Study of Oxidative–Reductive Potential Changes in the Enrichment of Oxidized Polymetallic Ores
Abstract
1. Introduction
- 1.
- Treatment of minerals with sulfur-containing reagents:
- Minerals are treated with sulfidizing agents such as sodium sulfide (Na2S), sodium or ammonium polysulfides, hydrogen sulfide (H2S), or other compounds that can interact with the oxidized surfaces.
- 2.
- Sulfidization reaction:
- The reagent interacts with the surface of oxidized minerals, forming a sulfide film on the surface. For example:
- As a result, the oxide and carbonate compounds of lead are converted into lead sulfide.
- 3.
- Fixation of the sulfide film:
- The sulfide film formed on the surface of the minerals improves their hydrophobicity, which facilitates the attachment of flotation reagents and air bubbles during flotation.
- 4.
- Flotation:
- The modified sulfide minerals are extracted by flotation using xanthates or other collector reagents that effectively interact with the sulfide surface.
2. Materials and Methods
3. Results and Discussion
- -
- Activation of zinc minerals using copper sulfate after sulfidization.
- -
- Flotation with other collectors—for example, fatty acids are often used for carbonate zinc minerals.
- -
- Hydrometallurgy of zinc.
- -
- Mechanochemical sulfidization—grinding oxidized ore with elemental sulfur.
- -
- Sulfidization roasting—roasting oxidized concentrates with the addition of sulfidizing agents followed by flotation.
- -
- Electrochemical sulfidization.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://dknews.kz/ru/ekonomika/105991-v-kazahstane-ezhegodno-proizvoditsya-300-tysyach-tonn (accessed on 2 March 2025).
- Merkibayev, Y.S. Processing of Poor Hard-to-Process Complex Lead-Zinc Ores and Enrichment Intermediate Products. Ph.D. Thesis, Satbayev University, Almaty, Kazakhstan, 2024; p. 187. Available online: https://official.satbayev.university/ru/protection (accessed on 20 February 2025).
- Nayak, A.; Jena, M.S.; Mandre, N.R. Beneficiation of lead–zinc ores—A review. Miner. Process. Extr. Metall. Rev. 2022, 43, 564–583. [Google Scholar] [CrossRef]
- Chen, Y.; Suna, Y.; Hana, Y. Efficient flotation separation of lead–zinc oxide ores using mineral sulfidation reconstruction technology: A review. Green Smart Min. Eng. 2024, 1, 175–189. [Google Scholar] [CrossRef]
- Turysbekov, D.; Semushkina, L.; Narbekova, S.; Mukhamedilova, A. On the Use of Environmentally Safe Sulfidizator in the Beneficiation of Ores. News Univ. Kyrg. 2019, 8, 24–29. [Google Scholar]
- Zhang, H.; Yu, H.; Sun, W.; Lin, S.; Zhang, C. Beneficiation of silver and silver-bearing lead–zinc ores: A review. Miner. Eng. 2024, 208, 108608. [Google Scholar] [CrossRef]
- Chepushtanova, T.A.; Motovilov, I.Y.; Merkibayev, Y.S.; Polyakov, K.V.; Gostu, S. Flotation studies of the middling product of lead-zinc ores with preliminary sulfidizing roasting of oxidized lead and zinc compounds. Kompleks. Ispolz. Miner. Syra=Complex Use Miner. Resour. 2022, 323, 77–83. [Google Scholar] [CrossRef]
- Motovilov, I.Y.; Barmenshinova, M.B.; Telkov, S.A.; Omar, R.S. Study of the chemical composition and assessment of the gravity beneficiation of oxidized polymetallic ores of the RK deposit. Min. J. Kazakhstan. 2024, 9, 51–58. Available online: https://minmag.kz/wp-content/uploads/2024/10/2409_51-58.pdf (accessed on 20 February 2025).
