Hydrogen Evolution in Battery Electric Vehicle Coolants During Accidental Leakage: The Impact of Corrosion Inhibitors and Electrical Conductivity
Abstract
Featured Application
Abstract
1. Introduction
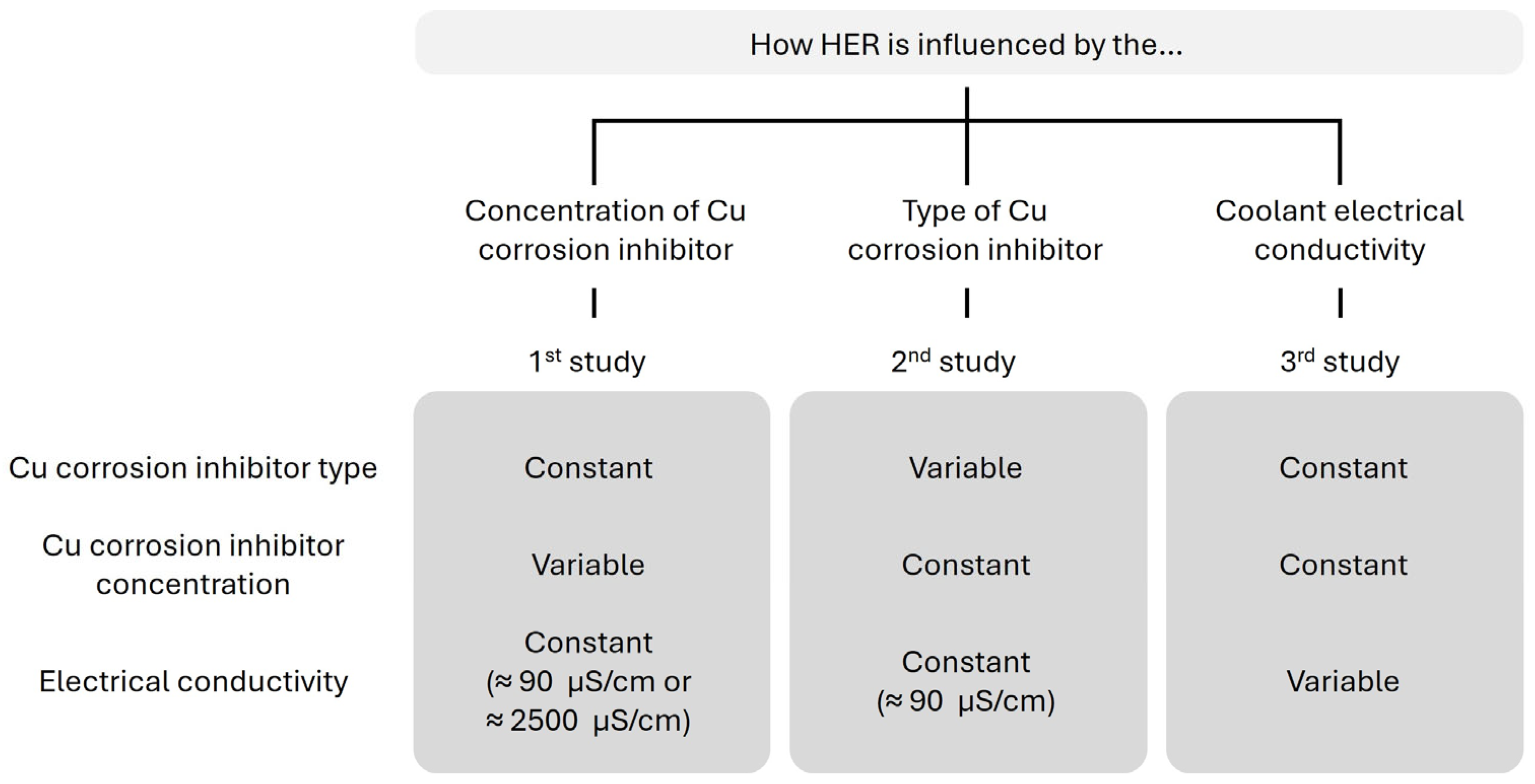
2. Materials and Methods
2.1. Materials Preparation
2.2. Solution Characterization
2.3. Electrochemical Characterization
2.4. Reproducibility
3. Results and Discussion
3.1. Effect of Copper Corrosion Inhibitor Concentration on HER Suppression
3.2. Effect of Different Copper Corrosion Inhibitors on HER Suppression
3.3. Effect of Coolant Electrical Conductivity on HER
3.4. Future Research Directions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bamdezh, M.A.; Molaeimanesh, G.R. The Path from Conventional Battery Thermal Management Systems to Hybrid Battery Thermal Management Systems for Electric Vehicles, Opportunities and Challenges. J. Energy Storage 2024, 100, 113160. [Google Scholar] [CrossRef]
- Anisha; Kumar, A. Identification and Mitigation of Shortcomings in Direct and Indirect Liquid Cooling-Based Battery Thermal Management System. Energies 2023, 16, 3857. [Google Scholar] [CrossRef]
- EV Specifications. Available online: https://www.evspecifications.com (accessed on 17 March 2025).
