Impact of HF-Free Synthesis Modification on Purity and Adsorption Performances of MOF MIL-100(Fe) for Gas Capture and Energy Storage Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrothermal Synthesis
- Washing procedure No. 1: The powder obtained from synthesis protocol No. 1 was dispersed in distilled water. Then, the solution was stirred at room temperature for different stirring times, namely 5 days, 10 days, and 15 days.
- Washing procedure No. 2: The powder obtained from synthesis protocol No. 1 was washed by stirring in hot water several times until the pH of the wastewater of washing became neutral. After that, the MOF was washed three times with hot ethanol. For each water wash, 700 mL of water with a temperature of 70 °C was used for 3 h. For the ethanol wash, 700 mL of ethanol was heated to 65 °C. All washing steps used a stirring speed of 200 rpm.
2.3. Chemo-Physical Characterization
2.4. H2 and CO2 Adsorption Measurements
2.5. Water Vapor Adsorption Measurements
3. Results and Discussion
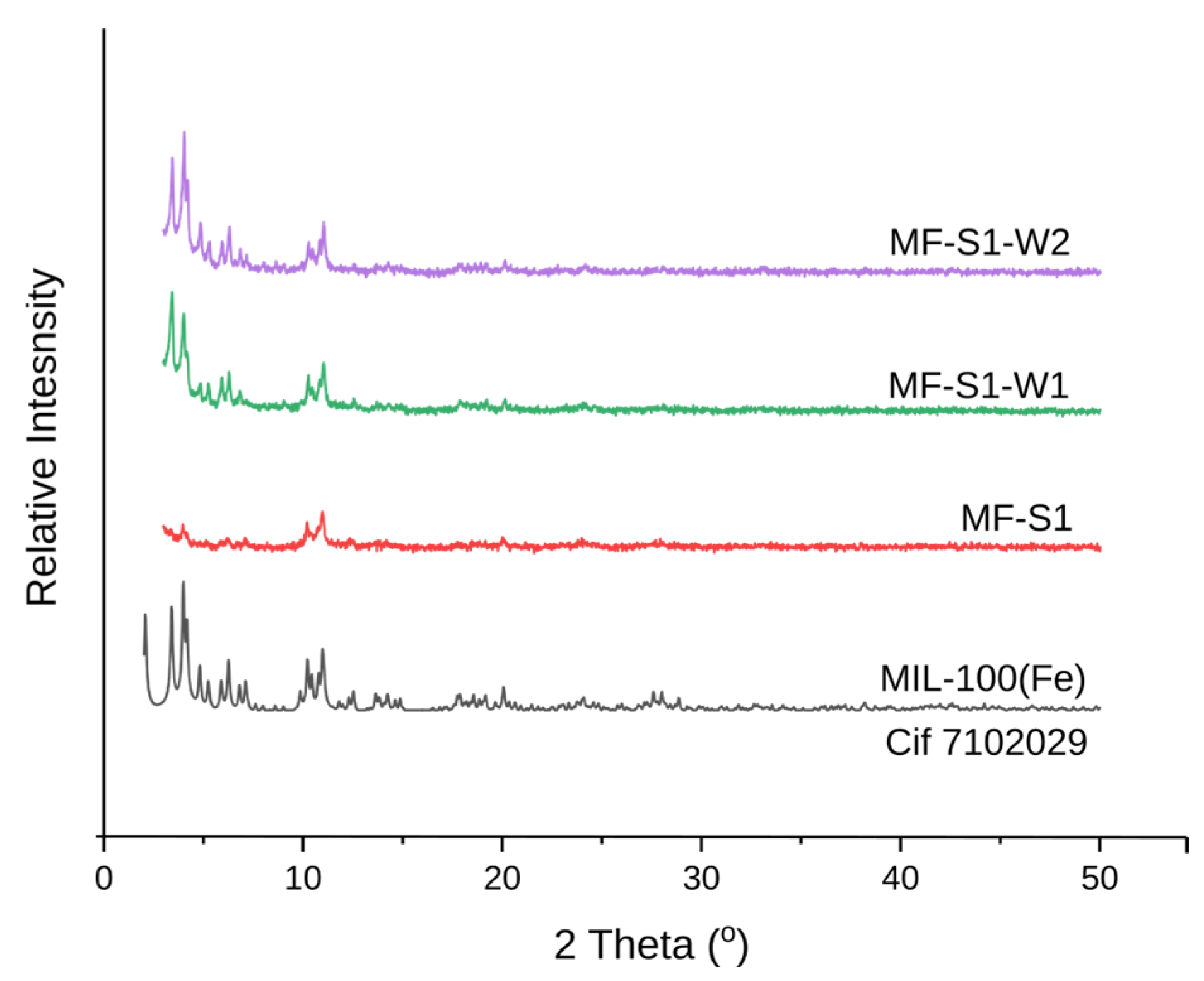
3.1. Impact of Hydrothermal Synthesis Under Different Conditions
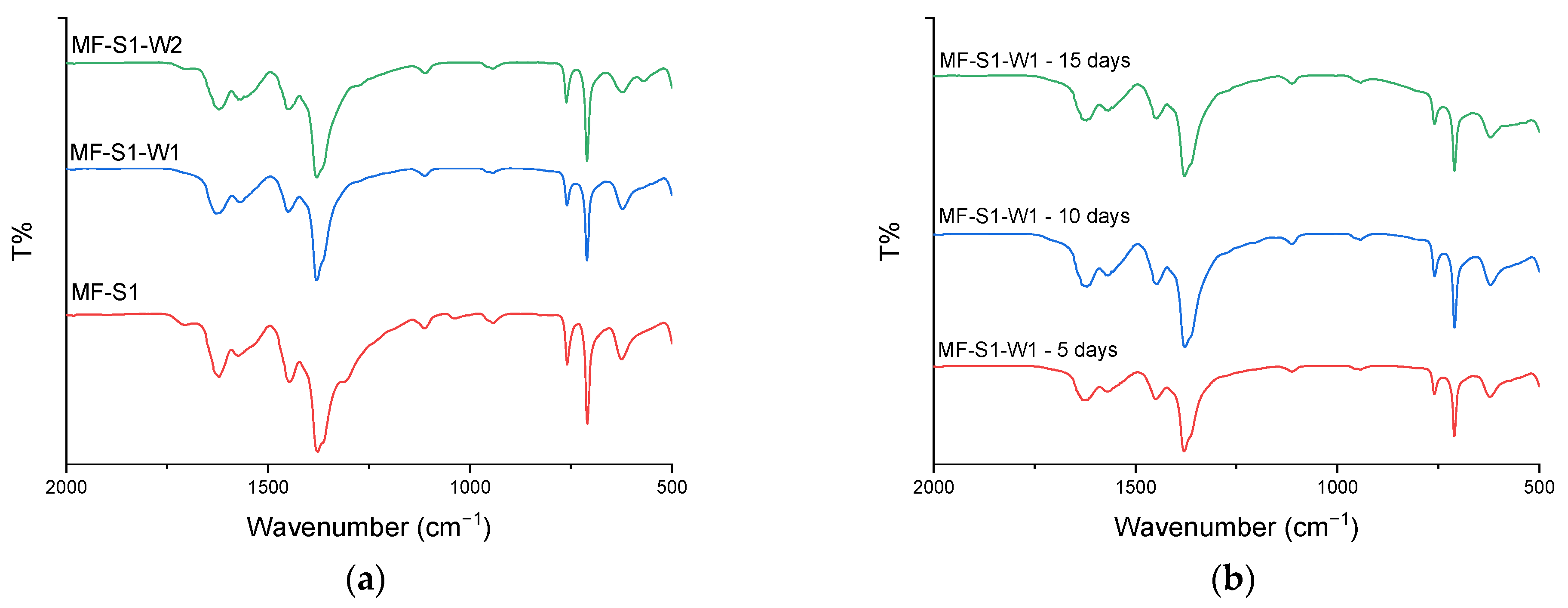
3.2. Impact of Washing Procedure
3.3. Adsorption Performances
4. Conclusions
- A high-quality MIL-100(Fe) MOF was successfully synthesized using an HF-free hydrothermal method, demonstrating a sustainable and environmentally friendly approach. Optimized synthesis conditions (Fe3+/(H3BTC)/water ratio of 1:0.67:56) and a 12 h reaction time were identified as crucial for maximizing the yield and purity of the MOF.
- Washing protocol No. 2 (multiple washes with hot water and ethanol) proved most effective in maintaining high crystallinity, surface area (1162 m2/g), and pore volume (0.377 cm3/g) while minimizing the presence of impurities. Washing protocol No. 1 (extended immersion in distilled water) enhanced crystallinity but led to a significant decrease in surface area and pore volume. This highlights the importance of optimizing both synthesis and washing procedures for obtaining high-quality MOFs.
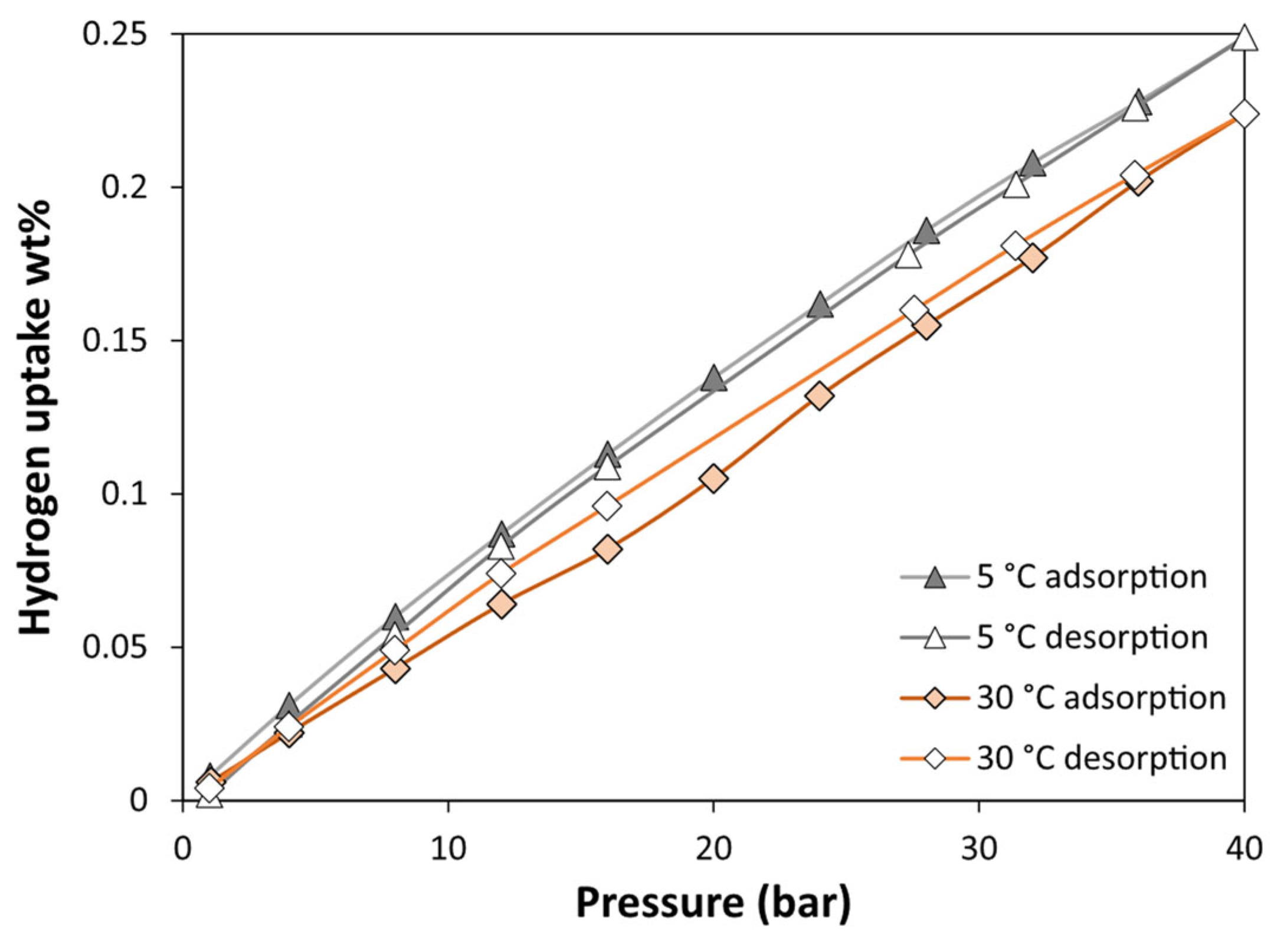
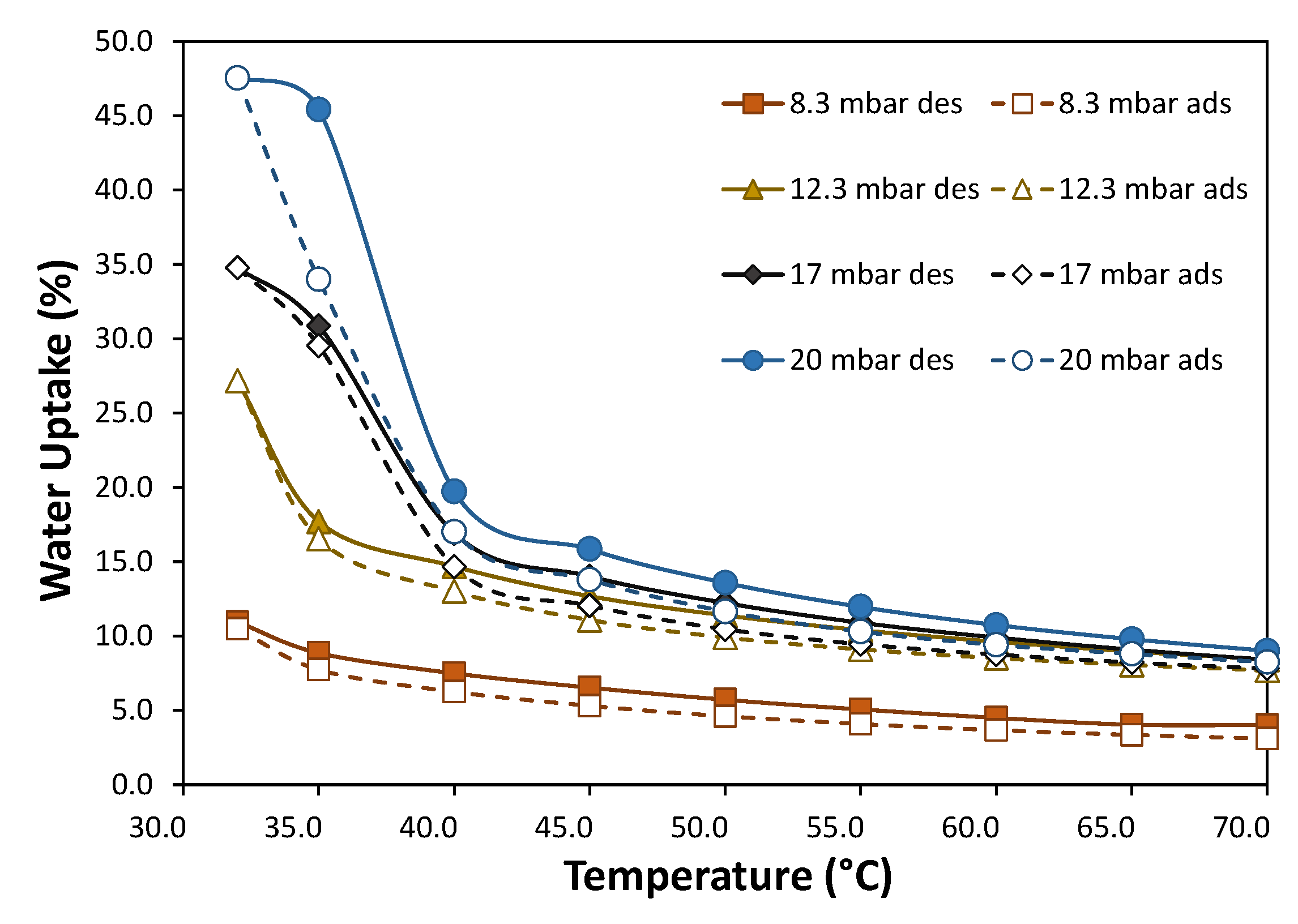
