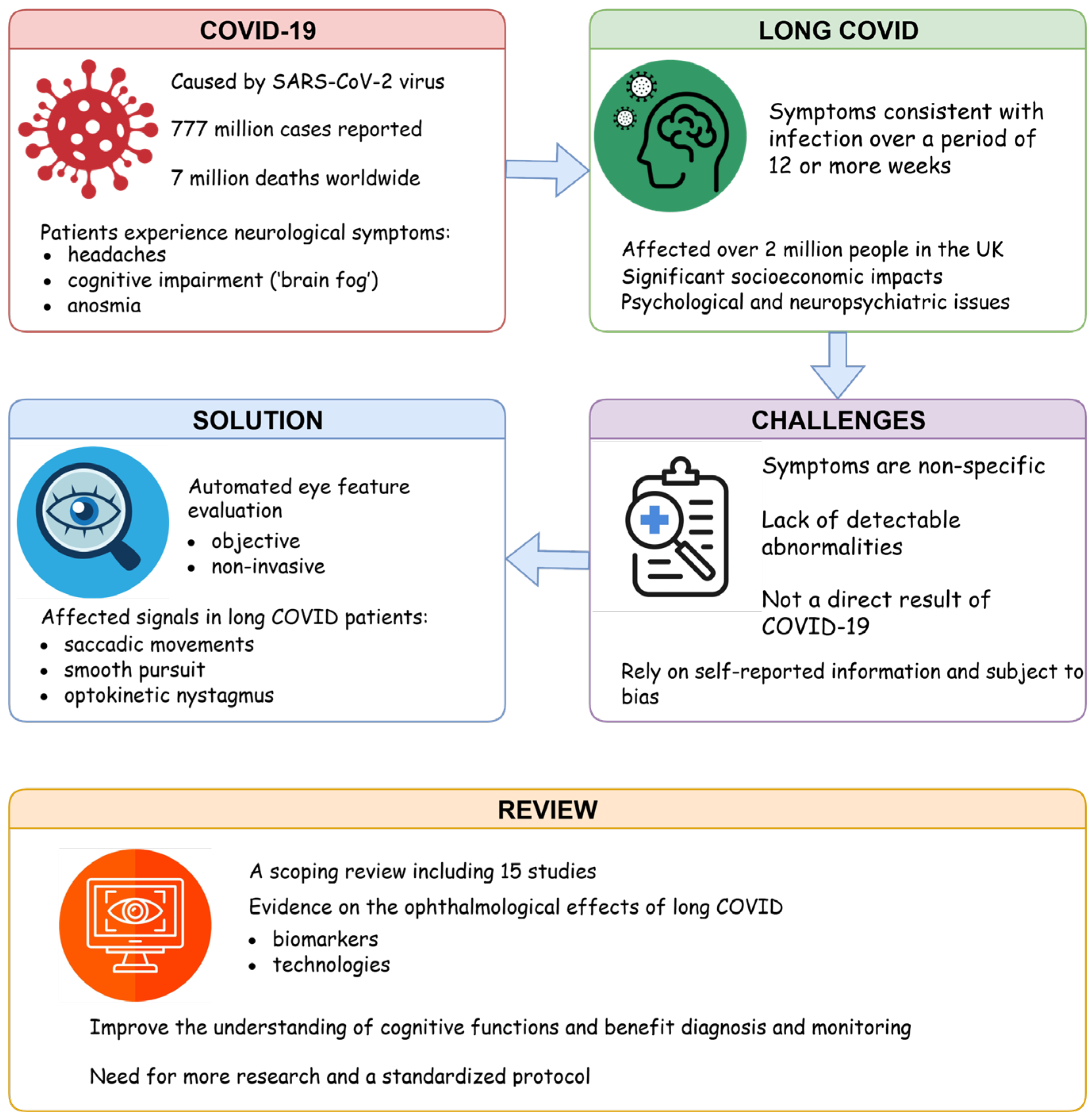
On the Quest for Ophthalmological Biomarkers for Long COVID: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Registration and Reporting
2.2. Search Strategy
2.3. Study Eligibility
- Studies of clinical populations being investigated for long COVID.
- Studies using automated technology for measuring the above symptoms.
- Studies reporting ophthalmological parameters and outcome measures relevant to the clinical management of patient populations.
- Studies on fundoscopy.
- Studies on post-vaccination populations.
- Studies on healthy populations (unless healthy control comparator data were reported separately in a study also reporting on a patient population).