- Tai, L.D.; Garud, K.S.; Hwang, S.-G.; Lee, M.-Y. A Review on Advanced Battery Thermal Management Systems for Fast Charging in Electric Vehicles. Batteries 2024, 10, 372. [Google Scholar] [CrossRef]
- Nilsson, E.J.K.; Runefors, M. Fire Hazards Associated with the Use of Water and Glycol as Coolants for Li-Ion Battery Systems. Fire Technol. 2025. [Google Scholar] [CrossRef]
- Naimi, Y.; Antar, A. Hydrogen Generation by Water Electrolysis. In Advances In Hydrogen Generation Technologies; Eyvaz, M., Ed.; InTech: Rijeka, Croatia, 2018; ISBN 978-1-78923-535-7. [Google Scholar]
- Farinazzo Bergamo Dias Martins, P.; Papa Lopes, P.; Ticianelli, E.A.; Stamenkovic, V.R.; Markovic, N.M.; Strmcnik, D. Hydrogen Evolution Reaction on Copper: Promoting Water Dissociation by Tuning the Surface Oxophilicity. Electrochem. Commun. 2019, 100, 30–33. [Google Scholar] [CrossRef]
- Marin, D.; Medicuti, F.; Teijeiro, C. An Electrochemistry Experiment: Hydrogen Evolution Reaction on Different Electrodes. J. Chem. Educ. 1994, 71, A277. [Google Scholar] [CrossRef]
- Liu, M.; Yoon, L.; Lee, S.; Wong, K. Highly Efficient Electrocatalytic Water Splitting Rational Design of Catalyst and Research Progress. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova, O.V., Torres-Martínez, L.M., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-11155-7. [Google Scholar]
- IEN Staff. Coolant Leak in Single Battery Pack the Probable Cause of Nikola Truck Fire. Industrial Equipment News, 14 August 2023. [Google Scholar]
- Ono, R.; Nifuku, M.; Fujiwara, S.; Horiguchi, S.; Oda, T. Minimum Ignition Energy of Hydrogen-Air Mixture: Effects of Humidity and Spark Duration. J. Electrostat. 2007, 65, 87–93. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, X.; Hao, H. A Review of Hydrogen-Air Cloud Explosions: The Fundamentals, Overpressure Prediction Methods, and Influencing Factors. Int. J. Hydrogen Energy 2023, 48, 13705–13730. [Google Scholar] [CrossRef]
- Blaufuß, M.; Wetzig, D. Proposed Standards and Methods for Leak Testing Lithium-Ion Battery Packs Using Glycol-Based Coolant with Empirically Derived Rejection Limits. SAE Int. J. Adv. Curr. Pract. Mobil. 2022, 4, 2012–2023. [Google Scholar] [CrossRef]
- Adhikari, N.; Bhandari, R.; Joshi, P. Thermal Analysis of Lithium-Ion Battery of Electric Vehicle Using Different Cooling Medium. Appl. Energy 2024, 360, 122781. [Google Scholar] [CrossRef]
- Shahroom, A.F.; Rahman, N.A.; Mansor, M.; Abd. Rahman, M.S. Modelling Analysis of Propylene Glycol as a Cooling Media for Battery Thermal Management System in Electric Vehicles. Arab. J. Sci. Eng. 2024. [Google Scholar] [CrossRef]
- Fateh, A.; Aliofkhazraei, M.; Rezvanian, A.R. Review of Corrosive Environments for Copper and Its Corrosion Inhibitors. Arab. J. Chem. 2020, 13, 481–544. [Google Scholar] [CrossRef]
- Bayaguud, A.; Fu, Y.; Zhu, C. Interfacial Parasitic Reactions of Zinc Anodes in Zinc Ion Batteries: Underestimated Corrosion and Hydrogen Evolution Reactions and Their Suppression Strategies. J. Energy Chem. 2022, 64, 246–262. [Google Scholar] [CrossRef]
- Nian, Q.; Zhang, X.; Feng, Y.; Liu, S.; Sun, T.; Zheng, S.; Ren, X.; Tao, Z.; Zhang, D.; Chen, J. Designing Electrolyte Structure to Suppress Hydrogen Evolution Reaction in Aqueous Batteries. ACS Energy Lett. 2021, 6, 2174–2180. [Google Scholar] [CrossRef]
- Alba-Molina, D.; Puente Santiago, A.R.; Giner-Casares, J.J.; Rodríguez-Castellón, E.; Martín-Romero, M.T.; Camacho, L.; Luque, R.; Cano, M. Tailoring the ORR and HER Electrocatalytic Performances of Gold Nanoparticles through Metal-Ligand Interfaces. J. Mater. Chem. A Mater. 2019, 7, 20425–20434. [Google Scholar] [CrossRef]
- ASTM D1125; Standard Test Methods for Electrical Conductivity and Resistivity of Water. ASTM: West Conshohocken, PA, USA, 2023.