- Ejtemaei, M.; Gharabaghi, M.; Irannajad, M. A review of zinc oxide mineral beneficiation using flotation method. Adv. Colloid Interface Sci. 2014, 206, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Chimonyo, W.; Corin, K.; Wiese, J.; O’Connor, C. Redox Potential Control During Flotation of Sulphide Minerals; Centre for Minerals Research, Department of Chemical Engineering, University of Cape Town: Cape Town, South Africa, 2015; Available online: https://www.researchgate.net/publication/283510716_Redox_potential_control_during_flotation_of_sulphide_minerals (accessed on 5 March 2025).
- Seksenova, N.; Bykov, R.; Mamyachenkov, S.; Daumanova, G.; Kozhakanova, M. Optimization of Conditions for Processing of Lead–Zinc Ores Enrichment Tailings of East Kazakhstan. Metals 2021, 11, 1802. [Google Scholar] [CrossRef]
- Oskembekov, I.M.; Bekturganov, N.S.; Katkeeva, G.L.; Burkitseterkyzy, G.; Gizatullina, D.R. Use of the sulphidation process in the processing of oxidised copper ores. KIMS 2017, 1, 16–22. Available online: https://kims-imio.com (accessed on 5 March 2025).
- Dospaev, M.M.; Figurinene, I.V.; Gabdullin, S.T. Combined electrochemical sulphidisation of difficult to enrich oxidised copper ores. Obogashchenie Rud. 2024, 19–24. [Google Scholar] [CrossRef]
- Oserov, T. Mechanochemical Synthesis of a Sulfidizer for Processing Copper Ores. Ph.D. Thesis, Satbayev University, Almaty, Kazakhstan, 2020; p. 142. Available online: https://official.satbayev.university (accessed on 24 April 2025).