2.4. Study Selection
2.5. Data Extraction and Synthesis
3. Results
3.1. Summary of Results
3.2. Study Characteristics
3.2.1. Study Types
3.2.2. Study Demographics
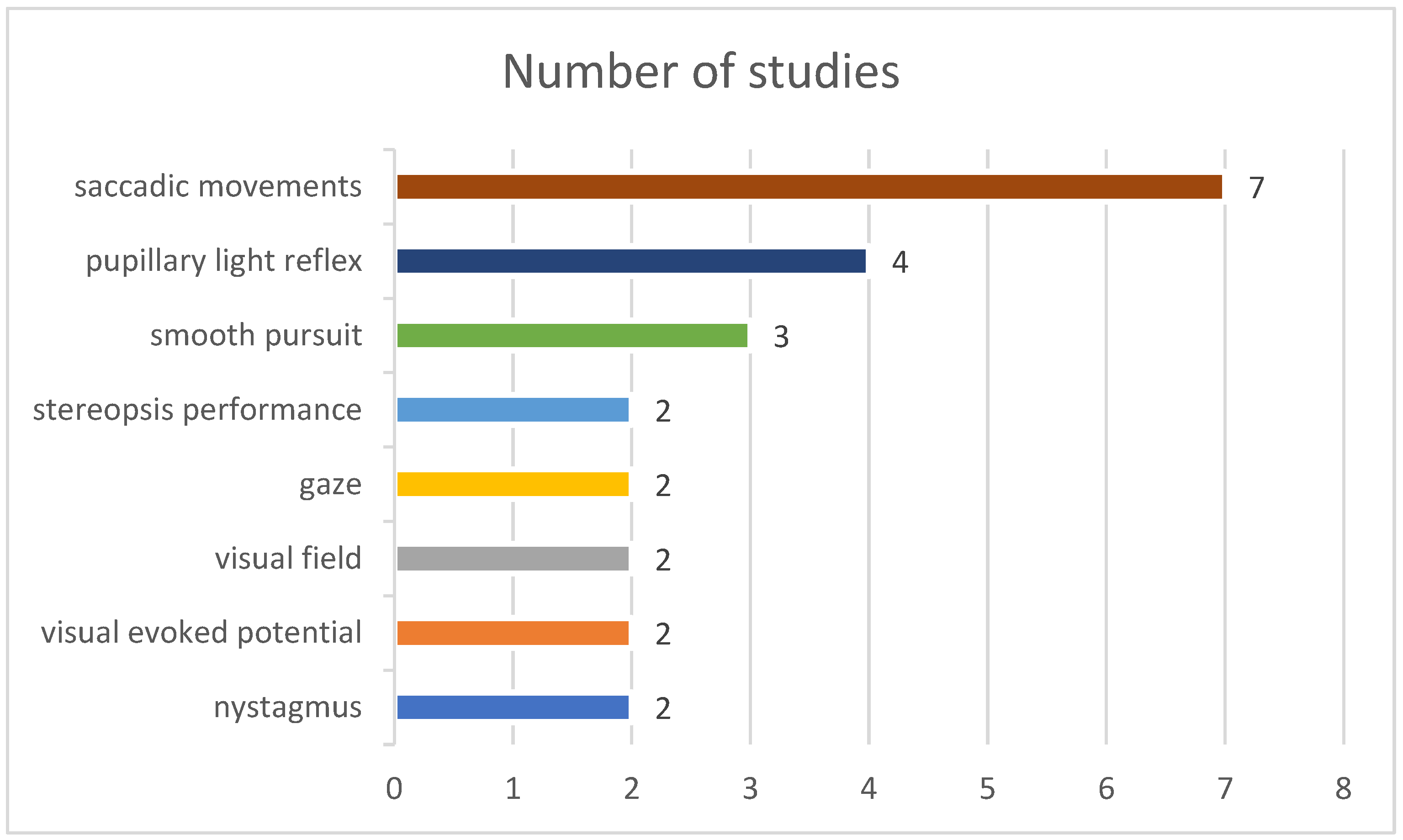
3.2.3. Ophthalmological Signal Types
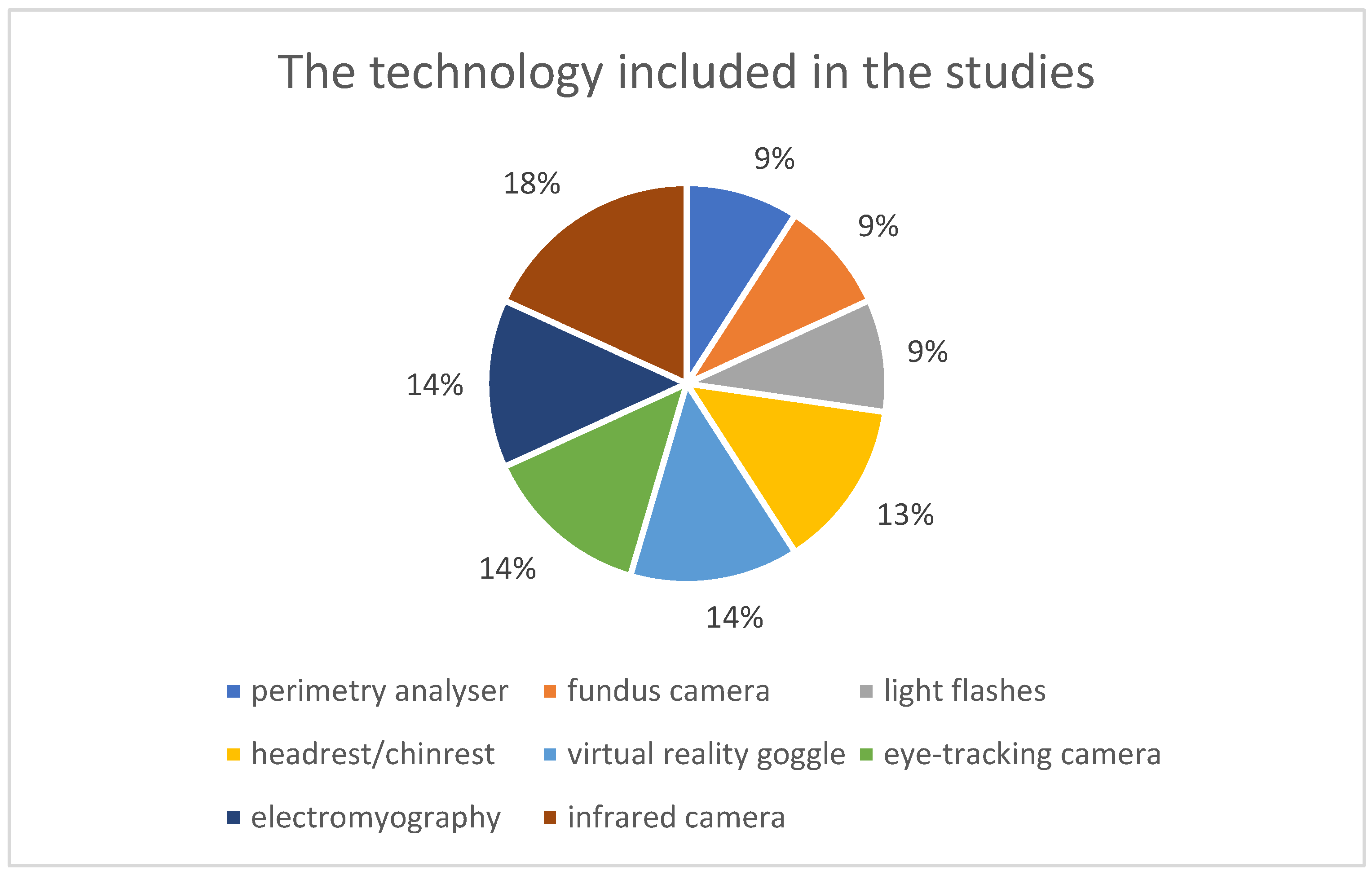
3.2.4. Technologies
3.2.5. Ophthalmological Abnormalities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDC | Centers for Disease Control and Prevention |
| CSF | cerebrospinal fluid |
| EOG | electroretinogram |
| ERG | electrooculogram |
| NICE | National Institute for Health and Care Excellence |
| TSD | time since diagnosis |
| PLR | pupillary light reflex |
| VEP | visual evoked potential |
| OVRT-C | Oculomotor Vestibular Reaction Time Cognitive test |
References
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 26 January 2023).