- ASTM D1287; Standard Test Method for pH of Engine Coolants and Antirusts. ASTM: West Conshohocken, PA, USA, 2020.
- Hollander, O.; May, R.C. The Chemistry of Azole Copper Corrosion Inhibitors in Cooling Waters. Corrosion 1985, 41, 39–45. [Google Scholar] [CrossRef]
- Walker, R. Corrosion Inhibition of Copper by Tolyltriazole. Corrosion 1976, 32, 339–341. [Google Scholar] [CrossRef]
- Ahmad, Z. Principles of Corrosion Engineering and Corrosion Control; Butterworth-Heinemann: Oxford, UK; Elsevier: Amsterdam, The Netherlands, 2006; ISBN 9780750659246. [Google Scholar]
- Schmitt, T.; Muhzer, E.S. Determination of 2-Mercaptobenzothiazole, Tolyltriazole and Benzotriazole in Coolant Formulations by Liquid Chromatography. Talanta 1981, 28, 777–779. [Google Scholar] [CrossRef]
- Antonijević, M.M.; Milić, S.M.; Petrović, M.B. Films Formed on Copper Surface in Chloride Media in the Presence of Azoles. Corros. Sci. 2009, 51, 1228–1237. [Google Scholar] [CrossRef]
- Loo, B.H.; Ibrahim, A.; Emerson, M.T. Analysis of Surface Coverage of Benzotriazole and 6-Tolyltriazole Mixtures on Copper Electrodes from Surface-Enhanced Raman Spectra. Chem. Phys. Lett. 1998, 287, 449–454. [Google Scholar] [CrossRef]
- Notoya, T.; Poling, G.W. Benzotriazole and Tolyltriazole as Corrosion Inhibitors for Copper and Brasses. Corros. Eng. 1981, 389, 381–389. [Google Scholar] [CrossRef]
- Marconato, J.C.; Bulhões, L.O.; Temperini, M.L. A Spectroelectrochemical Study of the Inhibition of the Electrode Process on Copper by 2-Mercaptobenzothiazole in Ethanolic Solutions. Electrochim. Acta 1998, 43, 771–780. [Google Scholar] [CrossRef]
- Altaf, F.; Qureshi, R.; Ahmed, S. Surface Protection of Copper by Azoles in Borate Buffers-Voltammetric and Impedance Analysis. J. Electroanal. Chem. 2011, 659, 134–142. [Google Scholar] [CrossRef]
- Lin, J.-Y.; West, A.C. Adsorption–Desorption Study of Benzotriazole in a Phosphate-Based Electrolyte for Cu Electrochemical Mechanical Planarization. Electrochim. Acta 2010, 55, 2325–2331. [Google Scholar] [CrossRef]
- Elhamid, M.H.A.; Ateya, B.G.; Pickering, H.W. Effect of Benzotriazole on the Hydrogen Absorption by Iron. J. Electrochem. Soc. 1997, 144, L58–L61. [Google Scholar] [CrossRef]
- Semat, H.; Katz, R. (Eds.) Electrical Conduction in Liquids and Solids. In Physics; Rinehart & Company: Lindsay, OK, USA, 1958. [Google Scholar]
- Anantharaj, S. Hydrogen Evolution Reaction on Pt and Ru in Alkali with Volmer-Step Promotors and Electronic Structure Modulators. Curr. Opin. Electrochem. 2022, 33, 100961. [Google Scholar] [CrossRef]
- Oelßner, W.; Berthold, F.; Guth, U. The IR Drop—Well-Known but Often Underestimated in Electrochemical Polarization Measurements and Corrosion Testing. Mater. Corros. 2006, 57, 455–466. [Google Scholar] [CrossRef]
- Orendorff, C.J.; Lamb, J.; Anna, L.; Steele, M. Recommended Practices for Abuse Testing Rechargeable Energy Storage Systems (RESSs); Sandia National Laboratories: Albuquerque, NM, USA, 2017. [Google Scholar]
- Perry, J.; Velotta, M. Simulated Battery Coolant Leak Test Apparatus—Development and Learnings from Testing. Available online: http://archive.today/2025.05.28-111002/https://connect.asminternational.org/indianapolischapter/events/event-description?CalendarEventKey=6b890f9e-7112-4150-93a1-01965daf03ad (accessed on 15 April 2025).