- Moimane, T.; Peng, Y. Sulphidisation of oxides and oxidised sulphides and adsorption of thiol collectors on the sulphidised products—A critical review. Adv. Colloid Interface Sci. 2022, 305, 102697. [Google Scholar] [CrossRef] [PubMed]
- Smaylov, B. Development of a Method of Enrichability Assessment and Modeling of Flotation Schemes for Processing of Hard-to-Enrich Lead-Zinc Ores. Doctoral Thesis Submitted for the Degree of Ph.D. in Engineering, MISIS, Moscow, Russia, 2018. Available online: https://xn--80apgmbdfl.xn--p1ai (accessed on 24 April 2025).
- Kalichini, M.; Corin, K.; O’Connor, C.; Simukanga, S. The role of pulp potential and the sulphidization technique in the recovery of sulphide and oxide copper minerals from a complex ore. J. South. Afr. Inst. Min. Metall. 2017, 117, 819–828. [Google Scholar] [CrossRef]
- Li, J.L.; Liu, S.Y.; Liu, D.W.; Liu, R.Z.; Liu, Z.C.; Jia, X.D.; Chang, T.C. Sulfidization mechanism in the flotation of cerussite: A heterogeneous solid-liquid reaction that yields PbCO3/PbS core–shell particles. Miner. Eng. 2020, 153, 106400. [Google Scholar] [CrossRef]
- Yuan, W.Y.; Li, J.H.; Zhang, Q.W.; Saito, F. Mechanochemical sulfidization of lead oxides by grinding with sulfur. Powder Technol. 2012, 230, 63–66. [Google Scholar] [CrossRef]
- Anthropova, I.G.; Merinov, A.A.; Gulyashinov, P.A.; Damdinov, B.B. Sulfidation of oxidized lead and zinc with pyrite-bearing lead-and-zinc ore. Physical and technical problems of mineral resources development. J. Min. Sci. 2023, 59, 465–474. [Google Scholar] [CrossRef]