- Alimohamadi, Y.; Sepandi, M.; Taghdir, M.; Hosamirudsari, H. Determine the most common clinical symptoms in COVID-19 patients: A systematic review and meta-analysis. J. Prev. Med. Hyg. 2020, 61, e304–e312. [Google Scholar] [PubMed]
- Ahmad, S.J.; Feigen, C.M.; Vazquez, J.P.; Kobets, A.J.; Altschul, D.J. Neurological Sequelae of COVID-19. J. Integr. Neurosci. 2022, 21, 77. [Google Scholar] [CrossRef]
- Wang, L.; Shen, Y.; Li, M.; Chuang, H.; Ye, Y.; Zhao, H.; Wang, H. Clinical manifestations and evidence of neurological involvement in 2019 novel coronavirus SARS-CoV-2: A systematic review and meta-analysis. J. Neurol. 2020, 267, 2777–2789. [Google Scholar] [CrossRef]
- Destras, G.; Bal, A.; Escuret, V.; Morfin, F.; Lina, B.; Josset, L. COVID-Diagnosis HCL Study Group. Systematic SARS-CoV-2 screening in cerebrospinal fluid during the COVID-19 pandemic. Lancet Microbe 2020, 1, 149. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lazartigues, E. Expression of ACE2 in Human Neurons Supports the Neuro-Invasive Potential of COVID-19 Virus. Cell Mol. Neurobiol. 2022, 42, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Maragakis, L. Coronavirus Diagnosis: What Should I Expect? Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/diagnosed-with-covid-19-what-to-expect (accessed on 19 April 2023).
- NICE. SIGN COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. Available online: https://www.nice.org.uk/guidance/ng188/resources/covid19-rapid-guideline-managing-the-longterm-effects-of-covid19-pdf-66142028400325 (accessed on 27 January 2023).
- National Institute for Health and Care Excellence. Long-Term Effects of Coronavirus (long COVID). Available online: https://cks.nice.org.uk/topics/long-term-effects-of-coronavirus-long-covid/ (accessed on 27 March 2023).
- Nittas, V.; Gao, M.; West, E.A.; Ballouz, T.; Menges, D.; Wulf Hanson, S.; Puhan, M.A. Long COVID Through a Public Health Lens: An Umbrella Review. Public Health Rev. 2022, 43, 1604501. [Google Scholar] [CrossRef]
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Debski, M.; Naing, T.K.P.; Garg, P.; Clark, A.; Ntatsaki, E.; Vassiliou, V.S. Risk Factors Associated With Post−COVID-19 Condition: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2023, 183, 566–580. [Google Scholar] [CrossRef]
- Sykes, D.L.; Holdsworth, L.; Jawad, N.; Gunasekera, P.; Morice, A.H.; Crooks, M.G. Post-COVID-19 Symptom Burden: What is Long-COVID and How Should We Manage It? Lung 2021, 199, 113–119. [Google Scholar] [CrossRef]
- Younger, D.S. Postmortem neuropathology in COVID-19: An update. Brain Pathol. 2023, 33, e13204. [Google Scholar] [CrossRef]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef]
- Zilberman-Itskovich, S.; Catalogna, M.; Sasson, E.; Elman-Shina, K.; Hadanny, A.; Lang, E.; Finci, S.; Polak, N.; Fishlev, G.; Korin, C.; et al. Hyperbaric oxygen therapy improves neurocognitive functions and symptoms of post-COVID condition: Randomized controlled trial. Sci. Rep. 2022, 12, 11252. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.L.; Clark, J.R.; Orban, Z.S.; Lim, P.H.; Szymanski, A.L.; Taylor, C.; DiBiase, R.M.; Jia, D.T.; Balabanov, R.; Ho, S.U.; et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized COVID-19 “long haulers”. Ann. Clin. Transl. Neurol. 2021, 8, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.-Y.; Saavedra-Pena, G.; Sodini, C.G.; Sze, V.; Heldt, T. Measuring Saccade Latency Using Smartphone Cameras. IEEE J. Biomed. Health Inform. 2020, 24, 885–897. [Google Scholar] [CrossRef]