- The MOF sample washed with a sequential hot water and ethanol washing procedure (MF-S1-W2) was identified as the most promising candidate for further gas adsorption studies due to its superior crystallinity, purity, and surface area. The optimized MIL-100(Fe) exhibited a remarkable hydrogen adsorption capacity, reaching 0.25 wt.% at 5 °C and 40 bar. The material demonstrated significant CO2 adsorption, reaching 32.6 wt.% at 5 °C and 10 bar and 23.9 wt.% at 30 °C and 10 bar. Heats of adsorption were calculated to be 5 kJ/mol for H2 and 20 kJ/mol for CO2. The MOF also displayed an excellent water vapor adsorption capacity, reaching a maximum of 47.5 wt.% at 20 mbar and 30 °C. The observed S-shaped adsorption isotherms and minimal hysteresis during desorption indicate the potential of this material for efficient water vapor sorption applications.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Felix Sahayaraj, A.; Joy Prabu, H.; Maniraj, J.; Kannan, M.; Bharathi, M.; Diwahar, P.; Salamon, J. Metal–Organic Frameworks (MOFs): The Next Generation of Materials for Catalysis, Gas Storage, and Separation. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1757–1781. [Google Scholar] [CrossRef]
- He, T.; Kong, X.-J.; Li, J.-R. Chemically Stable Metal–Organic Frameworks: Rational Construction and Application Expansion. Acc. Chem. Res. 2021, 54, 3083–3094. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Liou, K.-H.; Kang, D.-Y.; Chen, J.-J.; Lin, L.-C. Investigation of the Water Adsorption Properties and Structural Stability of MIL-100(Fe) with Different Anions. Langmuir 2018, 34, 4180–4187. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Wang, C.-C. MIL-100(Fe)-Based Functional Materials for Water Decontamination: A State of the Art Review. Prog. Nat. Sci. Mater. Int. 2023, 33, 386–406. [Google Scholar] [CrossRef]
- Zhong, G.; Liu, D.; Zhang, J. Applications of Porous Metal–Organic Framework MIL-100(M) (M = Cr, Fe, Sc, Al, V). Cryst. Growth Des. 2018, 18, 7730–7744. [Google Scholar] [CrossRef]
- Joseph, J.; Iftekhar, S.; Srivastava, V.; Fallah, Z.; Zare, E.N.; Sillanpää, M. Iron-Based Metal-Organic Framework: Synthesis, Structure and Current Technologies for Water Reclamation with Deep Insight into Framework Integrity. Chemosphere 2021, 284, 131171. [Google Scholar] [CrossRef]
- Severino, M.I.; Freitas, C.; Pimenta, V.; Nouar, F.; Pinto, M.L.; Serre, C. Cost Estimation of the Production of MIL-100(Fe) at Industrial Scale from Two Upscaled Sustainable Synthesis Routes. Ind. Eng. Chem. Res. 2025, 64, 2708–2718. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Zhang, J.; Tang, L.; Luo, L.; Zeng, G. Iron Containing Metal-Organic Frameworks: Structure, Synthesis, and Applications in Environmental Remediation. ACS Appl. Mater. Interfaces 2017, 9, 20255–20275. [Google Scholar] [CrossRef]