- Xie, H.; Sun, R.; Wu, J.; Feng, D.; Gao, L. A Case Study of Enhanced Sulfidization Flotation of Lead Oxide Ore: Influence of Depressants. Minerals 2020, 10, 95. [Google Scholar] [CrossRef]
- Chepushtanova, T.A.; Merkibayev, Y.S.; Mishra, B.; Kuldeyev, Y.I. Processing of the zinc-lead-bearing flotation middlings by sulfidizing roasting with pyrrhotites production by predicted properties. Non-Ferr. Met. 2022, 2, 15–24. [Google Scholar] [CrossRef]
- Antropova, I.; Merinov, A.; Gulyashinov, P. Pyrosulfidation of oxidized lead and zinc minerals alcohol-containing lead-zinc ore. Phys.-Tech. Probl. Min. 2023, 3, 132–141. [Google Scholar]
- Xojimuratova, X.B.; Abdusamieva, L.N. Method for Processing Sulphide-Oxidized Copper Ores with Copper and Silver Extraction. Eur. J. Innov. Nonform. Educ. 2024, 4, 510–513. [Google Scholar]

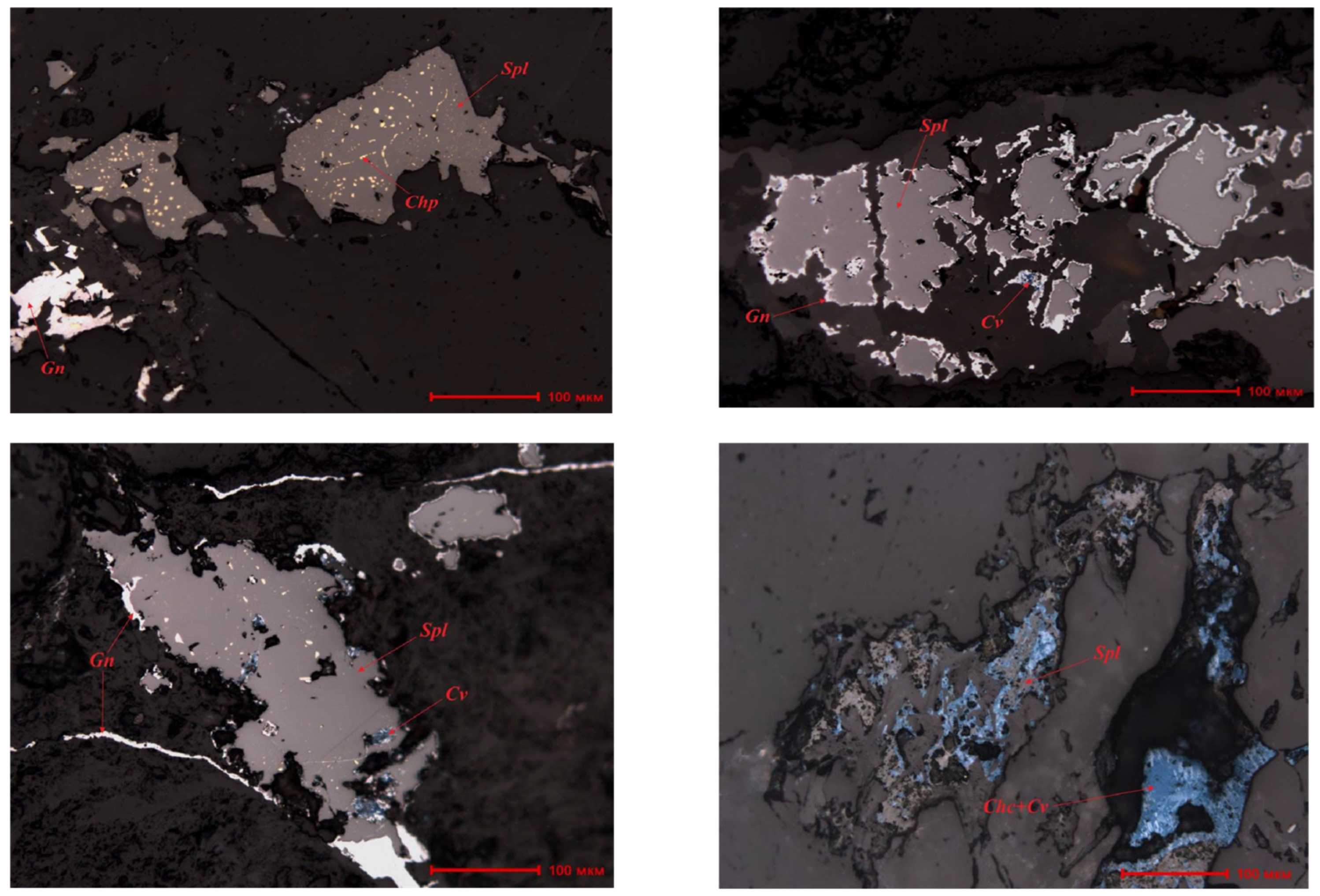
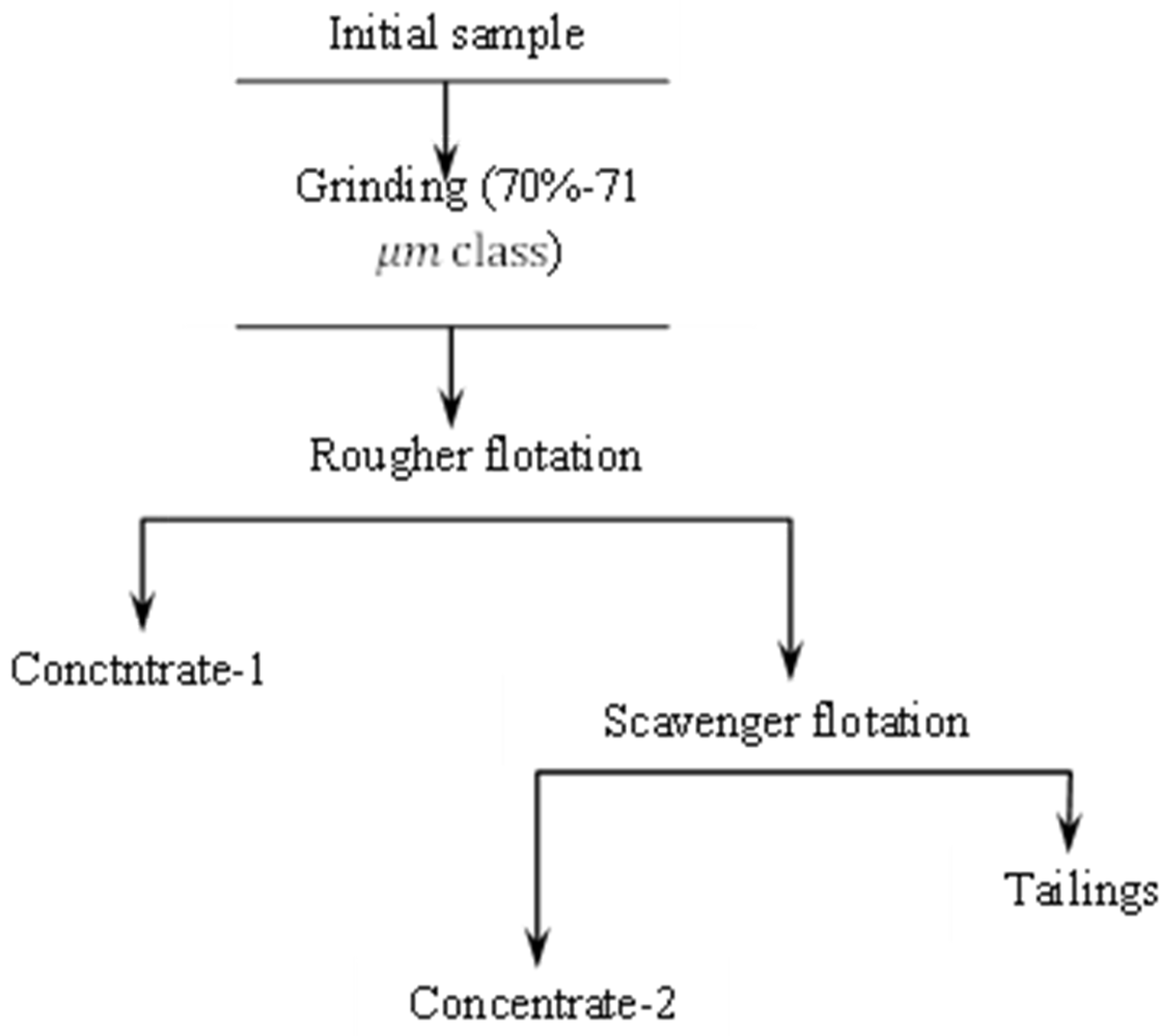
| Component | Cu | Pb | Zn | Fe | CaO | MgO | As |
|---|---|---|---|---|---|---|---|
| Mass Fraction, % | 0.02 | 0.90 | 0.70 | 6.42 | 1.33 | 3.35 | 0.023 |
| Component | SiO2 | Al2O3 | Au, g/ton | Au, g/ton | Na | S total | K |
| Mass Fraction, % | 55.23 | 26.34 | 0.51 | 6.1 | 0.48 | 0.17 | 3.1 |
| Mineral/Phase Group | Content (%) |
|---|---|
| Sulfide minerals (total): | 0.37 |
| Galena (PbS) | 0.16 |
| Sphalerite (ZnS) | 0.12 |
| Copper sulfide minerals (e.g., Cu2S) | 0.04 |
| Pyrite (FeS2) | 0.05 |
| Oxidized phases (total): | 6.47 |
| Oxidized and residual lead compounds | 0.89 |
| Oxidized and insoluble zinc compounds | 1.07 |