- Bueno, A.P.A.; Sato, J.R.; Hornberger, M. Eye tracking–The overlooked method to measure cognition in neurodegeneration? Neuropsychologia 2019, 133, 107191. [Google Scholar] [CrossRef]
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohana, M.; et al. Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef]
- Carbone, F.; Zamarian, L.; Rass, V.; Bair, S.; Ritter, M.; Beer, R.; Mahlknecht, P.; Heim, B.; Limmert, V.; Peball, M. Cognitive dysfunction 1 year after COVID-19: Evidence from eye tracking. Ann. Clin. Transl. Neurol. 2022, 9, 1826–1831. [Google Scholar] [CrossRef]
- Liotta, E.M.; Batra, A.; Clark, J.R.; Shlobin, N.A.; Hoffman, S.C.; Orban, Z.S.; Koralnik, I.J. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann. Clin. Transl. Neurol. 2020, 7, 2221–2230. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Lisy, K.; Porritt, K. Narrative Synthesis: Considerations and challenges. JBI Evid. Implement. 2016, 14, 201. [Google Scholar] [CrossRef]
- Bitirgen, G.; Korkmaz, C.; Zamani, A.; Iyisoy, M.S.; Kerimoglu, H.; Malik, R.A. Abnormal quantitative pupillary light responses following COVID-19. Int. Ophthalmol. 2022, 42, 2847–2854. [Google Scholar] [CrossRef]
- Abdelrahman, T.; Shafik, N. Video-nystagmography test findings in post COVID-19 patients. Hear Balance Commun. 2021, 19, 264–269. [Google Scholar] [CrossRef]
- Bellavia, S.; Scala, I.; Luigetti, M.; Brunetti, V.; Gabrielli, M.; Zileri Dal Verme, L.; Servidei, S.; Calabresi, P.; Frisullo, G.; Della Marca, G. Instrumental Evaluation of COVID-19 Related Dysautonomia in Non-Critically-Ill Patients: An Observational, Cross-Sectional Study. J. Clin. Med. 2021, 10, 5861. [Google Scholar] [CrossRef]
- Vinuela-Navarro, V.; Goset, J.; Aldaba, M.; Mestre, C.; Rovira-Gay, C.; Cano, N.; Ariza, M.; Delàs, B.; Garolera, M.; Vilaseca, M. Eye movements in patients with post-COVID condition. Biomed. Opt. Express 2023, 14, 3936–3949. [Google Scholar] [CrossRef]
- Kelly, K.M.; Anghinah, R.; Kullmann, A.; Ashmore, R.C.; Synowiec, A.S.; Gibson, L.C.; Manfrinati, L.; de Araújo, A.; Spera, R.R.; Brucki, S.M.D.; et al. Oculomotor, vestibular, reaction time, and cognitive tests as objective measures of neural deficits in patients post COVID-19 infection. Front. Neurol. 2022, 13, 919596. [Google Scholar] [CrossRef]
- García Cena, C.; Costa, M.C.; Saltarén Pazmiño, R.; Santos, C.P.; Gómez-Andrés, D.; Benito-León, J. Eye Movement Alterations in Post-COVID-19 Condition: A Proof-of-Concept Study. Sensors 2022, 22, 1481. [Google Scholar] [CrossRef]
- Koskderelioglu, A.; Eskut, N.; Ortan, P.; Ozdemir, H.O.; Tosun, S. Visual evoked potential and nerve conduction study findings in patients recovered from COVID-19. Neurol. Sci. 2022, 43, 2285–2293. [Google Scholar] [CrossRef]
- Johansson, J.; Möller, M.; Markovic, G.; Borg, K. Vision impairment is common in non-hospitalised patients with post-COVID-19 syndrome. Clin. Exp. Optom. 2024, 107, 324–331. [Google Scholar] [CrossRef]
- González-Vides, L.; Hernández-Verdejo, J.; Gómez-Pedrero, J.; Ruiz-Pomeda, A.; Cañadas-Suárez, P. Oculomotor Behaviour in Individuals with Long COVID-19. Clin. Rehabil. 2024, 38, 1372–1381. [Google Scholar] [CrossRef]
- Güttes, M.; Lucio, M.; Skornia, A.; Rühl, E.; Steußloff, F.; Zott, J.; Mardin, C.; Mehringer, W.; Ganslmayer, M.; Michelson, G.; et al. A case-control study of reaction time deficits in a 3D virtual reality in patients with Post-COVID syndrome. Sci. Rep. 2024, 14, 27204. [Google Scholar] [CrossRef]