- Horcajada, P.; Surblé, S.; Serre, C.; Hong, D.Y.; Seo, Y.K.; Chang, J.S.; Grenèche, J.M.; Margiolaki, I.; Férey, G. Synthesis and Catalytic Properties of MIL-100(Fe), an Iron(III) Carboxylate with Large Pores. Chem. Commun. 2007, 27, 2820–2822. [Google Scholar] [CrossRef]
- Seo, Y.K.; Yoon, J.W.; Lee, J.S.; Lee, U.H.; Hwang, Y.K.; Jun, C.H.; Horcajada, P.; Serre, C.; Chang, J.S. Large Scale Fluorine-Free Synthesis of Hierarchically Porous Iron(III) Trimesate MIL-100(Fe) with a Zeolite MTN Topology. Microporous Mesoporous Mater. 2012, 157, 137–145. [Google Scholar] [CrossRef]
- Shi, J.; Hei, S.; Liu, H.; Fu, Y.; Zhang, F.; Zhong, Y.; Zhu, W. Synthesis of MIL-100(Fe) at Low Temperature and Atmospheric Pressure. J. Chem. 2013, 2013, 792827. [Google Scholar] [CrossRef]
- Fang, Y.; Yang, Z.; Li, H.; Liu, X. MIL-100(Fe) and Its Derivatives: From Synthesis to Application for Wastewater Decontamination. Environ. Sci. Pollut. Res. 2020, 27, 4703–4724. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Wang, X.; Zhou, X.; Xiao, J.; Li, Z. Novel Room-Temperature Synthesis of MIL-100(Fe) and Its Excellent Adsorption Performances for Separation of Light Hydrocarbons. Chem. Eng. J. 2019, 355, 679–686. [Google Scholar] [CrossRef]
- Han, L.; Qi, H.; Zhang, D.; Ye, G.; Zhou, W.; Hou, C.; Xu, W.; Sun, Y. A Facile and Green Synthesis of MIL-100(Fe) with High-Yield and Its Catalytic Performance. New J. Chem. 2017, 41, 13504–13509. [Google Scholar] [CrossRef]
- Hong, D.Y.; Hwang, Y.K.; Serre, C.; Férey, G.; Chang, J.S. Porous Chromium Terephthalate MIL-101 with Coordinatively Unsaturated Sites: Surface Functionalization, Encapsulation, Sorption and Catalysis. Adv. Funct. Mater. 2009, 19, 1537–1552. [Google Scholar] [CrossRef]
- Tan, F.; Liu, M.; Li, K.; Wang, Y.; Wang, J.; Guo, X.; Zhang, G.; Song, C. Facile Synthesis of Size-Controlled MIL-100(Fe) with Excellent Adsorption Capacity for Methylene Blue. Chem. Eng. J. 2015, 281, 360–367. [Google Scholar] [CrossRef]
- Chakraborty, D.; Yurdusen, A.; Mouchaham, G.; Nouar, F.; Serre, C. Large-Scale Production of Metal–Organic Frameworks. Adv. Funct. Mater. 2024, 34, 2309089. [Google Scholar] [CrossRef]
- Lin, R.-B.; Zhang, Z.; Chen, B. Achieving High Performance Metal–Organic Framework Materials through Pore Engineering. Acc. Chem. Res. 2021, 54, 3362–3376. [Google Scholar] [CrossRef]