| Iron oxides (goethite, hematite) | 4.51 |
| Silicate minerals (total): | 84.39 |
| Mica group minerals | 53.44 |
| Quartz (SiO2) | 30.95 |
| Other phases (calcite, etc.) | 8.77 |
| Total | 100.0 |
| Particle Size Class, mm | Yield, % | Content, % | Recovery, % | ||
|---|---|---|---|---|---|
| Zn | Pb | Zn | Pb | ||
| −2 + 1 | 39.78 | 0.58 | 0.65 | 32.67 | 28.59 |
| −1 + 0.5 | 18.48 | 1.11 | 0.77 | 29.37 | 15.76 |
| −0.5 + 0.2 | 14.73 | 0.49 | 0.50 | 10.29 | 8.23 |
| −0.2 + 0.1 | 6.45 | 0.67 | 0.88 | 6.17 | 6.32 |
| −0.1 + 0.071 | 1.99 | 1.10 | 1.80 | 3.12 | 3.99 |
| −0.071 + 0.045 | 1.79 | 1.49 | 2.68 | 3.81 | 5.34 |
| −0.045 + 0 | 16.78 | 0.61 | 1.70 | 14.57 | 31.77 |
| The initial sample | 100.0 | 0.70 | 0.90 | 100.0 | 100.0 |
| Parameter | Content |
|---|---|
| Sodium and potassium, meq/L | 4.80 |
| Calcium, meq/L | 6.40 |
| Magnesium, meq/L | 3.40 |
| Chloride ion, meq/L | 0.50 |
| Sulfate ion, meq/L | 8.81 |
| Bicarbonate ion, meq/L | 5.20 |
| Nitrate ion, meq/L | 0.09 |
| Total hardness, °dH | 9.80 |
| Carbonate hardness, °dH | 5.20 |
| pH, units | 7.50 |
| Operation | Time (min) | Reagent Consumption (g/t) | |||
|---|---|---|---|---|---|
| Sodium Bisulfide | Thiocarbamate-Based Collector | Sodium Butyl Xanthate | Methyl Isobutyl Ketone (MIBK) | ||
| Total: | 0–900 | 5 | 165 | 10 | |
| Grinding, −0.071 mm, 70% | 10 | - | - | - | - |
| Rougher flotation | 10 | 0–900 | 5 | 120 | 5 |
| Scavenger flotation | 7 | - | - | 45 | 5 |
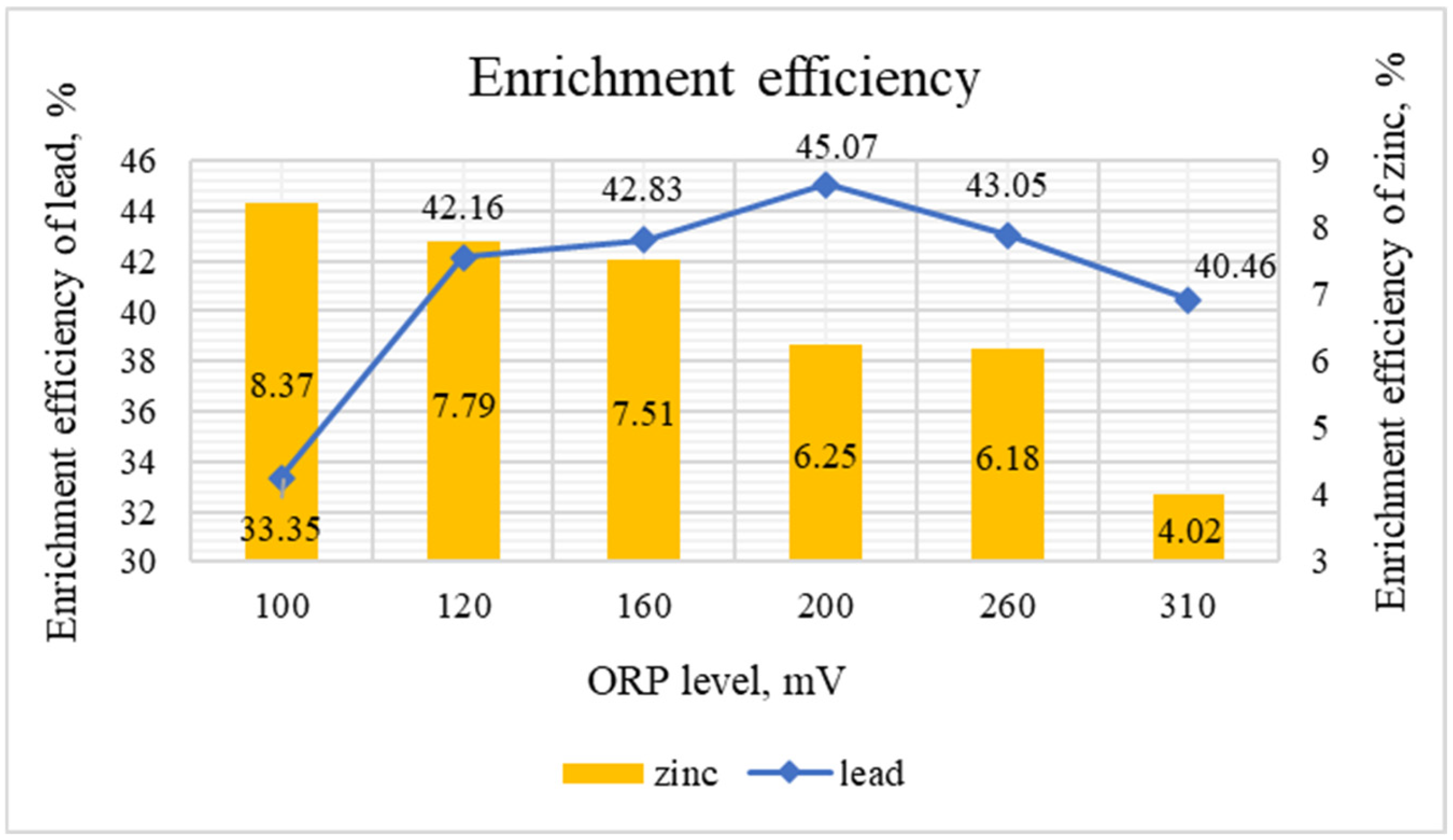