- Mehringer, W.; Stoeve, M.; Krauss, D.; Ring, M.; Steussloff, F.; Güttes, M.; Zott, J.; Hohberger, B.; Michelson, G.; Eskofier, B. Virtual reality for assessing stereopsis performance and eye characteristics in Post-COVID. Sci. Rep. 2023, 13, 13167. [Google Scholar] [CrossRef]
- Braceros, K.K.; Asahi, M.G.; Gallemore, R.P. Visual Snow-Like Symptoms and Posterior Uveitis following COVID-19 Infection. Case Rep. Ophthalmol. Med. 2021, 2021, 6668552. [Google Scholar] [CrossRef] [PubMed]
- De Giglio, L.; Sadun, F.; Roberti, C.; Polidori, L.; Gilardi, M.; Altavista, M.C.; Pennisi, E.M. Post-COVID simultaneous onset of Graves’ disease and ocular myasthenia gravis in a patient with a complex ocular motility impairment. Eur. J. Ophthalmol. 2023, 33, np49–np51. [Google Scholar] [CrossRef]
- Sabel, B.A.; Zhou, W.; Huber, F.; Schmidt, F.; Sabel, K.; Gonschorek, A.; Bilc, M. Non-invasive brain microcurrent stimulation therapy of long-COVID-19 reduces vascular dysregulation and improves visual and cognitive impairment. Restor. Neurol. Neurosci. 2021, 39, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Abreu, G.E.; Carreón-Rodriguez, A.; Zuñiga, S.; Pozo, D. Editorial: SARS-CoV-2 in neurodegenerative diseases. Front. Neurosci. 2024, 18, 1360234. [Google Scholar] [CrossRef]
- Monje, M.; Iwasaki, A. The neurobiology of long COVID. Neuron 2022, 110, 3484–3496. [Google Scholar] [CrossRef]
- Crunfli, F.; Carregari, V.C.; Veras, F.P.; Silva, L.S.; Nogueira, M.H.; Antunes, A.; Vendramini, P.H.; Valença, A.G.F.; Brandão-Teles, C.; Zuccoli, G.D.S.; et al. Morphological, cellular, and molecular basis of brain infection in COVID-19 patients. Proc. Natl. Acad. Sci. USA 2022, 119, e2200960119. [Google Scholar] [CrossRef]
- Horowitz, T.; Pellerin, L.; Zimmer, E.; Guedj, E. Brain fog in long COVID: A glutamatergic hypothesis with astrocyte dysfunction accounting for brain PET glucose hypometabolism. Med. Hypotheses 2023, 180, 111186. [Google Scholar] [CrossRef]
- Hosp, J.A.; Dressing, A.; Blazhenets, G.; Bormann, T.; Rau, A.; Schwabenland, M.; Thurow, J.; Wagner, D.; Waller, C.; Niesen, W.D.; et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain 2021, 144, 1263–1276. [Google Scholar] [CrossRef]
- Cauli, B.; Dusart, I.; Li, D. Lactate as a determinant of neuronal excitability, neuroenergetics and beyond. Neurobiol. Dis. 2023, 184, 106207. [Google Scholar] [CrossRef]
- Hepschke, J.L.; Seymour, R.A.; He, W.; Etchell, A.; Sowman, P.F.; Fraser, C.L. Cortical oscillatory dysrhythmias in visual snow syndrome: A magnetoencephalography study. Brain Commun. 2022, 4, fcab296. [Google Scholar] [CrossRef]
- Gollion, C. Cortical excitability in migraine: Contributions of magnetic resonance imaging. Rev. Neurol. 2021, 177, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Accornero, N.; Li Voti, P.; La Riccia, M.; Gregori, B. Visual evoked potentials modulation during direct current cortical polarization. Exp. Brain Res. 2007, 178, 261–266. [Google Scholar] [CrossRef]
- Balduz, M.; Fidancı, H. Visual evoked potential abnormalities in patients with COVID-19. Rev. Assoc. Med. Bras. 2024, 70, e20231061. [Google Scholar] [CrossRef]
- Bressler, S.L.; Menon, V. Large-scale brain networks in cognition: Emerging methods and principles. Trends Cogn. Sci. 2010, 14, 277–290. [Google Scholar] [CrossRef]
- Avery, M.C.; Krichmar, J.L. Neuromodulatory Systems and Their Interactions: A Review of Models, Theories, and Experiments. Front. Neural Circuits 2017, 11, 108. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. S2), 136–153. [Google Scholar] [CrossRef]