- Ji, Z.; Wang, H.; Canossa, S.; Wuttke, S.; Yaghi, O.M. Pore Chemistry of Metal–Organic Frameworks. Adv. Funct. Mater. 2020, 30, 2000238. [Google Scholar] [CrossRef]
- Yurduşen, A.; Yürüm, A.; Yürüm, Y. A Remarkable Increase in the Adsorbed H2 Amount: Influence of Pore Size Distribution on the H2 Adsorption Capacity of Fe-BTC. Int. J. Hydrogen Energy 2020, 45, 12394–12407. [Google Scholar] [CrossRef]
- Hirscher, M.; Panella, B. Nanostructures with High Surface Area for Hydrogen Storage. J. Alloys Compd. 2005, 404–406, 399–401. [Google Scholar] [CrossRef]
- Ghanbari, T.; Abnisa, F.; Wan Daud, W.M.A. A Review on Production of Metal Organic Frameworks (MOF) for CO2 Adsorption. Sci. Total Environ. 2020, 707, 135090. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.; Moslein, A.; Titov, K.; Taylor, J.D.; Rudic, S.; Tan, J.-C. Green Reconstruction of MIL-100 (Fe) in Water for High Crystallinity and Enhanced Guest Encapsulation. ACS Sustain. Chem. Eng. 2020, 8, 8247–8255. [Google Scholar] [CrossRef]
- Yao, J.; Han, H.; Yang, Y.; Song, Y.; Li, G. A Review of Recent Progress of Carbon Capture, Utilization, and Storage (CCUS) in China. Appl. Sci. 2023, 13, 1169. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Liang, C.; Carriveau, R.; Ting, D.S.-K.; Li, P.; Cen, H.; Xiong, W. Underwater Compressed Gas Energy Storage (UWCGES): Current Status, Challenges, and Future Perspectives. Appl. Sci. 2022, 12, 9361. [Google Scholar] [CrossRef]
- Sobhani, D.; Djahaniani, H.; Duong, A.; Kazemian, H. Efficient Removal of Microcystin-LR from Contaminated Water Using Water-Stable MIL-100(Fe) Synthesized under HF-Free Conditions. Environ. Sci. Pollut. Res. 2024, 31, 24512–24524. [Google Scholar] [CrossRef]
- Du, M.; Xu, G.; Zhang, J.; Guan, Y.; Guo, C.; Chen, Y. Hierarchically Porous MIL-100(Fe) with Large Mesopores for Cationic Dye Adsorption. J. Solid. State Chem. 2023, 322, 123950. [Google Scholar] [CrossRef]
- Fang, Y.; Wen, J.; Zeng, G.; Jia, F.; Zhang, S.; Peng, Z.; Zhang, H. Effect of Mineralizing Agents on the Adsorption Performance of Metal–Organic Framework MIL-100(Fe) towards Chromium(VI). Chem. Eng. J. 2018, 337, 532–540. [Google Scholar] [CrossRef]
- Zhang, F.; Shi, J.; Jin, Y.; Fu, Y.; Zhong, Y.; Zhu, W. Facile Synthesis of MIL-100(Fe) under HF-Free Conditions and Its Application in the Acetalization of Aldehydes with Diols. Chem. Eng. J. 2015, 259, 183–190. [Google Scholar] [CrossRef]