| Consumption of Na2S (g/t) and Redox Potential (mV) | Product | Yield, % | Content, % | Recovery, % | ||
|---|---|---|---|---|---|---|
| Zn | Pb | Zn | Pb | |||
| Test 1 | ||||||
| 0 (100) | Concentrate-1 | 3.45 | 1.92 | 7.43 | 9.46 | 28.47 |
| Concentrate-2 | 0.98 | 2.34 | 8.28 | 3.28 | 9.02 | |
| Σ Concentrate | 4.43 | 2.01 | 7.62 | 12.74 | 37.48 | |
| Tailings | 95.57 | 0.64 | 0.59 | 87.26 | 62.52 | |
| Total | 100.0 | 0.70 | 0.90 | 100.0 | 100.0 | |
| Test 2 | ||||||
| 500 (−120) | Concentrate-1 | 3.76 | 1.74 | 9.48 | 9.34 | 39.60 |
| Concentrate-2 | 1.09 | 2.08 | 5.81 | 3.24 | 7.03 | |
| Σ Concentrate | 4.85 | 1.82 | 8.65 | 12.58 | 46.63 | |
| Tailings | 95.15 | 0.64 | 0.51 | 87.42 | 53.37 | |
| Total | 100.0 | 0.70 | 0.90 | 100.0 | 100.0 | |
| Test 3 | ||||||
| 600 (−160) | Concentrate-1 | 4.02 | 1.63 | 9.13 | 9.36 | 40.76 |
| Concentrate-2 | 1.19 | 1.95 | 5.21 | 3.31 | 6.89 | |
| Σ Concentrate | 5.21 | 1.70 | 8.23 | 12.67 | 47.65 | |
| Tailings | 94.79 | 0.64 | 0.50 | 87.33 | 52.35 | |
| Total | 100.0 | 0.70 | 0.90 | 100.0 | 100.0 | |
| Test 4 | ||||||
| 700 (−200) | Concentrate-1 | 4.12 | 1.39 | 9.18 | 8.18 | 42.03 |
| Concentrate-2 | 1.28 | 1.87 | 5.65 | 3.42 | 8.03 | |
| Σ Concentrate | 5.40 | 1.50 | 8.34 | 11.60 | 50.07 | |
| Tailings | 94.60 | 0.65 | 0.48 | 88.40 | 49.93 | |
| Total | 100.0 | 0.70 | 0.90 | 100.0 | 100.0 | |
| Test 5 | ||||||
| 800 (−260) | Concentrate-1 | 4.08 | 1.42 | 8.86 | 8.28 | 40.18 |
| Concentrate-2 | 1.30 | 1.75 | 5.44 | 3.24 | 7.86 | |
| Σ Concentrate | 5.38 | 1.50 | 8.03 | 11.52 | 48.04 | |
| Tailings | 94.62 | 0.66 | 0.49 | 88.48 | 51.96 | |