- Bungenberg, J.; Hohenfeld, C.; Costa, A.S.; Heine, J.; Schwichtenberg, K.; Hartung, T.; Franke, C.; Binkofski, F.; Schulz, J.B.; Finke, C.; et al. Characteristic functional connectome related to Post-COVID-19 syndrome. Sci. Rep. 2024, 14, 4997. [Google Scholar] [CrossRef] [PubMed]
- Kesler, S.R.; Franco-Rocha, O.Y.; De La Torre Schutz, A.; Lewis, K.A.; Aziz, R.M.; Henneghan, A.M.; Melamed, E.; Brode, W.M. Altered functional brain connectivity, efficiency, and information flow associated with brain fog after mild to moderate COVID-19 infection. Sci. Rep. 2024, 14, 22094. [Google Scholar] [CrossRef] [PubMed]
- Scardua-Silva, L.; Amorim da Costa, B.; Karmann Aventurato, Í.; Batista Joao, R.; Machado de Campos, B.; Rabelo de Brito, M.; Bechelli, J.F.; Santos Silva, L.C.; Ferreira dos Santos, A.; Koutsodontis Machado Alvim, M.; et al. Microstructural brain abnormalities, fatigue, and cognitive dysfunction after mild COVID-19. Sci. Rep. 2024, 14, 1758. [Google Scholar] [CrossRef]
- Zhao, S.; Shibata, K.; Hellyer, P.J.; Trender, W.; Manohar, S.; Hampshire, A.; Husain, M. Rapid vigilance and episodic memory decrements in COVID-19 survivors. Brain Commun. 2022, 4, fcab295. [Google Scholar] [CrossRef]
- Fietz, J.; Pöhlchen, D.; Binder, F.P.; Czisch, M.; Sämann, P.G.; Spoormaker, V.I. Pupillometry tracks cognitive load and salience network activity in a working memory functional magnetic resonance imaging task. Hum. Brain Mapp. 2022, 43, 665–680. [Google Scholar] [CrossRef]
- Yeung, M.K.; Lee, T.L.; Han, Y.M.Y.; Chan, A.S. Prefrontal activation and pupil dilation during n-back task performance: A combined fNIRS and pupillometry study. Neuropsychologia 2021, 159, 107954. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Li, Y.; Kalwani, R.M.; Gold, J.I. Relationships between Pupil Diameter and Neuronal Activity in the Locus Coeruleus, Colliculi, and Cingulate Cortex. Neuron 2016, 89, 221–234. [Google Scholar] [CrossRef]
- Ebitz, R.B.; Moore, T. Selective Modulation of the Pupil Light Reflex by Microstimulation of Prefrontal Cortex. J. Neurosci. 2017, 37, 5008–5018. [Google Scholar] [CrossRef] [PubMed]
- Karahan, M.; Demirtaş, A.A.; Hazar, L.; Erdem, S.; Ava, S.; Dursun, M.E.; Keklikçi, U. Autonomic dysfunction detection by an automatic pupillometer as a non-invasive test in patients recovered from COVID-19. Graefes. Arch. Clin. Exp. Ophthalmol. 2021, 259, 2821–2826. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.A.; Chilcott, R.P. Eyeing up the Future of the Pupillary Light Reflex in Neurodiagnostics. Diagnostics 2018, 8, 19. [Google Scholar] [CrossRef]
- Colombo, J.; Weintraub, M.I.; Munoz, R.; Verma, A.; Ahmad, G.; Kaczmarski, K.; Santos, L.; DePace, N.L. Long COVID and the Autonomic Nervous System: The Journey from Dysautonomia to Therapeutic Neuro-Modulation through the Retrospective Analysis of 152 Patients. NeuroSci 2022, 3, 300–310. [Google Scholar] [CrossRef]
- Pouget, P. The cortex is in overall control of ‘voluntary’ eye movement. Eye 2015, 29, 241–245. [Google Scholar] [CrossRef]
- Ranti, C.; Jones, W.; Klin, A.; Shultz, S. Blink Rate Patterns Provide a Reliable Measure of Individual Engagement with Scene Content. Sci. Rep. 2020, 10, 8267. [Google Scholar] [CrossRef]
- Callara, A.L.; Greco, A.; Scilingo, E.P.; Bonfiglio, L. Neuronal correlates of eyeblinks are an expression of primary consciousness phenomena. Sci. Rep. 2023, 13, 12617. [Google Scholar] [CrossRef]

| Author&Year | N 1 | Controls | Male | Female | Age (Years) | Setting | Days Since Diagnosis (Days) 2 |
|---|---|---|---|---|---|---|---|