- Chávez, A.M.; Rey, A.; López, J.; Álvarez, P.M.; Beltrán, F.J. Critical Aspects of the Stability and Catalytic Activity of MIL-100(Fe) in Different Advanced Oxidation Processes. Sep. Purif. Technol. 2021, 255, 117660. [Google Scholar] [CrossRef]
- Asoufi, H.M.; Al-Antary, T.M.; Awwad, A.M. Green Route for Synthesis Hematite (α-Fe2O3) Nanoparticles: Toxicity Effect on the Green Peach Aphid, Myzus Persicae (Sulzer). Environ. Nanotechnol. Monit. Manag. 2018, 9, 107–111. [Google Scholar] [CrossRef]
- Hjiri, M. Highly Sensitive NO2 Gas Sensor Based on Hematite Nanoparticles Synthesized by Sol–Gel Technique. J. Mater. Sci. Mater. Electron. 2020, 31, 5025–5031. [Google Scholar] [CrossRef]
- Lestari, W.W.; Yunita, L.; Saraswati, T.E.; Heraldy, E.; Khafidhin, M.A.; Krisnandi, Y.K.; Arrozi, U.S.F.; Kadja, G.T.M. Fabrication of Composite Materials MIL-100(Fe)/Indonesian Activated Natural Zeolite as Enhanced CO2 Capture Material. Chem. Pap. 2021, 75, 3253–3263. [Google Scholar] [CrossRef]
- Zhou, M.; Tang, T.; Qin, D.; Cheng, H.; Wang, X.; Chen, J.; Wågberg, T.; Hu, G. Hematite Nanoparticle Decorated MIL-100 for the Highly Selective and Sensitive Electrochemical Detection of Trace-Level Paraquat in Milk and Honey. Sens. Actuators B Chem. 2023, 376, 132931. [Google Scholar] [CrossRef]
- Ambroz, F.; Macdonald, T.J.; Martis, V.; Parkin, I.P. Evaluation of the BET Theory for the Characterization of Meso and Microporous MOFs. Small Methods 2018, 2, 1800173. [Google Scholar] [CrossRef]
- Guesh, K.; Caiuby, C.A.D.; Mayoral, Á.; Díaz-García, M.; Díaz, I.; Sanchez-Sanchez, M. Sustainable Preparation of MIL-100(Fe) and Its Photocatalytic Behavior in the Degradation of Methyl Orange in Water. Cryst. Growth Des. 2017, 17, 1806–1813. [Google Scholar] [CrossRef]
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F. Is the Bet Equation Applicable to Microporous Adsorbents? In Studies in Surface Science and Catalysis; Llewellyn, P.L., Rodriquez-Reinoso, F., Rouqerol, J., Seaton, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 160, pp. 49–56. ISBN 978-0-444-52022-7. [Google Scholar]
- Delgado-Marín, J.J.; Narciso, J.; Ramos-Fernández, E.V. Effect of the Synthesis Conditions of MIL-100(Fe) on Its Catalytic Properties and Stability under Reaction Conditions. Materials 2022, 15, 6499. [Google Scholar] [CrossRef]