| Total | 100.0 | 0.70 | 0.90 | 100.0 | 100.0 | |
| Test 6 | ||||||
| 900 (−310) | Concentrate-1 | 5.42 | 0.97 | 6.73 | 7.49 | 40.52 |
| Concentrate-2 | 1.06 | 1.97 | 5.15 | 2.98 | 6.06 | |
| Σ Concentrate | 6.48 | 1.13 | 6.47 | 10.47 | 46.58 | |
| Tailings | 93.52 | 0.67 | 0.51 | 89.53 | 53.42 | |
| Total | 100.0 | 0.70 | 0.90 | 100.0 | 100.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mambetaliyeva, A.; Tussupbekova, T.; Sabirova, L.; Makasheva, G.; Rysbekov, K.; Barmenshinova, M. Study of Oxidative–Reductive Potential Changes in the Enrichment of Oxidized Polymetallic Ores. Appl. Sci. 2025, 15, 6091. https://doi.org/10.3390/app15116091
Mambetaliyeva A, Tussupbekova T, Sabirova L, Makasheva G, Rysbekov K, Barmenshinova M. Study of Oxidative–Reductive Potential Changes in the Enrichment of Oxidized Polymetallic Ores. Applied Sciences. 2025; 15(11):6091. https://doi.org/10.3390/app15116091
Chicago/Turabian StyleMambetaliyeva, Alima, Tansholpan Tussupbekova, Leyla Sabirova, Guldana Makasheva, Kanay Rysbekov, and Madina Barmenshinova. 2025. "Study of Oxidative–Reductive Potential Changes in the Enrichment of Oxidized Polymetallic Ores" Applied Sciences 15, no. 11: 6091. https://doi.org/10.3390/app15116091
APA StyleMambetaliyeva, A., Tussupbekova, T., Sabirova, L., Makasheva, G., Rysbekov, K., & Barmenshinova, M. (2025). Study of Oxidative–Reductive Potential Changes in the Enrichment of Oxidized Polymetallic Ores. Applied Sciences, 15(11), 6091. https://doi.org/10.3390/app15116091