| Carbone 2022 [20] | 78 | 23 | 41 | 37 | 55.2 ± 12.2 | University | 419.1 ± 26.0 |
| De Giglio 2023 [36] | 1 | 0 | 1 | 0 | 74 | Hospital | 60 |
| Kelly 2022 [28] | 377 | 300 | 245 | 132 | 28.4 ± 7.4 | Hospital and university | >28 |
| Bitirgen 2022 [24] | 65 | 30 | NA | NA | 42.5 ± 10.7 | University hospital | 120 (60–150) |
| Garcia 2022 [29] | 18 | 9 | 10 | 8 | 49.56 ± 9.14 | University hospital | NA |
| Abdelrahman 2021 [25] | 20 | 0 | 10 | 10 | 40 ± 9.58 | Hospital | NA |
| Bellavia 2021 [26] | 40 | 20 | 27 | 13 | 54.3 ± 16.7 | Hospital | NA |
| Koskderelioglu 2022 [30] | 120 | 44 | 48 | 72 | 39.0 ± 9.6 | University | 132 ± 66 (30–360) |
| Braceros 2021 [35] | 1 | 0 | 1 | 0 | 28 | Hospital | 30 |
| Sabel 2021 [37] | 2 | 0 | 0 | 2 | 56 ± 16 | Hospital | >28 |
| González-Vides 2024 [32] | 117 | 42 | 31 | 86 | 48.9 ± 11.15 | Hospital and university | 600 |
| Jan Johansson 2024 [31] | 38 | 0 | 9 | 29 | 46.8 ± 9.3 | Hospital | 660 (360–870) |
| Moritz Güttes 2024 [33] | 179 | 49 | 88 | 91 | 35.63 ± 11 | University hospital | 442.49 ± 215 |
| Vinuela-Navarro 2023 [27] | 85 | 20 | 22 | 63 | 49.03 ± 6.67 | Hospital | >28 |
| Mehringer 2023 [34] | 35 | 15 | 19 | 16 | 27.29 ± 3.84 | University | 389.25 ± 189.34 |
| Ophthalmological Feature | Abnormalities Detected | Related Literature |
|---|---|---|
| Saccadic feature | Higher saccade error rate | [20,28,29,31,33] |
| Prolonged saccade latency (reaction time) | [27,29,31,32,33] | |
| Pursuit feature | Smooth pursuit abnormal (disrupted or unstable) | [27,28] |
| Nystagmus feature | Nystagmus (optokinetic and positional) abnormal | [25,28] |
| Caloric weakness | [25] | |
| Pupillary reflex feature | Increased latency of pupil contraction | [24] |
| Reduced duration of pupil contraction | [24] | |
| Reduced latency of pupil dilation | [24] | |
| Higher dilatation velocity | [26] | |
| Higher absolute constriction amplitude | [26] | |
| Higher constriction index | [26] | |
| Higher baseline pupil diameter | [26] | |
| Reduced pupil constriction | [27] | |
| Reduced pupil dilation | [27] | |
| Visual field feature | Suppression of static visual field perimetry | [35] |
| Impaired peripheral field defects | [37] | |
| VEP feature | Reduced VEP * amplitude | [30,35] |
| Prolonged p100 latencies | [30] | |
| Abnormalities in several peripheral nerve measurements | [30] | |
| Stereopsis performance feature | Increased reaction time | [33,34] |
| Other features | Mild macular thickening | [35] |
| Abnormal ERG * implicit times | [35] | |
| Suppressed EOG * amplitude | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, W.; Statham, L.; Jammal, C.; Pecchia, L.; Hoad, D.; Piaggio, D. On the Quest for Ophthalmological Biomarkers for Long COVID: A Scoping Review. Appl. Sci. 2025, 15, 6126. https://doi.org/10.3390/app15116126
Su W, Statham L, Jammal C, Pecchia L, Hoad D, Piaggio D. On the Quest for Ophthalmological Biomarkers for Long COVID: A Scoping Review. Applied Sciences. 2025; 15(11):6126. https://doi.org/10.3390/app15116126
Chicago/Turabian StyleSu, Wanzi, Laura Statham, Carla Jammal, Leandro Pecchia, Damon Hoad, and Davide Piaggio. 2025. "On the Quest for Ophthalmological Biomarkers for Long COVID: A Scoping Review" Applied Sciences 15, no. 11: 6126. https://doi.org/10.3390/app15116126
APA StyleSu, W., Statham, L., Jammal, C., Pecchia, L., Hoad, D., & Piaggio, D. (2025). On the Quest for Ophthalmological Biomarkers for Long COVID: A Scoping Review. Applied Sciences, 15(11), 6126. https://doi.org/10.3390/app15116126