- Ahmed, I.; Jeon, J.; Khan, N.A.; Jhung, S.H. Synthesis of a Metal-Organic Framework, Iron-Benezenetricarboxylate, from Dry Gels in the Absence of Acid and Salt. Cryst. Growth Des. 2012, 12, 5878–5881. [Google Scholar] [CrossRef]
- Canioni, R.; Roch-Marchal, C.; Sécheresse, F.; Horcajada, P.; Serre, C.; Hardi-Dan, M.; Férey, G.; Grenèche, J.M.; Lefebvre, F.; Chang, J.S.; et al. Stable Polyoxometalate Insertion within the Mesoporous Metal Organic Framework MIL-100(Fe). J. Mater. Chem. 2011, 21, 1226–1233. [Google Scholar] [CrossRef]
- Qian, X.; Yadian, B.; Wu, R.; Long, Y.; Zhou, K.; Zhu, B.; Huang, Y. Structure Stability of Metal-Organic Framework MIL-53 (Al) in Aqueous Solutions. Int. J. Hydrogen Energy 2013, 38, 16710–16715. [Google Scholar] [CrossRef]
- Gong, F.; Wang, X.; Wang, P.; Xuan, L.; Li, Z.; Zhu, Y. Facile Synthesis of SAPO-34 with Excellent Methanol-to-Olefin Activity in a Short Time via a Conventional Hydrothermal Method. New J. Chem. 2020, 44, 10410–10417. [Google Scholar] [CrossRef]
- Yetgin, S.; Balkose, D. Calf Thymus DNA Characterization and Its Adsorption on Different Silica Surfaces. RSC Adv. 2015, 5, 57950–57959. [Google Scholar] [CrossRef]
- Forghani, M.; Azizi, A.; Livani, M.J.; Kafshgari, L.A. Adsorption of Lead(II) and Chromium(VI) from Aqueous Environment onto Metal-Organic Framework MIL-100(Fe): Synthesis, Kinetics, Equilibrium and Thermodynamics. J. Solid. State Chem. 2020, 291, 121636. [Google Scholar] [CrossRef]
- Chaturvedi, G.; Kaur, A.; Umar, A.; Khan, M.A.; Algarni, H.; Kansal, S.K. Removal of Fluoroquinolone Drug, Levofloxacin, from Aqueous Phase over Iron Based MOFs, MIL-100(Fe). J. Solid. State Chem. 2020, 281, 121029. [Google Scholar] [CrossRef]
- Cobian-Solorio, G.A.; Aguayo-Villarreal, I.A.; Rojas-Mayorga, C.K.; Muñiz-Valencia, R.; Emparan-Legaspi, M.J.; Davila Guzman, N.E. Non-Steroidal Anti-Inflammatory Drugs Adsorption from Aqueous Solution by MOFs MIL-100(Fe), ZIF-8 and UiO-66: Synthesis, Characterization, and Comparative Study. J. Mol. Struct. 2025, 1321, 139698. [Google Scholar] [CrossRef]
- Mohammadifard, Z.; Saboori, R.; Mirbagheri, N.S.; Sabbaghi, S. Heterogeneous Photo-Fenton Degradation of Formaldehyde Using MIL-100(Fe) under Visible Light Irradiation. Environ. Pollut. 2019, 251, 783–791. [Google Scholar] [CrossRef]
- Adhikari, A.K.; Lin, K.-S.; Chang, C.-S. Improved Hydrogen Storage Capacity by Hydrogen Spillover and Fine Structural Characterization of MIL-100 Metal Organic Frameworks. Res. Chem. Intermed. 2015, 41, 7655–7667. [Google Scholar] [CrossRef]
