1 | 0.303 |
| 42 | 3.7.2B1 | 2 | 2 | 5 | 0.771 | 94 | 1.4.2A2 | 6 | 1 | 1 | 0.303 |
| 43 | 3.7.3B1 | 2 | 2 | 5 | 0.771 | 95 | 2.2.1A11 | 6 | 1 | 1 | 0.303 |
| 44 | 2.5.1A6 | 6 | 1 | 4 | 0.753 | 96 | 2.2.1A7 | 6 | 1 | 1 | 0.303 |
| 45 | 3.5.1A10 | 6 | 1 | 4 | 0.753 | 97 | 2.2.1A8 | 6 | 1 | 1 | 0.303 |
| 46 | 3.5.1A5 | 6 | 1 | 4 | 0.753 | 98 | 2.2.1A9 | 6 | 1 | 1 | 0.303 |
| 47 | 2.5.1A4 | 3.667 | 1.667 | 4 | 0.741 | 99 | 1.3.1A4 | 5 | 1 | 1 | 0.258 |
| 48 | 3.7.1A3 | 2.286 | 2 | 4.571 | 0.74 | 100 | 2.2.1B6 | 5 | 1 | 1 | 0.258 |
| 49 | 2.5.1B10 | 3 | 1 | 5 | 0.737 | 101 | 2.3.1A4 | 5 | 1 | 1 | 0.258 |
| 50 | 2.5.1B11 | 3 | 1 | 5 | 0.737 | 102 | 2.3.1A5 | 5 | 1 | 1 | 0.258 |
| 51 | 2.5.1B5 | 3 | 1 | 5 | 0.737 | 103 | 2.3.1A6 | 5 | 1 | 1 | 0.258 |
| 52 | 2.5.1B8 | 3 | 1 | 5 | 0.737 | 104 | 3.4.1A8 | 5 | 1 | 1 | 0.258 |
| No. | Scenario 1 | Scenario 2 | Scenario 3 | Scenario 4 |
|---|---|---|---|---|
| Scenario 1 | 1.000 | 0.555 | 0.838 | 0.552 |
| Scenario 2 | 0.555 | 1.000 | 0.679 | 0.439 |
| Scenario 3 | 0.838 | 0.679 | 1.000 | 0.685 |
| Scenario 4 | 0.552 | 0.439 | 0.685 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phokee, W.; Chaiklieng, S.; Boriwan, P.; Phoka, T.; Vanoirbeek, J.; Chatpun, S. A Cascading Delphi Method-Based FMEA Risk Assessment Framework for Surgical Instrument Design: A Case Study of a Fetoscope. Appl. Sci. 2025, 15, 6203. https://doi.org/10.3390/app15116203
Phokee W, Chaiklieng S, Boriwan P, Phoka T, Vanoirbeek J, Chatpun S. A Cascading Delphi Method-Based FMEA Risk Assessment Framework for Surgical Instrument Design: A Case Study of a Fetoscope. Applied Sciences. 2025; 15(11):6203. https://doi.org/10.3390/app15116203
Chicago/Turabian StylePhokee, Wipharat, Sunisa Chaiklieng, Pornpimon Boriwan, Thanathorn Phoka, Jeroen Vanoirbeek, and Surapong Chatpun. 2025. "A Cascading Delphi Method-Based FMEA Risk Assessment Framework for Surgical Instrument Design: A Case Study of a Fetoscope" Applied Sciences 15, no. 11: 6203. https://doi.org/10.3390/app15116203
APA StylePhokee, W., Chaiklieng, S., Boriwan, P., Phoka, T., Vanoirbeek, J., & Chatpun, S. (2025). A Cascading Delphi Method-Based FMEA Risk Assessment Framework for Surgical Instrument Design: A Case Study of a Fetoscope. Applied Sciences, 15(11), 6203. https://doi.org/10.3390/app15116203