- GB 29743.2; Motor Vehicle Coolant-Part 2: Electric Vehicle Coolant. Standardization Administration of the People’s Republic of China: Beijing, China, 2025.





| Property | ICE Coolant | BEV Coolant |
|---|---|---|
| Base fluid | Water/Ethylene glycol mixture | Water/Ethylene glycol mixture |
| Water content (%) | 50 | 50 |
| Freezing point (°C) | −37 | −38 |
| Boiling point (°C) | 110 | 112 |
| Electrical conductivity (µS/cm) | 2000–8000 | <100 |
| Principal corrosion inhibitor | Organic acids | Organic acids |
| Corrosion inhibitor content (w%) | 1–6 | <1 |
| Neutralizing base | NaOH | Amine |
| pH | 7.0–9.0 | 7.0–9.0 |
| Density at 20 °C (g/mL) | 1.068 | 1.067 |
| Kinematic viscosity at 20 °C (mm2/s) | 4.3 | 3.9 |
| Thermal conductivity (W/m·K) | 0.42 | 0.42 |
| Heat capacity (kJ/kg·K) | 3.3 | 3.4 |
| Coolant | Tolyltriazole (Relative %) | pH | eConductivity at 25 °C (µS/cm) |
|---|---|---|---|
| BEV | 20 | 8.16 | 89.63 |
| 50 | 8.12 | 90.82 | |
| 100 | 8.11 | 86.43 | |
| 200 | 8.00 | 92.57 | |
| 430 | 8.01 | 109.14 | |
| ICE | 0 | 7.54 | 2520.0 |
| 50 | 7.62 | 2476.8 | |
| 100 | 7.54 | 2576.8 | |
| 200 | 7.50 | 2654.0 | |
| 430 | 8.40 | 2623.3 |
| Coolant | Cu Inhibitors | Inhibitor Content (Relative %) | pH | eConductivity at 25 °C (µS/cm) |
|---|---|---|---|---|
| BEV | TTZ | 100 | 8.11 | 86.43 |
| MBT-Na | 100 | 8.26 | 108.13 | |
| TTZ | 50 | 8.25 | 101.98 | |
| MBT-Na | 50 | |||
| BTZ | 100 | 8.14 | 97.60 | |
| TTZ | 50 | 8.10 | 95.31 | |
| BTZ | 50 |
| Coolant | Coolant Concentrate (w%) | Monoethylene Glycol (w%) | pH | eConductivity at 25 °C (µS/cm) |
|---|---|---|---|---|
| BEV | 100 | 0 | 8.11 | 86.43 |
| 75 | 25 | 8.05 | 71.05 | |
| 50 | 50 | 8.04 | 50.44 | |
| 25 | 75 | 7.95 | 28.70 | |
| 10 | 90 | 7.80 | 13.21 | |
| ICE | 100 | 0 | 7.54 | 2576.80 |
| 75 | 25 | 7.51 | 2040.70 | |
| 50 | 50 | 7.47 | 1430.60 | |
| 25 | 75 | 7.43 | 712.20 | |
| 10 | 90 | 7.31 | 299.10 | |
| 2.75 | 97.25 | 7.38 | 86.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sopchenski, L.; Clerick, S.; Buytaert, G.; Lievens, S.; Kalogiannis, T.; Hubin, A.; Terryn, H. Hydrogen Evolution in Battery Electric Vehicle Coolants During Accidental Leakage: The Impact of Corrosion Inhibitors and Electrical Conductivity. Appl. Sci. 2025, 15, 6168. https://doi.org/10.3390/app15116168
Sopchenski L, Clerick S, Buytaert G, Lievens S, Kalogiannis T, Hubin A, Terryn H. Hydrogen Evolution in Battery Electric Vehicle Coolants During Accidental Leakage: The Impact of Corrosion Inhibitors and Electrical Conductivity. Applied Sciences. 2025; 15(11):6168. https://doi.org/10.3390/app15116168
Chicago/Turabian StyleSopchenski, Luciane, Sander Clerick, Guy Buytaert, Serge Lievens, Theodoros Kalogiannis, Annick Hubin, and Herman Terryn. 2025. "Hydrogen Evolution in Battery Electric Vehicle Coolants During Accidental Leakage: The Impact of Corrosion Inhibitors and Electrical Conductivity" Applied Sciences 15, no. 11: 6168. https://doi.org/10.3390/app15116168
APA StyleSopchenski, L., Clerick, S., Buytaert, G., Lievens, S., Kalogiannis, T., Hubin, A., & Terryn, H. (2025). Hydrogen Evolution in Battery Electric Vehicle Coolants During Accidental Leakage: The Impact of Corrosion Inhibitors and Electrical Conductivity. Applied Sciences, 15(11), 6168. https://doi.org/10.3390/app15116168