- Villajos, J.A.; Orcajo, G.; Martos, C.; Botas, J.Á.; Villacañas, J.; Calleja, G. Co/Ni Mixed-Metal Sited MOF-74 Material as Hydrogen Adsorbent. Int. J. Hydrogen Energy 2015, 40, 5346–5352. [Google Scholar] [CrossRef]
- Orcajo, G.; Montes-Andrés, H.; Villajos, J.A.; Martos, C.; Botas, J.A.; Calleja, G. Li-Crown Ether Complex Inclusion in MOF Materials for Enhanced H2 Volumetric Storage Capacity at Room Temperature. Int. J. Hydrogen Energy 2019, 44, 19285–19293. [Google Scholar] [CrossRef]
- Nuhnen, A.; Janiak, C. A Practical Guide to Calculate the Isosteric Heat/Enthalpy of Adsorption via Adsorption Isotherms in Metal–Organic Frameworks, MOFs. Dalton Trans. 2020, 49, 10295–10307. [Google Scholar] [CrossRef]
- Alfe, M.; Policicchio, A.; Lisi, L.; Gargiulo, V. Solid Sorbents for CO2 and CH4 Adsorption: The Effect of Metal Organic Framework Hybridization with Graphene-like Layers on the Gas Sorption Capacities at High Pressure. Renew. Sustain. Energy Rev. 2021, 141, 110816. [Google Scholar] [CrossRef]
- Luo, Y.; Tan, B.; Liang, X.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. Dry Gel Conversion Synthesis of Hierarchical Porous MIL-100(Fe) and Its Water Vapor Adsorption/Desorption Performance. Ind. Eng. Chem. Res. 2019, 58, 7801–7807. [Google Scholar] [CrossRef]
- Said, S.A.M.; Qadir, N.U.; Mansour, R.B.; Mezghani, K.; Irshad, H.M. Synthesis and Water Sorption Properties of a Series of Exfoliated Graphene/MIL-100(Fe) Composites. RSC Adv. 2017, 7, 17353–17356. [Google Scholar] [CrossRef]
- Bahri, M.; Haghighat, F.; Kazemian, H.; Rohani, S. A Comparative Study on Metal Organic Frameworks for Indoor Environment Application: Adsorption Evaluation. Chem. Eng. J. 2017, 313, 711–723. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Freni, A.; Proverbio, E. Synthesis of SAPO-34 Zeolite Filled Macrocellular Foams for Adsorption Heat Pump Applications: A Preliminary Study. Appl. Therm. Eng. 2017, 124, 1312–1318. [Google Scholar] [CrossRef]
- Pajdak, A.; Kulakowska, A.; Liu, J.; Berent, K.; Kudasik, M.; Krzywanski, J.; Kalawa, W.; Sztekler, K.; Skoczylas, N. Accumulation and Emission of Water Vapor by Silica Gel Enriched with Carbon Nanotubes CNT-Potential Applications in Adsorption Cooling and Desalination Technology. Appl. Sci. 2022, 12, 5644. [Google Scholar] [CrossRef]





| Code | Ratio Fe(III):(H3BTC):Water | Synthesis Time |
|---|---|---|
| MF-S0 | 1:0.1:56 | 12 h |
| MF-S1 | 1:0.67:56 | 12 h |
| MF-S2 | 1:0.67:56 | 16 h |
| MF-S3 | 1:0.67:112 | 12 h |
| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | PSD (nm) | Density g/cm3 |
|---|---|---|---|---|
| MF-S1 | 1199 | 0.369 | 1.678 | 2.4679 |
| MF-S2 | 1067 | 0.313 | 1.633 | 2.5335 |
| MF-S3 | 500 | 0.208 | 1.417 | 2.4679 |
| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | PSD (nm) | Density (cm3/g) |
|---|---|---|---|---|
| MF-S1-W1—5 days | 953 | 0.295 | 1.533 | 2.5021 |
| MF-S1-W1—10 days | 905 | 0.262 | 1.509 | 2.5332 |
| MF-S1-W1—15 days | 871 | 0.257 | 1.509 | 2.5935 |
| MF-S1-W2 | 1162 | 0.377 | 1.524 | 2.6750 |
| Sorbent | BET Surface Area (m2/g) | Ref. |
|---|---|---|
| MIL-100(Fe) | 1162 | Our study |
| MIL-100(Fe) | 1000–1520 | [23,39,40] |
| MIL-53(Al) | 1027–1255 | [41] |
| SAPO-34 zeolite | 569–654 | [42] |
| Silica gel | 556 | [43] |
| Aerogel | 1055 | [43] |
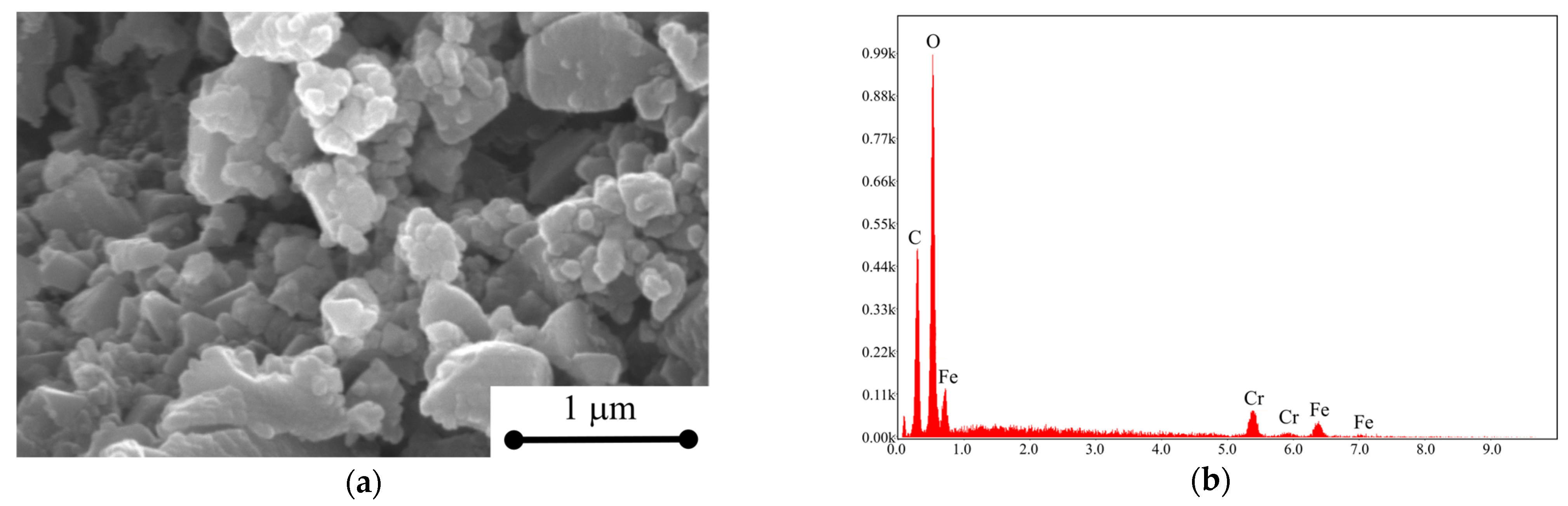
| Element | Weight % | Atomic % |
|---|---|---|
| C | 33.7 | 44.3 |
| O | 52.4 | 51.8 |
| Fe | 13.9 | 3.9 |
| Total | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idrees, M.; Mastronardo, E.; Piperopoulos, E.; Milone, C.; Calabrese, L. Impact of HF-Free Synthesis Modification on Purity and Adsorption Performances of MOF MIL-100(Fe) for Gas Capture and Energy Storage Applications. Appl. Sci. 2025, 15, 6097. https://doi.org/10.3390/app15116097
Idrees M, Mastronardo E, Piperopoulos E, Milone C, Calabrese L. Impact of HF-Free Synthesis Modification on Purity and Adsorption Performances of MOF MIL-100(Fe) for Gas Capture and Energy Storage Applications. Applied Sciences. 2025; 15(11):6097. https://doi.org/10.3390/app15116097
Chicago/Turabian StyleIdrees, Muhtadi, Emanuela Mastronardo, Elpida Piperopoulos, Candida Milone, and Luigi Calabrese. 2025. "Impact of HF-Free Synthesis Modification on Purity and Adsorption Performances of MOF MIL-100(Fe) for Gas Capture and Energy Storage Applications" Applied Sciences 15, no. 11: 6097. https://doi.org/10.3390/app15116097
APA StyleIdrees, M., Mastronardo, E., Piperopoulos, E., Milone, C., & Calabrese, L. (2025). Impact of HF-Free Synthesis Modification on Purity and Adsorption Performances of MOF MIL-100(Fe) for Gas Capture and Energy Storage Applications. Applied Sciences, 15(11), 6097. https://doi.org/10.3390/app15116097










